-
Cadmium (Cd) is a major toxic metal and environmental pollutant, targeting the kidney, lung, liver, testes, and ovaries, after either acute or chronic exposure, and causing serious toxicity damage, specifically nephrotoxicity[1-3]. Cadmium is also a potent carcinogen in several tissues and has been listed by the IARC as a human carcinogen[4].
Several studies have demonstrated that cadmium-induced toxicity damage may be closely related to oxidative stress. The accumulation of cadmium in the body can induce excessive amounts of reactive oxygen species (ROS) that elicit oxidative stress damage, resulting in an imbalance between oxidative stress and antioxidant defense system and cell apoptosis[5, 6]. Therefore, oxidative stress is considered to be an important toxicological mechanism of cadmium.
Considering the important role of oxidative stress in the toxicological mechanism of cadmium, with the deepening of understanding, how the body regulates oxidative stress through self-regulation has become a hotspot in the study of toxicological mechanism of cadmium. It has recently been reported that nuclear factor E2-related factor 2 (Nrf2) is a central regulator of cellular antioxidant stress, which can effectively scavenge free radicals, fight carcinogenic intermediates, regulate the expression of antioxidant enzymes, and play an important role in cell-mediated defense against oxidative stress[7]. Under normal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), which functions as a negative regulator and drives Nrf2 toward rapid degradation through the ubiquitin proteasome system[8]. Under oxidative stress condition, Nrf2 dissociates from Keap1 and translocates into the nucleus where it forms a heterodimer with Maf protein and binds to the antioxidant response element (ARE)[9] and further regulates the expression of its major phase Ⅱ enzymes, such as glutathione S-transferase subunit-P1 (GST-P1), glutamate cysteine ligase catalytic subunit (GCLC), NAD(P)H:quinine oxidoreductase 1 (NQO1), and heme oxygenase 1 (HO-1), to exert protection against chemical toxicity and enhance the antioxidant activity of cells and tissues[10-12]. Regarding the effects of cadmium on the Nrf2-ARE signaling pathway, there have been some achievements in recent years; however, the regulation mechanism of cadmium on the Nrf2-ARE signaling pathway has not yet been completely elucidated, thus necessitating additional research.
Recent investigation has indicated that, in addition to oxidative stress, cadmium causes endoplasmic reticulum stress (ERS) in vitro and in vivo, which also plays a critical role in the toxic effects of cadmium[13, 14]. The endoplasmic reticulum is a vital organelle and is the site of protein synthesis and folding. When cells are stimulated by different intensities in vitro and in vivo, unfolded or misfolded proteins in the endoplasmic reticulum will accumulate in higher amounts and induce unfolded protein response (UPR) and endoplasmic reticulum stress (ERS)[15]. BiP is strongly involved in the UPR, which is activated to offset stress-induced accumulation of unfolded or misfolded proteins in the ER lumen. In this cellular stress response, BiP binds to unfolded proteins, preventing their aggregation and then refolding proteins in an ATP-dependent pattern[16]. Furthermore, three ER stress receptor proteins, namely, PKR-like ER kinase (PERK), inositol-requiring kinase 1 (IRE1), and activating transcription factor 6 (ATF6), which are normally inhibited due to their association with BiP, are activated as BiP releases them to bind with unfolded or misfolded ER proteins[16]. PERK, an important factor in the UPR, belongs to type Ⅰ transmembrane protein. Under ERS condition, activation of PERK leads to the phosphorylation of eukaryotic translation initiation factor 2ɑ (eIF2ɑ), which leads to a general inhibition of protein synthesis and reduces the burden of endoplasmic reticulum[13]. Recent studies have demonstrated that Nrf2, a PERK substrate, can be directly phosphorylated by PERK, and PERK leads to the nuclear accumulation of Nrf2 and induces the transcription of Nrf2 target genes[17]. These findings indicate that ERS is closely related to the Nrf2 signaling pathway. Our previous study reported that cadmium can induce ERS and activate Nrf2 signaling pathway in the kidneys of rats[18]. However, in cadmium-induced toxicity, the regulation mechanism of ERS on the Nrf2 signaling pathway still remains unclear. Therefore, this study was conducted with a focus on the regulation of ERS on the Nrf2 signaling pathway by investigating the effect of PERK on the expression of Nrf2 signaling pathway.
The aim of this study was to further investigate the regulation mechanism of ERS on the Nrf2 signaling pathway by combining PERK and Nrf2 in the kidneys of rats. More specifically, we applied an ERS activator and an inhibitor to upregulate and downregulate the expression levels of endoplasmic reticulum stress-related factors, respectively, and then observe the expression changes of Nrf2, thereby studying the regulation of ERS on the Nrf2 signaling pathway. This would be helpful to provide a theoretical basis and strategies for the prevention and treatment of human damage occurring due to cadmium exposure.
-
Cadmium chloride (CdCl2), tauroursodeoxycholic acid (TUDCA), and bacitracin were purchased from Sigma-Aldrich (USA). Antibodies for Nrf2, Bip, PERK, and β-actin were purchased from Proteintech Group (USA). Peroxidase-conjugated goat anti-mouse IgG and peroxidase-conjugated goat anti-rabbit IgG were purchased from Proteintech Group (USA). Prestained Protein Marker was purchased from Thermo Fisher Scientific (USA). Reverse transcription reagents were purchased from TaKaRa (Japan). SYBR green RT-PCR kit was purchased from Applied Biosystems (USA). RIPA lysis buffer was purchased from Beyotime (China). All other chemicals were of reagent grade.
-
Healthy male and female SD rats, weighing 180-220 g, were obtained from Guangdong Provincial Medical Laboratory Animal Center, China. The laboratory animal production license number was SCXK(Yue)2013-0002, and the experimental animal license was SYXK(Yue)2014-0137. All animal treatment procedures were approved by the Ethical Committee of Guangzhou Quality Supervision and Testing Institute (serial number: 2016-03-08). The animals were kept under standard laboratory conditions and were supplied with standard pelleted diet and water. The SD rats were quarantined for 7 days before the experiment, and healthy rats were selected for the experiment.
-
The experiment was designed to include the control group, CdCl2-alone groups, ERS activator bacitracin pretreatment groups, and ERS inhibitor TUDCA pretreatment groups. All reagents were injected into the animals by the intraperitoneal route at a dose of 10 mL/kg body weight for 48 h.
The rats were divided into 12 groups of six animals each. Treatments were done as follows:
Group 1: Control group. Rats were injected with 0.9% physiological saline.
Groups 2-4: CdCl2 groups. Rats were injected at doses of 5, 10, or 20 μmol/kg body weight (bw) CdCl2 for 48 h.
Group 5: Bacitracin group. Rats were injected at a dose of 50.0 mg/kg body weight bacitracin for 48 h.
Groups 6-8: Bacitracin+CdCl2 groups. Rats were pre-administered with bacitracin (50.0 mg/kg·bw) for 120 min before they were injected with CdCl2 (5, 10, or 20 μmol/kg·bw) for 48 h.
Group 9: TUDCA group. Rats were injected at a dose of 50.0 mg/kg·bw TUDCA for 48 h.
Groups 10-12: TUDCA+CdCl2 groups. Rats were pre-administered with TUDCA (50.0 mg/kg·bw) for 120 min before they were injected with CdCl2 (5, 10, or 20 μmol/kg·bw) for 48 h.
-
Total RNA was extracted from the rat kidney tissues using TRIzol. The total RNA was reverse-transcribed into cDNA using TaKaRa reverse transcription reagents. The expression level of target genes was detected by RT-PCR using Applied Biosystems SYBR green RT-PCR kit on Applied Biosystems 7500HT Fast Real-Time PCR System. The PCR conditions were as follows: initial denaturation at 95 ℃ for 2 min, followed by 40 cycles at 95 ℃ for 15 s, and 60 ℃ for 1 min. The differences between samples were determined using the comparative Ct method. The levels of Bip, PERK, ATF4, Nrf2, GCLC, GST-P1, HO-1, and NQO1 mRNA were measured. The sequences of the primers used in this study are listed in Table 1.
Table 1. Primer Sequences of Bip, PERK, ATF4, Nrf2, GST-P1, GCLC, HO-1, NQO1, and GAPDH
cDNA Primer Sequences Bip F 5'-CATCACGCCGTCCTATGTCG-3' R 5'-CGTCAAAGACCGTGTTCTCG-3' PERK F 5'-GGAAACGAGAGCCGGATTTATT-3' R 5'-ACTATGTCCATTATGGCAGCTTC-3' ATF4 F 5'-ATGACCGAAATGAGCTTCCTG-3' R 5'-GCTGGAGAACCCATGAGGT-3' Nrf2 F 5'-TCAGCGACGGAAAGAGTATGA-3' R 5'-CCACTGGTTTCTGACTGGATGT-3' GST-P1 F 5'-GGCCCACCTAGCCATCAATG-3' R 5'-CGCTGAATGCAGTTGAAGATGT-3' GCLC F 5'-GGAGACCAGAGTATGGGAGTT-3' R 5'-CCGGCGTTTTCGCATGTTG-3' HO-1 F 5'-GAGCAGAACCAGCCTGAACTA-3' R 5'-GGTACAAGGAAGCCATCACCA-3' NQO1 F 5'-ACCCCACTCTATTTTGCTCC-3' R 5'-ACTTACTCCTTTTCCCATCCTC-3' GAPDH F 5'-CTGGGCTACACTGAGCACC-3' R 5'-AAGTGGTCGTTGAGGGCAATG-3' -
The kidney tissues were lysed with RIPA lysis buffer containing 1 mmol/L PMSF. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a PVDF transfer membrane, and blocked with 5% skim milk in TBST buffer for 1 h. The membranes were incubated overnight with anti-Nrf2, anti-Bip, anti-PERK, and anti-β-actin antibodies at 4 ℃. Then, the membranes were washed three times in TBST and incubated with peroxidase-conjugated secondary antibody for 1 h at room temperature. The membranes were again washed three times in TBST, and the blots were developed using ECL chemiluminescence solutions. Band intensities were quantified by the Tanon 5200 Multi fluorescence image analysis system (Tanon, Shanghai, China).
-
Data were expressed as mean ± standard deviation. Data analysis was conducted using one-way ANOVA, and comparisons between groups were performed using Student-Newman-Keuls (SNK) post hoc test. SPSS 17.0 was used for all statistical analyses. Values were considered to be statistically significant when P < 0.05.
-
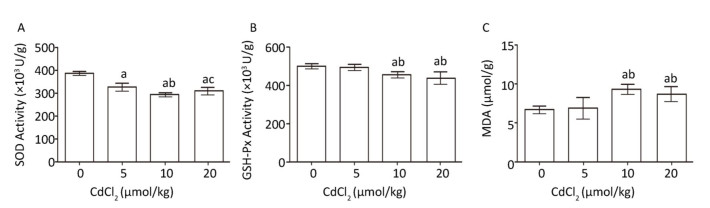
To examine the effect of cadmium on oxidative stress in the kidneys of rats, the activities of SOD and GSH-Px and the MDA content were analyzed using kits. The results revealed that after 48-h treatment with cadmium, there was a significant decrease in the activity of SOD (P < 0.05) when exposed to 5, 10, and 20 μmol/kg Cd compared to that in the control group (Figure 1A). The activity of GSH-Px was significantly decreased (P < 0.05), whereas the MDA content obviously increased (P < 0.05), in the 10 and 20 μmol/kg dose groups compared to those in the control group and the 5 μmol/kg dose group (Figure 1B and C).

Figure 1. Effect of cadmium on oxidative stress in kidneys of rats. (A) SOD activity, (B) GSH-Px activity, (C) MDA content. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
-
To investigate the effect of cadmium on ERS in the kidneys of rats, RT-PCR and western blot analysis were performed to examine the mRNA expression levels of Bip, PERK, and ATF4 and the protein expression levels of Bip and PERK. The results showed that Cd treatment significantly increased (P < 0.05) the mRNA expression levels of Bip, PERK, and ATF4 in all the Cd-exposed groups, especially in the high-dose group, and the mRNA expression levels of Bip, PERK, and ATF4 were significantly increased (Figure 2A, B, and C). The mRNA expression of ATF4 showed a gradually increasing trend with the increase in the Cd exposure dose (Figure 2C).
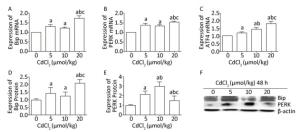
Figure 2. Effect of cadmium on endoplasmic reticulum stress in kidneys of rats. The mRNA expression levels of Bip (A), PERK (B), and ATF4 (C) were measured using RT-PCR analysis. Western blot analysis was performed to examine the protein expression levels of Bip (D) and PERK (E). (F) The protein expression levels of Bip and PERK. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
The protein expression of Bip was significantly increased (P < 0.05) in the Cd-exposed groups compared to that in the control group (Figure 2D and F). However, the protein level of PERK significantly increased initially and then decreased (P < 0.05), and in the medium-dose group, the protein expression of PERK was higher than that in the other groups (Figure 2E and F).
-
The mRNA level of Nrf2 was obviously increased (P < 0.05) in the Cd-exposed groups compared to that in the control group, and the mRNA expression of Nrf2 exhibited a gradually increasing trend with the increase in the Cd exposure dose (Figure 3A). The protein expression of Nrf2 was significantly increased (P < 0.05) in the 10 μmol/kg dose group compared to that in the control group. However, in the 20 μmol/kg dose group, the protein expression of Nrf2 was significantly lower (P < 0.05) than that in the 10 μmol/kg dose group (Figure 3F and G).
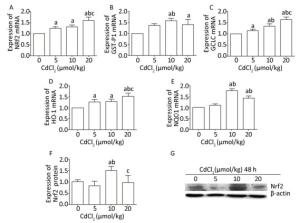
Figure 3. Effect of cadmium on Nrf2 signaling pathway in the kidneys of rats. The mRNA expression levels of Nrf2 (A), GST-P1 (B), GCLC (C), HO-1 (D), and NQO1 (E) were measured using RT-PCR analysis. Western blot analysis was performed to examine the protein expression of Nrf2 (F). (G) The protein expression of Nrf2. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
Cd treatment significantly increased (P < 0.05) the mRNA expression levels of GST-P1 and NQO1 in the 10 and 20 μmol/kg dose groups, whereas in the 20 μmol/kg dose group, the mRNA expression levels of GST-P1 and NQO1 were slightly decreased compared to those in the 10 μmol/kg dose group (Figure 3B and E). The mRNA levels of GCLC and HO-1 in the Cd-exposed groups were significantly higher (P < 0.05) than those in the control group, and in the high-dose group, the mRNA expression levels of GCLC and HO-1 were the highest (P < 0.05) (Figure 3C and D).
-
To evaluate the regulation of ERS on the Nrf2 signaling pathway, we applied bacitracin and TUDCA to upregulate and downregulate the expression of endoplasmic reticulum stress-related factors, respectively, and then observe the expression changes of Nrf2. Results showed that after pretreatment with bacitracin, Cd obviously increased (P < 0.05) the mRNA expression of Bip in the 5, 10, and 20 μmol/kg dose group compared to that in the bacitracin reagent group, and the mRNA expression of Bip in the 10 μmol/kg dose bacitracin+Cd group was significantly higher (P < 0.05) than that in the same-dose Cd treatment group (Figure 4A). The protein expression of Bip was significantly increased (P < 0.05) in the high-dose group compared to that in the bacitracin reagent group. However, in the 5 μmol/kg dose bacitracin+Cd group, the protein expression of Bip was significantly decreased (P < 0.05) compared to that in the same-dose Cd treatment group (Figure 4D and G).
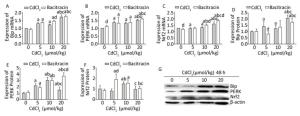
Figure 4. Effects of cadmium on endoplasmic reticulum stress and Nrf2 in kidneys of rats after pretreatment with bacitracin. The mRNA expression levels of Bip (A), PERK (B), and Nrf2 (C) were measured using RT-PCR analysis. Western blot analysis was conducted to examine the protein expression levels of Bip (D), PERK (E), and Nrf2 (F). The protein expression levels of Bip, PERK, and Nrf2 (G). Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group. dIndicates statistically significant differences compared with the same-dose Cd treatment group.
The mRNA level of PERK was obviously increased (P < 0.05) in the 5, 10, and 20 μmol/kg dose bacitracin+Cd groups compared to that in the bacitracin reagent group, and the mRNA level of PERK in the bacitracin reagent group was higher (P < 0.05) than that in the blank control group (Figure 4B). The protein expression of PERK was significantly increased (P < 0.05) in all the bacitracin+Cd treatment groups compared to that in the bacitracin reagent group. In the 20 μmol/kg dose bacitracin+Cd group, the protein expression of PERK was obviously higher (P < 0.05) than that in the 20 μmol/kg dose Cd treatment group (Figure 4E and G).
After pretreatment with bacitracin, the mRNA expression of Nrf2 was significantly increased (P < 0.05) in the 10 and 20 μmol/kg dose groups compared to that in the bacitracin reagent and 5 μmol/kg dose bacitracin+Cd groups, and in the 10 and 20 μmol/kg dose bacitracin+Cd groups, the mRNA expression of Nrf2 was significantly higher (P < 0.05) than that in the same-dose Cd treatment groups (Figure 4C). The protein level of Nrf2 was significantly increased (P < 0.05) in the low- and medium-dose bacitracin+Cd groups compared to that in the bacitracin reagent group. Especially, in the low dose bacitracin+Cd group, the protein expression of Nrf2 was markedly increased (P < 0.05) compared to that in the same-dose Cd treatment group (Figure 4F and G).
-
After pretreatment with TUDCA, Cd exposure significantly increased (P < 0.05) the mRNA and protein expression levels of Bip in the 5, 10, and 20 μmol/kg dose groups. In the high-dose (20 μmol/kg) TUDCA+Cd group, the mRNA and protein expression levels of Bip were found to be significantly lower (P < 0.05) than those in the 20 μmol/kg dose Cd treatment group. At the same time, the protein expression of Bip was significantly decreased (P < 0.05) in the TUDCA reagent and 5 μmol/kg dose TUDCA+Cd groups compared to that in the blank control group (Figure 5A, D, and G).
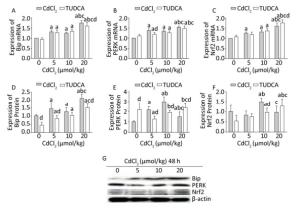
Figure 5. Effects of cadmium on endoplasmic reticulum stress and Nrf2 in kidneys of rats after pretreatment with TUDCA. The mRNA expression levels of Bip (A), PERK (B), and Nrf2 (C) were measured using RT-PCR analysis. Western blot analysis was used to examine the protein expression levels of Bip (D), PERK (E), and Nrf2 (F). The protein expression levels of Bip, PERK, and Nrf2 (G). Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group. dIndicates statistically significant differences compared with the same-dose Cd treatment group.
The mRNA expression of PERK was markedly increased (P < 0.05) in the 10 and 20 μmol/kg dose TUDCA+Cd groups compared to that in the TUDCA reagent group. After pretreatment with TUDCA, the mRNA expression of PERK was significantly decreased (P < 0.05) in the 5 μmol/kg dose TUDCA+Cd group compared to that in the 5 μmol/kg dose Cd treatment group (Figure 5B). The protein expression of PERK showed an abnormal increase (P < 0.05) in the TUDCA reagent group, and in the 5 and 10 μmol/kg dose TUDCA+Cd groups, the protein expression was markedly lower (P < 0.05) than that in the same-dose Cd treatment groups. However, in the 20 μmol/kg dose TUDCA+Cd group, the protein level of PERK was higher (P < 0.05) than that in the 20 μmol/kg dose Cd treatment group (Figure 5E and G).
The mRNA level of Nrf2 was significantly increased (P < 0.05) in the 10 and 20 μmol/kg dose TUDCA+Cd groups compared to that in the TUDCA reagent group. After pretreatment with TUDCA, Cd exposure did not decrease the mRNA expression of Nrf2 compared to that in the same-dose Cd treatment groups, whereas it increased the mRNA expression of Nrf2 in the 20 μmol/kg dose TUDCA+Cd group compared to that in the 20 μmol/kg dose Cd treatment group (Figure 5C). The protein expression of Nrf2 was significantly increased (P < 0.05) in the 10 and 20 μmol/kg dose TUDCA+Cd groups compared to that in the TUDCA reagent group, and in the 10 μmol/kg dose TUDCA+Cd group, the protein expression of Nrf2 was obviously lower (P < 0.05) than that in the 10 μmol/kg dose Cd treatment group (Figure 5F and G).
-
Occupational cadmium exposure and environmental cadmium contamination can cause cadmium poisoning, in which kidney damage is the primary clinical manifestation[19, 20]. It is generally believed that the toxicological effects of cadmium are closely related to oxidative stress[21]. Recent studies have reported that ERS can induce oxidative stress. It is worth noting that the endoplasmic reticulum signal factor PERK can directly activate Nrf2, which is a key factor in the body's endogenous antioxidant stress, and thus plays a role in antioxidative stress[22]. However, in combination with PERK and Nrf2, the regulation mechanism of ERS on the Nrf2 signaling pathway in cadmium-induced toxicity has not yet been reported. In the present study, we examined the effects of cadmium on endoplasmic reticulum stress and Nrf2 signaling pathway in the kidneys of rats and applied an activator and an inhibitor of ERS to investigate the regulation of ERS on the Nrf2 signaling pathway. Our results demonstrated that Cd exposure led to oxidative stress in the kidneys of rats and upregulated the expression of ERS-related factors and Nrf2 signaling pathway-related factors, and especially in the 10 and 20 μmol/kg dose Cd treatment groups, the expression changes were particularly obvious. On the one hand, after pretreatment with bacitracin, Cd exposure upregulated the expression of ERS-related factors to a certain extent, and at higher doses, the mRNA expression of Nrf2 was increased, but at a low dose, the changes were not significant. On the other hand, the co-treatment of TUDCA and Cd was able to reduce the level of ERS to a certain extent; however, there were no significant changes in the expression of Nrf2.
Several studies have reported that oxidative stress is an important component of the mechanism of cadmium poisoning. Cadmium can induce the production of oxygen free radicals, interfere with the balance between oxidative and antioxidant systems in the body, and produce MDA and inhibit the activities of antioxidant enzymes[23-25]. This study demonstrated that the cadmium treatment groups exhibited an inhibition of SOD and GSH-Px activities and an increase in the expression of MDA at medium and high doses. Nrf2 is a critical regulator of the cellular antioxidant response[26]. Chen et al.[7] demonstrated that treatment with 10 μmol/L Cd for 5 h resulted in a significant increase in the Nrf2-ARE-binding activity. In addition, a number of in vitro and in vivo studies have shown that downstream phase Ⅱ detoxification enzymes are regulated by Nrf2, and activated Nrf2 can upregulate the transcriptional expression of phase Ⅱ detoxification enzymes such as GST-P1, GCLC, HO-1, and NQO1, thereby responding to oxidative stress[27-29]. Our study results are largely consistent with previous research, wherein the administration of Cd significantly increased the expression of Nrf2 and further upregulated the expression of downstream phase Ⅱ detoxification enzymes. However, at high doses, the protein expression of Nrf2 was significantly decreased, which was inconsistent with the mRNA expression of Nrf2. It is speculated that the high-dose of Cd caused the kidneys of rats to be in a state of severe oxidative stress, thereby increasing the consumption of Nrf2 protein to alleviate the oxidative damage. This result further confirms that Nrf2-ARE signaling is a crucial regulator for cells to maintain the oxidant and antioxidant balance.
Some studies have reported that cadmium-induced ER stress and significantly upregulated ER stress markers GRP78/Bip and then activated the PERK-eIF2α pathway, which results in selective induction of the transcription factor ATF4[30, 31]. Similar results were observed in our study. The cadmium treatment groups exhibited a significant increase in the expression levels of Bip, PERK, and ATF4. These results indicate that cadmium not only induces oxidative stress but also causes ERS, both of which are important mechanisms of the toxicological effects of cadmium[32].
A majority of studies have confirmed that PERK is closely related to the expression of Nrf2[17, 22, 33]. However, there is a lack of studies on the PERK-Nrf2 signaling pathway in terms of the mechanism of cadmium toxicity. To investigate the regulation of ERS on the Nrf2 signaling pathway in cadmium toxicity, the ERS activator bacitracin[34, 35] and the inhibitor TUDCA[36, 37] were used to upregulate and downregulate the expression levels of endoplasmic reticulum stress-related factors, respectively, and then observe the expression changes of Nrf2. Our results revealed that after pretreatment with bacitracin, the level of ERS was not significantly increased in each dose group; however, in the 20 μmol/kg dose bacitracin+Cd group, the expression of PERK protein was significantly increased compared to that in the same-dose Cd treatment group, and at this dose, the expression of Nrf2 mRNA was also increased. This result suggests that PERK can activate Nrf2, and ERS is involved in the regulation of the Nrf2 signaling pathway. After pretreatment with TUDCA, the level of ERS was downregulated significantly. In the 5 and 10 μmol/kg TUDCA+Cd groups, the expression of PERK protein was decreased significantly compared to that in the same-dose Cd treatment group. However, at these doses, the expression change of Nrf2 mRNA was not obvious, and PERK had little effect on the regulation of Nrf2. In addition, in the 20 μmol/kg dose TUDCA+Cd group, the expression of PERK protein was abnormally increased compared to that in the same-dose Cd treatment group. Moreover, at this dose, there was a similar increase in the expression of Nrf2 mRNA. Currently, several studies have demonstrated that PERK can activate Nrf2 and initiate Nrf2 signaling pathway. Some studies have also indicated that the lack of PERK will block the transcriptional activation of Nrf2[17]. However, the results of this study demonstrated that a decrease in the expression of PERK did not significantly inhibit the activity of Nrf2. This result is inconsistent with previous research. We found that ERS is involved in the positive regulation of the Nrf2 signaling pathway, but it had little effect on the negative regulation of the Nrf2 signaling pathway. The specific mechanism underlying this phenomenon still needs further investigation.
In summary, under the experimental conditions of this study, cadmium can result in ERS and oxidative stress in the kidneys of rats, activate Nrf2, and upregulate the transcriptional expression of phase Ⅱ detoxification enzymes. Furthermore, ERS has a positive regulation effect on the Nrf2 signaling pathway, but has little effect on the negative regulation of the Nrf2 signaling pathway in cadmium toxicity. However, there are some limitations in our research. For example, our study used acute toxicity experiment, and it may be more reasonable to investigate the regulation mechanism of cadmium toxicity using a chronic toxicity experiment. Therefore, additional research is needed to verify the results of this study. Furthermore, we also intend to investigate the feedback regulation of ERS by the Nrf2 signaling pathway in cadmium-induced renal toxicity in rats.
doi: 10.3967/bes2019.001
Induction of Endoplasmic Reticulum Stress by Cadmium and Its Regulation on Nrf2 Signaling Pathway in Kidneys of Rats
-
Abstract:
Objective This study was conducted to investigate the regulation of endoplasmic reticulum stress on Nrf2 signaling pathway in the kidneys of rats. Methods Rats were divided into twelve groups of six animals each. Some groups were pre-administered with bacitracin or tauroursodeoxycholic acid (TUDCA), and all of them were treated with 5-20 μmol/kg cadmium (Cd) for 48 h. The oxidative stress levels were analyzed using kits. The mRNA and protein expression levels of endoplasmic reticulum stress-related factors and Nrf2 signaling pathway-related factors were determined using RT-PCR and western blot. Results Cd exposure resulted in oxidative stress in the kidneys of rats and upregulated the expression of endoplasmic reticulum stress (ERS)-related factors and Nrf2 signaling pathway-related factors, especially at doses of 10 and 20 μmol/kg Cd, and the expression changes were particularly obvious. Moreover, after pretreatment with bacitracin, Cd upregulated the expression of ERS-related factors to a certain extent and, at higher doses, increased the mRNA expression of Nrf2. After pretreatment with TUDCA, Cd reduced the level of ERS to a certain extent; however, at these doses, there were no significant changes in the expression of Nrf2. Conclusion Cadmium can result in ERS and oxidative stress in the kidneys of rats, activate Nrf2, and upregulate the transcriptional expression of phase Ⅱ detoxification enzymes under these experimental conditions. ERS has a positive regulation effect on Nrf2 signaling pathway but has little effect on the negative regulation of Nrf2 signaling pathway in cadmium toxicity. 注释:1) AUTHORS' CONTRIBUTIONS: 2) CONFLICT OF INTEREST: -
Figure 1. Effect of cadmium on oxidative stress in kidneys of rats. (A) SOD activity, (B) GSH-Px activity, (C) MDA content. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
Figure 2. Effect of cadmium on endoplasmic reticulum stress in kidneys of rats. The mRNA expression levels of Bip (A), PERK (B), and ATF4 (C) were measured using RT-PCR analysis. Western blot analysis was performed to examine the protein expression levels of Bip (D) and PERK (E). (F) The protein expression levels of Bip and PERK. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
Figure 3. Effect of cadmium on Nrf2 signaling pathway in the kidneys of rats. The mRNA expression levels of Nrf2 (A), GST-P1 (B), GCLC (C), HO-1 (D), and NQO1 (E) were measured using RT-PCR analysis. Western blot analysis was performed to examine the protein expression of Nrf2 (F). (G) The protein expression of Nrf2. Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group.
Figure 4. Effects of cadmium on endoplasmic reticulum stress and Nrf2 in kidneys of rats after pretreatment with bacitracin. The mRNA expression levels of Bip (A), PERK (B), and Nrf2 (C) were measured using RT-PCR analysis. Western blot analysis was conducted to examine the protein expression levels of Bip (D), PERK (E), and Nrf2 (F). The protein expression levels of Bip, PERK, and Nrf2 (G). Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group. dIndicates statistically significant differences compared with the same-dose Cd treatment group.
Figure 5. Effects of cadmium on endoplasmic reticulum stress and Nrf2 in kidneys of rats after pretreatment with TUDCA. The mRNA expression levels of Bip (A), PERK (B), and Nrf2 (C) were measured using RT-PCR analysis. Western blot analysis was used to examine the protein expression levels of Bip (D), PERK (E), and Nrf2 (F). The protein expression levels of Bip, PERK, and Nrf2 (G). Data are expressed as mean ± SD, n = 6. aIndicates statistically significant differences compared with the control group. bIndicates statistically significant differences compared with the 5 μmol/kg dose group. cIndicates statistically significant differences compared with the 10 μmol/kg dose group. dIndicates statistically significant differences compared with the same-dose Cd treatment group.
Table 1. Primer Sequences of Bip, PERK, ATF4, Nrf2, GST-P1, GCLC, HO-1, NQO1, and GAPDH
cDNA Primer Sequences Bip F 5'-CATCACGCCGTCCTATGTCG-3' R 5'-CGTCAAAGACCGTGTTCTCG-3' PERK F 5'-GGAAACGAGAGCCGGATTTATT-3' R 5'-ACTATGTCCATTATGGCAGCTTC-3' ATF4 F 5'-ATGACCGAAATGAGCTTCCTG-3' R 5'-GCTGGAGAACCCATGAGGT-3' Nrf2 F 5'-TCAGCGACGGAAAGAGTATGA-3' R 5'-CCACTGGTTTCTGACTGGATGT-3' GST-P1 F 5'-GGCCCACCTAGCCATCAATG-3' R 5'-CGCTGAATGCAGTTGAAGATGT-3' GCLC F 5'-GGAGACCAGAGTATGGGAGTT-3' R 5'-CCGGCGTTTTCGCATGTTG-3' HO-1 F 5'-GAGCAGAACCAGCCTGAACTA-3' R 5'-GGTACAAGGAAGCCATCACCA-3' NQO1 F 5'-ACCCCACTCTATTTTGCTCC-3' R 5'-ACTTACTCCTTTTCCCATCCTC-3' GAPDH F 5'-CTGGGCTACACTGAGCACC-3' R 5'-AAGTGGTCGTTGAGGGCAATG-3' -
[1] Lee YJ, Lee GJ, Baek BJ, et al. Cadmium-induced up-regulation of aldo-keto reductase 1C3 expression in human nasal septum carcinoma RPMI-2650 cells:Involvement of reactive oxygen species and phosphatidylinositol 3-kinase/Akt. Environ Toxicol Pharmacol, 2011; 31, 469-78. doi: 10.1016/j.etap.2011.03.006 [2] Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol, 2009; 238, 209-14. doi: 10.1016/j.taap.2009.01.029 [3] Yu R, He L, Chen X. Effects of Cadmium on Hepatocellular DNA Damage, Proto-Oncogene Expression and Apoptosis in Rats. Biomed Environ Sci, 2007; 20, 146-53. [4] Waalkes M. Cadmium carcinogenesis. Mutat Res, 2003; 533, 107-20. doi: 10.1016/j.mrfmmm.2003.07.011 [5] Chen S, Ren Q, Zhang J, et al. N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol Appl Neurobiol, 2014; 40, 759-77. doi: 10.1111/nan.2014.40.issue-6 [6] Wang Y, Wu Y, Luo K, et al. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem Toxicol, 2013; 58, 61-7. doi: 10.1016/j.fct.2013.04.013 [7] Chen J, Shaikh ZA. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol Appl Pharmacol, 2009; 241, 81-9. doi: 10.1016/j.taap.2009.07.038 [8] Zheng JL, Yuan SS, Wu CW, et al. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat Toxicol, 2016; 180, 36-44. doi: 10.1016/j.aquatox.2016.09.012 [9] Shinkai Y, Kimura T, Itagaki A, et al. Partial contribution of the Keap1-Nrf2 system to cadmium-mediated metallothionein expression in vascular endothelial cells. Toxicol Appl Pharmacol, 2016; 295, 37-46. doi: 10.1016/j.taap.2016.01.020 [10] Chen M, Li X, Fan R, et al. Selenium antagonizes cadmium-induced apoptosis in chicken spleen but not involving Nrf2-regulated antioxidant response. Ecotoxicol Environ Saf, 2017; 145, 503-10. doi: 10.1016/j.ecoenv.2017.08.001 [11] Staitieh BS, Egea EE, Fan X, et al. Activation of Alveolar Macrophages with Interferon-gamma Promotes Antioxidant Defenses via the Nrf2-ARE Pathway. J Clin Cell Immunol, 2015; 6, 365. [12] Usami H, Kusano Y, Kumagai T, et al. Selective induction of the tumor marker glutathione S-transferase P1 by proteasome inhibitors. J Biol Chem, 2005; 280, 25267-76. doi: 10.1074/jbc.M501014200 [13] Kitamura M, Hiramatsu N. The oxidative stress:endoplasmic reticulum stress axis in cadmium toxicity. Biometals, 2010; 23, 941-50. doi: 10.1007/s10534-010-9296-2 [14] Liu L, Yang B, Cheng Y, et al. Ameliorative Effects of Selenium on Cadmium-Induced Oxidative Stress and Endoplasmic Reticulum Stress in the Chicken Kidney. Biol Trace Elem Res, 2015; 167, 308-19. doi: 10.1007/s12011-015-0314-7 [15] Jin Y, Zhang S, Tao R, et al. Oral exposure of mice to cadmium (Ⅱ), chromium (Ⅵ) and their mixture induce oxidative-and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ Toxicol, 2016; 31, 693-705. doi: 10.1002/tox.v31.6 [16] Shirriff CS, Heikkila JJ. Characterization of cadmium chloride-induced BiP accumulation in Xenopus laevis A6 kidney epithelial cells. Comp Biochem Physiol C Toxicol Pharmacol, 2017; 191, 117-28. doi: 10.1016/j.cbpc.2016.10.003 [17] Cullinan SB, Zhang D, Hannink M, et al. Nrf2 Is a Direct PERK Substrate and Effector of PERK-Dependent Cell Survival. Mol Cell Biol, 2003; 23, 7198-209. doi: 10.1128/MCB.23.20.7198-7209.2003 [18] Chen Z, Chen J, Wu L, et al. Effects of Cadmium on PERK-Nrf2 Signaling Pathway in Kidney of Rats. Acta Med Univ Sci Technol Huazhong, 2018; 47, 59-63. (In Chinese). [19] Dai W, Chen H, Yu R, et al. Effects of cadmium on telomerase activity, expressions of TERT, c-myc and P53, and apoptosis of rat hepatocytes. J Huazhong Univ Sci Technolog Med Sci, 2010; 30, 709-13. doi: 10.1007/s11596-010-0645-8 [20] Wallin M, Sallsten G, Lundh T, et al. Low-level cadmium exposure and effects on kidney function. Occup Environ Med, 2014; 71, 848-54. doi: 10.1136/oemed-2014-102279 [21] Hagar H, Al Malki W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats:role of oxidative stress and caspase-3. Environ Toxicol Pharmacol, 2014; 37, 803-11. doi: 10.1016/j.etap.2014.02.013 [22] Sun F, Li X, Yang C, et al. A role for PERK in the mechanism underlying fluoride-induced bone turnover. Toxicology, 2014; 325, 52-66. doi: 10.1016/j.tox.2014.07.006 [23] El-Boshy ME, Risha EF, Abdelhamid FM, et al. Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol, 2015; 29, 104-10. doi: 10.1016/j.jtemb.2014.05.009 [24] Unsal C, Kanter M, Aktas C, et al. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol Ind Health, 2015; 31, 1106-15. doi: 10.1177/0748233713486960 [25] Veljkovic AR, Nikolic RS, Kocic GM, et al. Protective effects of glutathione and lipoic acid against cadmium-induced oxidative stress in rat's kidney. Ren Fail, 2012; 34, 1281-7. doi: 10.3109/0886022X.2012.723661 [26] Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med, 2004; 37, 433-41. doi: 10.1016/j.freeradbiomed.2004.04.033 [27] Li SA, Jiang WD, Feng L, et al. Dietary myo-inositol deficiency decreased the growth performances and impaired intestinal physical barrier function partly relating to nrf2, jnk, e2f4 and mlck signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol, 2017; 67, 475-92. doi: 10.1016/j.fsi.2017.06.032 [28] Liu M, Reddy NM, Higbee EM, et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemiareperfusion injury in mice. Kidney Int, 2014; 85, 134-41. doi: 10.1038/ki.2013.357 [29] Park JH, Choi JW, Ju EJ, et al. Antioxidant and Anti-Inflammatory Activities of a Natural Compound, Shizukahenriol, through Nrf2 Activation. Molecules, 2015; 20, 15989-6003. doi: 10.3390/molecules200915989 [30] Yan M, Zhang Y, Qin H, et al. Cytotoxicity of CdTe quantum dots in human umbilical vein endothelial cells:the involvement of cellular uptake and induction of pro-apoptotic endoplasmic reticulum stress. Int J Nanomedicine, 2016; 11, 529-42. [31] Yokouchi M, Hiramatsu N, Hayakawa K, et al. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ, 2007; 14, 1467-74. doi: 10.1038/sj.cdd.4402154 [32] Nair AR, Degheselle O, Smeets K, et al. Cadmium-Induced Pathologies:Where Is the Oxidative Balance Lost (or Not)? Int J Mol Sci, 2013; 14, 6116-43. doi: 10.3390/ijms14036116 [33] Zhu YF, Li XH, Yuan ZP, et al. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur J Pharmacol, 2015; 762, 239-46. doi: 10.1016/j.ejphar.2015.06.002 [34] Kim JY, Ko AR, Hyun HW, et al. PDI regulates seizure activity via NMDA receptor redox in rats. Sci Rep, 2017; 7, 42491. doi: 10.1038/srep42491 [35] Muller C, Bandemer J, Vindis C, et al. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal, 2013; 18, 731-42. doi: 10.1089/ars.2012.4577 [36] Ishimura S, Furuhashi M, Mita T, et al. Reduction of endoplasmic reticulum stress inhibits neointima formation after vascular injury. Sci Rep, 2014; 4, 6943. [37] Yan F, Li J, Chen J, et al. Endoplasmic reticulum stress is associated with neuroprotection against apoptosis via autophagy activation in a rat model of subarachnoid hemorrhage. Neurosci Lett, 2014; 563, 160-5. doi: 10.1016/j.neulet.2014.01.058 -




 下载:
下载:



 Quick Links
Quick Links