-
Uranium (U) is a severely poisonous environmental risk factor with three isotopes (238U, 235U, and 234U). The uranium content in the environment has gradually increased, exposing the general population to uranium mining, nuclear testing, and the nuclear industry[1,2]. Soluble uranium (VI) in the aquatic environment can enter the blood circulation of humans (animals) via various exposure routes such as dermal wounding, inhalation, and ingestion, and subsequently accumulates in target organs[3]. Renal failure is a prominent feature of uranium toxicity. Uranium-induced excess reactive oxygen species (ROS) trigger oxidative stress and abnormalities in the antioxidant system of kidney proximal tubule cells, ultimately resulting in nephrotoxicity[4,5]. Considering that ROS and antioxidant activities are valid markers of oxidative stress, researchers have used effective exogenous antioxidants[6] such as melatonin[7], zinc[8], bacterial exopolysaccharides[9], Ginkgo biloba extracts[10], and dietary fish oil[11].
ROS not only directly react with biological macromolecular proteins and DNA, causing cellular damage, but also attack polyunsaturated fatty acids in the biological membrane, inducing lipid peroxidation to form unstable lipid peroxides, which are subsequently decomposed to produce a series of carbonyl group-containing active aldehydes called secondary cytotoxic messengers[12–14]. To date, more than 20 active aldehydes have been identified during lipid peroxidation, among which the most toxic, 4-hydroxynonenal (4-HNE), and abundant malondialdehyde (MDA) are the most representative[15–16]. Under physiological conditions or low oxidative stress, 4-HNE forms a complex with reduced glutathione (GSH) through a non-enzymatic reaction. Then, glutathione S-transferase promotes this reaction and the complex is expelled from cells. Under moderate or high oxidative stress, 4-HNE is detoxified through aldehyde dehydrogenase 2 (ALDH2)- or alcohol dehydrogenase (ADH1)-mediated metabolic pathway[17]. Aldo-keto reductase (AKR7A1) catalyzes the reduction of various active aldehydes to their corresponding alcohols with low or no toxicity and is a key route for clearing active aldehydes[16–18].
Uranium can promote lipid peroxidation in kidney tissue homogenates or cells from animal models, resulting in an increased generation of MDA[8–11,19,20] and thiobarbituric acid reactive substances (TBARS)[7,21]. 4-HNE is also associated with uranium-induced neurotoxicity in rats[22]. MDA and 4-HNE accumulation mediate nephrotoxicity triggered by various stimuli such as nicotine[23], cisplatin[24,25], aflatoxin[26], and cadmium[27]. MDA and 4-HNE irreversibly undergo random nonspecific addition reactions with a wide range of proteins or DNA to generate aldehyde-protein or aldehyde-DNA adducts, resulting in carbonyl stress, which is involved in the cytotoxicity of various cell types caused by alcohol[28] and smoking[29]. Scientists are exploring effective carbonyl-trapping agents to attenuate or prevent carbonyl stress-mediated biological toxicity and disease[30].
Hydrogen sulfide (H2S) is a novel gas neurotransmitter that can freely pass through a membrane. It plays an important role in the regulation of the physiological and pathological functions of the kidney in mammals. Two major enzymes namely, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), participate in endogenous H2S biosynthesis[31,32]. H2S can attenuate kidney damage caused by various toxicants such as uranium, cisplatin, gentamicin, and doxorubicin by increasing antioxidant activity and decreasing ROS levels[33–35]. The protective effects of H2S against oxidative damage involve the transcriptional activator Nuclear Factor E2-related Factor 2 (Nrf2). Under physiological conditions, Nrf2 anchors to the cytoplasm via Kelch-like ECH-associated protein 1 (Keap1). Upon oxidative or electrophilic stress, Nrf2 dissociates from Keap1, translocates into the nucleus, combines with antioxidant response elements (AREs) present in the promoter regions of target genes, and regulates the expression of antioxidant and phase II detoxifying enzymes, resulting in the alleviation of oxidative stress[36]. By inducing these cytoprotective genes, short-term Nrf2 activation protects against oxidative stress-mediated kidney damages[37]. However, constitutive Nrf2 activation not only fails to provide a protective function, but also has some adverse effects, such as cadmium-induced nephrotoxicity[38] and various cancers or diseases triggered by cadmium, arsenic, lead, mercury, nickel, and chromium[39]. The dual role of Nrf2 activation may be present under the circumstances of toxic metal exposure.
Here, we propose that uranium treatment of the rat NRK-52E cells induces lipid peroxidation-mediated carbonyl stress. H2S attenuates the uranium-triggered cytotoxicity by counteracting carbonyl stress. The antagonistic mechanism of H2S may involve the Nrf2-regulated CBS/H2S axis and aldehyde-metabolizing enzymes.
-
Depleted uranyl (VI) acetate was purchased from Shanghai Yien Chemical Technology Co., Ltd. (China). The uranium solution was prepared as described in our previous report[33]. GYY4137 (No. SML0100) and sulforaphane (SFP) (No. S4441) were purchased from Sigma-Aldrich (USA). Aminoguanidine (AG) (No. A151036) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (China). Tempol (No. HY-100561) was purchased from MedChemExpress (USA).
We purchased six antibodies against Nrf2 (No. 66504-1-lg), Keap1 (No. 10503-2-AP), CBS (No. 14787-1-AP), ADH1 (No. 66939-1-lg), ALDH2 (No. 15310-1AP), and AKR7A1 (No. 13209-1-AP) from Proteintech Group Inc. (China); two antibodies against 4-HNE (No. Ab46545) and MDA (No. Ab27643) from Abcam (USA); and two reference antibodies against β-tubulin (No. ABL1030), and Histone H3 (No. ABL1070) from Abbkine Technology Co., Ltd. (China).
The corresponding assay kits for MDA (No. A003-1), GSH (No. A006-1-1), oxidized glutathione (GSSG) (No. A061-2-1), protein carbonylation (PCO) (No. A087-1-1), ROS (No. E004-1-1), BCA (No. A006-1-1), and NE-PER nuclear and cytoplasmic extraction reagents (No. P0028) were obtained from Nanjing Jiancheng Biological Co., Ltd. (China). The TRIzol reagent (No. R411-01), a cDNA synthesis kit (No. R111-01), and TaqPro Universal SYBR qPCR Master Mix (No. Q712-02) were purchased from Nanjing Nuoweizan Biotechnology Co., Ltd (China). Rat ELISA assay kits for TBARS (No. F40591-A), 4-HNE (No. F9058-A), and 8-hydroxydeoxyguanosine (8-OhdG) (No. F3100-A) were purchased from the China Fankew Company.
-
The culture conditions, methods, and steps used for the rat NRK-52E cell line were identical to those described in our previously published paper[40].
-
To determine the effects of uranium on lipid peroxidation-mediated carbonyl stress, the NRK-52E cells were divided into four groups. The cells were exposed to 0, 100, 200, or 400 µmol/L uranium for 24 h. Three uranium concentrations were obviously under the IC50 value of uranium (approximately 500 µmol/L) in the NRK-52E cells[41].
To investigate whether H2S antagonizes uranium-induced carbonyl stress during oxidative stress, the NRK-52E cells were divided into four groups. The cells in the normal group were cultured for 24 h without treatment. The cells in the GYY4137 (Tempol or AG) treatment group were treated with 600 µmol/L GYY4137 (6 mmol/L Tempol or 1 mmol/L AG). The cells in the uranium-exposed group were intoxicated with 400 µmol/L uranium for 24 h. The cells in the GYY4137 (Tempol or Tempol plus AG) and uranium cotreatment group were contaminated with 400 µmol/L uranium for 24 h after pretreatment with 600 µmol/L GYY4137 for 30 min (6 mmol/L Tempol alone for 2 h or 6 mmol/L Tempol plus 1 mmol/L AG together for 2 h).
To explore the protective mechanisms by which H2S attenuates uranium-induced carbonyl stress during oxidative stress, the NRK-52E cells were classified into six groups. The cells in the normal group were cultured for 24 h without treatment. The cells in the SFP administration group were treated with 5 µmol/L SFP. The cells in the CBS siRNA treatment group were treated with 5 µmol/L CBS siRNA. The cells in the uranium-treated group were exposed to 400 µmol/L uranium for 24 h. The cells in the SFP and uranium cotreatment group were exposed to 400 µmol/L uranium for 24 h after pretreatment with 5 µmol/L SFP for 2 h. The cells in the CBS siRNA, SFP, and uranium cotreatment group were contaminated with 400 µmol/L uranium for 24 h after they were administered with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h. All the experiments in our study were performed in triplicate.
-
The NRK-52E cells were seeded at a density of approximately 5,000 cells/well in a 96-well plate. Cell viability was determined using CCK-8 assay following the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader (EPOCH2, BioTek, USA). Cell viability was calculated as a percentage of the control level.
-
According to the instruction of the reagent kit, the total, nuclear, and cytoplasmic proteins were prepared from the NRK-52E cells in different experimental groups and stored at –80 °C for the following experiments. The protein concentration was estimated using bovine serum albumin as a standard protein, based on the BCA kit manufacturer’s instructions.
-
The intracellular ROS concentration was assessed by staining with the fluorescent probe DCFH-DA, according to the manufacturer’s instructions. The fluorescence intensity of ROS was observed and analyzed using a fluorescence microscope (PD80, Olympus, Japan) with Image-Pro Plus software 6.0. ROS levels are shown as percentages of the control levels.
-
Based on the manufacturer’s instructions of the respective kits, the levels of TBARS and 4-HNE were detected using a double-antibody, one-step sandwich enzyme-linked immunosorbent assay. The MDA concentration was determined using the thiobarbituric acid colorimetric method. The absorbance was measured at 450 nm for TBARS and 4-HNE and 532 nm for MDA using a microplate reader. The results of the lipid peroxidation products are expressed as nmol/mg of protein.
-
The PCO and 8-OHdG levels were estimated according to manufacturer’s instructions of the corresponding kits. Absorbance was measured at 370 nm for PCO and 450 nm for 8-OHdG using a microplate reader. The PCO and 8-OHdG levels are expressed as nmol/mg of protein.
-
The GSH and GSSG levels were determined by spectrophotometry according to the manufacturer’s instructions of the respective kits. The absorbance was measured at 420 nm for GSH and 412 nm for GSSG using a microplate reader. The GSH and GSSG levels are expressed as nmol/mg of protein.
-
Zinc acetate trapping and N, N-dimethyl-p-phenylenediamine methods were used to determine the endogenous H2S concentration in the NRK-52E cells. The experimental conditions, methods, and steps were identical to those used in our previous study[33]. The results are presented as nmol/mg of protein.
-
After Annexin V-FITC/PI staining, the NRK-52E cell apoptosis was detected using flow cytometry. The specific detection conditions, methods, and steps were the same as previously described[40]. The number of double-positive nuclei was calculated as a percentage of the total nuclei per field.
-
Based on the nucleotide sequence of the rat CBS mRNA (GenBank No: NP_036654), three pairs of CBS siRNAs were designed using the Primer Blast Website and synthesized by Shanghai Gene Pharma Co., Ltd. (China). After the preliminary experiment, we ultimately selected the pair of CBS siRNAs with the highest inhibitory rate (approximately 55%), the sequences of which are as follows: sense, 5’-CCAUUGACCUGCUAAAC UUTT-3’; and antisense, 5’-AAGUUUAGCAGUCAAGGTT-3’.
According to the kit instructions, 250 μL of Opti-MEM and 5 µmol/L CBS siRNA were mixed lightly in an enzyme-free EP tube. Simultaneously, 250 μL of Opti-MEM and 5 μL of GP-transfer-Mate were mixed in another enzyme-free EP tube and incubated at 25 °C for 5 min. Subsequently, different dilutions of the two EP tubes were gently added and the mixture was incubated at 25 °C for 20 min. When the cell density in the six-well plate reached approximately 60%–70%, kidney cells were transfected with the abovementioned mixture and subsequently cultured in DMEM supplemented with 10% fetal bovine serum and 1% dual antibiotics (penicillin and streptomycin) in a constant-temperature carbon dioxide incubator (37 °C) for 48 h. Finally, total proteins were isolated from the cells to determine the transfection effect. Scrambled and glyceraldehydes-3-phosphate dehydrogenase siRNAs provided by the supplier were used as negative and positive controls, respectively.
-
Total RNA was extracted from kidney cells using the TRIzol reagent according to the manufacturer’s instructions, and the concentration was determined by spectrophotometry. Total RNA (1 μg) was transcribed into cDNA using a cDNA synthesis kit. The total volume of the real-time PCR mixture (20 μL) consisted of 10 μL of SYBR premix, 2 μL of forward and reverse primers, 2 μL of cDNA, and 6 μL of nuclease-free water. PCR amplification was performed using a fluorescence quantitative PCR instrument (C1000 touch). The amplification procedure involved initial denaturation (94 °C, 5 min) and 40 cycles of denaturation (94 °C, 30 s), annealing (55 °C, 30 s), and extension (72 °C, 30 s). The specific primer sequences used to target the rat ADH1, ALDH2, and AKR7A1 genes are listed in Table 1. β-actin was used as an internal reference gene. The relative mRNA expression levels of the target/reference genes were calculated using the standard 2-ΔΔCt method.
Table 1. Primer sequences for the rat ADH1, ALDH2, and AKR7A1 genes
Gene GenBank No. Primer sequence (5’ to 3’) ADH1 NM_019286.4 F 5’- CGTGCTGGAAAGAGTATCCGT-3’
R 5’- GGAGTCAGAAACCGGGAAGG-3’ALDH2 XM_032886762.1 F 5’- GCTGACAAGTACCACGGGAA-3’
R 5’- CGGGAAGTTCCACGGAATGA-3’AKR7A1 NM_013215.2 F 5’- TACACTTTCCAGACCACGGC -3’
R 5’- CTCCACAAACTTGCCCTCCT-3’β-actin NP_112406.1 F 5’-CCCGCGAGTACAACCTTCTT-3’
R 5’-TCATCCATGGCGAACTGGTG-3’Note. ADH1: alcohol dehydrogenase; ALDH2: aldehyde dehydrogenase 2; AKR7A1: Aldo-keto reductase; β-actin: an internal reference gene. -
Total samples (100 μg for 4-HNE), 60 μg for nuclear proteins, or 50 μg for cytoplasmic proteins were electrophoresed, transferred to the PVDF membranes, and washed three times with TBST. The membrane was then completely immersed in primary antibody-containing solution at 4 °C for 16 h. The antibody dilutions used were as follows: MDA (1:2,000), 4-HNE (1:500), ADH1 (1:3,000), ALDH2 (1:2,000), AKR7A1 (1:1,000), Nrf2 (1:2,500), Keap1 (1:3,000), CBS (1:2,000), β-tubulin (1:1,000), and Histone H3 (1:2,000). The membrane was incubated with the secondary antibody-containing solution at 25 °C for 1 h. HRP-conjugated goat anti-mouse IgG was used for the detection of four antibodies against ADH1, Nrf2, histone H3, and β-tubulin. Horseradish peroxidase-conjugated goat anti-rabbit IgG was used for the remaining six antibodies. The protein bands were visualized using a chemiluminescence imager (ChemiDoc™ XRS+, Bole, USA). The gray value of the protein band was detected using ImageJ software (1.46r). The ratio of the gray values of the bands between the target and reference proteins was considered to indicate normalization.
-
Data analysis was performed using IBM SPSS Statistics 23 software. Normality was detected using the Kolmogorov—Smirnov method, and the homogeneity of variance was tested using the Levene method. When the data were normally distributed and had homogeneous variance, one-way analysis of variance was used to analyze the data, and the differences between the two groups were compared using the least significant difference test. Data are expressed as the mean ± standard deviation (x ± SD) of three independent experiments. P < 0.05 was considered to indicate a statistically significant difference.
-
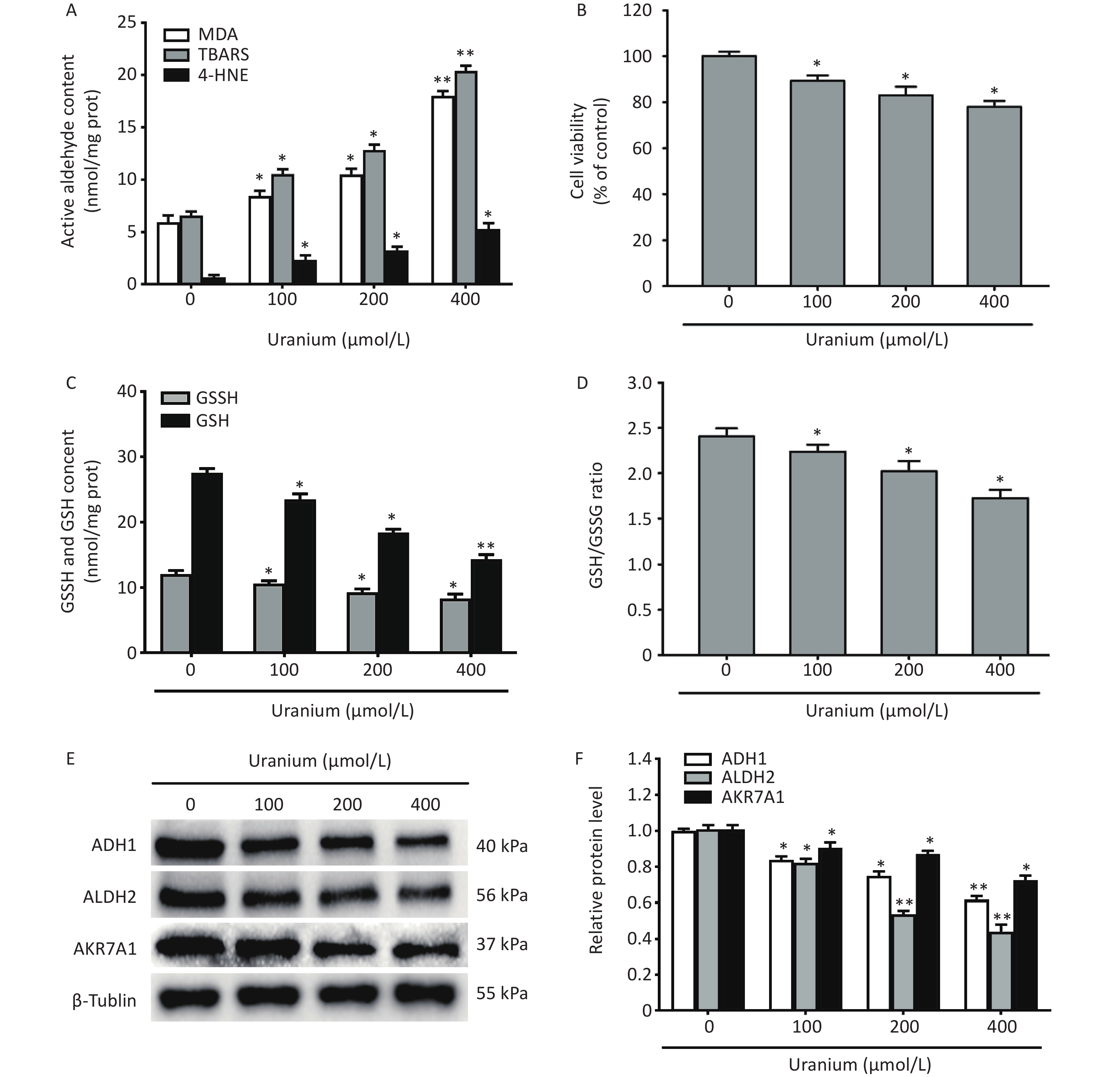
To investigate the effects of uranium on cell viability, the generation of reactive aldehydes, the levels of GSH and GSSG, and the expression of aldehyde-metabolizing enzymes, the NRK-52E cells were treated with different concentrations of depleted uranyl acetate (100, 200, and 400 µmol/L) for 24 h.
Compared with those in the control group, uranium significantly increased the contents of TBARS, MDA, and 4-HNE at a significantly greater rate, and cell viability was lower in the uranium-treated group (*P < 0.05, **P < 0.01) (Figure 1A and B). Moreover, compared to those in the control cells, uranium evidently decreased the levels of GSH and GSSG and the ratio of GSH to GSSG (*P < 0.05, **P < 0.01) (Figure 1C and D). Similarly, compared with those in the control group, the expression of ADH1, ALDH2, and AKR7A1 in the uranium-treated cells was significantly reduced in a concentration-dependent manner (*P < 0.05, **P < 0.01) (Figure 1E and F).
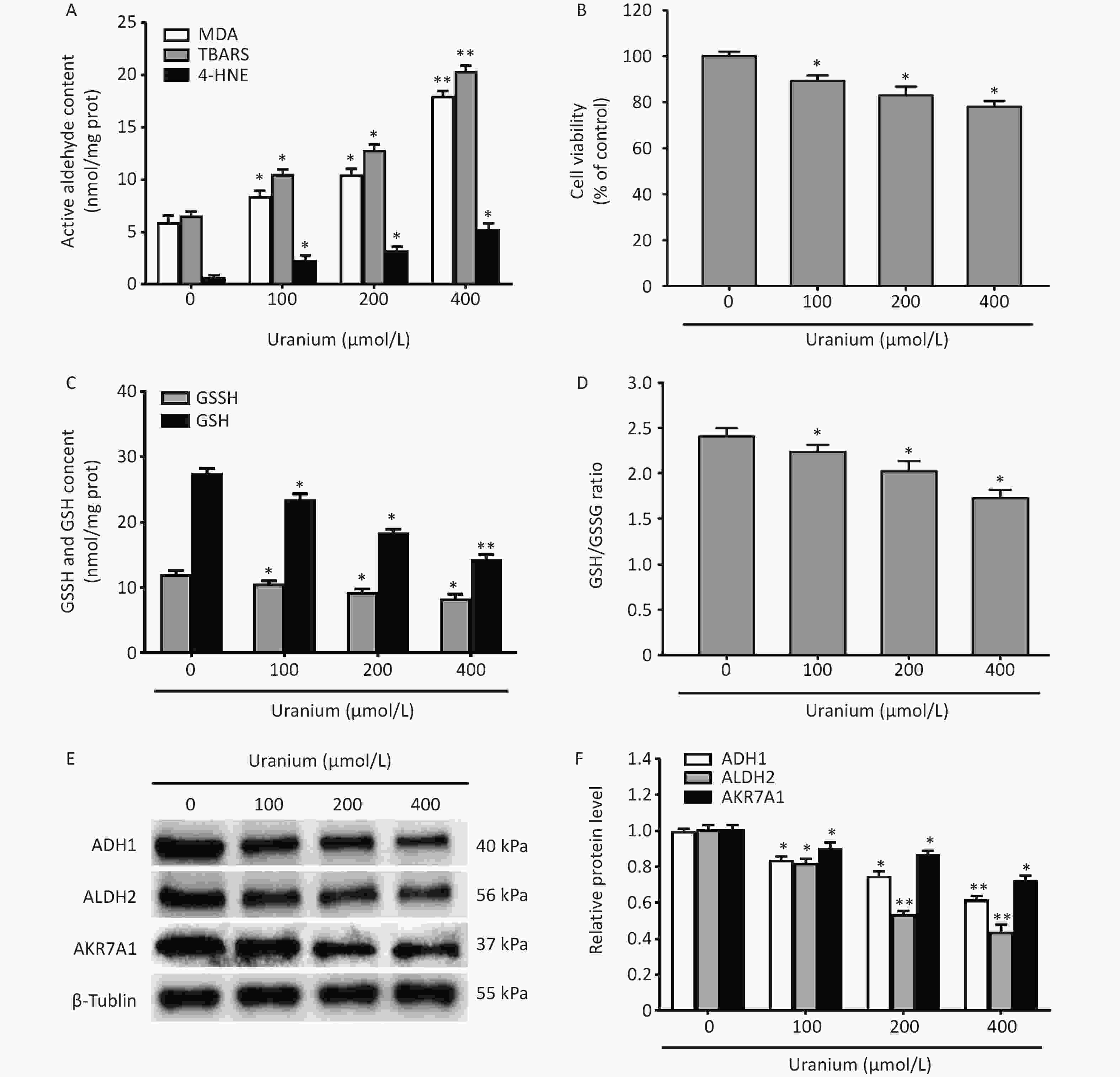
Figure 1. Effects of uranium on the contents of active aldehydes, cell viability, levels of reduced and oxidized glutathione, and expression of aldehyde-metabolizing enzymes in the NRK-52E cells. After kidney cells were exposed to depleted uranyl acetate for 24 h, the contents of thiobarbituric acid reactive substances and 4-hydroxynonenal were detected by double-antibody one-step sandwich enzyme-linked immunosorbent assay. The abundant malondialdehyde level was tested using thiobarbituric acid colorimetric method (A). Cell viability was determined by CCK-8 method (B). Spectrophotometry was used to measure the contents of glutathione (GSH) and oxidized glutathione (GSSG) (C) and the ratio of GSH to GSSG levels is shown (D). Western blots was adopted to determine the expression of alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase with an internal reference β-tubulin (E, F). *P < 0.05, **P < 0.01 compared to the control cells (n = 3).
-
To determine whether uranium triggered the formation of aldehyde-protein adducts and damage to proteins and DNA in kidney cells, different concentrations of depleted uranyl acetate (100, 200, and 400 µmol/L) were used to contaminate the NRK-52E cells for 24 h. Western blotting was used to detect the formation of MDA- and 4-HNE-protein adducts. We also measured carbonyl/oxidative stress markers, including protein carbonylation (PCO, a product of non-enzymatic irreversible carbonyl modification of proteins) and 8-hydroxydeoxyguanosine (8-OHdG), a product of ROS-induced DNA oxidative damage.
We observed that a variety of proteins were modified by MDA and 4-HNE at the three uranium concentrations compared to those in the control cells (Figure 2A and B). Compared to those in the control group, the PCO content in the uranium treatment group significantly increased with increasing uranium concentration (*P < 0.05, **P < 0.01) (Figure 2C), indicating that uranium exposure results in intracellular protein carbonylation. Compared to those in the control cells, the 8-OHdG levels in the uranium-exposed group were significantly higher (*P < 0.05, **P < 0.01), indicating that uranium treatment caused intracellular DNA oxidative damage. The degree of DNA oxidative injury was positively correlated with uranium concentration (Figure 2D).
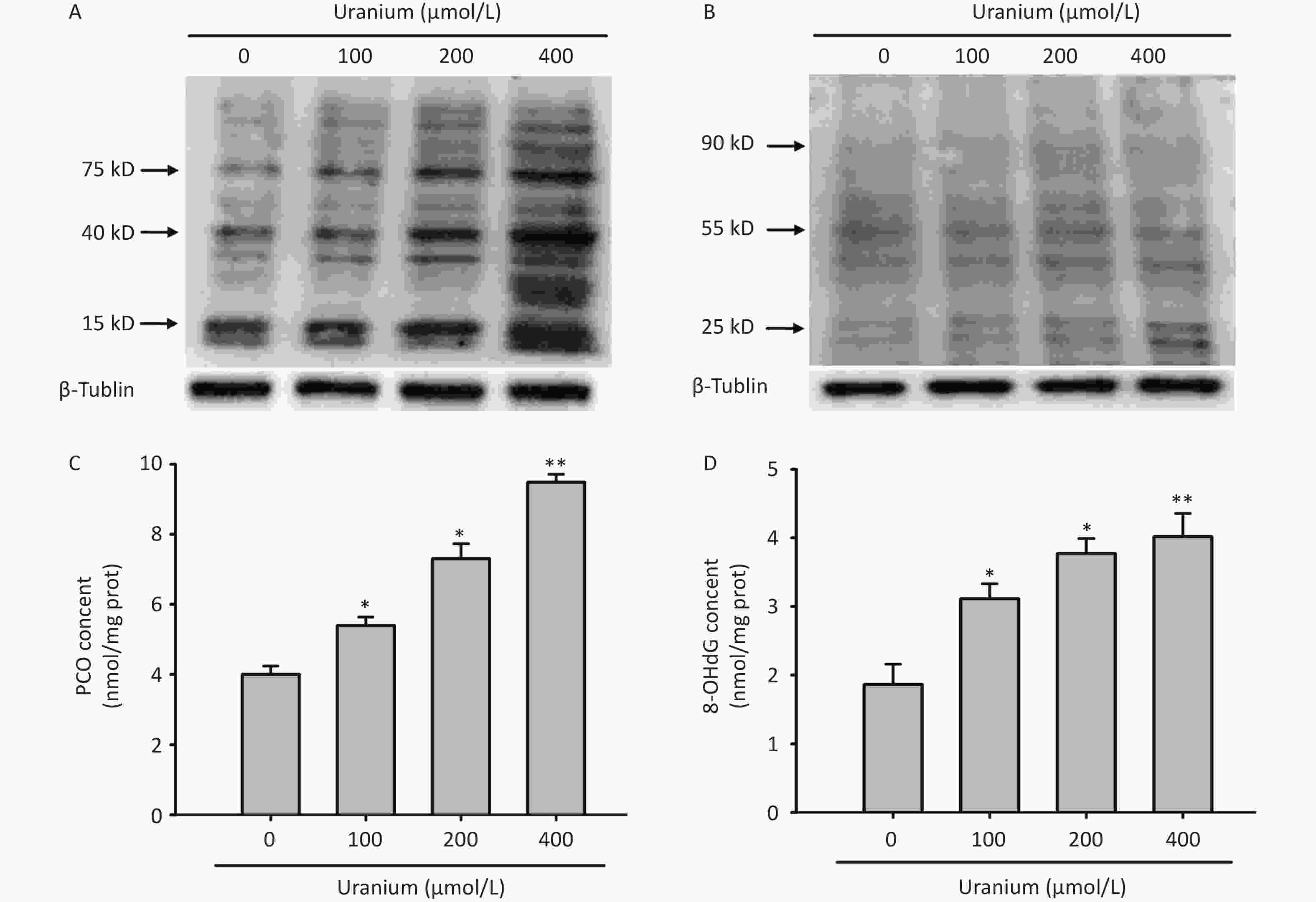
Figure 2. Effects of uranium on the formation of abundant malondialdehyde (MDA)- and 4-hydroxynonenal (4-HNE)-protein adducts and contents of protein carbonylation (PCO) and 8-hydroxydeoxyguanosine (8-OHdG) in the NRK-52E cells. After kidney cells were intoxicated by depleted uranyl acetate for 24 h, Western blots was used to examine the production of MDA- and 4-HNE-protein adducts with an internal reference β-tubulin (A, B). The PCO content was determined by 2, 4-dinitrophenylhydrazine colorimetric method (C). The 8-OHdG level was tested using double-antibody sandwich enzyme-linked immunosorbent assay (D). *P < 0.05, **P < 0.01 compared with the control group (n = 3).
-
To determine whether active aldehydes were involved in uranium-induced oxidative damage in kidney cells, we pretreated the NRK-52E cells with 6 mmol/L Tempol (a specific inhibitor of ROS) alone for 2 h or with 6 mmol/L Tempol and 1 mmol/L AG (a carbonyl capture agent) together for 2 h before they were intoxicated with 400 µmol/L uranium for 24 h.
As expected (Figure 3A–C), uranium treatment significantly increased the ROS levels and decreased cell viability compared with those in the control treatment (*P < 0.05, **P < 0.01). Although Tempol effectively cleared the uranium-induced ROS, it did not significantly restore cell viability compared to that in the uranium-exposed cells. Cell viability was significantly greater in the Tempol and AG co-treatment group than that in the uranium-treated group (#P < 0.05), indicating that active aldehydes may be involved in uranium-triggered cytotoxicity.
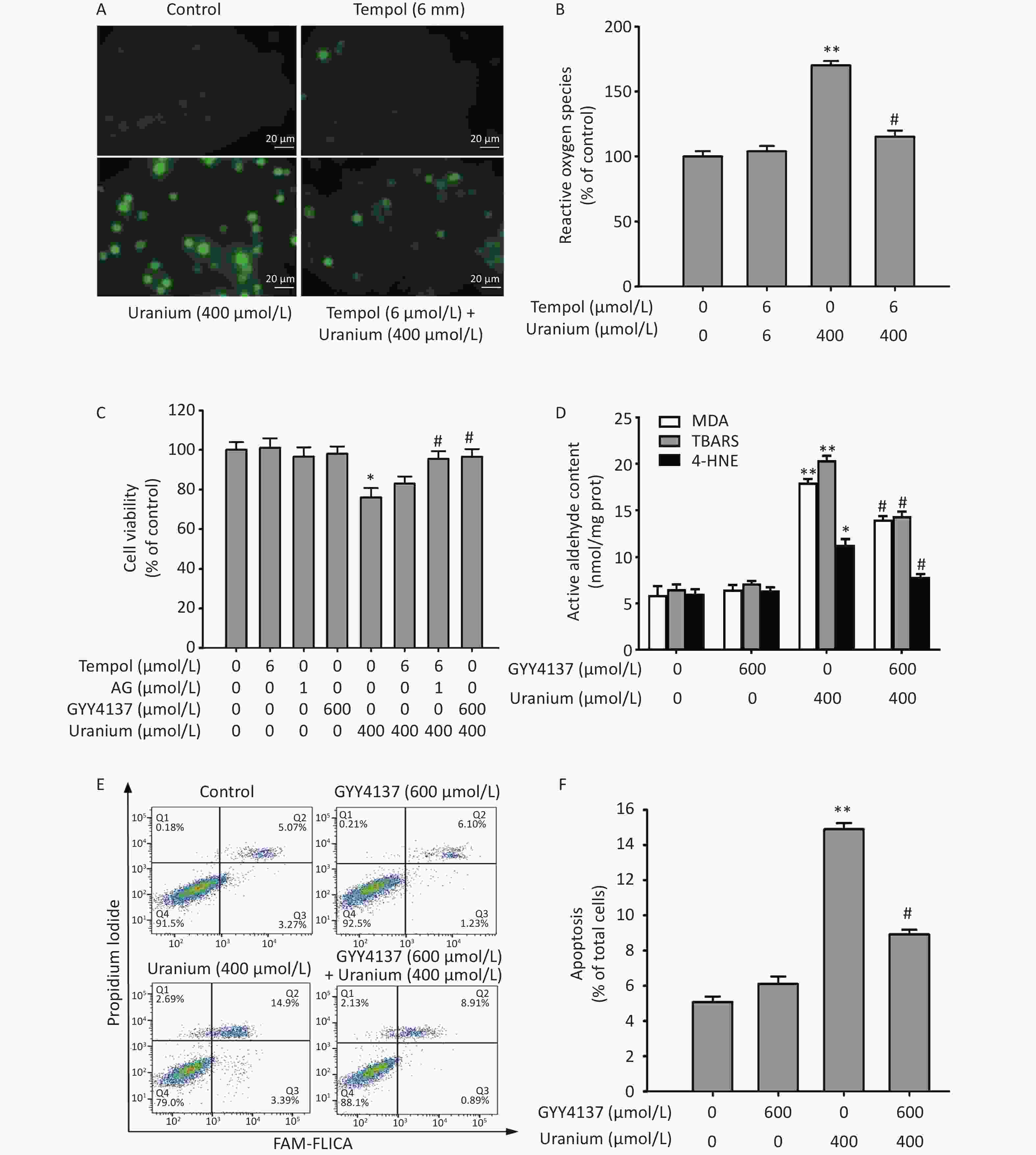
Figure 3. Effects of GYY4137 (a slow-releasing donor of H2S) on the contents of active aldehydes, apoptosis, and cell viability in the uranium-intoxicated NRK-52E cells. After they were pretreated with 600 µmol/L GYY4137 for 30 min (6 mmol/L Tempol alone for 2 h, or 6 mmol/L Tempol plus 1 mmol/L aminoguanidine together for 2 h), kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ROS level was measured by fluorescent probe DCFH-DA staining method (A, B). Cell viability was determined by CCK-8 method (C). The levels of thiobarbituric acid reactive substances and 4-hydroxynonenal were determined using double antibody one-step sandwich enzyme-linked immunosorbent assay and abundant malondialdehyde content was measured using thiobarbituric acid colorimetric method (D). Apoptosis was detected by flow cytometry (E–F). Scale bar, 20 μm, *P < 0.05, **P < 0.01 compared with the normal cells, #P < 0.05 compared with the uranium-exposed group (n = 3).
The antagonistic effects of H2S on uranium-induced aldehyde production, apoptosis, and cytotoxicity were explored. The NRK-52E cells were pretreated with 600 µmol/L GYY4137 (a slow-releasing donor of H2S) for 30 min and then intoxicated with 400 µmol/L depleted uranyl acetate for 24 h. We determined cell viability, apoptosis rate, and reactive aldehyde levels. As shown in Figure 3C–F, uranium significantly increased the cytotoxicity, apoptosis ratio, and levels of active aldehydes in the NRK-52E cells compared with those in the control group (*P < 0.05, **P < 0.01). However, GYY4137 treatment effectively reversed the uranium-induced increase in the contents of active aldehydes and the apoptosis rate, and decreased cell viability compared to those in the uranium-treated cells (#P < 0.05). These results suggest that H2S antagonizes active aldehydes, apoptosis, and cytotoxicity, in addition to eliminating ROS in the uranium-treated kidney cells.
-
To investigate whether H2S attenuates uranium-induced aldehyde-protein adducts and oxidative damage to proteins and DNA, we pretreated the NRK-52E cells with 600 µmol/L GYY4137 for 30 min and exposed them to 400 µmol/L depleted uranyl acetate for 24 h. As expected (Figure 4), compared with those in the control cells, uranium evidently promoted the generation of MDA- and 4-HNE-protein adducts. Additionally, compared to the control treatment, uranium significantly increased the levels of PCO and 8-OHdG (**P < 0.01). However, compared to the uranium treatment, GYY4137 administration effectively decreased the uranium-triggered formation of MDA- and 4-HNE-protein adducts, and uranium increased the contents of PCO and 8-OHdG (#P < 0.05).
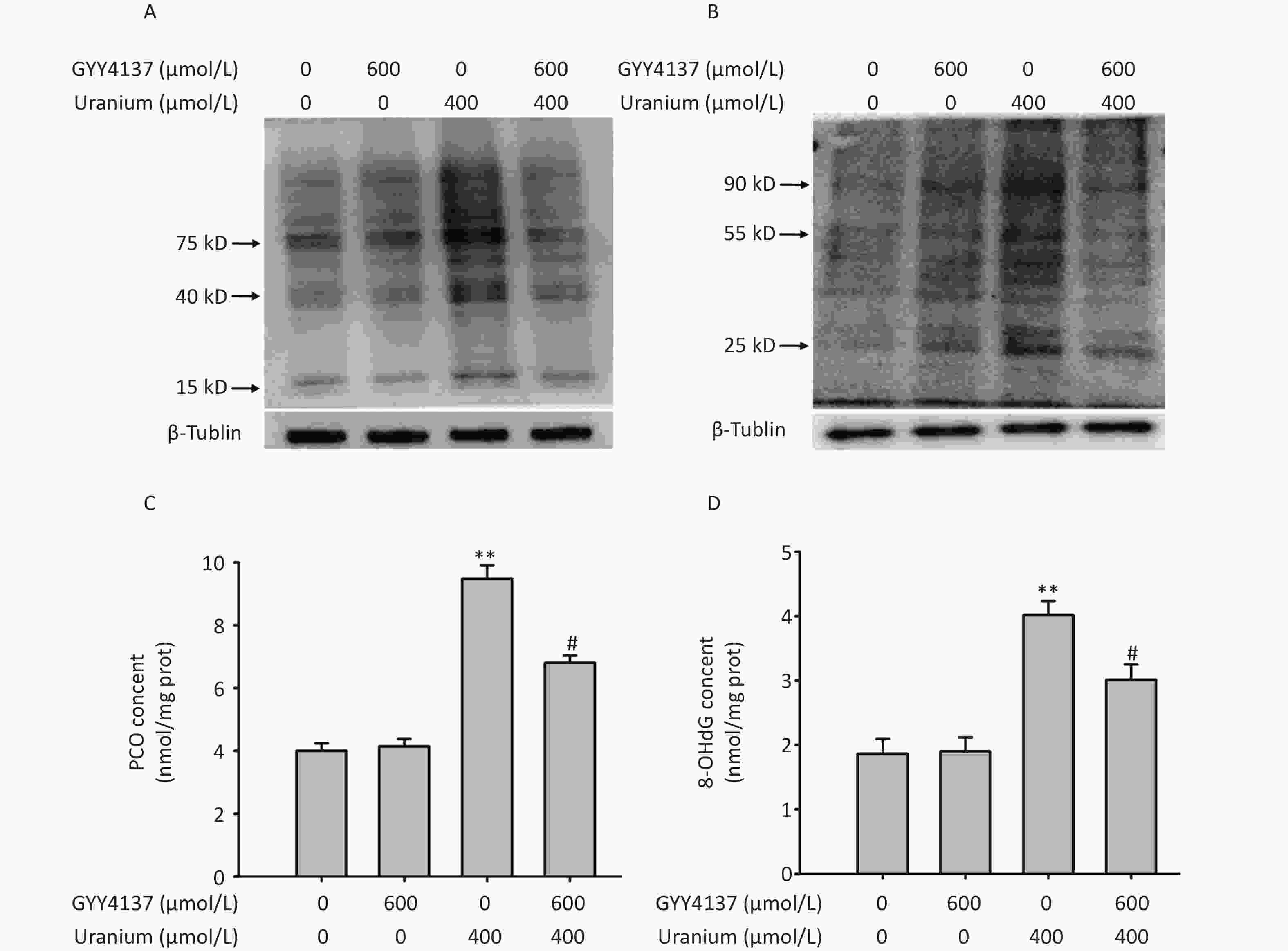
Figure 4. Effects of GYY4137 (a slow-releasing donor of H2S) on the production of abundant malondialdehyde (MDA)- and 4-hydroxynonenal (4-HNE)-protein adducts and levels of protein carbonylation (PCO) and 8-hydroxydeoxyguanosine (8-OHdG) in the uranium-exposed NRK-52E cells. After they were pretreated with 600 μmol/L GYY4137 for 30 min, kidney cells were intoxicated with 400 µmol/L depleted uranyl acetate for 24 h. Western blots was used to detect the formation of MDA- and 4-HNE-protein adducts with an internal reference β-tubulin (A, B). The PCO content was determined using 2, 4-dinitrophenylhydrazine colorimetric method (C). The 8-OHdG content was tested by double-antibody one-step sandwich enzyme-linked immunosorbent assay (D). **P < 0.01 compared with the normal group, #P < 0.05 compared to the uranium-intoxicated group (n = 3).
-
To investigate whether H2S mediated the regulatory effects of the transcription factor Nrf2 on the expression of CBS and aldehyde-metabolizing enzymes during uranium-induced carbonyl stress, we pretreated the NRK-52E cells with 600 µmol/L GYY4137 for 30 min and exposed them to 400 µmol/L depleted uranyl acetate for 24 h.
Compared to the control group, uranium exposure significantly decreased Nrf2 expression and nuclear translocation, and increased the levels of cytoplasmic Nrf2 and Keap1 (*P < 0.05, **P < 0.01). However, co-treatment with GYY4137 and uranium effectively triggered Nrf2 activation and nuclear translocation and reduced the expression of Keap1 and cytoplasmic Nrf2 compared to the uranium-treated cells. The application of GYY4137 effectively reversed the decrease in the ratio of nuclear to cytoplasmic Nrf2 compared with that in the uranium-treated group (#P < 0.05) (Figure 5A–C).
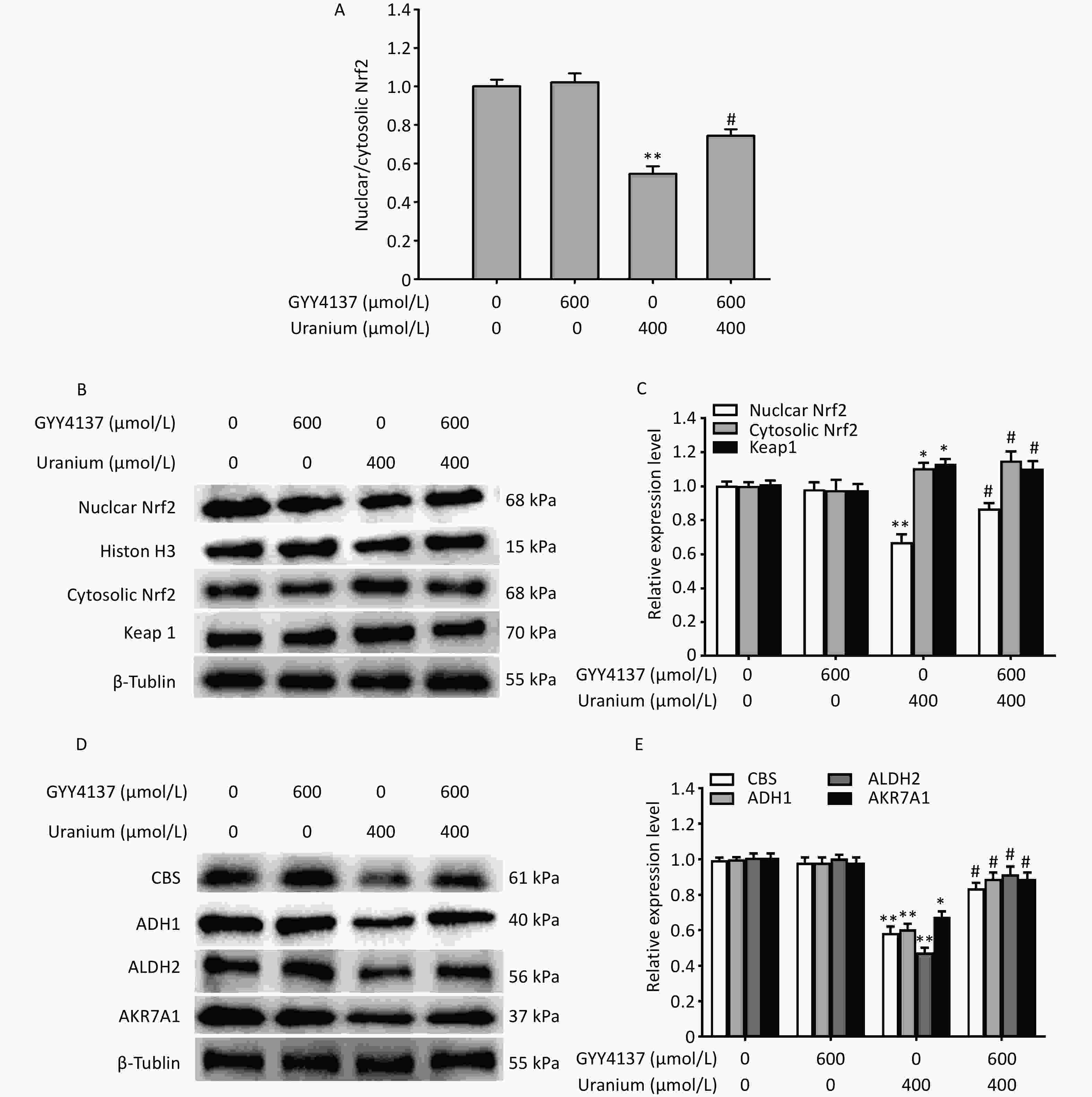
Figure 5. Effects of GYY4137 (a slow-releasing donor of H2S) on the expression of nuclear and cytoplasmic nuclear factor E2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), cystathionine β-synthase (CBS), and aldehyde-metabolizing enzymes in the uranium-intoxicated NRK-52E cells. After they were pretreated with 600 µmol/L GYY4137 for 30 min, kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ratio of nuclear to cytoplasmic Nrf2 was calculated (A). Western blotting was adopted to determine the expression of nuclear and cytoplasmic Nrf2, Keap1, CBS, alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase (B–E). Respective histone H3 and β-tubulin were used as the internal references for nuclear and cytoplasmic (total) protein expression. *P < 0.05, **P < 0.01 compared with the normal group, #P < 0.05 compared with the uranium-exposed cells (n = 3).
Moreover, compared to the control treatment, uranium significantly decreased the expression of CBS, ADH1, ALDH2, and AKR7A1 (*P < 0.05, **P < 0.01). However, the administration of GYY4137 to the uranium-treated cells effectively reversed the decrease in the expression of CBS and aldehyde-metabolizing enzymes compared to the uranium-exposed group (#P < 0.05) (Figure 5D–E). Taken together, these findings show that H2S promotes Nrf2 activation and nuclear translocation, accompanied by the reversal of the decrease in the expression of CBS and aldehyde-metabolizing enzymes induced by uranium.
-
To determine whether the CBS/H2S axis was involved in Nrf2 activation, nuclear translocation, and the expression of aldehyde-metabolizing enzymes during uranium-triggered carbonyl stress, the NRK-52E cells were intoxicated with 400 µmol/L depleted uranyl acetate after they were pretreated with 5 µmol/L SFP (an Nrf2 agonist) for 2 h and then 5 µmol/L CBS siRNA for 24 h.
As expected (Figure 6A–D), treatment with CBS siRNA or uranium alone significantly decreased CBS expression, endogenous H2S content, Nrf2 level, nuclear translocation, and the ratio of nuclear to cytoplasmic Nrf2 and increased the levels of Keap1 and cytoplasmic Nrf2 compared to those in the control group (*P < 0.05, **P < 0.01). The administration of SFP effectively abolished the above-mentioned uranium-induced changes in expression compared to those in the uranium-exposed cells (#P < 0.05). However, the application of CBS siRNA significantly abrogated the SFP-induced increase in CBS expression, endogenous H2S content, Nrf2 activation, and nuclear translocation, as well as the decrease in the expression of Keap1 and cytoplasmic Nrf2 in the uranium-contaminated cells compared to those in the SFP and uranium co-treatment group. Concomitantly, treatment with CBS siRNA effectively abolished the SFP-induced increase in the nuclear to cytoplasmic Nrf2 ratio in the uranium-intoxicated cells compared to that in the SFP- and uranium-co-treated cells (&P < 0.05, &&P < 0.01).
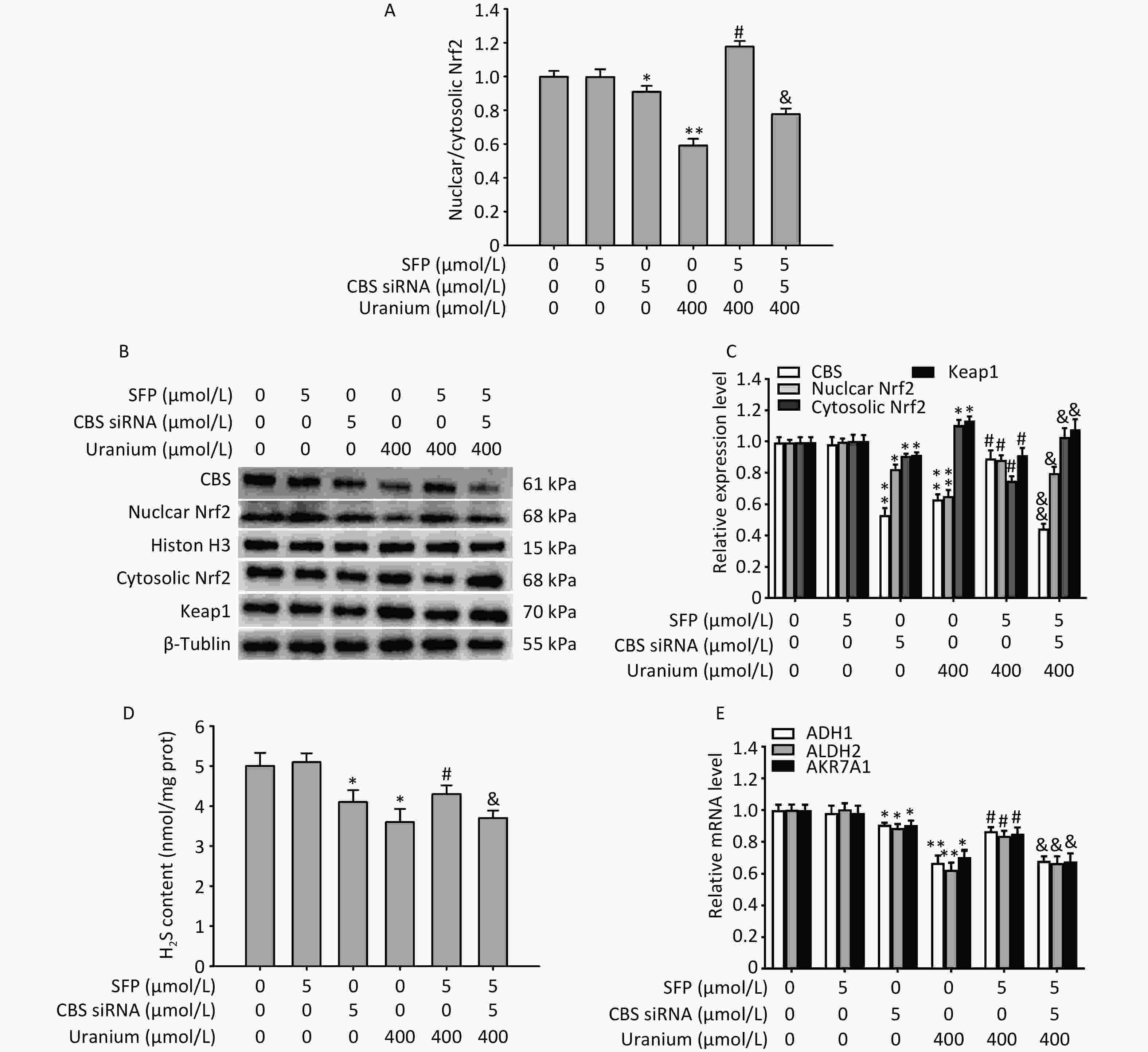
Figure 6. Effects of cystathionine β-synthase (CBS) siRNA on the expression of CBS, nuclear and cytoplasmic nuclear factor E2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), and aldehyde-metabolizing enzymes in the NRK-52E cells cotreated by uranium and sulforaphane (SFP, an Nrf2 agonist). After they were pretreated with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h, kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ratio of nuclear to cytoplasmic Nrf2 was shown (A). Western blots was used to determine the expression of CBS, nuclear and cytoplasmic Nrf2, and Keap1. Respective Histone H3 and β-tubulin were used as the internal references for the expression of respective nuclear and cytoplasmic proteins (B, C). Zinc acetate trapping and N, N-dimethyl-p-phenylenediamine methods were adopted to detect the content of endogenous H2S (D). Real-time PCR was adopted to examine the mRNA expression of alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase (E). *P < 0.05, **P < 0.01 compared with the control group. #P < 0.05 compared to the uranium-exposed cells. &P < 0.05, &&P < 0.01 compared to the GYY4137 and uranium cotreatment group (n = 3).
Moreover, treatment with CBS siRNA or uranium alone significantly decreased the mRNA expression of ADH1, ALDH2, and AKR7A1 compared with that in the normal cells (*P < 0.05, **P < 0.01). Administration of SFP significantly abrogated the decrease in the mRNA expression of aldehyde-metabolizing enzymes compared to that in the uranium-intoxicated cells (#P < 0.05). However, the application of CBS siRNA significantly reversed the SFP-induced increase in the mRNA levels of aldehyde-metabolizing enzymes in the uranium-intoxicated cells when compared to the SFP and uranium co-treatment group (&P < 0.05, &&P < 0.01) (Figure 6E). Taken together, our results suggest that the CBS/H2S axis may be, at least partially, involved in Nrf2 activation and nuclear translocation and in the mRNA expression of aldehyde-metabolizing enzymes.
-
To determine whether the CBS/H2S axis mediates the antagonistic effects of Nrf2 activation and nuclear translocation on the levels of reactive aldehydes, apoptosis, and cytotoxicity during uranium-induced carbonyl stress, the NRK-52E cells were pretreated with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h before they were intoxicated with 400 µmol/L depleted uranyl acetate for 24 h.
As expected (Figure 7), treatment with CBS siRNA or uranium alone significantly increased the levels of reactive aldehydes and the apoptosis ratio and decreased cell viability compared to those in the control group (*P < 0.05, **P < 0.01). The administration of SFP effectively cancelled the abovementioned uranium-affected indicators compared to those in the uranium-exposed cells (#P < 0.05). However, compared to the SFP and uranium co-treatment, CBS siRNA efficiently abolished the antagonistic effects of SFP on the reactive aldehyde content, apoptotic ratio, and decrease in cell viability in the uranium-intoxicated cells (&P < 0.05). These results imply that the CBS/H2S system is at least partially involved in the attenuation of uranium-induced changes in reactive aldehyde levels, apoptosis, and cytotoxicity through Nrf2 activation and nuclear translocation.
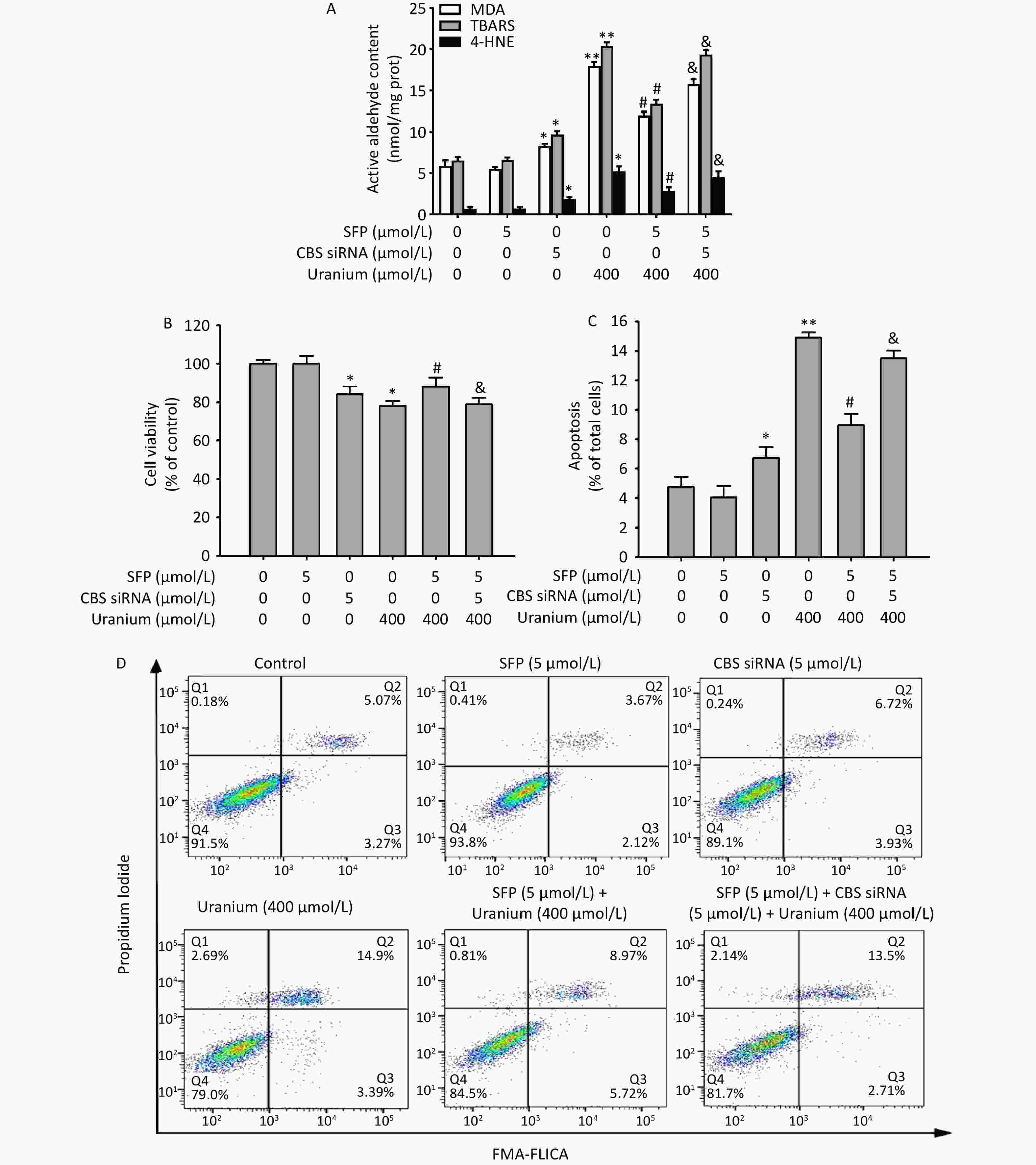
Figure 7. Effects of cystathionine β-synthase (CBS) siRNA on the contents of reactive aldehydes, apoptosis ratio, and cell viability in the NRK-52E cells cotreated by uranium and sulforaphane (SFP, an Nrf2 agonist). After they were pretreated with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h, kidney cells were exposed to 400 μmol/L depleted uranyl acetate for 24 h. The contents of thiobarbituric acid reactive substances and 4-hydroxynonenal were determined by double-antibody one-step sandwich enzyme-linked immunosorbent assay. The abundant malondialdehyde level was measured by thiobarbituric acid colorimetric method (A). Cell viability was tested by CCK-8 method (B). Cell apoptosis was detected by flow cytometry (C, D). *P < 0.05, **P < 0.01 compared to the control group, #P < 0.05 compared to the uranium-intoxicated group, &P < 0.05 compared with the GYY4137 and uranium cotreated cells (n = 3).
-
Owing to the slow and steady release of H2S at physiological pH and temperature, GYY4137 has been widely adopted as a cytoprotective antioxidant in various cellular and animal models of human diseases such as cancer, inflammation, and acute kidney injury[42,43]. In the present study, uranium intoxication in kidney cells was shown to induce carbonyl stress during oxidative stress, which is characterized by ROS production, GSH depletion, and DNA oxidative damage, all of which were alleviated by GYY4137. Many studies have indicated that H2S induces antioxidant enzymes to antagonize ROS triggered by various stimuli, including uranium, in a variety of cells and animal models[40,44], which may be involved in the defense mechanism of H2S against oxidative stress.
In addition to ROS, active aldehydes are now considered one of the most crucial mechanisms by which environmental toxicants induce biological toxicity and multiple human diseases such as cardiovascular, neurological, digestive, and respiratory disorders[18]. Active aldehydes and ROS may influence each other to form an “active aldehyde-ROS” axis, increasing the complexity of the intervention strategy against carbonyl/oxidative damage[17,45]. A related study has shown that reactive aldehydes decrease concomitantly with decreasing ROS levels in the menadione-treated hamster V79-4 cells[46]. In the present study, uranium increased the levels of carbonyl stress markers, apoptosis, and cytotoxicity. However, GYY4137 reversed the effects of uranium on these parameters. Importantly, H2S ameliorates 1-methy-4-phenylpyridinium ion -induced carbonyl stress by decreasing the levels of intracellular ROS, MDA, and 4-HNE[47]. Therefore, our data indicate that H2S alleviates cytotoxicity by antagonizing reactive aldehydes and ROS in the uranium-treated kidney cells.
H2S post-translationally modifies many proteins with intracellular functions via persulfhydration[48]. H2S is considered a Keap1-Cys151-preferring inducer[49]. H2S triggers Nrf2 activation and subsequently alleviates methylglyoxal-induced carbonyl stress in the SH-SY5Y cells[50,51]. Although the metabolic pathways associated with MDA and 4-HNE differ significantly in various cell types, protein carbonylation and adducts disturb cellular homeostasis and adaptive responses and are involved in Nrf2 signaling[52,53]. In this study, aldehyde-protein adducts were not specific molecular weight bands, but rather affected the post-translational modification of proteins[54]. The expression of ADH1, ALDH2, and AKR7A1 decreased owing to a decrease in Nrf2 activation and nuclear translocation mediated by uranium-induced oxidative stress. However, GYY4137 effectively reversed the uranium-induced decrease in Nrf2 levels and nuclear translocation, concomitantly reversing the uranium-induced decrease in the expression of the three aldehyde-metabolizing enzymes and the uranium-induced formation of aldehyde-protein adducts. Our results confirm that H2S regulates the metabolism of 4-HNE and MDA by activating Nrf2/ARE signaling, which induces the expression of aldehyde-metabolizing enzymes.
In addition, H2S contains a sulfhydryl group in its molecular structure, which directly eliminates exogenous 4-HNE and acrolein (another type of active aldehyde) through chemical reactions and reduces the generation of carbonyl proteins and DNA adducts[55–57]. A reasonable explanation for this is that H2S is an effective carbonyl scavenger that inhibits the accumulation of aldehyde adducts, mainly by neutralizing aldehyde groups and promoting aldehyde excretion. This mechanism is similar to that of GSH detoxification by 4-HNE[17,30]. Thus, the mechanism including the induction of aldehyde-metabolizing enzymes and direct chemical reactions may contribute to the defense of H2S against carbonyl stress. From the above, it is inferred that H2S scavengers not only ROS but also reactive aldehydes, which constitutes the “dual line of defense against carbonyl/oxidative stress”, resulting in the antagonism of the “active aldehyde-ROS” axis.
To date, the regulatory mechanism of CBS expression in physiological and pathological kidney function has not been fully elucidated in mammals[32]. Two independent reports by the same group indicated that oxidative stress decreased Nrf2 expression and nuclear translocation[58], CBS expression, and H2S levels[59] in kidney tissue homogenates from a rat chronic kidney disease model, suggesting a possible correlation between Nrf2 expression, nuclear translocation, and the CBS/H2S system. In the present study, treatment with SFN (an Nrf2 agonist) reversed the uranium-induced decrease in Nrf2 expression and nuclear translocation. Inhibition of CBS expression by CBS siRNA reversed the effects of the SFN-enhanced Nrf2 activation on the uranium-decreased nuclear-to-cytoplasmic Nrf2 ratio and mRNA expression of aldehyde-metabolizing enzymes. Moreover, the inhibition of CBS expression by CBS siRNA reversed the antagonistic effects of the SFN-enhanced Nrf2 activation and nuclear translocation on the uranium-induced reactive aldehydes, apoptotic ratio, and cytotoxicity. Considering the multiple pathways involved in endogenous H2S biosynthesis[32], our results suggest that the CBS/H2S axis may partially modulate the Nrf2 levels and nuclear translocation involved in uranium-induced carbonyl stress. Additionally, Nrf2 expression mediates H2S generation in the uranium-treated NRK-52E cells[60]. Exogenous H2S triggers Nrf2 activation and subsequently upregulates the CBS/H2S axis under various toxicological conditions[61–63]. Therefore, there may be a feedback loop between Nrf2 expression/activation and the upregulation of the CBS/H2S axis. The promoter region of the rat CBS gene contains multiple potential cis-acting elements homologous to the ARE of Nrf2-controlled target genes, whose functions may be similar to those of the ARE[64]. We further speculated that the specificity and inducibility of the CBS gene promoter may exist in a variety of animal cells, tissues, and species under various stimuli. Recently, Nrf2-targeting sequences at the CSE gene promoter were identified in the human primary small airway epithelial cells infected with respiratory syncytial virus[65]. An experiment in our laboratory is underway to determine the combination of Nrf2 with precise cis-acting elements of the CBS gene promoter during uranium-induced carbonyl stress.
In conclusion, H2S induces Nrf2 activation and nuclear translocation, which regulates the expression of aldehyde-metabolizing enzymes responsible for active aldehyde metabolism. Simultaneously, Nrf2 controls the endogenous CBS/H2S axis, which in turn, at least partially, further promotes Nrf2 activation and nuclear translocation. These events form a cycle-modulating mode of H2S alleviation of uranium-induced carbonyl stress, apoptosis, and cytotoxicity in kidney cells (Figure 8).
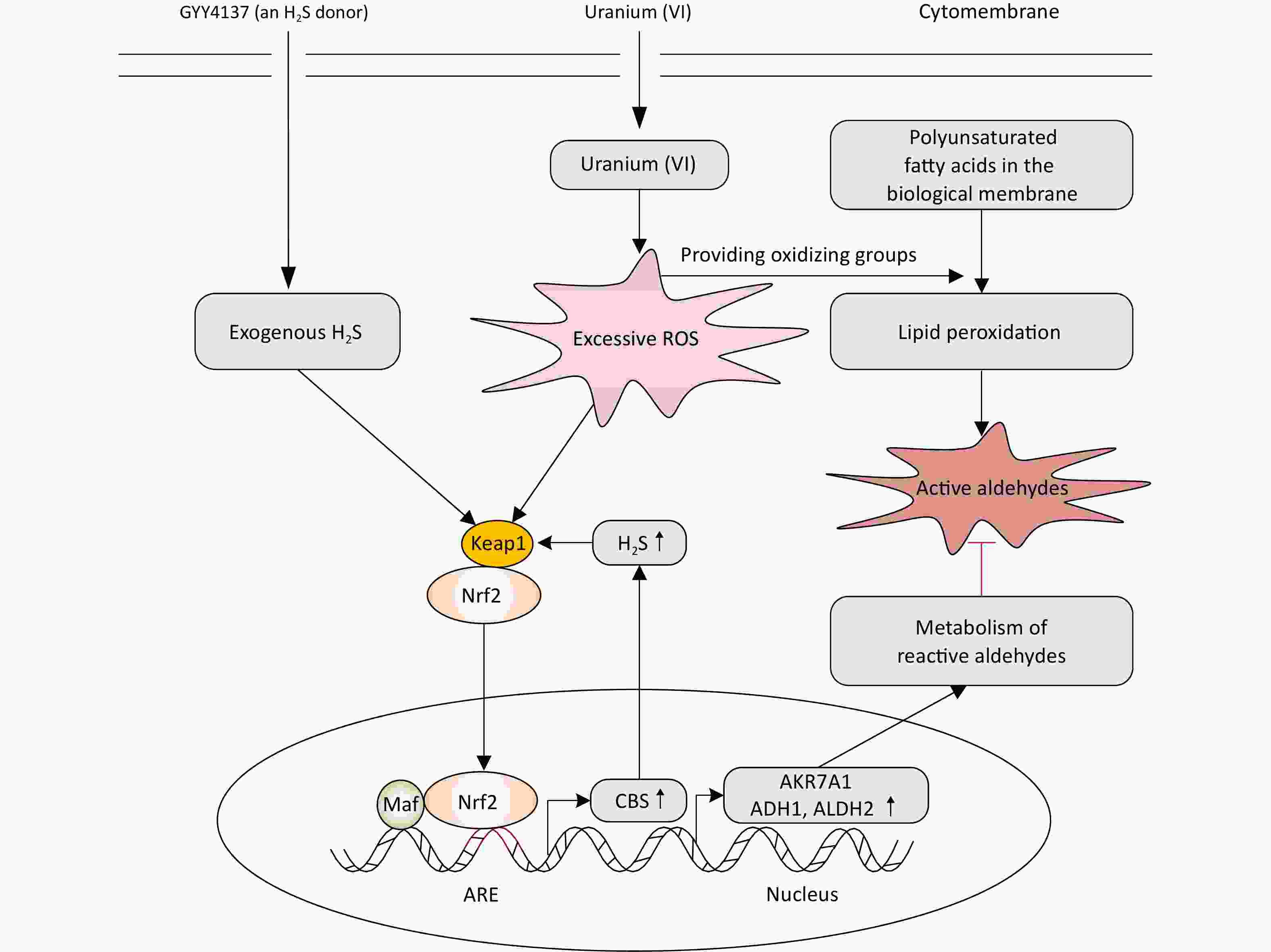
Figure 8. Mechanism of H2S on antagonizing uranium (VI)-induced reactive aldehydes and kidney cells cytotoxicity. Uranium (VI) enters kidney cells and triggers reactive oxygen species production, which provides oxidative groups to cause lipid peroxidation of polyunsaturated fatty acids to generate reactive aldehydes. The application of exogenous H2S activates the expression and nuclear translocation of transcription activator nuclear factor E2-related factor 2 (Nrf2), promotes the recognition and binding of Nrf2 with cis-acting elements also called antioxidant response elements present at the promoters of target genes, and finally initiates the expression of aldehyde metabolizing enzymes. Simultaneously, Nrf2 activated by H2S regulates cystathionine β-synthase (CBS) expression responsible for endogenous H2S formation. That is, Nrf2 controls the CBS/H2S system which in turn activates itself. This constitutes the cyclic regulatory mode of H2S on alleviating the reactive aldehydes-mediated kidney cells cytotoxicity induced by uranium.
doi: 10.3967/bes2025.021
Hydrogen Sulfide Alleviates Lipid Peroxidation-Mediated Carbonyl Stress in Uranium-Intoxicated Kidney Cells via Nrf2/ARE Signaling
-
Abstract:
Objective To explore the protective effects and underlying mechanisms of H2S against lipid peroxidation-mediated carbonyl stress in the uranium-treated NRK-52E cells. Methods Cell viability was evaluated using CCK-8 assay. Apoptosis was measured using flow cytometry. Reagent kits were used to detect carbonyl stress markers malondialdehyde, 4-hydroxynonenal, thiobarbituric acid reactive substances, and protein carbonylation. Aldehyde-protein adduct formation and alcohol dehydrogenase, aldehyde dehydrogenase 2, aldo-keto reductase, nuclear factor E2-related factor 2 (Nrf2), and cystathionine β-synthase (CBS) expression were determined using western blotting or real-time PCR. Sulforaphane (SFP) was used to activate Nrf2. RNA interference was used to inhibit CBS expression. Results GYY4137 (an H2S donor) pretreatment significantly reversed the uranium-induced increase in carbonyl stress markers and aldehyde-protein adducts. GYY4137 effectively restored the uranium-decreased Nrf2 expression, nuclear translocation, and ratio of nuclear to cytoplasmic Nrf2, accompanied by a reversal of the uranium-decreased expression of CBS and aldehyde-metabolizing enzymes. The application of CBS siRNA efficiently abrogated the SFP-enhanced effects on the expression of CBS, Nrf2 activation, nuclear translocation, and ratio of nuclear to cytoplasmic Nrf2 and concomitantly reversed the SFP-enhanced effects of the uranium-induced mRNA expression of aldehyde-metabolizing enzymes. Simultaneously, CBS siRNA reversed the SFP-mediated alleviation of the uranium-induced increase in reactive aldehyde levels, apoptosis rates, and uranium-induced cell viability. Conclusion H2S induces Nrf2 activation and nuclear translocation, which modulates the expression of aldehyde-metabolizing enzymes and the CBS/H2S axis. Simultaneously, the Nrf2-controlled CBS/H2S axis may at least partially promote Nrf2 activation and nuclear translocation. These events form a cycle-regulating mode through which H2S attenuates the carbonyl stress-mediated NRK-52E cytotoxicity triggered by uranium. -
Key words:
- Hydrogen sulfide (H2S) /
- Carbonyl stress /
- Lipid peroxidation /
- Uranium /
- Nephrotoxicity /
- Nrf2
All authors declare that there is no potential controversy of interest in this paper.
This study is exempt from ethical review.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. Effects of uranium on the contents of active aldehydes, cell viability, levels of reduced and oxidized glutathione, and expression of aldehyde-metabolizing enzymes in the NRK-52E cells. After kidney cells were exposed to depleted uranyl acetate for 24 h, the contents of thiobarbituric acid reactive substances and 4-hydroxynonenal were detected by double-antibody one-step sandwich enzyme-linked immunosorbent assay. The abundant malondialdehyde level was tested using thiobarbituric acid colorimetric method (A). Cell viability was determined by CCK-8 method (B). Spectrophotometry was used to measure the contents of glutathione (GSH) and oxidized glutathione (GSSG) (C) and the ratio of GSH to GSSG levels is shown (D). Western blots was adopted to determine the expression of alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase with an internal reference β-tubulin (E, F). *P < 0.05, **P < 0.01 compared to the control cells (n = 3).
Figure 2. Effects of uranium on the formation of abundant malondialdehyde (MDA)- and 4-hydroxynonenal (4-HNE)-protein adducts and contents of protein carbonylation (PCO) and 8-hydroxydeoxyguanosine (8-OHdG) in the NRK-52E cells. After kidney cells were intoxicated by depleted uranyl acetate for 24 h, Western blots was used to examine the production of MDA- and 4-HNE-protein adducts with an internal reference β-tubulin (A, B). The PCO content was determined by 2, 4-dinitrophenylhydrazine colorimetric method (C). The 8-OHdG level was tested using double-antibody sandwich enzyme-linked immunosorbent assay (D). *P < 0.05, **P < 0.01 compared with the control group (n = 3).
Figure 3. Effects of GYY4137 (a slow-releasing donor of H2S) on the contents of active aldehydes, apoptosis, and cell viability in the uranium-intoxicated NRK-52E cells. After they were pretreated with 600 µmol/L GYY4137 for 30 min (6 mmol/L Tempol alone for 2 h, or 6 mmol/L Tempol plus 1 mmol/L aminoguanidine together for 2 h), kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ROS level was measured by fluorescent probe DCFH-DA staining method (A, B). Cell viability was determined by CCK-8 method (C). The levels of thiobarbituric acid reactive substances and 4-hydroxynonenal were determined using double antibody one-step sandwich enzyme-linked immunosorbent assay and abundant malondialdehyde content was measured using thiobarbituric acid colorimetric method (D). Apoptosis was detected by flow cytometry (E–F). Scale bar, 20 μm, *P < 0.05, **P < 0.01 compared with the normal cells, #P < 0.05 compared with the uranium-exposed group (n = 3).
Figure 4. Effects of GYY4137 (a slow-releasing donor of H2S) on the production of abundant malondialdehyde (MDA)- and 4-hydroxynonenal (4-HNE)-protein adducts and levels of protein carbonylation (PCO) and 8-hydroxydeoxyguanosine (8-OHdG) in the uranium-exposed NRK-52E cells. After they were pretreated with 600 μmol/L GYY4137 for 30 min, kidney cells were intoxicated with 400 µmol/L depleted uranyl acetate for 24 h. Western blots was used to detect the formation of MDA- and 4-HNE-protein adducts with an internal reference β-tubulin (A, B). The PCO content was determined using 2, 4-dinitrophenylhydrazine colorimetric method (C). The 8-OHdG content was tested by double-antibody one-step sandwich enzyme-linked immunosorbent assay (D). **P < 0.01 compared with the normal group, #P < 0.05 compared to the uranium-intoxicated group (n = 3).
Figure 5. Effects of GYY4137 (a slow-releasing donor of H2S) on the expression of nuclear and cytoplasmic nuclear factor E2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), cystathionine β-synthase (CBS), and aldehyde-metabolizing enzymes in the uranium-intoxicated NRK-52E cells. After they were pretreated with 600 µmol/L GYY4137 for 30 min, kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ratio of nuclear to cytoplasmic Nrf2 was calculated (A). Western blotting was adopted to determine the expression of nuclear and cytoplasmic Nrf2, Keap1, CBS, alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase (B–E). Respective histone H3 and β-tubulin were used as the internal references for nuclear and cytoplasmic (total) protein expression. *P < 0.05, **P < 0.01 compared with the normal group, #P < 0.05 compared with the uranium-exposed cells (n = 3).
Figure 6. Effects of cystathionine β-synthase (CBS) siRNA on the expression of CBS, nuclear and cytoplasmic nuclear factor E2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), and aldehyde-metabolizing enzymes in the NRK-52E cells cotreated by uranium and sulforaphane (SFP, an Nrf2 agonist). After they were pretreated with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h, kidney cells were exposed to 400 µmol/L depleted uranyl acetate for 24 h. The ratio of nuclear to cytoplasmic Nrf2 was shown (A). Western blots was used to determine the expression of CBS, nuclear and cytoplasmic Nrf2, and Keap1. Respective Histone H3 and β-tubulin were used as the internal references for the expression of respective nuclear and cytoplasmic proteins (B, C). Zinc acetate trapping and N, N-dimethyl-p-phenylenediamine methods were adopted to detect the content of endogenous H2S (D). Real-time PCR was adopted to examine the mRNA expression of alcohol dehydrogenase, aldehyde dehydrogenase 2, and aldo-keto reductase (E). *P < 0.05, **P < 0.01 compared with the control group. #P < 0.05 compared to the uranium-exposed cells. &P < 0.05, &&P < 0.01 compared to the GYY4137 and uranium cotreatment group (n = 3).
Figure 7. Effects of cystathionine β-synthase (CBS) siRNA on the contents of reactive aldehydes, apoptosis ratio, and cell viability in the NRK-52E cells cotreated by uranium and sulforaphane (SFP, an Nrf2 agonist). After they were pretreated with 5 µmol/L SFP for 2 h and then 5 µmol/L CBS siRNA for 24 h, kidney cells were exposed to 400 μmol/L depleted uranyl acetate for 24 h. The contents of thiobarbituric acid reactive substances and 4-hydroxynonenal were determined by double-antibody one-step sandwich enzyme-linked immunosorbent assay. The abundant malondialdehyde level was measured by thiobarbituric acid colorimetric method (A). Cell viability was tested by CCK-8 method (B). Cell apoptosis was detected by flow cytometry (C, D). *P < 0.05, **P < 0.01 compared to the control group, #P < 0.05 compared to the uranium-intoxicated group, &P < 0.05 compared with the GYY4137 and uranium cotreated cells (n = 3).
Figure 8. Mechanism of H2S on antagonizing uranium (VI)-induced reactive aldehydes and kidney cells cytotoxicity. Uranium (VI) enters kidney cells and triggers reactive oxygen species production, which provides oxidative groups to cause lipid peroxidation of polyunsaturated fatty acids to generate reactive aldehydes. The application of exogenous H2S activates the expression and nuclear translocation of transcription activator nuclear factor E2-related factor 2 (Nrf2), promotes the recognition and binding of Nrf2 with cis-acting elements also called antioxidant response elements present at the promoters of target genes, and finally initiates the expression of aldehyde metabolizing enzymes. Simultaneously, Nrf2 activated by H2S regulates cystathionine β-synthase (CBS) expression responsible for endogenous H2S formation. That is, Nrf2 controls the CBS/H2S system which in turn activates itself. This constitutes the cyclic regulatory mode of H2S on alleviating the reactive aldehydes-mediated kidney cells cytotoxicity induced by uranium.
Table 1. Primer sequences for the rat ADH1, ALDH2, and AKR7A1 genes
Gene GenBank No. Primer sequence (5’ to 3’) ADH1 NM_019286.4 F 5’- CGTGCTGGAAAGAGTATCCGT-3’
R 5’- GGAGTCAGAAACCGGGAAGG-3’ALDH2 XM_032886762.1 F 5’- GCTGACAAGTACCACGGGAA-3’
R 5’- CGGGAAGTTCCACGGAATGA-3’AKR7A1 NM_013215.2 F 5’- TACACTTTCCAGACCACGGC -3’
R 5’- CTCCACAAACTTGCCCTCCT-3’β-actin NP_112406.1 F 5’-CCCGCGAGTACAACCTTCTT-3’
R 5’-TCATCCATGGCGAACTGGTG-3’Note. ADH1: alcohol dehydrogenase; ALDH2: aldehyde dehydrogenase 2; AKR7A1: Aldo-keto reductase; β-actin: an internal reference gene. -
[1] Bjørklund G, Semenova Y, Pivina L, et al. Uranium in drinking water: a public health threat. Arch Toxicol, 2020; 94, 1551−60. doi: 10.1007/s00204-020-02676-8 [2] Zhao B, Sun ZX, Guo YD, et al. Occurrence characteristics of uranium mineral-related substances in various environmental media in China: a critical review. J Hazard Mater, 2023; 441, 129856. doi: 10.1016/j.jhazmat.2022.129856 [3] Gao N, Huang ZH, Liu HQ, et al. Advances on the toxicity of uranium to different organisms. Chemosphere, 2019; 237, 124548. doi: 10.1016/j.chemosphere.2019.124548 [4] Ma MH, Wang RX, Xu LN, et al. Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades. Environ Int, 2020; 145, 106107. doi: 10.1016/j.envint.2020.106107 [5] Guéguen Y, Frerejacques M. Review of knowledge of uranium-induced kidney toxicity for the development of an adverse outcome pathway to renal impairment. Int J Mol Sci, 2022; 23, 4397. doi: 10.3390/ijms23084397 [6] Yue YC, Li MH, Wang HB, et al. The toxicological mechanisms and detoxification of depleted uranium exposure. Environ Health Prev Med, 2018; 23, 18. doi: 10.1186/s12199-018-0706-3 [7] Bellés M, Linares V, Luisa Albina M, et al. Melatonin reduces uranium-induced nephrotoxicity in rats. J Pineal Res, 2007; 43, 87−95. doi: 10.1111/j.1600-079X.2007.00447.x [8] Hao YH, Ren J, Liu J, et al. The protective role of zinc against acute toxicity of depleted uranium in rats. Basic Clin Pharmacol Toxicol, 2012; 111, 402−10. doi: 10.1111/j.1742-7843.2012.00910.x [9] Nagaraj K, Devasya RP, Bhagwath AA. Exopolysaccharide produced by Enterobacter sp. YG4 reduces uranium induced nephrotoxicity. Int J Biol Macromol, 2016; 82, 557−61. doi: 10.1016/j.ijbiomac.2015.11.020 [10] Yapar K, Çavuşoğlu K, Oruç E, et al. Protective role of Ginkgo biloba against hepatotoxicity and nephrotoxicity in uranium-treated mice. J Med Food, 2010; 13, 179−88. doi: 10.1089/jmf.2009.0028 [11] Priyamvada S, Khan SA, Khan MW, et al. Studies on the protective effect of dietary fish oil on uranyl-nitrate-induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot Essent Fatty Acids, 2010; 82, 35−44. doi: 10.1016/j.plefa.2009.10.009 [12] Rodnick KJ, Holman RW, Ropski PS, et al. A perspective on reagent diversity and non-covalent binding of reactive carbonyl species (RCS) and effector reagents in non-enzymatic glycation (NEG): mechanistic considerations and implications for future research. Front Chem, 2017; 5, 39. doi: 10.3389/fchem.2017.00039 [13] Van Bussel BCT, Van de Poll MCG, Schalkwijk CG, et al. Increased dicarbonyl stress as a novel mechanism of multi-organ failure in critical illness. Int J Mol Sci, 2017; 18, 346. doi: 10.3390/ijms18020346 [14] Irazabal MV, Torres VE. Reactive oxygen species and redox signaling in chronic kidney disease. Cells, 2020; 9, 1342. doi: 10.3390/cells9061342 [15] Onyango AN. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem Phys Lipids, 2012; 165, 777−86. doi: 10.1016/j.chemphyslip.2012.09.004 [16] Laskar AA, Younus H. Aldehyde toxicity and metabolism: the role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab Rev, 2019; 51, 42−64. doi: 10.1080/03602532.2018.1555587 [17] Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev, 2014; 2014, 360438. [18] Eckl PM, Bresgen N. Genotoxicity of lipid oxidation compounds. Free Radic Biol Med, 2017; 111, 244−52. doi: 10.1016/j.freeradbiomed.2017.02.002 [19] Kathren RL, Burklin RK. Acute chemical toxicity of uranium. Health Phys, 2008; 94, 170−79. doi: 10.1097/01.HP.0000288043.94908.1f [20] Hao YH, Huang JW, Gu Y, et al. Metallothionein deficiency aggravates depleted uranium-induced nephrotoxicity. Toxicol Appl Pharmacol, 2015; 287, 306−15. doi: 10.1016/j.taap.2015.06.019 [21] Linares V, Bellés M, Albina ML, et al. Assessment of the pro-oxidant activity of uranium in kidney and testis of rats. Toxicol Lett, 2006; 167, 152−61. doi: 10.1016/j.toxlet.2006.09.004 [22] Lestaevel P, Romero E, Dhieux B, et al. Different pattern of brain pro-/anti-oxidant activity between depleted and enriched uranium in chronically exposed rats. Toxicology, 2009; 258, 1−9. doi: 10.1016/j.tox.2008.12.021 [23] Arany I, Hall S, Dixit M. Age-dependent sensitivity of the mouse kidney to chronic nicotine exposure. Pediatr Res, 2017; 82, 822−8. doi: 10.1038/pr.2017.153 [24] Domitrović R, Cvijanović O, Pugel EP, et al. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology, 2013; 310, 115−23. doi: 10.1016/j.tox.2013.05.015 [25] Potočnjak I, Broznić D, Kindl M, et al. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-κB activation. Food Chem Toxicol, 2017; 107, 215−25. doi: 10.1016/j.fct.2017.06.043 [26] Eraslan G, Sarıca ZS, Bayram LÇ, et al. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int, 2017; 24, 27931−41. doi: 10.1007/s11356-017-0232-7 [27] Kuang WH, Zhang X, Zhu WF, et al. Ligustrazine modulates renal cysteine biosynthesis in rats exposed to cadmium. Environ Toxicol Pharmacol, 2017; 54, 125−32. doi: 10.1016/j.etap.2017.07.003 [28] Heymann HM, Gardner AM, Gross ER. Aldehyde-induced DNA and protein adducts as biomarker tools for alcohol use disorder. Trends Mol Med, 2018; 24, 144−55. doi: 10.1016/j.molmed.2017.12.003 [29] Alamil H, Galanti L, Heutte N, et al. Genotoxicity of aldehyde mixtures: profile of exocyclic DNA-adducts as a biomarker of exposure to tobacco smoke. Toxicol Lett, 2020; 331, 57−64. doi: 10.1016/j.toxlet.2020.05.010 [30] Davies SS, Zhang LS. Reactive carbonyl species scavengers-novel therapeutic approaches for chronic diseases. Curr Pharmacol Rep, 2017; 3, 51−67. doi: 10.1007/s40495-017-0081-6 [31] Peleli M, Zampas P, Papapetropoulos A. Hydrogen sulfide and the kidney: physiological roles, contribution to pathophysiology, and therapeutic potential. Antioxid Redox Signal, 2022; 36, 220−43. doi: 10.1089/ars.2021.0014 [32] Zuhra K, Augsburger F, Majtan T, et al. Cystathionine-β-synthase: molecular regulation and pharmacological inhibition. Biomolecules, 2020; 10, 697. doi: 10.3390/biom10050697 [33] Zheng JF, Zhao TT, Yuan Y, et al. Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-κB pathways. Chem Biol Interact, 2015; 242, 353−62. doi: 10.1016/j.cbi.2015.10.021 [34] Lobb I, Sonke E, Aboalsamh G, et al. Hydrogen sulphide and the kidney: important roles in renal physiology and pathogenesis and treatment of kidney injury and disease. Nitric Oxide, 2015; 46, 55−65. doi: 10.1016/j.niox.2014.10.004 [35] Feliers D, Lee HJ, Kasinath BS. Hydrogen sulfide in renal physiology and disease. Antioxid Redox Signal, 2016; 25, 720−31. doi: 10.1089/ars.2015.6596 [36] Corsello T, Komaravelli N, Casola A. Role of hydrogen sulfide in NRF2- and sirtuin- dependent maintenance of cellular redox balance. Antioxidants, 2018; 7, 129. doi: 10.3390/antiox7100129 [37] Fan RF, Li ZF, Zhang D, et al. Involvement of Nrf2 and mitochondrial apoptotic signaling in trehalose protection against cadmium-induced kidney injury. Metallomics, 2020; 12, 2098−107. doi: 10.1039/d0mt00213e [38] Fan RF, Tang KK, Wang ZY, et al. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology, 2021; 464, 152999. doi: 10.1016/j.tox.2021.152999 [39] Buha A, Baralić K, Djukic-Cosic D, et al. The role of toxic metals and metalloids in Nrf2 signaling. Antioxidants, 2021; 10, 630. doi: 10.3390/antiox10050630 [40] Yi J, Yuan Y, Zheng JF, et al. Hydrogen sulfide alleviates uranium-induced kidney cell apoptosis mediated by ER stress via 20S proteasome involving in Akt/GSK-3β/Fyn-Nrf2 signaling. Free Radical Res, 2018; 52, 1020−9. doi: 10.1080/10715762.2018.1514603 [41] Thiébault C, Carrière M, Milgram S, et al. Uranium induces apoptosis and is genotoxic to normal rat kidney (NRK-52E) proximal cells. Toxicol Sci, 2007; 98, 479−87. doi: 10.1093/toxsci/kfm130 [42] Nin DS, Idres SB, Song ZJ, et al. Biological effects of morpholin-4-Ium 4 methoxyphenyl (morpholino) phosphinodithioate and other phosphorothioate-based hydrogen sulfide donors. Antioxid Redox Signal, 2020; 32, 145−58. doi: 10.1089/ars.2019.7896 [43] Whiteman M, Perry A, Zhou ZM, et al. Phosphinodithioate and phosphoramidodithioate hydrogen sulfide donors. In: Moore PK, Whiteman M. Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide. Springer. 2015, 337-63. [44] Cirino G, Szabo C, Papapetropoulos A. Physiological roles of hydrogen sulfide in mammalian cells, tissues, and organs. Physiol Rev, 2023; 103, 231−76. [45] Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int, 2018; 93, 803−13. doi: 10.1016/j.kint.2017.11.034 [46] Li D, Ferrari M, Ellis EM. Human aldo–keto reductase AKR7A2 protects against the cytotoxicity and mutagenicity of reactive aldehydes and lowers intracellular reactive oxygen species in hamster V79-4 cells. Chem Biol Interact, 2012; 195, 25−34. doi: 10.1016/j.cbi.2011.09.007 [47] Xiao F, Zhang P, Chen AH, et al. Hydrogen sulfide inhibits MPP+-induced aldehyde stress and endoplasmic reticulum stress in PC12 cells: involving upregulation of BDNF. Exp Cell Res, 2016; 348, 106−14. doi: 10.1016/j.yexcr.2016.09.006 [48] Filipovic MR, Zivanovic J, Alvarez B, et al. Chemical biology of H2S signaling through persulfidation. Chem Rev, 2018; 118, 1253−337. doi: 10.1021/acs.chemrev.7b00205 [49] Suzuki T, Takahashi J, Yamamoto M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol Cells, 2023; 46, 133−41. doi: 10.14348/molcells.2023.0028 [50] Koike S, Kayama T, Yamamoto S, et al. Polysulfides protect SH-SY5Y cells from methylglyoxal-induced toxicity by suppressing protein carbonylation: a possible physiological scavenger for carbonyl stress in the brain. NeuroToxicology, 2016; 55, 13−9. doi: 10.1016/j.neuro.2016.05.003 [51] Koike S, Nishimoto S, Ogasawara Y. Cysteine persulfides and polysulfides produced by exchange reactions with H2S protect SH-SY5Y cells from methylglyoxal-induced toxicity through Nrf2 activation. Redox Biol, 2017; 12, 530−9. doi: 10.1016/j.redox.2017.03.020 [52] Kumar M, Sandhir R. Neuroprotective effect of hydrogen sulfide in hyperhomocysteinemia is mediated through antioxidant action involving Nrf2. Neuromol Med, 2018; 20, 475−90. doi: 10.1007/s12017-018-8505-y [53] Milkovic L, Zarkovic N, Marusic Z, et al. The 4-hydroxynonenal-protein adducts and their biological relevance: are some proteins preferred targets? Antioxidants, 2023; 12, 856. [54] Gęgotek A, Skrzydlewska E. Biological effect of protein modifications by lipid peroxidation products. Chem Phys Lipids, 2019; 221, 46−52. doi: 10.1016/j.chemphyslip.2019.03.011 [55] Laggner H, Gmeiner BMK. Investigating the role of H₂S in 4-HNE scavenging. Methods Enzymol, 2015; 555, 3−18. [56] Mao ZM, Huang YR, Li BQ, et al. Hydrogen sulfide as a potent scavenger of toxicant acrolein. Ecotoxicol Environ Saf, 2022; 229, 113111. doi: 10.1016/j.ecoenv.2021.113111 [57] Schreier SM, Muellner MK, Steinkellner H, et al. Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res, 2010; 17, 249−56. doi: 10.1007/s12640-009-9099-9 [58] Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol, 2010; 298, F662−71. doi: 10.1152/ajprenal.00421.2009 [59] Aminzadeh MA, Vaziri ND. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol Dial Transplant, 2012; 27, 498−504. doi: 10.1093/ndt/gfr560 [60] Yuan Y, Zheng JF, Zhao TT, et al. Uranium-induced rat kidney cell cytotoxicity is mediated by decreased endogenous hydrogen sulfide (H2S) generation involved in reduced Nrf2 levels. Toxicol Res, 2016; 5, 660−73. doi: 10.1039/C5TX00432B [61] Meng WQ, Pei ZP, Feng YW, et al. Neglected role of hydrogen sulfide in sulfur mustard poisoning: Keap1 S-sulfhydration and subsequent Nrf2 pathway activation. Sci Rep, 2017; 7, 9433. doi: 10.1038/s41598-017-09648-6 [62] Liu N, Lin XL, Huang CY. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br J Cancer, 2020; 122, 279−92. doi: 10.1038/s41416-019-0660-x [63] Mohammed RA, Mansour SM. Sodium hydrogen sulfide upregulates cystathionine β-synthase and protects striatum against 3-nitropropionic acid-induced neurotoxicity inrats. J Pharm Pharmacol, 2021; 73, 310−21. doi: 10.1093/jpp/rgaa072 [64] Ebert B, Kisiela M, Malátková P, et al. Regulation of human carbonyl reductase 3 (CBR3; SDR21C2) expression by Nrf2 in cultured cancer cells. Biochemistry, 2010; 49, 8499−511. doi: 10.1021/bi100814d [65] Jamaluddin M, de Mello AH, Tapryal N, et al. NRF2 regulates cystathionine Gamma-lyase expression and activity in primary airway epithelial cells infected with respiratory syncytial virus. Antioxidants, 2022; 11, 1582. doi: 10.3390/antiox11081582 -




 下载:
下载:



 Quick Links
Quick Links