-
Approximately 1.5 million HIV-infected women become pregnant every year in the world[1]. Additionally, 1.8 million new infections reported globally in 2016, of which 160,000 were children younger than 15 years who infected through mother-to-child transmission (MTCT)[2]. The number of people living with HIV is increasing in developing countries, where this infection predominantly affects younger people who desire to bear children[3]. Data from the HIV/AIDS Comprehensive Response Information Management System in China demonstrate a rapid increase in the population of female patients aged 15-49 years in Guangdong Province[4]. The largest number of HIV-infected pregnant women resides in Guangzhou, the capital city of Guangdong Province.
In the 1994 ACTG 076 trial demonstrated that administration of the single antiretroviral drug zidovudine could reduce perinatal HIV transmission in infants by nearly 70%[5]. Accordingly, World Health Organization (WHO) began to recognize the importance of using antiretroviral therapy (ART) during pregnancy to block HIV perinatal transmission. Programs intended to prevent perinatal HIV transmission subsequently updated the maternal ART regimens every few years. Compared with mono-therapy, a 3-drug ART more effectively reduced the vertical transmission of HIV[6,7] without readily triggering the emergence of viral resistance[8], which was more conducive to maternal health. Thus, mono-therapy has been classified as alternative rather than first-line regimen[9]. WHO updated the indications and programs for ART administration to pregnant women in 2010, 2013, and 2015[10-12].
The increasing availability of combination antiretroviral therapy (cART) has significantly reduced the number of new pediatric HIV infections and improved both maternal and infant survival[13]. Accordingly, such regimens represent one of the most successful public health responses to HIV. Still, the success of these programs has led to new problems regarding the potential adverse effects of fetal exposure to antiretroviral drugs and HIV[14,15]. Although the association between untreated advanced AIDS and adverse pregnancy outcomes is well documented[16], some studies[17-22] also suggest the risks of ART during pregnancy, including possible increases in the rates of preterm delivery, low birth weight and other adverse pregnancy outcomes. However, other studies found no significant differences in associations of ART regimens with serious adverse fetal outcomes[23,24]. In other words, the safety evidence is insufficient and conflicting, and the findings vary by the class of antiretroviral agents, which exhibit different placental and pharmaco-kinetic transference characteristics[25].
Until recently, birth data of ART-exposed pregnancies were mostly derived from studies conducted in developed countries and African populations. In addition, most studies investigated antiretroviral agents not widely used in low-and middle-income countries, and many focused only on pregnancy outcomes but did not monitor the effects of these drugs on early infant growth. It is critical to monitor the rates of adverse outcomes among HIV-infected pregnant women receiving various ART regimens to determine whether adverse outcomes differ among regimen types[15]. Therefore, we examined the effects of in utero HIV and ART exposure on adverse birth outcomes and early infant growth (4 weeks postpartum) in Guangzhou, China.
-
This prospective cohort study was conducted within the Integrated Prevention of MTCT (IPMTCT) program, a national system of HIV, syphilis and hepatitis B testing, treatment and surveillance that includes all pregnant women in China. This system covers all medical institutions, and all pregnant women are offered free opt-out HIV testing during their first prenatal visit in Guangzhou. If a patient is diagnosed with HIV, more detailed information is retrieved from the system and double-checked. Pregnant women living with HIV are offered free ART and follow-up care until delivery.
Infants born to HIV-infected mothers are subjected to high-risk neonatal management and are followed by pediatricians at 1, 3, 6, 9, 12, and 18 months of age. These infants are provided with free antiretroviral prophylaxis, HIV testing during early infancy, HIV-antibody testing and physical examinations involving standard procedures to establish the infection status. Infants undergo early-infant HIV testing and a physical examination by pediatricians at 1 and 3 months of age, respectively.
The present study included all HIV-infected pregnant women reported to the IPMTCT system in Guangzhou between October 2009 and May 2018. HIV-infected pregnant women who elected to terminate their pregnancy were excluded. Any adverse pregnancy outcomes of women with singleton pregnancy were analyzed.
The study was conducted in accordance with the China IPMTCT guidelines. All study protocols were approved by the Center for National Women’s and Children’s Health, China CDC. Informed consent was obtained from all participating mothers.
-
The treatment regimen for the prevention of MTCT of HIV in Guangzhou was updated three times. By 2015, all HIV-positive pregnant women began receiving zidovudine-based cART (zidovudine, lamivudine and lopinavir/ritonavir) or efavirenz- based cART (tenofovir, lamivudine and efavirenz). Before 2015, two regimens were provided: a preventive antiviral regimen and a therapeutic antiviral regimen according to the maternal condition. The specific medication plans are listed in Table 1. After ART initiation, clinical follow-ups were conducted through an integrated primary care service that provided antenatal and HIV care.
Table 1. Description of the three maternal medication plans
Year Prophylaxis regimen Treatment regimen Regimen / medication period Regimen Eligibility Regimen Eligibility From Feb. 2011, to Aug. 2011 Recommended regimen From gestational week 28 to labor: AZT 300 mg bid At labor: immediately AZT 300 mg + NVP 200 mg + 3TC 150 mg oral, and then AZT 300 mg every 3 hours, 3TC 150 mg every 12 hours Postpartum: AZT 300 mg bid + 3TC 150 mg bid for 1 week No indication for ART, and did not receive ART previously AZT + 3TC +
NVP/EFVViral load > 1,000 copies/mL Minimum-level plan NVP 200 mg post-labor, once From Sep. 2011 to June 2015 Pregnancy (≥ 14 w) and labor AZT 300 mg + 3TC 150 mg + LPV/r 400/100 mg, bid or AZT 300 mg + 3TC 150 mg, bid, +EFV 600 mg, qd CD4 cell count
> 350 cells/mL and/or WHO stage I/II diseaseAZT 300 mg + 3TC 150 mg, bid, + EFV 600 mg, qd, as early as possible CD4 cell count ≤ 350 cells/mL and/or WHO stage III/IV disease Postpartum 1. Artificial feeding, stop ART after delivery 2. Breastfeeding, continue ART till 1 week after stopping breastfeeding Labor A single dose of NVP 200 mg, and AZT 300 mg + 3TC 150 mg, bid, until the end of delivery Pregnant women with confirmed HIV infection during labor who fed their babies artificially AZT 300 mg + 3TC 150 mg + NVP 200 mg, bid, as early as possible CD4 cell count < 250 cells/mL and/or WHO stage III/IV disease Postpartum AZT 300 mg + 3TC 150 mg, bid, for 7 days Option 1 AZT + 3TC + LPV/r or AZT + 3TC + EFV (usage and dosage as listed above), 1 week after breastfeeding cessation Pregnant women with confirmed with HIV infection during labor who breastfed their babies Option 2 A single dose of NVP 200 mg, and AZT 300 mg + 3TC 150 mg, bid, until the end of delivery; Continued AZT 300 mg + 3TC 150 mg, bid, post-labor for 7 days. After July 2015 AZT 300 mg bid + 3TC 300 mg qd + LPV/r 400/1,000 mg bid Pregnant women who did not receive ART during pregnancy or labor, regardless of CD4 level and stage; start ART immediately. TDF 300 mg qd + 3TC 300 mg qd + EFV 600 mg qd 1. If virus suppression is ideal, retain the original regimen. 2. If virus suppression is not ideal, adjust the ART regimen. Pregnant women who have received ART before pregnancy; evaluate effect based on the viral load. Note. ART, antiretroviral therapy; AZT, Zidovudine; NVP, evirapine; 3TC, Lamivudine; EFV, efavirenz; LPV/r, Lopinavir/ritonavir; TDF, Tenofovir; qd, once-daily; bid, twice-daily. All HIV-exposed infants were prescribed once-daily nevirapine or twice-daily zidovudine within 6-12 hours after birth, which continued for 4-12 weeks depending on the MTCT risk assessment. All infants included in this study were fed artificially.
The study participants were categorized into three groups based on the medication regimens used by the pregnant women: (1) cART, a combination of three or more antiretroviral drugs; (2) ART comprising one or two antiviral drugs, referred to as ‘mono/dual ART’ or (3) no treatment.
-
The outcomes of interest were the composite adverse pregnancy outcomes, including at least one of the following: (1) preterm birth, defined as a live birth before a gestational age of 37 weeks; (2) small for gestational age (SGA), defined as a live infant birthweight of less than the 10th percentile by gestational age and sex (based on the Intergrowth-21st Project Standards[26]); (3) congenital anomaly detected by the nurse midwife during neonatal examination or structural screening via B-ultrasound; (4) stillbirth, defined as fetal death occurring before/during labor (based on a 1-min Apgar score of 0); (5) spontaneous abortion, defined as a spontaneous pregnancy loss at or before 20 weeks of gestation and (6) ectopic pregnancy.
The following main outcomes were used to evaluate the adverse effects of in utero HIV and ART exposure on early infants: (1) infant mortality, defined as the death of live-born infant within 1 year old. (2) MTCT of HIV: as per the China IPMTCT guideline, HIV-exposed infants are diagnosed via virological testing at 4 and 12 weeks of age. A positive virological test indicates that the infant is HIV-infected, whereas a negative test indicates the need for repeated serological HIV antibody testing at 12 and 18 months of age. If both subsequent HIV antibody tests are positive, the child is considered HIV-infected. (3) Stunting, defined as a length-for-age Z score (LAZ) by sex of less than -2. (4) Underweight, defined as a weight-for-age Z score (WAZ) by sex of less than -2. (5) Wasting, defined as a weight-for-length Z score (WLZ) by sex of less than -2. We calculated the infant LAZ, WAZ, and WLZ based on Fenton[27] and WHO[28] growth reference standards, using a corrected age for infants born prior to 37 completed weeks of gestation.
-
The socio-demographic and clinical characteristics of HIV-positive mothers, including age, parity, educational level, occupation, marital status, household registration, maternal CD4 count and HIV viral load during the third trimester, mode of delivery and ART regimens, were collected. Information about their infants, including birthdate, gestational age, sex, birthweight, birthlength, birth defects, neonatal diseases, antiretroviral prophylaxis, early infant diagnosis and HIV antibody testing, weights and heights at all follow-up time-points were recorded using a national standardized format with assistance from medical professionals. All maternal CD4 counts and HIV viral loads during the third trimester were tested at Guangzhou No. 8 People’s Hospital, a single specialized infectious disease hospital in Guangdong Province.
The accuracy of all case cards was evaluated. The gestational age at birth was expressed as completed weeks and based on the first- or second-trimester ultrasound test. In the absence of a recorded ultrasound result, the last menstrual period was used to calculate gestational age. The infant birthlength and birthweight were abstracted from clinical records. Trained nurses used a firm recumbent stadiometer to measure infant lengths to the nearest 0.1 cm and weights to the nearest 10 grams at all subsequent study visits.
-
A descriptive analysis was used to describe the socio-demographic information of the study participants. The chi-square test was used to describe and compare categorical socio-demographic data and clinical characteristics between different groups of participants receiving different treatment regimens. The t-test, analysis of variance and non-parametric test were used to compare continuous data and characteristics among different groups according to the results of normal distribution tests. The risks of adverse pregnancy outcomes and early infant growth outcomes associated with the treatment regimens were estimated using a logistic regression adjusted for the mother’s education level (primary or no education, junior high school, senior high school and higher, missing or unknown), ethnicity (Han, Others), parity (primiparous, parous), residence registration (Guangdong Province, other province/country), gestational age (weeks) at the first antenatal care visit and number of antenatal care visits. The mode of delivery (spontaneous labor, elective caesarean section, emergency caesarean section), gestational age at delivery (days), birthweight (grams) and infant antiretroviral prophylaxis (nevirapine, zidovudine, unmedicated) were also included as covariates when analyzing the risks of early infant growth outcomes. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA) and the Statistical Package for Social Sciences version 20 software package for Windows (SPSS Inc, Chicago, IL, USA). All reported P
values are based on a two-sided test with a significance level of α = 0.05. -
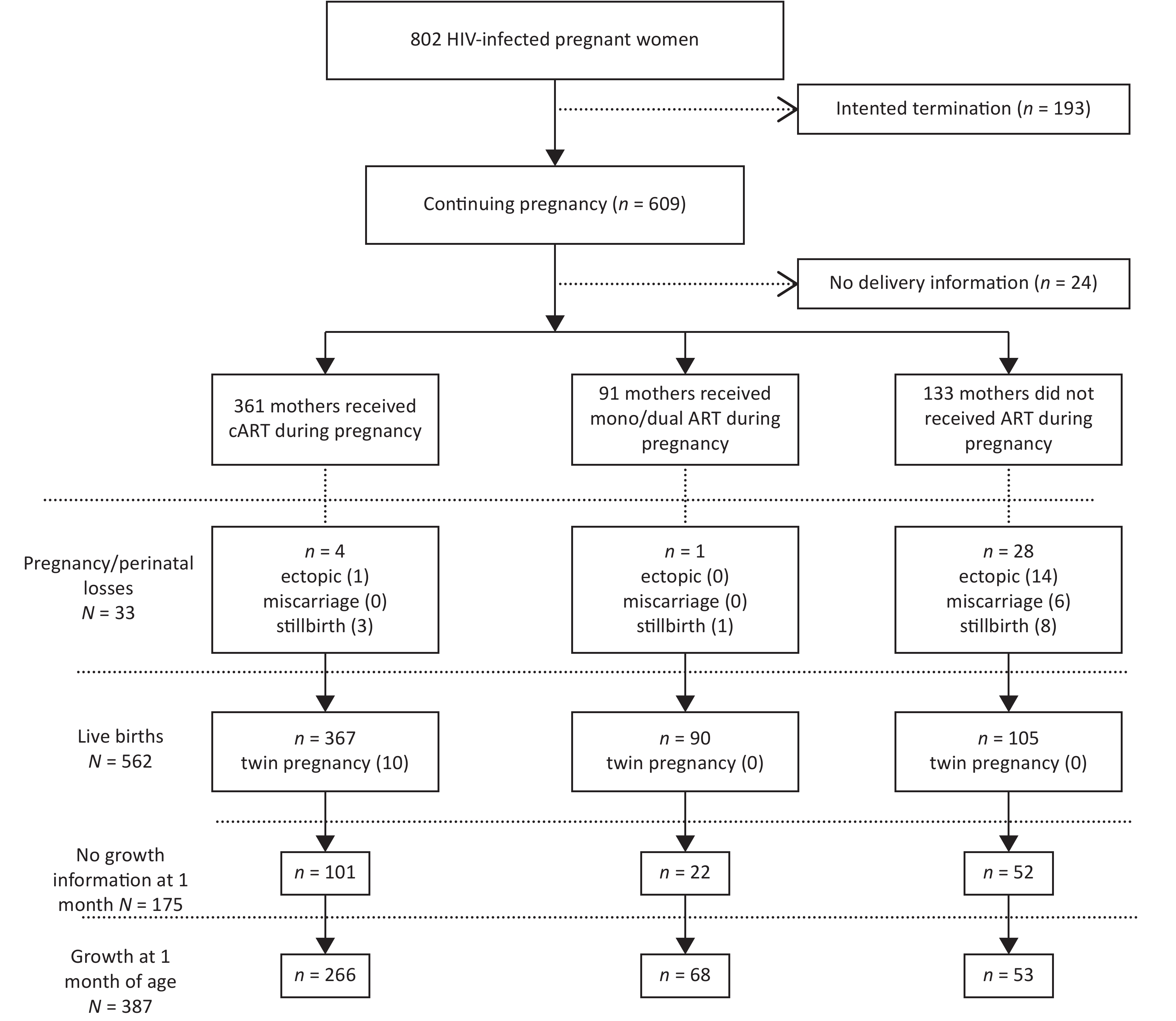
From October 2009 to May 2018, 802 pregnant women with HIV were recruited in this study. Of these women, 361 received cART during pregnancy, 91 received mono/dual ART and 133 did not receive ART. Of the 562 total live births, 6 infants were infected with HIV via MTCT (Figure 1).
No significant differences in the maternal age, marital status and newborn’s sex were observed between the treatment regimens (Table 2). However, pregnant women who had not received ART were more likely to exhibit the following characteristics: non-Han ethnicity, lower education level, residence in another province/country, multiparous, first antenatal care at a later date, less antenatal care, a lower CD4 count during pregnancy, lower rate of selective cesarean section, later delivery, heavier birthweight of her baby and reduced likelihood of infant antiretroviral prophylaxis. HIV pregnant women receiving cART and those with mono/dual drug therapy began ART at similar gestational ages and had similar durations of ART during pregnancy.
Table 2. Maternal, pregnancy and delivery characteristics stratified by antiretroviral regimen during pregnancy
Characteristics cART (N = 361) Mono/dual ART (N = 91) No treatmen (N = 133) P-value Mother’s age in years (mean ± SD) 29.8 ± 5.0 29.0 ± 5.1 28.6 ± 6.0 0.07 Ethnicity, n (%) < 0.01 Han 329 (91.1) 84 (92.3) 103 (77.4) Others 32 (8.9) 7 (7.7) 30 (22.6) Mother’s education, n (%) < 0.01 Primary or no education 36 (10.0) 14 (15.4) 27 (20.3) Junior high school 179 (49.6) 42 (46.2) 57 (42.9) High school and higher 121 (33.5) 26 (28.6) 30 (22.6) Missing or unknown 25 (6.9) 9 (9.9) 19 (14.3) Mother’s occupation, n (%) 0.03 Housewife or none 190 (52.6) 48 (52.7) 66 (49.6) Physical work 106 (29.4) 33 (36.3) 36 (27.1) Office work 20 (5.5) 6 (6.6) 4 (3.0) Missing or unknown 45 (12.5) 4 (4.4) 27 (20.3) Marital status, n (%) 0.10 Single 57 (15.8) 15 (16.5) 32 (24.1) Married 304 (84.2) 76 (83.5) 101 (75.9) Household registration, n (%) 0.02 Guangdong Province 191 (52.9) 44 (48.4) 52 (39.1) Other province/country 170 (47.1) 47 (51.6) 81 (60.9) Parity, n (%) < 0.01 Primiparous 283 (78.4) 74 (81.3) 84 (63.2) Parous 78 (21.6) 17 (18.7) 49 (36.8) Gestational week at the first antenatal care in weeks
(mean ± SD)19.0 ± 9.1 21.3 ± 10.2 26.6 ± 12.8 < 0.01* Gestational age starting ART in weeks (mean ± SD) 20.7 ± 9.7 21.6 ± 10.9 − 0.45 Duration of ART during pregnancy in weeks (mean ± SD) 16.7 ± 9.9 15.9 ± 10.4 − 0.48 Number of antenatal care visits (mean ± SD) 4.7 ± 2.7 3.8 ± 3.0 0.5 ± 1.2 < 0.01* Last CD4 count (cells/μL) during pregnancy, n (%) < 0.01 < 350 84 (23.3) 19 (20.9) 11 (8.3) ≥ 350 135 (37.4) 30 (33.0) 5 (3.8) Missing or unknown 142 (39.3) 42 (46.2) 117 (88.0) Mode of delivery, n (%) < 0.01 Spontaneous labor 39 (10.8) 11 (12.1) 63 (56.8) Elective Caesarean section 265 (73.6) 64 (70.3) 13 (11.7) Emergency Caesarean section 56 (15.6) 16 (17.6) 35 (31.5) Gestational age at delivery in days (mean ± SD) 265.3 ± 10.4 265.3 ± 14.0 269.2 ± 20.8 < 0.01* Birth weight in grams (mean ± SD) 2777.3 ± 449.0 2737.4 ± 432.5 3027.2 ± 521.6 < 0.01 Newborn’s sex, n (%) 0.29 Male 83 (56.1) 41 (45.6) 54 (51.4) Female 65 (43.9) 49 (54.4) 51 (48.6) Infant antiretroviral prophylaxis, n (%) < 0.01 Nevirapine 113 (76.9) 62 (68.9) 18 (17.1) Zidovudine 33 (22.4) 21 (23.3) 26 (24.8) Unmedicated 1 (0.7) 7 (7.8) 61 (58.1) Note. Mean ± SD = mean ± standard deviation; *Non-parametric test was used. Overall, 202 (35.1%) of all HIV-infected women experienced an adverse pregnancy outcome, and 121 (31.3%) of all infants included in this analysis exhibited adverse effects on early growth at 4 weeks of age. The rates of adverse pregnancy outcomes, spontaneous abortion, ectopic pregnancy, stillbirth, infant death and perinatal HIV infection were higher among women not receiving ART, compared to those receiving cART or mono/dual ART (P < 0.05). However, the rate of SGA was higher among women receiving cART, compared to those not receiving treatment (P < 0.05) (Table 3). Meanwhile, no significant differences in congenital anomalies, preterm birth, adverse early growth, stunting, underweight and wasting at 1 month of age were observed among the three treatment groups.
Table 3. Comparison of adverse pregnancy outcomes and adverse early growth effects stratified by antiretroviral regimen during pregnancy
Outcomes cART (n = 361) Mono/dual AR (n = 91) No treatment (n = 133) P-value Adverse pregnancy outcomes, n (%) 112 (31.9) 31 (34.1) 59 (44.4) 0.04 Spontaneous abortion 0 0 6 (4.5) − Ectopic pregnancy 1 (0.3) 0 14 (10.5) − Stillbirth 3 (0.9) 1 (1.1) 8 (7.1) < 0.01 Congenital anomaly 2 (0.6) 2 (2.2) 2 (1.8) 0.33 Preterm birth 44 (12.5) 15 (16.5) 23 (20.5) 0.33 SGA 77 (21.9) 17 (18.7) 14 (10.5) 0.02 Adverse early growth outcomes, n (%) 87 (32.7) 17 (25.0) 17 (32.1) 0.47 Infant death 1 (0.3) 0 3 (2.7) < 0.01 HIV infection via MTCT 1 (0.3) 0 5 (4.4) − Stunting 41 (15.4) 12 (17.1) 2 (3.9) 0.07 Underweight 34 (12.7) 6 (8.6) 5 (9.8) 0.57 Wasting 35 (13.1) 4 (5.7) 6 (11.8) 0.23 Note. SGA, small for gestational age; MTCT, mother-to-child transmission. The relationships among ART initiation timing of HIV-infected pregnant women and adverse outcomes suggested that 1-month-old infants of participants who started ART in the second or third trimester were more likely to be wasting than those of participants starting ART in the first trimester or delivery/postpartum (P < 0.05) (Table 4). No significant differences were observed in adverse pregnancy outcomes, infant death, HIV infection via MTCT, stunting and underweight at 1 month of age among the four groups of ART initiating timings.
Table 4. Comparison of adverse pregnancy outcomes and adverse early growth effects stratified byART initiation timing
Outcomes 1st Trimester
(n = 89)2nd Trimester
(n = 247)3rd Trimester
(n = 84)Delivery/postpartum
(n = 39)P-value Adverse pregnancy outcomes, n (%) 37 (41.6) 86 (34.8) 26 (31.0) 12 (30.8) 0.46 Ectopic pregnancy 1 (1.1) 0 0 0 0.28 Stillbirth 0 1 (0.4) 0 3 (7.7) 0.24 Congenital anomaly 1 (1.1) 2 (0.8) 0 1 (2.6) 0.47 Preterm birth 12 (13.6) 45 (18.2) 8 (9.5) 6 (16.2) 0.27 SGA 26 (29.5) 52 (21.1) 20 (23.8) 5 (13.5) 0.21 Adverse early growth outcomes, n (%) 17 (23.3) 67 (36.6) 16 (28.6) 3 (14.3) 0.05 Infant death 0 0 0 1 (2.6) 0.28 HIV infection via MTCT 0 0 0 1 (2.6) 0.28 Stunting 13 (17.8) 32 (17.1) 7 (12.5) 1 (5.0) 0.49 Underweight 10 (13.7) 22 (11.8) 7 (12.5) 1 (5.0) 0.82 Wasting 2 (2.7) 29 (15.5) 8 (14.3) 0 0.01 Note. SGA, small for gestational age; MTCT, mother-to-child transmission. In the unadjusted analyses and model 1, women who received cART had a significantly lower risk of any adverse pregnancy outcome [odds ratio (OR) = 0.59, 95% confidence interval (CI): 0.39-0.89; adjusted OR (aOR) = 0.53, 95% CI: 0.35-0.81] and higher risk of SGA (OR = 1.92, 95% CI: 1.04-3.56; aOR = 1.87, 95% CI: 1.00-3.51), compared to women not receiving treatment. After adjustment for pregnancy covariates, however, no differences in any adverse pregnancy outcomes or SGA were observed among women receiving different antiviral regimens Table 5). Neither the unadjusted nor adjusted analyses indicated differences in any adverse early growth outcomes of 4-week-old infants born to women receiving different antiviral regimens.
Table 5. Adverse pregnancy outcomes and adverse early infant growth effects at 4 weeks of age stratified by antiretroviral regimens during pregnancy
Items cART Mono/dual ART No treatment Any adverse pregnancy outcome Number of women, n (%) 112/351 (31.9%) 31/91 (34.1%) 59/133 (44.4%) Crude Model, OR (95% CI) 0.59 (0.39, 0.89) 0.65 (0.37, 1.13) 1 (ref) Model 1a, OR (95% CI) 0.53 (0.35, 0.81) 0.60 (0.34, 1.05) 1 (ref) Model 2b, OR (95% CI) 1.16 (0.64, 2.08) 1.28 (0.65, 2.51) 1 (ref) SGA Number of women, n (%) 77/351 (21.9) 17/91 (18.7) 14/133 (10.5) Crude Model, OR (95% CI) 1.92 (1.04, 3.56) 1.51 (0.70, 3.27) 1 (ref) Model 1a, OR (95% CI) 1.87 (1.00, 3.51) 1.47 (0.67, 3.22) 1 (ref) Model 2b, OR (95% CI) 2.10 (0.99, 4.44) 1.53 (0.64, 3.66) 1 (ref) Any adverse early growth Number of children, n (%) 87/266 (32.7%) 17/68 (25.0%) 17/53 (32.1%) Crude Model, OR (95% CI) 1.03 (0.55, 1.94) 0.71 (0.32, 1.57) 1 (ref) Model 1a, OR (95% CI) 1.07 (0.56, 2.03) 0.75 (0.33, 1.69) 1 (ref) Model 2b, OR (95% CI) 1.18 (0.55, 2.57) 0.81 (0.33, 2.00) 1 (ref) Model 3c, OR (95% CI) 1.10 (0.39, 3.11) 0.71 (0.23, 2.23) 1 (ref) Note. SGA, small for gestational age. aModel 1: covariates mother’s education, ethnicity, household registration, parity; bModel 2: covariates in model 1, and gestational week at the first antenatal care, number of antenatal care; cModel 3: covariates in model 2, and Mode of delivery, gestational age at delivery, birthweight and infant antiretroviral prophylaxis. -
This study contributes to the limited body of evidence regarding the effects of ART regimens on mother-infant pairs in China and the safety and effectiveness of ART regimens during pregnancy. Our findings suggest that the rates of spontaneous abortion, ectopic pregnancy, stillbirth, infant death and HIV infection of infants via MTCT were higher among women who did not receive ART, suggesting that a failure to provide ART to HIV-infected pregnant women may have more serious consequences. In addition, although we observed no significant effect of the treatment regimen type on adverse early infant growth, the rate of SGA was higher among women receiving cART, compared with other regimens.
The correlation between preterm birth and ART is often debated. Our study found a low rate of prematurity and no statistically significant relationship between preterm birth and the use of different ART regimens, consistent with previously published findings[29-31]. Although recent studies suggested that a protease inhibitor (PI)-based ART regimen may increase the risk of preterm birth[17,32], our further analysis did not confirm this association (The rates of preterm birth were 15.4%, 15.5%, 16.7%, and 16.2% for HIV-infected women receiving NNRTI-based cART, receiving PI-based cART, receiving mono/dual ART and receiving no treatment, respectively. Data was shown in Table 6. Some studies demonstrated fetal exposure to antiretroviral drugs increased the risks of preterm delivery and other adverse pregnancy outcomes[15,21]. Differences in the types of antiviral drugs, timing of ART initiation and maternal HIV disease stage prior to conception may have a stronger influence on the risk of preterm birth than the ART regimen type. In addition, the causes of preterm birth are multifactorial, and associated risk factors include the socioeconomic status, maternal body-mass index, maternal smoking, under-nutrition, environmental exposure and maternal infection[33].
Table 6. Association of preterm birth with NNRTI-based cART, PI-based cART, Mono/dual ARTand no treatment
Item Preterm birth Term birth P-value NNRTI-based cART 23 126 0.99 PI-based cART 34 186 mono/dual ART 15 75 No treatment 17 88 Few studies that investigated the effects of in utero exposure to ART and the occurrence of SGA births have yielded inconclusive results. Our study suggested that exposure to cART during pregnancy was associated with a higher risk of SGA when compared with no treatment. Erika Aaron found that women who received a non-nucleoside reverse-transciptase inhibitors-based regimen (NNRTI-based cART) were less likely to have a SGA neonate, compared to women receiving a PI-based regimen[34]. However, after adjusting for socio-demographic factors, medication and HIV disease severity, no association of ART regimen type was associated with SGA. Still, one study conducted in Botswana found an association of SGA with cART during pregnancy[30]. The ATHENA cohort suggested that the use of triple-drug ART, particularly a PI-based regimen, prior to conception was associated with SGA[35]. Other studies reported no evidence of an association between ART during pregnancy and SGA at delivery[31,36,37].
A systematic review reported inconclusive findings regarding the effects of in utero HIV and ART exposure on postnatal weight gain in HIV-exposed infants[38]. A smaller study conducted in China found no effect of maternal cART use during pregnancy on infant growth at 18 months of age[39], consistent with our findings. Still, other studies demonstrated that HIV-exposed infants exhibited poorer growth than their HIV-unexposed counterparts[40,41]. One study conducted in Botswana suggested that children exposed to cART in utero had significantly lower length-for-age and weight-for-age Z scores, compared to those exposed to zidovudine monotherapy[29]. Another study conducted in South Africa found no association between the duration of Tenofovir exposure in utero and the early linear growth of infants[42]. These inconsistent findings may be attributed to the use of different antiviral drugs by the mothers, the evaluation of infants in different age groups and differences in the feeding modes and nutritional statuses during early infancy.
Our study had several strengths, including the reported effects of in utero HIV and ART exposure on pregnancy outcomes and early infant growth at 4 weeks-age. Such research is rare in China, where a huge number of HIV-infected women have yet to receive ART in compliance with the implemented PMTCT. We comprehensively discussed the effects of three groups of mothers−those receiving no treatment, mono/dual ART or cART−to inform target populations and policy makers for advance the universal access.
However, our study also had some limitations. First, the study used an observational design, rather than a randomized controlled clinical trial design, to address whether the uses of different ART regimens would be associated with adverse pregnancy outcomes and adverse early infant growth. Some unmeasured confounders may have biased our results. We could not compare other outcomes such as the maternal viral load at the time of labor, maternal nutrition, maternal BMI and the induced or spontaneous status of preterm birth. However, several studies of pregnant HIV-infected women documented associations between poor nutrition, a low maternal BMI and advanced maternal HIV disease[43,44]. Second, few women with HIV underwent CD4 count analyses during the third trimester, as the new ART treatment guidelines do not require this test prior to ART initiation. However, this deficiency would randomly appear among the three groups. Third, growth information was not available for 175 of 562 newborns (31.1%), which may have biased the research results. Although 90% of infants received early HIV testing in recent years, the body length, weight and other indicators were not measured in many children because their guardians considered the physical growth of infants to be unimportant. Finally, this study focused only on the short-term effects of pregnancy outcomes and infant growth at 4 weeks’ postpartum and could not address the long-term effects of intrauterine exposure to ART.
In conclusion, the administration of ART to HIV-infected pregnant women will remain a global high-priority issue, as even mono/dual ART can improve pregnancy outcomes and infant survival in resource-limited countries. The reduction of serious adverse fetal and infant outcomes and the risk of MTCT are critical issues. Further studies are needed to monitor the short-and long-term health effects of HIV and ART exposure and thus optimize antiretroviral therapy during pregnancy.
-
We thank all pregnant women and their children for their participation in the study. We are grateful to the obstetric and pediatric care providers who have assisted the implementation of the IPMTCT program.
-
LIN Sui Fang and MA Ying Hua conceptualized and designed the study; HU Fang carried out the whole program, performed the initial analyses, drafted the initial manuscript; LIANG Jing Jing interpreted the data, critically reviewed the manuscript; LU Jian Jun coordinated and supervised data collection; HU Yi Fei critically reviewed the manuscript; HU Yan coordinated and supervised data collection; YU Jia coordinated and supervised data collection; ZOU Xing Wen coordinated and supervised data collection.
doi: 10.3967/bes2019.092
Effects of Antiretroviral Therapy and HIV Exposure in Utero on Adverse Pregnancy and Infant Outcomes: A Prospective Cohort Study in Guangzhou, China
-
Abstract:
Objective This study aimed to evaluate the effects of in-utero exposure to HIV and ART on pregnancy outcome and early growth of children. Methods This cohort study enrolled 802 HIV-infected pregnant women between October 2009 and May 2018 in Guangzhou, China. The women were assigned to receive combination ART (cART) or mono/dual ART or no treatment. The primary outcomes were the combined endpoints of any adverse pregnancy outcome [including ectopic pregnancy, spontaneous abortion, stillbirth, preterm birth, small for gestational age (SGA)] and adverse early growth outcome (including infant death, HIV infection of mother-to-child transmission, and underweight, wasting and stunting of infants at 4 weeks of age). Results Adverse pregnancy outcomes occurred in 202 (35.1%) of all enrolled HIV-infected women, and 121 (31.3%) of all infants exhibited adverse effects on early growth at 4 weeks of age. The rates of adverse pregnancy outcomes, spontaneous abortion, ectopic pregnancy, stillbirth, infant death and perinatal HIV infection were higher among women not receiving ART, compared to those treated with cART or mono/dual ART (P < 0.05). However, women treated with cART had a higher rate of SGA, compared to untreated women (P < 0.05). No differences in early infant growth were observed among the different treatment regimens. Conclusion Our findings underscore the essentiality of prioritizing HIV-positive pregnant women for ART, as even mono/dual ART available in resource-limited countries could improve pregnancy outcomes and infant survival. -
Table 1. Description of the three maternal medication plans
Year Prophylaxis regimen Treatment regimen Regimen / medication period Regimen Eligibility Regimen Eligibility From Feb. 2011, to Aug. 2011 Recommended regimen From gestational week 28 to labor: AZT 300 mg bid At labor: immediately AZT 300 mg + NVP 200 mg + 3TC 150 mg oral, and then AZT 300 mg every 3 hours, 3TC 150 mg every 12 hours Postpartum: AZT 300 mg bid + 3TC 150 mg bid for 1 week No indication for ART, and did not receive ART previously AZT + 3TC +
NVP/EFVViral load > 1,000 copies/mL Minimum-level plan NVP 200 mg post-labor, once From Sep. 2011 to June 2015 Pregnancy (≥ 14 w) and labor AZT 300 mg + 3TC 150 mg + LPV/r 400/100 mg, bid or AZT 300 mg + 3TC 150 mg, bid, +EFV 600 mg, qd CD4 cell count
> 350 cells/mL and/or WHO stage I/II diseaseAZT 300 mg + 3TC 150 mg, bid, + EFV 600 mg, qd, as early as possible CD4 cell count ≤ 350 cells/mL and/or WHO stage III/IV disease Postpartum 1. Artificial feeding, stop ART after delivery 2. Breastfeeding, continue ART till 1 week after stopping breastfeeding Labor A single dose of NVP 200 mg, and AZT 300 mg + 3TC 150 mg, bid, until the end of delivery Pregnant women with confirmed HIV infection during labor who fed their babies artificially AZT 300 mg + 3TC 150 mg + NVP 200 mg, bid, as early as possible CD4 cell count < 250 cells/mL and/or WHO stage III/IV disease Postpartum AZT 300 mg + 3TC 150 mg, bid, for 7 days Option 1 AZT + 3TC + LPV/r or AZT + 3TC + EFV (usage and dosage as listed above), 1 week after breastfeeding cessation Pregnant women with confirmed with HIV infection during labor who breastfed their babies Option 2 A single dose of NVP 200 mg, and AZT 300 mg + 3TC 150 mg, bid, until the end of delivery; Continued AZT 300 mg + 3TC 150 mg, bid, post-labor for 7 days. After July 2015 AZT 300 mg bid + 3TC 300 mg qd + LPV/r 400/1,000 mg bid Pregnant women who did not receive ART during pregnancy or labor, regardless of CD4 level and stage; start ART immediately. TDF 300 mg qd + 3TC 300 mg qd + EFV 600 mg qd 1. If virus suppression is ideal, retain the original regimen. 2. If virus suppression is not ideal, adjust the ART regimen. Pregnant women who have received ART before pregnancy; evaluate effect based on the viral load. Note. ART, antiretroviral therapy; AZT, Zidovudine; NVP, evirapine; 3TC, Lamivudine; EFV, efavirenz; LPV/r, Lopinavir/ritonavir; TDF, Tenofovir; qd, once-daily; bid, twice-daily. Table 2. Maternal, pregnancy and delivery characteristics stratified by antiretroviral regimen during pregnancy
Characteristics cART (N = 361) Mono/dual ART (N = 91) No treatmen (N = 133) P-value Mother’s age in years (mean ± SD) 29.8 ± 5.0 29.0 ± 5.1 28.6 ± 6.0 0.07 Ethnicity, n (%) < 0.01 Han 329 (91.1) 84 (92.3) 103 (77.4) Others 32 (8.9) 7 (7.7) 30 (22.6) Mother’s education, n (%) < 0.01 Primary or no education 36 (10.0) 14 (15.4) 27 (20.3) Junior high school 179 (49.6) 42 (46.2) 57 (42.9) High school and higher 121 (33.5) 26 (28.6) 30 (22.6) Missing or unknown 25 (6.9) 9 (9.9) 19 (14.3) Mother’s occupation, n (%) 0.03 Housewife or none 190 (52.6) 48 (52.7) 66 (49.6) Physical work 106 (29.4) 33 (36.3) 36 (27.1) Office work 20 (5.5) 6 (6.6) 4 (3.0) Missing or unknown 45 (12.5) 4 (4.4) 27 (20.3) Marital status, n (%) 0.10 Single 57 (15.8) 15 (16.5) 32 (24.1) Married 304 (84.2) 76 (83.5) 101 (75.9) Household registration, n (%) 0.02 Guangdong Province 191 (52.9) 44 (48.4) 52 (39.1) Other province/country 170 (47.1) 47 (51.6) 81 (60.9) Parity, n (%) < 0.01 Primiparous 283 (78.4) 74 (81.3) 84 (63.2) Parous 78 (21.6) 17 (18.7) 49 (36.8) Gestational week at the first antenatal care in weeks
(mean ± SD)19.0 ± 9.1 21.3 ± 10.2 26.6 ± 12.8 < 0.01* Gestational age starting ART in weeks (mean ± SD) 20.7 ± 9.7 21.6 ± 10.9 − 0.45 Duration of ART during pregnancy in weeks (mean ± SD) 16.7 ± 9.9 15.9 ± 10.4 − 0.48 Number of antenatal care visits (mean ± SD) 4.7 ± 2.7 3.8 ± 3.0 0.5 ± 1.2 < 0.01* Last CD4 count (cells/μL) during pregnancy, n (%) < 0.01 < 350 84 (23.3) 19 (20.9) 11 (8.3) ≥ 350 135 (37.4) 30 (33.0) 5 (3.8) Missing or unknown 142 (39.3) 42 (46.2) 117 (88.0) Mode of delivery, n (%) < 0.01 Spontaneous labor 39 (10.8) 11 (12.1) 63 (56.8) Elective Caesarean section 265 (73.6) 64 (70.3) 13 (11.7) Emergency Caesarean section 56 (15.6) 16 (17.6) 35 (31.5) Gestational age at delivery in days (mean ± SD) 265.3 ± 10.4 265.3 ± 14.0 269.2 ± 20.8 < 0.01* Birth weight in grams (mean ± SD) 2777.3 ± 449.0 2737.4 ± 432.5 3027.2 ± 521.6 < 0.01 Newborn’s sex, n (%) 0.29 Male 83 (56.1) 41 (45.6) 54 (51.4) Female 65 (43.9) 49 (54.4) 51 (48.6) Infant antiretroviral prophylaxis, n (%) < 0.01 Nevirapine 113 (76.9) 62 (68.9) 18 (17.1) Zidovudine 33 (22.4) 21 (23.3) 26 (24.8) Unmedicated 1 (0.7) 7 (7.8) 61 (58.1) Note. Mean ± SD = mean ± standard deviation; *Non-parametric test was used. Table 3. Comparison of adverse pregnancy outcomes and adverse early growth effects stratified by antiretroviral regimen during pregnancy
Outcomes cART (n = 361) Mono/dual AR (n = 91) No treatment (n = 133) P-value Adverse pregnancy outcomes, n (%) 112 (31.9) 31 (34.1) 59 (44.4) 0.04 Spontaneous abortion 0 0 6 (4.5) − Ectopic pregnancy 1 (0.3) 0 14 (10.5) − Stillbirth 3 (0.9) 1 (1.1) 8 (7.1) < 0.01 Congenital anomaly 2 (0.6) 2 (2.2) 2 (1.8) 0.33 Preterm birth 44 (12.5) 15 (16.5) 23 (20.5) 0.33 SGA 77 (21.9) 17 (18.7) 14 (10.5) 0.02 Adverse early growth outcomes, n (%) 87 (32.7) 17 (25.0) 17 (32.1) 0.47 Infant death 1 (0.3) 0 3 (2.7) < 0.01 HIV infection via MTCT 1 (0.3) 0 5 (4.4) − Stunting 41 (15.4) 12 (17.1) 2 (3.9) 0.07 Underweight 34 (12.7) 6 (8.6) 5 (9.8) 0.57 Wasting 35 (13.1) 4 (5.7) 6 (11.8) 0.23 Note. SGA, small for gestational age; MTCT, mother-to-child transmission. Table 4. Comparison of adverse pregnancy outcomes and adverse early growth effects stratified byART initiation timing
Outcomes 1st Trimester
(n = 89)2nd Trimester
(n = 247)3rd Trimester
(n = 84)Delivery/postpartum
(n = 39)P-value Adverse pregnancy outcomes, n (%) 37 (41.6) 86 (34.8) 26 (31.0) 12 (30.8) 0.46 Ectopic pregnancy 1 (1.1) 0 0 0 0.28 Stillbirth 0 1 (0.4) 0 3 (7.7) 0.24 Congenital anomaly 1 (1.1) 2 (0.8) 0 1 (2.6) 0.47 Preterm birth 12 (13.6) 45 (18.2) 8 (9.5) 6 (16.2) 0.27 SGA 26 (29.5) 52 (21.1) 20 (23.8) 5 (13.5) 0.21 Adverse early growth outcomes, n (%) 17 (23.3) 67 (36.6) 16 (28.6) 3 (14.3) 0.05 Infant death 0 0 0 1 (2.6) 0.28 HIV infection via MTCT 0 0 0 1 (2.6) 0.28 Stunting 13 (17.8) 32 (17.1) 7 (12.5) 1 (5.0) 0.49 Underweight 10 (13.7) 22 (11.8) 7 (12.5) 1 (5.0) 0.82 Wasting 2 (2.7) 29 (15.5) 8 (14.3) 0 0.01 Note. SGA, small for gestational age; MTCT, mother-to-child transmission. Table 5. Adverse pregnancy outcomes and adverse early infant growth effects at 4 weeks of age stratified by antiretroviral regimens during pregnancy
Items cART Mono/dual ART No treatment Any adverse pregnancy outcome Number of women, n (%) 112/351 (31.9%) 31/91 (34.1%) 59/133 (44.4%) Crude Model, OR (95% CI) 0.59 (0.39, 0.89) 0.65 (0.37, 1.13) 1 (ref) Model 1a, OR (95% CI) 0.53 (0.35, 0.81) 0.60 (0.34, 1.05) 1 (ref) Model 2b, OR (95% CI) 1.16 (0.64, 2.08) 1.28 (0.65, 2.51) 1 (ref) SGA Number of women, n (%) 77/351 (21.9) 17/91 (18.7) 14/133 (10.5) Crude Model, OR (95% CI) 1.92 (1.04, 3.56) 1.51 (0.70, 3.27) 1 (ref) Model 1a, OR (95% CI) 1.87 (1.00, 3.51) 1.47 (0.67, 3.22) 1 (ref) Model 2b, OR (95% CI) 2.10 (0.99, 4.44) 1.53 (0.64, 3.66) 1 (ref) Any adverse early growth Number of children, n (%) 87/266 (32.7%) 17/68 (25.0%) 17/53 (32.1%) Crude Model, OR (95% CI) 1.03 (0.55, 1.94) 0.71 (0.32, 1.57) 1 (ref) Model 1a, OR (95% CI) 1.07 (0.56, 2.03) 0.75 (0.33, 1.69) 1 (ref) Model 2b, OR (95% CI) 1.18 (0.55, 2.57) 0.81 (0.33, 2.00) 1 (ref) Model 3c, OR (95% CI) 1.10 (0.39, 3.11) 0.71 (0.23, 2.23) 1 (ref) Note. SGA, small for gestational age. aModel 1: covariates mother’s education, ethnicity, household registration, parity; bModel 2: covariates in model 1, and gestational week at the first antenatal care, number of antenatal care; cModel 3: covariates in model 2, and Mode of delivery, gestational age at delivery, birthweight and infant antiretroviral prophylaxis. Table 6. Association of preterm birth with NNRTI-based cART, PI-based cART, Mono/dual ARTand no treatment
Item Preterm birth Term birth P-value NNRTI-based cART 23 126 0.99 PI-based cART 34 186 mono/dual ART 15 75 No treatment 17 88 -
[1] Piske M, Budd MA, Qiu AQ, et al. Neurodevelopmental outcomes and in-utero antiretroviral exposure in HIV-exposed uninfected children. AIDS, 2018; 32, 2583−92. [2] Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS update. Ending AIDS: progress towards the 90-90-90 targets. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS), July 2017. http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf. [2019-6-12] [3] Bokharaei-Salim F, Kalantari S, Gholamypour Z, et al. Investigation of the effects of a prevention of mother-to-child HIV transmission program among Iranian neonates. Arch Virol, 2018; 163, 1179−85. doi: 10.1007/s00705-017-3661-1 [4] Chen Fangfang, Qin Qianqian, Cai Chang, et al. Spatial-temporal distribution of newly detected HIV/AIDS cases among aged 15 years or older women in China, 2010-2016. Chin J Epidemiol, 2018; 39, 739−44. (In Chinese) [5] Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med, 1994; 331, 1173−80. doi: 10.1056/NEJM199411033311801 [6] The Petra study team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet, 2002; 359, 1178−86. doi: 10.1016/S0140-6736(02)08214-4 [7] Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr, 2002; 29, 484−94. doi: 10.1097/00042560-200204150-00009 [8] Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med, 2004; 351, 217−28. doi: 10.1056/NEJMoa033500 [9] Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet, 2011; 378, 282−84. doi: 10.1016/S0140-6736(10)62303-3 [10] World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach (2010 version). Geneva: World Health Organization, November 2010. http://www.who.int/hiv/pub/mtct/guidelines/en/.[2019-6-12] [11] World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, June 2013. http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [2019-6-12] [12] World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach-2nd edition, December 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/. [2019-6-12]. [13] Bailey H, Zash R, Rasi V, et al. HIV treatment in pregnancy. The Lancet HIV, 2018; 5, e457−e67. doi: 10.1016/S2352-3018(18)30059-6 [14] Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol, 2017; 46, 1678−89. doi: 10.1093/ije/dyx136 [15] Luzuriaga K, Mofenson LM. Challenges in the Elimination of Pediatric HIV-1 Infection. N Engl J Med, 2016; 374, 761−70. doi: 10.1056/NEJMra1505256 [16] Wedi CO, Kirtley S, Hopewell S, et al. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. The Lancet HIV, 2016; 3, e33−48. doi: 10.1016/S2352-3018(15)00207-6 [17] Favarato G, Townsend CL, Bailey H, et al. Protease inhibitors and preterm delivery: another piece in the puzzle. AIDS, 2018; 32, 243−52. [18] Chetty T, Thorne C, Coutsoudis A. Preterm delivery and small-for-gestation outcomes in HIV-infected pregnant women on antiretroviral therapy in rural South Africa: Results from a cohort study, 2010-2015. PloS One, 2018; 13, e019280−21. [19] Zash R, Souda S, Chen JY, et al. Reassuring Birth Outcomes With Tenofovir/Emtricitabine/Efavirenz Used for Prevention of Mother-to-Child Transmission of HIV in Botswana. J Acquir Immune Defic Syndr, 2016; 71, 428−36. doi: 10.1097/QAI.0000000000000847 [20] Shapiro R, Dryden-Peterson S, Powis K, et al. Hidden in plain sight: HIV, antiretrovirals, and stillbirths. Lancet, 2016; 387, 1994−5. doi: 10.1016/S0140-6736(16)30459-7 [21] Saleska JL, Turner AN, Maierhofer C, et al. Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with HIV-1 in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr, 2018; 79, 1−9. doi: 10.1097/QAI.0000000000001770 [22] Li N, Sando MM, Spiegelman D, et al. Antiretroviral Therapy in Relation to Birth Outcomes among HIV-infected Women: A Cohort Study. J Infect Dis, 2016; 213, 1057−64. doi: 10.1093/infdis/jiv389 [23] Masaba R, Borkowf CB, Girde S, et al. Adverse fetal and infant outcomes among HIV-infected women who received either NNRTI- or PI-based ART for PMTCT. AIDS, 2018; 32, 1625−32. doi: 10.1097/QAD.0000000000001816 [24] Heffron R, Mugo N, Hong T, et al. Pregnancy outcomes and infant growth among babies with in utero exposure to tenofovir-based pre-exposure prophylaxis for HIV prevention. AIDS, 2018; 32, 1707−13. doi: 10.1097/QAD.0000000000001867 [25] Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. The Lancet HIV, 2017; 4, e21−e30. doi: 10.1016/S2352-3018(16)30195-3 [26] Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet, 2014; 384, 857−68. doi: 10.1016/S0140-6736(14)60932-6 [27] Fenton TR, Sauve RS. Using the LMS method to calculate Z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr, 2007; 61, 1380−5. doi: 10.1038/sj.ejcn.1602667 [28] World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. WHO Multicentre Growth Reference Study Group 2006, http://www.who.int/childgrowth/standards/technical_report/en/. [2017-9-12]. [29] Powis KM, Smeaton L, Hughes MD, et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS, 2016; 30, 211−20. doi: 10.1097/QAD.0000000000000895 [30] Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis, 2012; 206, 1695−705. doi: 10.1093/infdis/jis553 [31] Dadabhai S, Gadama L, Chamanga R, et al. Pregnancy Outcomes in the Era of Universal Antiretroviral Treatment in Sub-Saharan Africa (POISE Study). J Acquir Immune Defic Syndr, 2019; 80, 7−14. doi: 10.1097/QAI.0000000000001875 [32] Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis, 2012; 54, 1348−60. doi: 10.1093/cid/cis198 [33] Thorne C, Aebi-Popp K. Beyond prevention of mother-to-child HIV transmission. The lancet HIV, 2016; 3, e5−6. doi: 10.1016/S2352-3018(15)00243-X [34] Aaron E, Bonacquisti A, Mathew L, et al. Small-for-gestational-age births in pregnant women with HIV, due to severity of HIV disease, not antiretroviral therapy. Infectious diseases in obstetrics and gynecology, 2012; 2012, 135030. [35] Snijdewind IJM, Smit C, Godfried MH, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PloS one, 2018; 13, e0191389. doi: 10.1371/journal.pone.0191389 [36] Phiri K, Williams PL, Dugan KB, et al. Antiretroviral Therapy Use During Pregnancy and the Risk of Small for Gestational Age Birth in a Medicaid Population. Pediatr Infect Dis J, 2015; 34, e169−75. doi: 10.1097/INF.0000000000000712 [37] Briand N, Mandelbrot L, Le Chenadec J, et al. No relation between in-utero exposure to HAART and intrauterine growth retardation. AIDS, 2009; 23, 1235−43. doi: 10.1097/QAD.0b013e32832be0df [38] Jao J, Abrams EJ. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. The Pediatr Infect Dis J, 2014; 33, 734−40. doi: 10.1097/INF.0000000000000224 [39] HE Yan, LUO Yan, DING Yi-ling, et al. Effect of highly active anti-retroviral therapy on prevention of mother to child transmission of HIV and on infant growth and development. Chin J Pre Med, 2011; 45, 912−15. (In Chinese) [40] Rosala-Hallas A, Bartlett JW, Filteau S. Growth of HIV-exposed uninfected, compared with HIV-unexposed, Zambian children: a longitudinal analysis from infancy to school age. BMC Pediat, 2017; 17, 80. doi: 10.1186/s12887-017-0828-6 [41] Omoni AO, Ntozini R, Evans C, et al. Child Growth According to Maternal and Child HIV Status in Zimbabwe. Pediatr Infect Dis J, 2017; 36, 869−76. doi: 10.1097/INF.0000000000001574 [42] S MlR, Jao J, Brittain K, et al. Tenofovir exposure in utero and linear growth in HIV-exposed, uninfected infants. AIDS, 2017; 31, 97−104. doi: 10.1097/QAD.0000000000001302 [43] Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. J Infect Dis, 2013; 207, 612−21. doi: 10.1093/infdis/jis728 [44] Marazzi MC, Palombi L, Nielsen-Saines K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS, 2011; 25, 1611−8. doi: 10.1097/QAD.0b013e3283493ed0 -





 下载:
下载:



 Quick Links
Quick Links