-
Human immunodeficiency virus (HIV) is a serious public health concern. Globally, 1.7 million (1.3–2.1 million) children aged 0–14 were living with HIV at the end of 2021, 90% of whom lived in low- and middle-income countries. Acquired Immune Deficiency Syndrome (AIDS) remains the leading cause of mortality among children worldwide, resulting in 98,000 (67,000−140,000) child deaths due to HIV-related illnesses worldwide in 2021[1]. Over 50% of HIV-infected infants die within two years of age in the absence of treatment[2], indicating rapid disease progression and a high mortality rate. Early initiation of antiretroviral therapy (ART) in infants reduces the risk of severe morbidity by 75% and mortality by 76%[3]. Therefore, timely antiretroviral treatment is urgently needed for children with HIV infection.
Expanded access to ART has led to a steep decrease in the number of children dying from HIV-related causes worldwide. China’s National Free Antiretroviral Treatment Program (NFATP) was fully implemented nationally in 2003 and has significantly reduced deaths in children with HIV. The cost of second-line ART is higher than that of first-line[4], and most children with HIV infection in China receive NFATP-recommended first-line ART regimens. The recommended first-line ART regimens of children with HIV infection comprises two nucleoside reverse transcriptase inhibitors (NRTI) plus either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a protease inhibitor (PI) (abacavir [ABC] or zidovudine [AZT]) plus lamivudine (3TC) and (efavirenz [EFV] or nevirapine [NVP] or ritonavir-boosted lopinavir [LPV/r]). The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1060 study conducted a randomized trial of initial therapy with AZT and 3TC plus either NVP or LPV/r in children with HIV infection and compared short-term (2–36 months) or long-term (5 years) outcomes in six African countries. Antiretroviral treatment with AZT and 3TC plus LPV/r resulted in better outcomes than treatment with AZT and 3TC plus NVP[5-7]. However, the drug (LPV/r, NVP, or EFV) crucial for reducing mortality, and guidelines to choose the optimal ART regimen in large cohorts of children with HIV infection, according to the recommended first-line ART regimens of NFATP in China, is unclear.
In this study, we retrospectively collected data on children with HIV infection receiving first-line ART in Guangxi from the Guangxi Center for Disease Prevention and Control and electronic medical records from the Check Hospital of Guangxi, and evaluated the effects of different first-line ART regimens on mortality among children with HIV infection in Guangxi.
The study included children no more than 14 years of age with ART initiation, had complete follow-up records, started ART between Nov 16, 2004, and Mar 22, 2018, and were treated with the baseline ART regimen: AZT+3TC+LPV/r (LPV/r-based), AZT+3TC+NVP (NVP-based), or AZT+3TC+EFV (EFV-based). Participants were excluded if they were treated with regimens other than LPV/r-, NVP-, or EFV-based regimens. In total, 653 children with HIV infection who were initiated on ART between November 2004 and March 2018 in Guangxi, China, were screened. Of these, 212 children who did not receive AZT+3TC+LPV/r / NVP / EFV were excluded from the study. Finally, 441 children met the inclusion criteria. Of these, 131, 158, and 152 patients received LPV/r-based, NVP-based regimen and EFV-based regimen, respectively. This study was approved by the Human Research Ethics Committee of Guangxi Medical University. The identities and information of all participants were stored in the hospital’s medical record system, which is a secure password-protected system that is not open to the public or unauthorized users.
Data including baseline characteristics, clinical and laboratory information, treatment outcomes, and follow-up times were collected. The monitoring schedule for ART was baseline, half-month, 1-month, and 2-month, then changed to follow-up every three months. The data collected included the CD4+ T cell count, World Health Organization (WHO) clinical stage, time from clinical diagnosis to ART initiation, opportunistic infection, and ART regimens. The primary outcome of the analysis was mortality. Death and mortality rates were compared among children with HIV infection who received different first-line ART. Follow-up data were collected until May 25, 2018. The observation period ranged from the ART initiation to the date of death.
Categorical variables are described as frequency while quantitative variables are expressed as the median ± interquartile range (IQR). Chi-square tests for categorical variables were used to compare the characteristics among the three ART regimen groups. Kaplan-Meier analysis was used to assess the cumulative mortality of all children with HIV infection, and statistical testing of differences was performed using the log-rank test or Breslow test. Univariate and multivariate Cox regression analyses were performed to explore factors associated with death. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 26.0 (SPSS Inc. Chicago, USA) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, California, USA). All statistical tests were two-tailed. The P values less than 0.05 were considered statistically significant.
Together, these children contributed 2,183.66 person-years of follow-up, with a median of 4.84 person-year (IQR 2.09–7.33). The baseline characteristics of the 441 enrolled patients are summarized in Supplementary Table S1 (available in www.besjournal.com). A total of 39.2% were diagnosed with HIV, 34.7% started ART at 25–60 months, 67.6% were diagnosed with HIV-to-ART initiation at no more than 6 months, and 52.4% were male. More than half of the children (57.4%) had the WHO HIV clinical stage I disease. 59.2% children with a CD4+ T cell count ≤ 500 cells/μL, 66.2% children with a CD8+ T cell count > 1,138 cells/μL.
Table S1. Characteristics of children with HIV infection receiving ART, n (%)
Variables Study Population (n = 441) Age at HIV diagnosis, months ≤ 24 124 (28.1) 25–60 173 (39.2) > 60 141 (32.0) Unknown 3 (0.7) Time period between HIV diagnosis and ART initiation, months ≤ 6 298 (67.6) > 6 140 (31.7) Unknown 3 (0.7) Age at ART initiation, months ≤ 24 80 (18.1) 25–60 153 (34.7) > 60 208 (47.2) Sex Male 231 (52.4) Female 210 (47.6) WHO clinical stage I 253 (57.4) II 45 (10.2) III 87 (19.7) IV 56 (12.7) Baseline CD4+ T cell count, cells/µL ≤ 500 261 (59.2) > 500 173 (39.2) Baseline CD8+ T cell count, cells/µL ≤ 760 65 (14.7) 761–1,138 76 (17.2) > 1,138 292 (66.2) Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. WHO, World Health Organization. ART regimens were associated with mortality in HIV-infected children (Table 1). Of the total children, the AIDS-related cumulative mortality rate was 6.1% (27/441) and the mortality density was 12.4 per 1,000 person-years (95% confidence interval [CI] 7.8–16.9) in the overall cohort. In children with HIV infection treated with an LPV/r-based regimen, the mortality rate was 3.1% (4/131) or 5.6 per 1000 person-years (95% CI 0.2–10.9). In those with NVP-based regimen, the mortality rate was 10.8% (17/158), or 20.2 per 1000 person-years (95% CI 10.8–29.5). In those with EFV-based regimen, the mortality rate was 3.9% (6/152), or 9.6 per 1000 person-years (95% CI 2.1–17.1). The log-rank test (P = 0.010) showed that mortality was significantly different among the three groups (Table 1). Survival analyses also showed that the cumulative mortality of children with HIV infection in the NVP-based regimen group was higher than that in the LPV/r- or EFV-based regimens at all evaluated time points (log-rank test, P = 0.010; Figure 1).
Table 1. Comparison of mortality of children with HIV infection with baseline ART regimen
Group Total children, n Dead, n (%) χ2 P* Person-years Dead/1,000 Persons-years (95%CI) P# LPV/r-based regimen 131 4 (3.1) 714.72 5.6 (0.2–10.9) NVP-based regimen 158 17 (10.8) 842.55 20.2 (10.8–29.5) EFV-based regimen 152 6 (3.9) 626.40 9.6 (2.1–17.1) Total 441 27 (6.1) 9.309 0.010 2183.67 12.4 (7.8–16.9) 0.010 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. CI, confidence interval. *P, by Chi-squared test.#P, by Log-rank test. 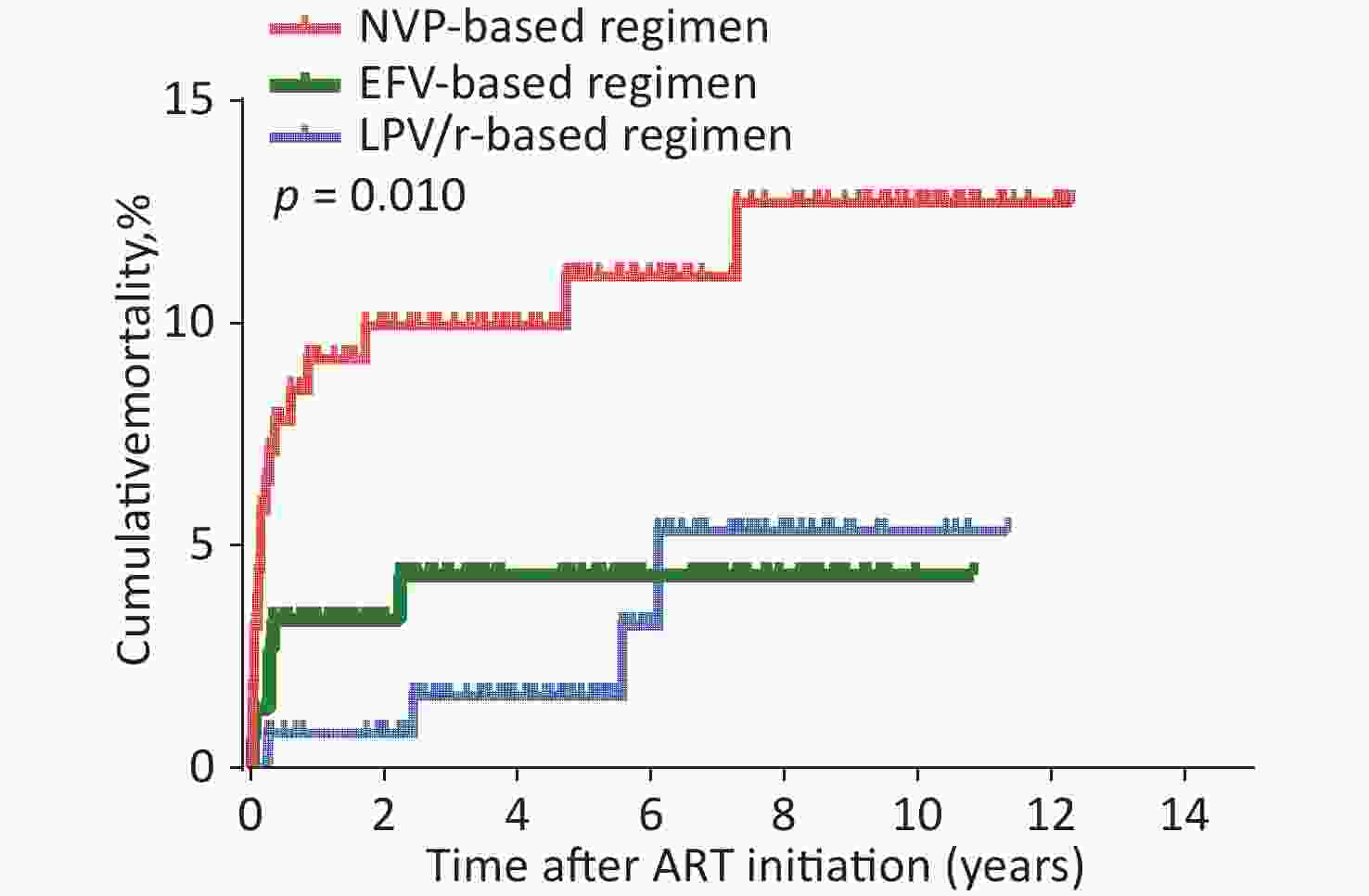
Figure 1. Kaplan-Meier analysis of cumulative mortality of children with HIV infection, grouped by baseline ART regimen. The statistical significance was measured by log-rank test. ART, antiretroviral therapy, HIV, human immunodeficiency virus.
Univariate and multivariate Cox regression analyses were used to identify factors that affected mortality (Supplementary Table S2, available in www.besjournal.com). As shown in Table 2, univariate Cox regression analysis showed that mortality was significantly higher in the NVP-based regimen than in the LPV/r-based regimen [hazard ratio (HR) = 3.782, 95% CI 1.272–11.248, P = 0.017]. No significant difference was observed in mortality between LPV/r- and EFV-based regimen (HR = 1.415, 95% CI 0.399–5.019, P = 0.591). After adjustment for a collection of pre-defined and forward-selection variables (including age at HIV diagnosis, time period between HIV diagnosis and ART initiation, age at ART initiation, sex, WHO clinical stage, baseline CD4+ T cell count, baseline CD8+ T cell count, baseline ART regimen, TB infection, and opportunistic infection), the NVP-based regimen still had a significantly higher mortality rate than the LPV/r-based regimen [adjusted hazard ratio (aHR) = 4.350, 95% CI 1.193–15.860, P = 0.026]. There was also no significant difference in mortality between the LPV/r- and EFV-based regimens (aHR = 1.702, 95% CI 0.396–7.320, P = 0.475) (Table 2 and Supplementary Table S2). Collectively, these data provide strong evidence of the clinical impact of NVP-based regimens on the mortality of HIV/AIDS children receiving ART compared with LPV/r- or EFV-based regimens.
Table 2. Effect of baseline ART regimen on mortality among children with HIV infection receiving ART
Group Total children, n Dead, n (%) HR*(95% CI) pHR* aHR#(95% CI) paHR# LPV/r-based regimen 131 4 (3.1) 1 − 1 − NVP-based regimen 158 17 (10.8) 3.782 (1.272-11.248) 0.017 4.350 (1.193-15.860) 0.026 EFV-based regimen 152 6 (3.9) 1.415 (0.399-5.019) 0.591 1.702 (0.396-7.320) 0.475 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. *HR: hazard ratio. #aHR: adjusted hazard ratio, adjusted for age at HIV diagnosis, time period between HIV diagnosis and ART initiation, age at ART initiation, sex, WHO clinical stage, baseline CD4+ T cell count, baseline CD8+ T cell count, baseline ART regimens, TB infection, opportunistic infection. Table S2. Factors associated with mortality among HIV-infected children
Factors HR (95% CI) PHR aHR (95% CI) PaHR Age at HIV diagnosis, months ≤ 24 1 − 1 − 25–60 0.717 (0.298−1.724) 0.458 0.348 (0.080−1.510) 0.159 > 60 0.684 (0.259−1.802) 0.442 1.005 (0.060−16.952) 0.997 Time period between HIV diagnosis and ART initiation, months ≤ 6 1 − 1 − > 6 0.793 (0.335−1.880) 0.599 1.368 (0.398−4.700) 0.619 Age at ART initiation, months ≤ 24 1 − 1 − 25–60 1.124 (0.427−2.959) 0.813 2.718 (0.484−15.261) 0.256 > 60 0.567 (0.196−1.642) 0.296 0.375 (0.020−7.054) 0.512 Sex Male 1 − 1 − Female 1.440 (0.674−3.079) 0.346 1.852 (0.825−4.157) 0.135 WHO clinical stage I/II 1 − 1 − III/IV 9.332 (3.530−24.668) < 0.001 8.223 (2.583−26.180) 0.000 Baseline CD4+ T cell count, cells/µL ≤ 500 1 − 1 − > 500 0.197 (0.059−0.656) 0.008 0.396 (0.104−1.514) 0.176 Baseline CD8+ T cell count, cells/µL ≤ 760 1 − 1 − 761–1,138 1.765 (0.543−5.737) 0.345 3.324 (0.913−12.108) 0.069 > 1,138 0.628 (0.204−1.932) 0.417 0.862 (0.248−2.999) 0.815 Baseline ART regimens LPV/r-based 1 − 1 − NVP-based 3.782 (1.272−11.248) 0.017 4.350 (1.193−15.860) 0.026 EFV-based 1.415 (0.399−5.019) 0.591 1.702 (0.396−7.320) 0.475 TB infection Yes 1 − 1 − No 0.373 (0.112−1.240) 0.108 0.705 (0.188−2.648) 0.605 Opportunistic infection Yes 1 − 1 − No 0.310 (0.145−0.661) 0.002 1.182 (0.466−3.002) 0.725 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. TB, tuberculosis. HR, hazard ratio. aHR, adjusted hazard ratio. Several studies have confirmed an association between mortality and WHO clinical stage. WHO clinical stage III/IV is associated with an increased risk of death[8,9]. Factors associated with mortality in children remaining in care included clinical stage (HR = 10.13; 95% CI 2.25, 45.58 comparing WHO stage I/II with III/IV)[10]. In this study, advanced WHO clinical stage was independently associated with higher mortality (WHO clinical stage III/IV: aHR = 8.223, 95% CI 2.583–26.180, P < 0.001; reference to WHO clinical stage I/II) (Supplementary Table S2). As shown in Supplementary Table S3 (available in www.besjournal.com), the highest mortality (23.3%, or 45.1/1,000 person-years, 95% CI 22.1–68.2, P < 0.001) was observed in NVP-based regimen with WHO clinical stage III/IV. Similarly, in the EFV-based regimen, mortality with WHO clinical stage III/IV (9.8%, or 22.1/1,000 person-years, 95% CI 3.2–41.0, P = 0.009) was significantly higher than children with WHO clinical stage I/II. The Kaplan-Meier chart showed that WHO clinical stage III/IV patients had significantly higher cumulative mortality than WHO clinical stage I/II patients in all three baseline ART regimen subgroups (Supplementary Figure S1A–C, available in www.besjournal.com).
Table S3. The mortality of children with HIV infection, group by ART regimenand WHO clinical stage
Baseline ART regimen WHO clinical stage Total children, n Dead, n (%) Person-years Dead/1,000 Persons-years (95% CI) P# LPV/r-based I/II 99 1 (1.0) 498.25 2.0 (−1.8−5.8) 0.056 III/IV 32 3 (9.4) 216.47 13.9 (−1.4−29.1) NVP-based I/II 98 3 (3.1) 532.27 5.6 (−0.6−11.9) < 0.001 III/IV 60 14 (23.3) 310.28 45.1 (22.1−68.2) EFV-based I/II 101 1 (1.0) 400.47 2.5 (−2.3−7.3) 0.009 III/IV 51 5 (9.8) 225.93 22.1 (3.2–41.0) Total 441 27 (6.1) 2183.67 12.4 (7.8−16.9) Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavi. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. #P by log-rank test. 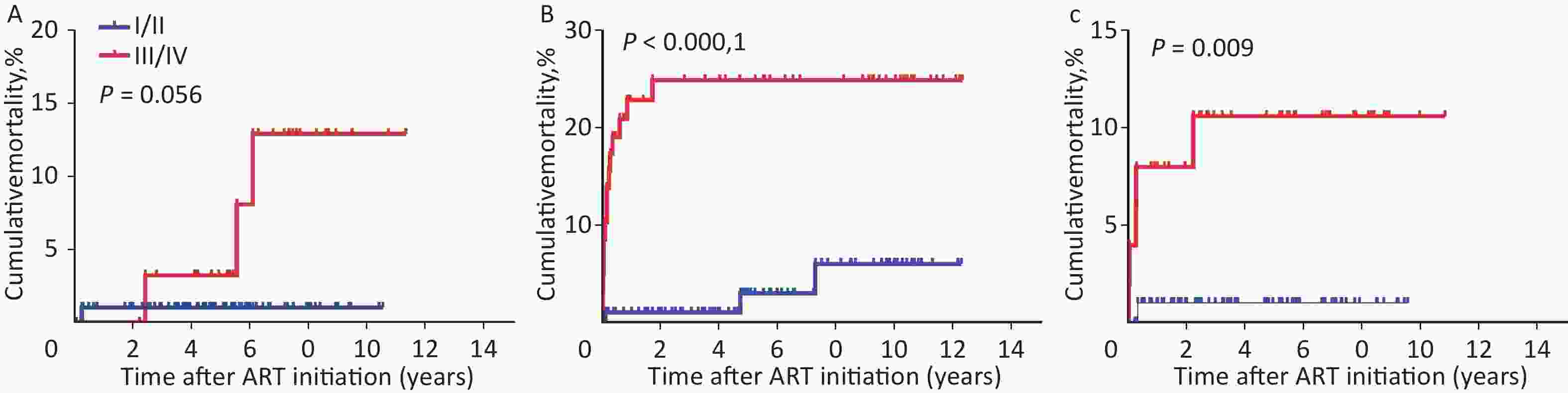
Figure S1. Kaplan-Meier analysis of cumulative mortality in HIV-infected children, grouped by ART regimen and WHO clinical stage. (A) Children received LPV/r-based regimen. (B) Children received NVP-based regimen. (C) Children received EFV-based regimen. The statistical significance was measured by log-rank test. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization.
As the baseline WHO clinical stage was a major factor influencing mortality, we evaluated the effect of different baseline ART regimens on mortality in the two subgroups of WHO clinical stages I/II or III/IV using the Kaplan-Meier method. In WHO clinical stage III/IV, the cumulative mortality in the NVP-based regimen was significantly higher than that in the LPV/r- or EFV-based regimens (Supplementary Figure S2(A–B), available in www.besjournal.com, P = 0.018). As shown in Supplementary Table S4–S5 (available in www.besjournal.com), in WHO clinical stage III/IV, the highest death rate (23.3%, or 45.1/1,000 person-years, 95% CI 22.1–68.2, P = 0.018) was found in NVP-based regimen compared with LPV/r- or EFV-based regimen. There was also no significant difference in mortality between the LPV/r- and EFV-based regimens (P > 0.05), indicating that the WHO clinical stage does not affect the relationship between the baseline ART regimen and mortality. Notably, different baseline ART regimens were most significant in the WHO clinical stage III/IV subgroup. This provides further support for the finding that if different baseline ART regimens have an effect on mortality, the highest effect of the NVP-based regimen would be expected to be most profound in the patient group with baseline WHO clinical stage III/IV. In addition, we found that the mortality rates between LPV/r-based regimen and EFV-based regimen were similar, which means that we could choose LPV/r- or EFV-based regimens regardless of the baseline WHO clinical stage.
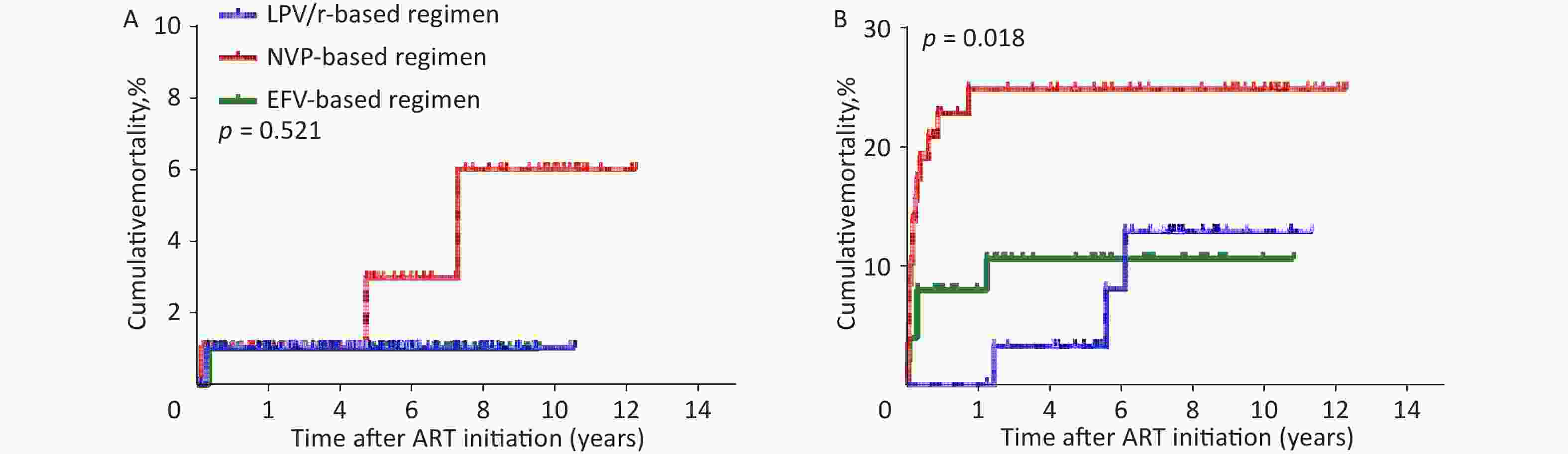
Figure S2. Kaplan-Meier analysis of cumulative mortality of children with HIV infection, grouped by WHO clinical stage. (A) children with HIV infection were in WHO clinical stage I/II. (B) children with HIV infection were in WHO clinical stage III/IV. HIV, human immunodeficiency virus, WHO, World Health Organization.
Table S4. Comparison of mortality of HIV-infected children, group by WHO clinical stage
WHO clinical stage Baseline ART regimen Total children, n Dead, n (%) Person-years Dead/1000 Persons-years (95% CI) p I/II LPV/r-based 99 1 (1.0) 498.25 2.0 (−1.8 to 5.8) 0.521 NVP-based 98 3 (3.1) 532.27 5.6 (−0.6 to 11.9) EFV-based 101 1 (1.0) 400.47 2.5 (−2.3 to 7.3) III/IV LPV/r-based 32 3 (9.4) 216.47 13.9 (−1.4 to 29.1) 0.018 NVP-based 60 14 (23.3) 310.28 45.1 (22.1 to 68.2) EFV-based 51 5 (9.8) 225.93 22.1 (3.2 to 41.0) Total 441 27 (6.1) 2183.67 12.4 (7.8 to 16.9) Note. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. Table S5. Comparison of mortality of HIV-infected children receiving different ART regimen, group by WHO clinical stage
WHO clinical stage ART regimen LPV/r-based NVP-based EFV-based χ2 p χ2 p χ2 p I/II LPV/r-based − − 0.853 0.356 0.001 0.980 NVP-based 0.853 0.356 − − 0.682 0.409 EFV-based 0.001 0.980 0.682 0.409 − − III/IV LPV/r-based − − 5.193 0.023 0.889 0.346 NVP-based 5.193 0.023 − − 3.978 0.046 EFV-based 0.889 0.346 3.978 0.046 − − Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. In addition to the inherent limitations of this retrospective study, there were other limitations to our research. First, this study was conducted only in Guangxi and thus may not be representative of other regions in China. Second, AZT-based ART regimens (AZT+3TC+LPV/r / NVP / EFV) and ABC-based ART regimens (ABC+3TC+LPV/r / NVP / EFV) are first-line ART regimens according to the NFATP. Only children with HIV/AIDS who received AZT-based ART regimens (AZT+3TC+LPV/r / NVP / EFV) were included in the study, whereas those who received ABC-based ART regimens (ABC+3TC+LPV/r / NVP / EFV) were excluded. Therefore, we could not estimate the treatment effects of ABC-based ART regimens. Finally, there may be a possibility of regimen changesduring treatment; this study was based on ART regimen initiation and did not analyze children with regimen changes.
In this large retrospective cohort study, we estimated the mortality associated with different baseline ART regimens in children with HIV infection who started ART in Southern China. Our study highlights the important contribution of higher mortality associated with NVP-based regimens in children with HIV/AIDS in WHO clinical stage III/IV. Simultaneously, EFV-and LPV/r-based regimens have the same security. This provides a basis for the selection of baseline ART regimens that are associated with fewer deaths in children with HIV/AIDS, especially in those with WHO clinical stage III/IV. In future, we will increase the number of ABC-based ART regimens to identify the safest first-line drugs for children with HIV.
We express our gratitude to all staff from the Guangxi Center for Disease Prevention and Control, and Check Hospital of Guangxi, Guangxi, China, for their collection and provision of epidemiological data on local HIV/AIDS children.
The authors declare that they have no conflict of interest.
doi: 10.3967/bes2023.137
Effects of Differential First-Line Antiretroviral Therapy (ART) Regimens on Mortality among HIV/AIDS Children in Southwest China: A 15-year Retrospective Cohort Study
-
&These authors contributed equally to this work.
注释: -
S1. Kaplan-Meier analysis of cumulative mortality in HIV-infected children, grouped by ART regimen and WHO clinical stage. (A) Children received LPV/r-based regimen. (B) Children received NVP-based regimen. (C) Children received EFV-based regimen. The statistical significance was measured by log-rank test. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization.
S2. Kaplan-Meier analysis of cumulative mortality of children with HIV infection, grouped by WHO clinical stage. (A) children with HIV infection were in WHO clinical stage I/II. (B) children with HIV infection were in WHO clinical stage III/IV. HIV, human immunodeficiency virus, WHO, World Health Organization.
S1. Characteristics of children with HIV infection receiving ART, n (%)
Variables Study Population (n = 441) Age at HIV diagnosis, months ≤ 24 124 (28.1) 25–60 173 (39.2) > 60 141 (32.0) Unknown 3 (0.7) Time period between HIV diagnosis and ART initiation, months ≤ 6 298 (67.6) > 6 140 (31.7) Unknown 3 (0.7) Age at ART initiation, months ≤ 24 80 (18.1) 25–60 153 (34.7) > 60 208 (47.2) Sex Male 231 (52.4) Female 210 (47.6) WHO clinical stage I 253 (57.4) II 45 (10.2) III 87 (19.7) IV 56 (12.7) Baseline CD4+ T cell count, cells/µL ≤ 500 261 (59.2) > 500 173 (39.2) Baseline CD8+ T cell count, cells/µL ≤ 760 65 (14.7) 761–1,138 76 (17.2) > 1,138 292 (66.2) Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. WHO, World Health Organization. Table 1. Comparison of mortality of children with HIV infection with baseline ART regimen
Group Total children, n Dead, n (%) χ2 P* Person-years Dead/1,000 Persons-years (95%CI) P# LPV/r-based regimen 131 4 (3.1) 714.72 5.6 (0.2–10.9) NVP-based regimen 158 17 (10.8) 842.55 20.2 (10.8–29.5) EFV-based regimen 152 6 (3.9) 626.40 9.6 (2.1–17.1) Total 441 27 (6.1) 9.309 0.010 2183.67 12.4 (7.8–16.9) 0.010 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. CI, confidence interval. *P, by Chi-squared test.#P, by Log-rank test. Table 2. Effect of baseline ART regimen on mortality among children with HIV infection receiving ART
Group Total children, n Dead, n (%) HR*(95% CI) pHR* aHR#(95% CI) paHR# LPV/r-based regimen 131 4 (3.1) 1 − 1 − NVP-based regimen 158 17 (10.8) 3.782 (1.272-11.248) 0.017 4.350 (1.193-15.860) 0.026 EFV-based regimen 152 6 (3.9) 1.415 (0.399-5.019) 0.591 1.702 (0.396-7.320) 0.475 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. *HR: hazard ratio. #aHR: adjusted hazard ratio, adjusted for age at HIV diagnosis, time period between HIV diagnosis and ART initiation, age at ART initiation, sex, WHO clinical stage, baseline CD4+ T cell count, baseline CD8+ T cell count, baseline ART regimens, TB infection, opportunistic infection. S2. Factors associated with mortality among HIV-infected children
Factors HR (95% CI) PHR aHR (95% CI) PaHR Age at HIV diagnosis, months ≤ 24 1 − 1 − 25–60 0.717 (0.298−1.724) 0.458 0.348 (0.080−1.510) 0.159 > 60 0.684 (0.259−1.802) 0.442 1.005 (0.060−16.952) 0.997 Time period between HIV diagnosis and ART initiation, months ≤ 6 1 − 1 − > 6 0.793 (0.335−1.880) 0.599 1.368 (0.398−4.700) 0.619 Age at ART initiation, months ≤ 24 1 − 1 − 25–60 1.124 (0.427−2.959) 0.813 2.718 (0.484−15.261) 0.256 > 60 0.567 (0.196−1.642) 0.296 0.375 (0.020−7.054) 0.512 Sex Male 1 − 1 − Female 1.440 (0.674−3.079) 0.346 1.852 (0.825−4.157) 0.135 WHO clinical stage I/II 1 − 1 − III/IV 9.332 (3.530−24.668) < 0.001 8.223 (2.583−26.180) 0.000 Baseline CD4+ T cell count, cells/µL ≤ 500 1 − 1 − > 500 0.197 (0.059−0.656) 0.008 0.396 (0.104−1.514) 0.176 Baseline CD8+ T cell count, cells/µL ≤ 760 1 − 1 − 761–1,138 1.765 (0.543−5.737) 0.345 3.324 (0.913−12.108) 0.069 > 1,138 0.628 (0.204−1.932) 0.417 0.862 (0.248−2.999) 0.815 Baseline ART regimens LPV/r-based 1 − 1 − NVP-based 3.782 (1.272−11.248) 0.017 4.350 (1.193−15.860) 0.026 EFV-based 1.415 (0.399−5.019) 0.591 1.702 (0.396−7.320) 0.475 TB infection Yes 1 − 1 − No 0.373 (0.112−1.240) 0.108 0.705 (0.188−2.648) 0.605 Opportunistic infection Yes 1 − 1 − No 0.310 (0.145−0.661) 0.002 1.182 (0.466−3.002) 0.725 Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. TB, tuberculosis. HR, hazard ratio. aHR, adjusted hazard ratio. S3. The mortality of children with HIV infection, group by ART regimenand WHO clinical stage
Baseline ART regimen WHO clinical stage Total children, n Dead, n (%) Person-years Dead/1,000 Persons-years (95% CI) P# LPV/r-based I/II 99 1 (1.0) 498.25 2.0 (−1.8−5.8) 0.056 III/IV 32 3 (9.4) 216.47 13.9 (−1.4−29.1) NVP-based I/II 98 3 (3.1) 532.27 5.6 (−0.6−11.9) < 0.001 III/IV 60 14 (23.3) 310.28 45.1 (22.1−68.2) EFV-based I/II 101 1 (1.0) 400.47 2.5 (−2.3−7.3) 0.009 III/IV 51 5 (9.8) 225.93 22.1 (3.2–41.0) Total 441 27 (6.1) 2183.67 12.4 (7.8−16.9) Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavi. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. #P by log-rank test. S4. Comparison of mortality of HIV-infected children, group by WHO clinical stage
WHO clinical stage Baseline ART regimen Total children, n Dead, n (%) Person-years Dead/1000 Persons-years (95% CI) p I/II LPV/r-based 99 1 (1.0) 498.25 2.0 (−1.8 to 5.8) 0.521 NVP-based 98 3 (3.1) 532.27 5.6 (−0.6 to 11.9) EFV-based 101 1 (1.0) 400.47 2.5 (−2.3 to 7.3) III/IV LPV/r-based 32 3 (9.4) 216.47 13.9 (−1.4 to 29.1) 0.018 NVP-based 60 14 (23.3) 310.28 45.1 (22.1 to 68.2) EFV-based 51 5 (9.8) 225.93 22.1 (3.2 to 41.0) Total 441 27 (6.1) 2183.67 12.4 (7.8 to 16.9) Note. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. S5. Comparison of mortality of HIV-infected children receiving different ART regimen, group by WHO clinical stage
WHO clinical stage ART regimen LPV/r-based NVP-based EFV-based χ2 p χ2 p χ2 p I/II LPV/r-based − − 0.853 0.356 0.001 0.980 NVP-based 0.853 0.356 − − 0.682 0.409 EFV-based 0.001 0.980 0.682 0.409 − − III/IV LPV/r-based − − 5.193 0.023 0.889 0.346 NVP-based 5.193 0.023 − − 3.978 0.046 EFV-based 0.889 0.346 3.978 0.046 − − Note. ART, antiretroviral therapy. HIV, human immunodeficiency virus. LPV/r, ritonavir-boosted lopinavir. NVP, nevirapine. EFV, efavirenz. WHO, World Health Organization. -
[1] WHO. Summary of the global HIV epidemic, 2022. Geneve: World Health Organization. https://www.who.int/images/default-source/departments/hiv/summary-of-the-global-hiv-epidemic-2021.png?sfvrsn=73ac5b6a_9. [2022-05-13 [2] Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet, 2004; 364, 1236−43. doi: 10.1016/S0140-6736(04)17140-7 [3] Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med, 2008; 359, 2233−44. doi: 10.1056/NEJMoa0800971 [4] Mulisa D, Tesfa M, Kassa GM, et al. Determinants of first line antiretroviral therapy treatment failure among adult patients on ART at central Ethiopia: un-matched case control study. BMC Infect Dis, 2019; 19, 1024. doi: 10.1186/s12879-019-4651-6 [5] Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med, 2010; 363, 1510−20. doi: 10.1056/NEJMoa1000931 [6] Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med, 2012; 366, 2380−9. doi: 10.1056/NEJMoa1113249 [7] Barlow-Mosha L, Angelidou K, Lindsey J, et al. Nevirapine- versus Lopinavir/ritonavir-based antiretroviral therapy in HIV-infected infants and young children: long-term follow-up of the IMPAACT P1060 randomized trial. Clin Infect Dis, 2016; 63, 1113−21. doi: 10.1093/cid/ciw488 [8] Angdembe MR, Rai A, Bam K, et al. Predictors of mortality in adult people living with HIV on antiretroviral therapy in Nepal: A retrospective cohort study, 2004-2013. PLoS One, 2019; 14, e0215776. doi: 10.1371/journal.pone.0215776 [9] Kibuuka H, Musingye E, Mwesigwa B, et al. Predictors of all-cause mortality among people with human immunodeficiency virus (HIV) in a prospective cohort study in East Africa and Nigeria. Clin Infect Dis, 2022; 75, 657−64. doi: 10.1093/cid/ciab995 [10] Ebissa G, Deyessa N, Biadgilign S. Predictors of early mortality in a cohort of HIV-infected children receiving high active antiretroviral treatment in public hospitals in Ethiopia. AIDS Care, 2015; 27, 723−30. doi: 10.1080/09540121.2014.997180 -
 23156+Supplementary Materials.pdf
23156+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links