-
The side effects of ionizing radiation (IR) in patients receiving radiotherapy and the risks of exposure to IR for nuclear workers are inevitable. The methods to protect and mitigate radiation-induced injury in humans are still not adequate. High-dose IR can result in severe acute radiation syndrome (ARS)[1]. Depletion of peripheral blood cells and immunosuppression are the main characteristics of ARS[1]. Several compounds can reduce radiation-induced hematopoietic injury. Prolonged administration of hematopoietic growth factors and cytokines could exhaust the reservoir of hematopoietic stem and progenitor cells, as well as causing proinflammatory and immunogenic effects[2]. With the exception of several cytokines, such as granulocyte colony-stimulating factor (FDA approved for treatment of accidental radiation-induced hematopoietic syndrome), no other drugs have been approved by the FDA for protection against hematopoietic injury[3]. Therefore, there is an urgent need to develop agents to ameliorate radiation-induced hematopoietic and immunological injury to protect against and mitigate the effects of IR exposure.
The extracellular matrix is a major component of the hematopoietic microenvironment, and changes in its structure or function may contribute to development of hematological disorders[4]. Matrix metalloproteinases (MMPs) modulate the proliferation, differentiation, and migration of hematopoietic stem/progenitor cells of different lineages through extracellular matrix proteolysis and release of growth factors/cytokines[5]. In addition, radiation-induced increase of MMP activity causes endothelial cell apoptosis and bone marrow endothelial cells play a critical role in the regulation of hematopoiesis[6]. Therefore, inhibition of MMPs may provide a novel approach for hematopoiesis regulation in myeloproliferative or thrombotic disorders.
Ilomastat is a broad-spectrum MMP inhibitor. It can inhibit human skin fibroblast interstitial collagenase, human neutrophil interstitial gelatinase A and B, and stromelysin[7]. Charrier et al. reported that ACE inhibitors can be used to decrease the hematopoietic toxicity of irradiation[8]. There is also evidence that ilomastat ameliorates oxidative and nitrosative stress in injury models[9]. In the present study, we aimed to establish the radioprotective efficacy of ilomastat in ameliorating ARS and the protective and mitigative effects on survival of mice after total body irradiation (TBI).
All animals were kept in the Laboratory Animal Center, Academy of Military Medical Sciences (Beijing, China) for 1 week prior to initiation of the study. Ilomastat (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in Tween-80, PEG4000, absolute ethanol and distilled water. The drug concentration was 10, 50, or 150 mg/kg. The mice were administered ilomastat 2 h before mice were exposed to 6 Gy γ-radiation (a conventional dose for most radiation protection or mitigation research). Blood samples were collected from the experimental mice on days 1, 5, 10, 20, and 30 post-TBI to analyze the various hematological parameters. For survival analysis, mice were received TBI with 4-12 Gy γ-rays. The results were expressed as the mean ± standard deviation (SD) from at least three independent mice. The data were analyzed by Student's t-test or one-way analysis of variance. P < 0.05 was considered statistically significant.
All mice treated with different dosed of ilomastat showed faster recovery, especially at day 20, for peripheral blood cell count compared to the irradiation alone group (Figure 1A). In particular, ilomastat accelerated recovery of the white blood cell WBC count in the peripheral blood of irradiated mice. The red blood cell (RBC) count was significantly recovered at day 20 after TBI when the mice were administered 10 mg/kg ilomastat 2 h prior to TBI. In contrast, the RBC count decreased to a minimum at day 20, and then recovered at day 30 in the mice that received irradiation without ilomastat (Figure 1B). Similarly, the hemoglobin levels in the irradiated mice without Ilomastat pretreatment decreased at days 5 and 10, and reached a minimum at day 20. In mice pretreated with ilomastat, there was rapid recovery of hemoglobin (Figure 1C). The platelet levels in the irradiation alone group reached a minimum at day 10 and failed to fully recover at day 20 post-TBI. Ilomastat pretreatment significantly recovered the platelet levels in the irradiated mice at days 10 and 20 (Figure 1D). Pretreatment with 10 mg/kg ilomastat significantly increased lymphocyte count at day 20 post-TBI, compared with the irradiation alone group (P < 0.001, Figure 1E). Pretreatment with 10 and 50 mg/kg ilomastat significantly increased the number of neutrophils at days 1-20 post-TBI (P < 0.001, Figure 1F). The RBC and hemoglobin levels recovered faster with 10 mg/kg ilomastat compared with other doses. Therefore, to reduce drug consumption and any burden on the mice, we primarily used 10 mg/kg for subsequent protection/mitigation experiments.

Figure 1. Ilomastat mitigates TBI-induced pancytopenia in mice. Mice were treated with ilomastat 2 h before TBI with 6 Gy γ-rays. Blood samples were removed at days 1, 5, 10, 20, and 30 post-TBI, and white blood cells (A), RBCs (B), hemoglobin (C), platelets (D), lymphocytes (E), and neutrophils (F) were counted (n = 8). All error bars indicate standard error of the mean (SEM). #, *, △P < 0.05; ##, **, △△P < 0.01; ###, ***, △△△P < 0.001 versus vehicle group. TBI, total body irradiation.
We investigated whether ilomastat pretreatment increased survival of mice after TBI. When the mice were treated with 10 mg/kg ilomastat 2 h prior to irradiation, survival was 90% at day 30 post-TBI, compared to 50% in mice treated with 6 Gy TBI alone (Figure 2A). To assess further the protective efficacy after higher doses of irradiation, we investigated survival of mice pretreated with 10 mg/kg ilomastat within 30 days after 9 Gy TBI (Figure 2B). In this case, ilomastat had a significant protective effect on the survival of mice after TBI. Ilomastat still had a radioprotective effect in mice that received 12 Gy TBI (P < 0.05), although survival at day 18 was 0% (Figure 2C). Ilomastat pretreatment had a protective effect on mice after all doses of TBI (4-12 Gy) (Figure 2D).
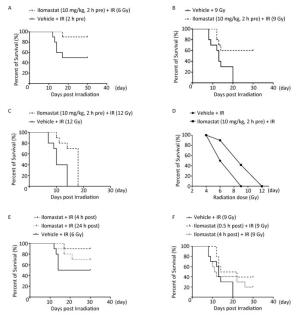
Figure 2. Ilomastat reduces lethality of irradiated mice. Ten mice in each group were administered ilomastat or vehicle 2 h before 4-12 Gy γ-ray TBI and monitored for survival till 30 days. Kaplan-Meier survival curves of mice administered 10 mg/kg ilomastat 2 h before exposure to 6 Gy TBI (A). Kaplan-Meier survival curves of mice administered different doses of ilomastat 2 h before 9 Gy γ-ray TBI (B). Kaplan-Meier survival curves of mice treated with 10 mg/kg ilomastat after 12 Gy γ-ray TBI (C). (D) Dose-response effect of TBI on mouse survival. Survival of mice administered 10 mg/kg ilomastat at 4 and 24 h after 6 (E) and 9 (F) Gy γ-ray TBI.
Our results demonstrated that pretreatment with ilomastat improved survival of mice after TBI. Thus, we sought to determine the mitigatory effects of ilomastat on mice after irradiation. Mice were subjected to 6 and 9 Gy TBI, and we administered 10 mg/kg ilomastat at 0.5, 4, and 24 h post-TBI. Survival was 90% and 70% at day 30 after 6 Gy TBI in mice treated with ilomastat at 4 and 24 h, respectively (Figure 2E). Survival was 40% and 20% at day 30 after 9 Gy TBI in mice treated with ilomastat at 0.5 and 4 h, respectively (Figure 2F). These results demonstrate the mitigatory effects of ilomastat when administered early on irradiation injury, although the survival rates were lower than for pretreatment with ilomastat (10 mg/kg, 90% at day 30 after 6 Gy TBI).
The Mantel-Cox (log-rank) and Gehan-Breslow-Wilcoxon tests for comparison of survival of mice after different doses of TBI plus different treatments with and without ilomastat are shown in Supplementary Table 1 (available in www.besjournal. com). Treatment with ilomastat 2 h prior to irradiation was the optimal time of administration, thus, it was selected to investigate the protective mechanism of ilomastat against radiation injury.
Group P-value Log-rank (Mantel-Cox) Gehan-Breslow-Wilcoxon 10 mg/kg (2 h pre) + 6 Gy 0.0437* 0.0386* 10 mg/kg (2 h pre) + 12 Gy 0.0015** 0.0022** 10 mg/kg (4 h post) + 6 Gy 0.0442* 0.0342* 10 mg/kg (24 h post) + 6 Gy 0.3214 0.2652 10 mg/kg (2 h pre) + 9 Gy 0.0114* 0.0272* 10 mg/kg (0.5 h post) + 9 Gy 0.0347* 0.0706 10 mg/kg (4 h post) + 9 Gy 0.3276 0.7613 Note. Pre, treatment with Ilomastat before IR; Post, treatment with Ilomastat after IR; *P < 0.05 vs. vehicle group; **P < 0.01 vs. vehicle group. Table Supplementary Table 1. Mantel-Cox (log-rank) and Gehan-Breslow-Wilcoxon Tests for Comparing Survival Responses between TBI and TBI + Ilomastat
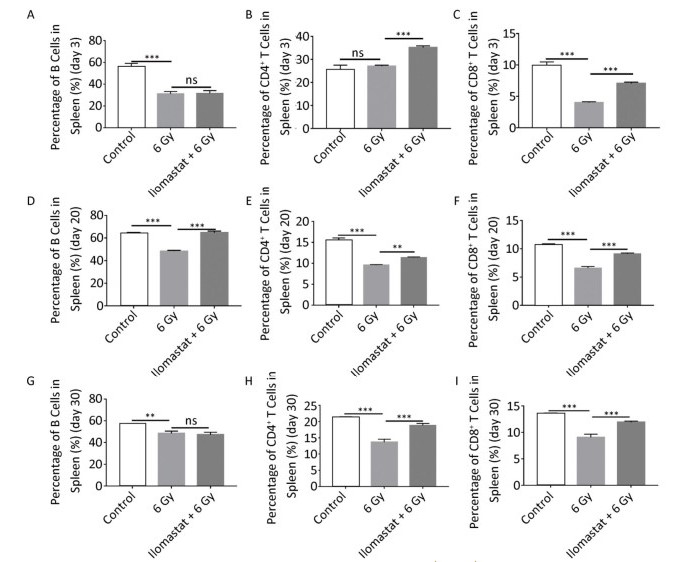
We examined the effects of ilomastat on proliferation of CD4+ T cells, CD8+ T cells, and B cells in the spleen using flow cytometry. C57BL/6 mice were irradiated with 6 Gy and splenic mononuclear cells were analyzed after 3, 20, and 30 days. Although radiation caused a large decline in the percentage of spleen cells, the degree of cytoreduction varied among the different cell subtypes. The CD4+ and CD8+ T lymphocyte subsets demonstrated differential radiosensitivity at day 3: the proportion of CD8+ T cells in the irradiated mice decreased by twofold compared to the control group (Figure 3C, P < 0.01), whereas the proportion of CD4+ T cells remained unchanged (Figure 3B). The proportion of CD8+ T cells in the spleen from mice with ilomastat pretreatment plus irradiation was significantly higher than that in mice treated with irradiation alone (P < 0.01). The proportion of B (CD45+) cells declined from 55.81% in controls to 31.05% in the irradiated mice by day 3. Mice that received 6 Gy TBI had significantly lower levels of B cells compared with control mice at all times (Figure 3A, D, G, P < 0.01). The proportions of B cells and CD4+/CD8+ T cells in the spleen in irradiated mice pretreated with ilomastat were significantly higher than those in mice treated with irradiation alone at day 20 (Figure 3D, F, P < 0.01). At day 30 post-TBI, there was no difference in the proportion of B cells between mice treated with irradiation alone and those pretreated with ilomastat (Figure 3G). However, the proportions of CD4+ and CD8+ T cells in the spleen from mice treated with ilomastat plus irradiation were still significantly higher than those from mice that were treated with irradiation alone (Figure 3H, I, P < 0.01). These results suggest that ilomastat pretreatment improves the proliferation of CD4+/CD8+ T cells and B cells in irradiated mice.
Figure 3. Ilomastat pretreatment improves proliferation of CD4+/CD8+ T cell in irradiated mice. Mice were pretreated with vehicle or 10 mg/kg ilomastat 2 h before exposure to 6 Gy γ-rays. Mice were killed and spleen lymphocytes were collected at days 3, 20, and 30 after TBI. Single cells were stained with APC-CD45R/B220, PE-CD4, FITC-CD8, and PerCP-Cy5-CD3e antibodies. The fractions of B cells are presented (A, D, G; at days 3, 20, and 30, respectively), CD4+ T cells (B, E, H; at days 3, 20, and 30, respectively) and CD8+ T cells (C, F, I; at days 3, 20, and 30, respectively). The data are expressed as mean ± SD (n = 8). All error bars indicate SEM. **P < 0.01; ***P < 0.001.
Some new radioprotective agents have recently been reported[3]. With increasing research about stem cells and extracellular vesicles, novel strategies to improve hematopoietic damage after irradiation have been found. BM-derived mesenchymal stromal cells without additional stem cell transplantation can result in long-term survival of lethally irradiated recipients. Extracellular vesicles can act as an efficient and immediate treatment option after irradiation injuries[10].
Studies in vitro have demonstrated that different lymphoid subsets have opposing effects on hematopoietic cell growth[11]. Hematopoietic recovery in irradiated animals may be influenced by the number of surviving recovery-enhancing and recovery-inhibiting cell types. T lymphocytes consist of two distinct subpopulations: a radioresistant population that can enhance hematopoietic recovery, and a radiosensitive population that can suppress it. The relationship of such subpopulations to the immunoregulatory CD4+ helper and CD8+ suppressor T lymphocytes, however, has not been determined. However, it has been recently reported that CD4+ T cells are stimulators of normal hematopoiesis and recovery following TBI. Our results indicated that ilomastat protected against radiation-induced damage by stimulating the proliferation and differentiation of immune cells. Therefore, the immunostimulatory role of ilomastat may be important for its radioprotective efficacy.
This study clearly demonstrated that ilomastat pretreatment effectively mitigated radiation-induced hematopoietic injury and accelerated recovery of peripheral blood cells. Most importantly, ilomastat enhanced immunoprotection by upregulating the proportions of B and T lymphocytes in the spleen. Survival of mice after TBI indicated the potential of ilomastat to protect and mitigate against the effects of exposure to IR. However, further studies are necessary to elucidate the cascade of events following lymphocyte activation by ilomastat to understand the detailed mechanism of ilomastat-mediated immunoregulation post-TBI.
HTML
a Military Medical Scientific and Technological Project for the 'Twelfth Five-year Plan' AWS15J007
grants from the National Key Scientific Instrument and Equipment Development Project of China 2012YQ03014210
the National Natural Science Foundation of China 11635013
the National Natural Science Foundation of China 81173572
grants from the National Key Scientific Instrument and Equipment Development Project of China 2012YQ03014211
the Foundation of General Logistics Department of PLA DWS16J007








 Quick Links
Quick Links
 DownLoad:
DownLoad: