-
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. Metabolic syndrome (MetS) is characterized by a clustering of cardiovascular risk factors, including abdominal obesity, elevated triglyceride levels, low high-density lipoprotein (HDL) cholesterol levels, high blood pressure, and elevated fasting glucose levels[1]. MetS is associated with an increased burden of cardiovascular diseases and other chronic diseases, particularly among women[2-4]. In China, the age-standardized prevalence of MetS has been reported to be approximately 17.8% in women and 9.8% in men[5]. Studies have mentioned that it would be of great significance for female individuals to understand the role of childbearing and childrearing to prevent cardiovascular diseases[6,7].
Pregnancy is an important stage for women, which is accompanied by a series of changes in sex hormone levels, hemodynamics, and glycolipid metabolism that are associated with metabolic syndrome[8]. Parity is the total number of live births. Although previous studies have investigated the association between parity and MetS, the findings have been inconsistent, which might be limited by small sample size or particularly selected populations[9-10]. The aim of the present study was to evaluate the associations between parity and MetS and its components among a cohort of women in Northern China.
Furthermore, it is known that overweight is associated with an increased risk for chronic diseases; however, body weight is an important modifiable risk factor compared with other unmodifiable factors, such as age and gender. In Chinese women, the age-standardized prevalence of overweight was found to be approximately 31.1%[5], indicating that obesity would be another concern causing psychological and physical distress. In this study, we also performed subgroup analyzes according to body mass index (BMI) categories, aiming to investigate whether BMI had effects on the association between parity and MetS.
-
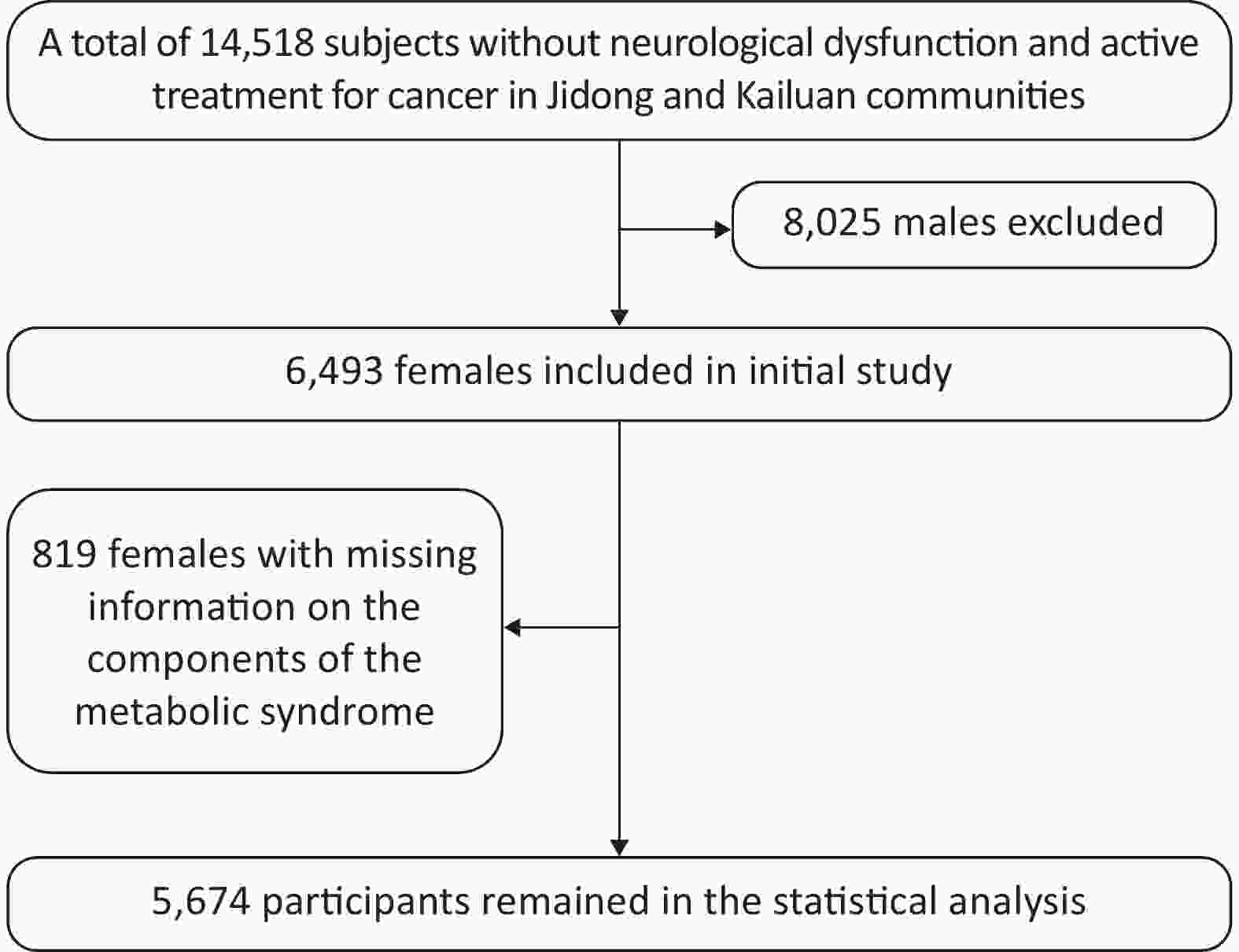
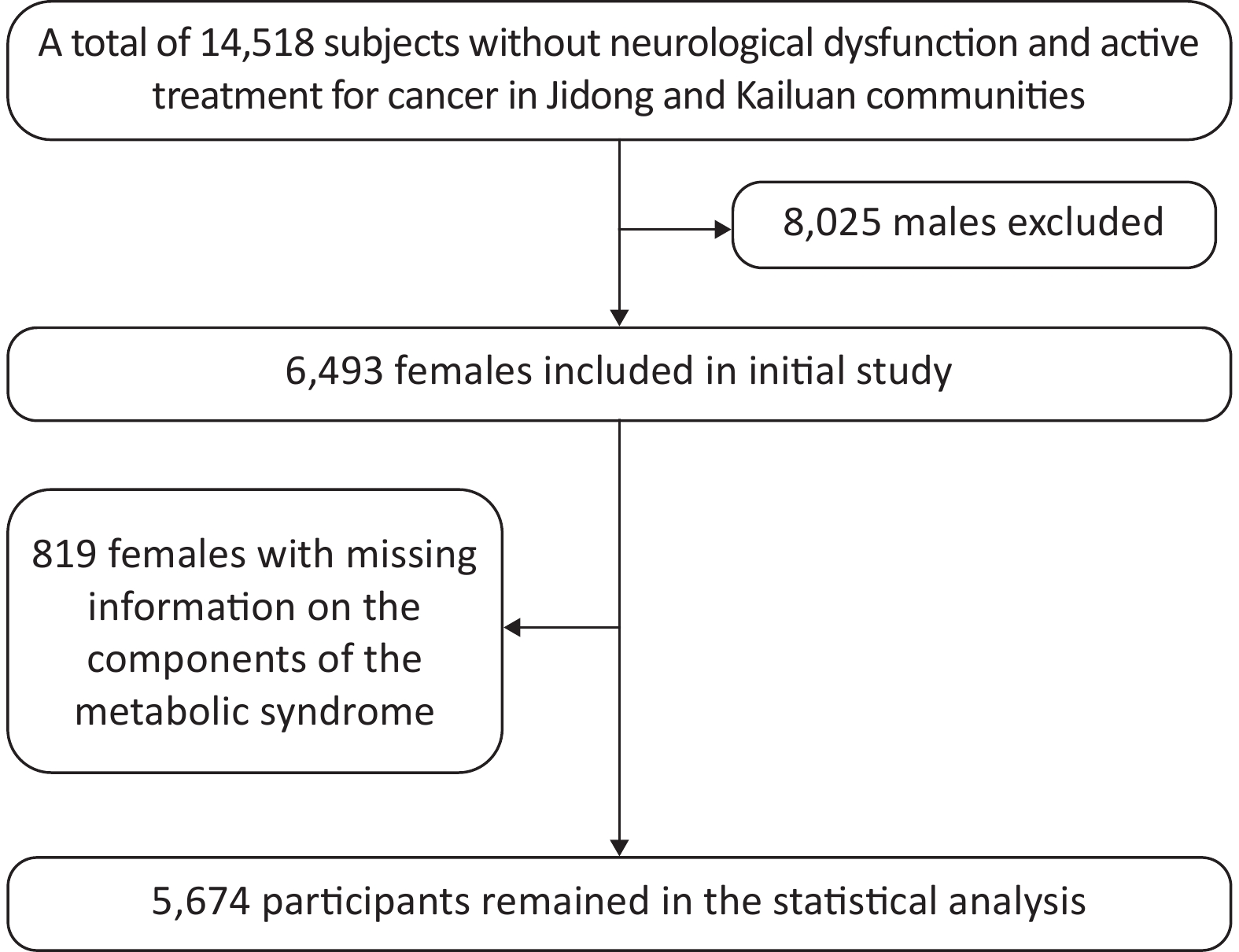
A total of 14,518 participants were recruited from Jidong and Kailuan communities (Tangshan city, Hebei, Northern China) from 2012 to 2014 as described in our previous studies[11-13]. The final study sample included 5,674 participants for statistical analysis after the exclusion of 8,025 male individuals and 819 female individuals with missing information on the components of MetS (Figure 1). This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Jidong Oilfield Inc Medical Center and Kailuan General Hospital. Written informed consent was obtained from all participants. All the experiments described in this study were performed in accordance with the approved guidelines.
-
A standardized, structured questionnaire was administered by well-trained research staff to collect information about the subjects’ demographic characteristics, socioeconomic status, marital status, menopausal status, cardiovascular risk factors, and medical history.
During the clinical examination, anthropometric measurements were obtained by certified observers using standard protocols and techniques. Three blood pressure measurements were obtained from the subjects after a 5-min rest in the seated position using an automatic sphygmomanometer, and the second and third readings were averaged and recorded. Participants were advised to avoid cigaret smoking, consumption of alcohol and caffeinated beverages, and exercise for at least 30 min before their blood pressure measurements. Body weight and height were measured twice during the examination. Body weight was measured in light indoor clothing without shoes to the nearest 0.1 kg, and height was measured without shoes to the nearest mm using a stadiometer. BMI was calculated as body weight in kilograms divided by height in square meters. Obesity was defined as a BMI of ≥ 25.0 kg/m2, according to the WHO definitions[5,14,15]. Waist circumference was measured at 1 cm above the navel to the nearest mm[14]. The physical activity of the participants was divided into the following three categories: (1) inactive, nearly none; (2) moderately active, 1–149 min/week of moderate intensity or 1–74 min/week of vigorous intensity; and (3) active, ≥ 150 min/week of moderate intensity or ≥ 75 min/week of vigorous intensity.
Venous blood samples were collected after overnight fasting for 10–12 h. The levels of serum lipids, including triglycerides, total cholesterol, low-density lipoprotein cholesterol, and high HDL cholesterol, and plasma glucose were determined using an autoanalyzer (AU400; Olympus, Tokyo, Japan) according to the manufacturer’s instructions at the central laboratory of Jidong Oilfield Hospital and Kailuan General Hospital.
The components of MetS were defined according to the following guidelines from the US National Cholesterol Education Program and modified for Asian populations: (1) abdominal obesity (waist circumference ≥ 80 cm); (2) elevated triglyceride levels (≥ 1.7 mmol/L); (3) low HDL cholesterol levels (< 1.0 mmol/L); (4) high blood pressure (≥ 130 mmHg systolic or ≥ 85 mmHg diastolic or use of antihypertensive medications or previously diagnosed hypertension); and (5) elevated fasting glucose levels (≥ 6.1 mmol/L or use of antidiabetic medications or previously diagnosed type 2 diabetes). A combination of three or more of these factors was defined as MetS[5,16-17].
Parity was then classified into four categories as zero (nulliparity), one, two, and three or more, and women with three or more children were combined into one group. The detailed protocol was consistent with our previous research[18].
-
Comparisons among parity groups were made using one-way ANOVA for continuous variables and Chi-square test for categorical variables. Nonparametric methods were used to compare ordinal variables and variables with skewed distribution. The associations between parity and MetS and its components were investigated using logistic regression models. Subgroup analyzes according to BMI and age were also performed using logistic regression models. Results of the logistic regression models were presented as odds ratio (OR) with 95% confidence interval (CI). Potential confounders, including age, postmenopausal status, marital status, current smoking, alcohol use, oral contraceptive use, physical activity, education level, and income, were adjusted in the models. The parity of one was considered as the reference in all models. Associations with a P value of < 0.05 were considered to be statistically significant. All statistical analyzes were performed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
-
Table 1 shows the baseline characteristics of participants stratified by parity. The study population consisted of women with parity of zero (9.1%), one (72.9%), two (13.0%), and three or more (5.0%). There was a significant increase in age with parity (P < 0.001). Multiple lifestyle factors, including current smoking, alcohol use, and physical activity, exhibited a significant difference across the parity groups (all with P < 0.001). Higher proportion of postmenopausal status and use of contraceptive drugs was associated with increasing parity (both with P < 0.001). Socioeconomic status, measured using education level and mean equivalent household income, also demonstrated a statistical difference among the parity groups (both with P < 0.001).
Table 1. Baseline characteristics of participants stratified by parity
Characteristics Overall Parity group P value 0 1 2 ≥ 3 Number, n (%) 5,674 513 (9.1) 4,138 (72.9) 739 (13.0) 284 (5.0) Age, year 45.4 ± 12.7 28.2 ± 6.2 43.7 ± 9.7 58.5 ± 8.8 67.3 ± 8.0 < 0.001 Age at menopause, year 49.8 ± 4.0 44.4 ± 7.3 49.5 ± 4.0 50.3 ± 3.7 50.0 ± 4.3 < 0.001 Postmenopause, n (%) 1,844 (32.5) 5 (1.0) 1,024 (24.8) 564 (76.3) 251 (88.4) < 0.001 Married, n (%) 5,311 (93.6) 292 (56.9) 4,042 (97.7) 714 (96.6) 263 (92.6) < 0.001 Current smoking, n (%) 120 (2.1) 5 (1.0) 75 (1.8) 24 (3.3) 16 (5.6) < 0.001 Alcohol use, n (%) 203 (3.6) 29 (5.7) 154 (3.7) 19 (2.6) 1 (0.4) < 0.001 Antihypertensive medication, n (%) 659 (11.6) 2 (0.4) 336 (8.1) 214 (29.0) 107 (37.7) < 0.001 Insulin or oral hypoglycemic drug, n (%) 206 (3.6) 1 (0.2) 109 (2.6) 70 (9.5) 26 (9.2) < 0.001 Antilipemic agent, n (%) 81 (1.4) 0 45 (1.1) 19 (2.6) 17 (6.0) < 0.001 Oral contraceptives, n (%) 148 (2.6) 4 (0.8) 91 (2.2) 33 (4.5) 20 (7.0) < 0.001 Estrogen replacement therapy, n (%) 35 (0.6) 1 (0.2) 27 (0.7) 5 (0.7) 2 (0.7) 0.647 Physical activity, n (%) < 0.001 Inactive 2,017 (35.6) 178 (34.7) 1,513 (36.6) 238 (32.2) 88 (31.0) Moderate active 1,062 (18.7) 103 (20.1) 829 (20.0) 86 (11.6) 44 (15.5) Active 2,595 (45.7) 232 (45.2) 1,796 (43.4) 415 (56.2) 152 (53.5) Education level, n (%) < 0.001 Illiteracy/primary school 319 (5.6) 1 (0.2) 86 (2.1) 148 (20.0) 84 (29.6) Middle/high school 2,705 (47.7) 73 (14.2) 1,960 (47.4) 501 (67.8) 171 (60.2) College or above 2,650 (46.7) 439 (85.6) 2,092 (50.5) 90 (12.2) 29 (10.2) Income, ¥/month, n (%) < 0.001 ≤ 3,000 3,194 (56.3) 159 (31.0) 2,221 (53.7) 584 (79.0) 230 (81.0) 3,001–5,000 2,153 (38.0) 304 (59.3) 1,667 (40.3) 134 (18.1) 48 (16.9) > 5,000 327 (5.7) 50 (9.7) 250 (6.0) 21 (2.9) 6 (2.1) -
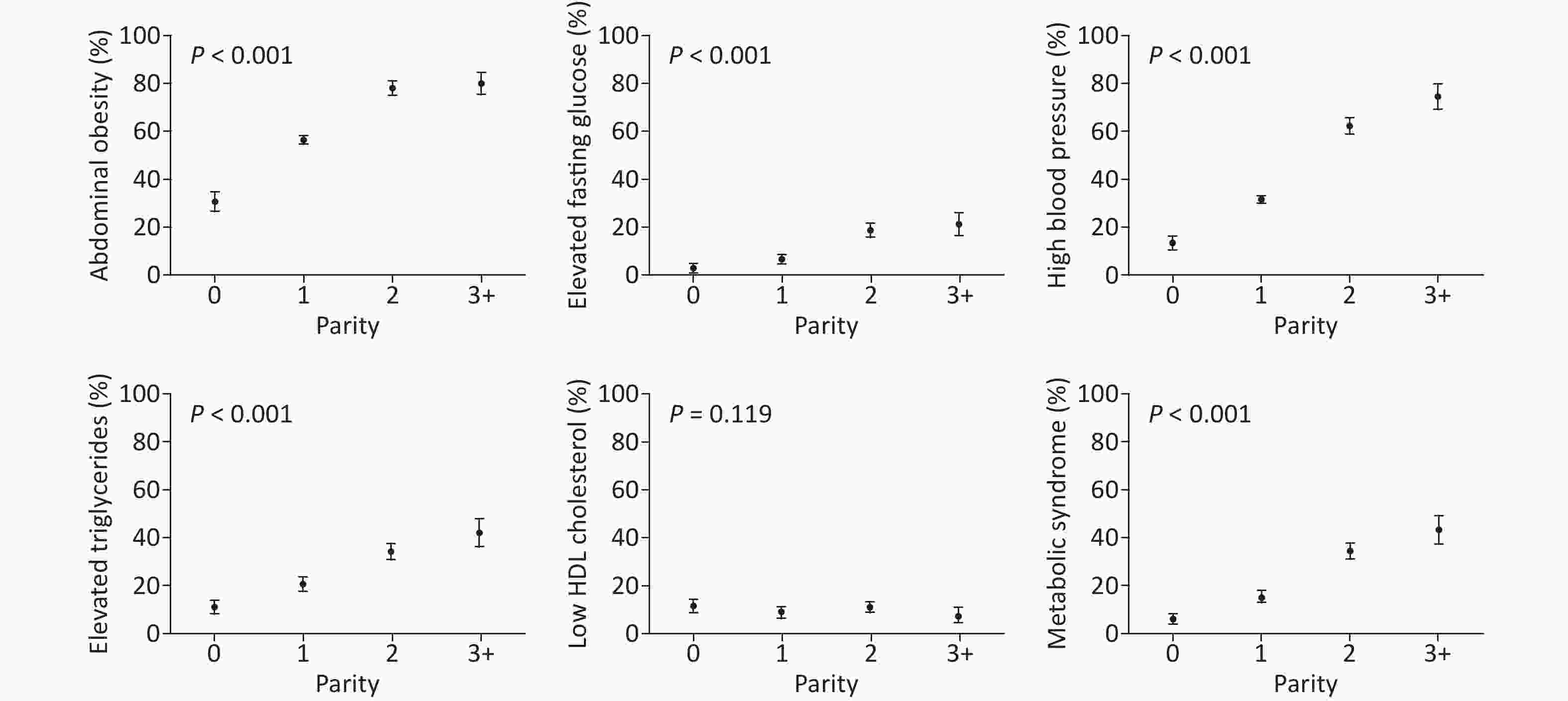
Figure 2 shows the prevalence of MetS and its components according to parity. Among the parity groups, nulliparous women had the lowest prevalence, and those with parity of three or more had the highest prevalence of abdominal obesity (81%), elevated fasting glucose levels (21%), high blood pressure (75%), elevated triglyceride levels (43%), and MetS (44%). The prevalence of MetS and its components appeared to increase progressively with parity (all with P < 0.001, Figure 2). However, a similar pattern was not observed for low HDL cholesterol levels (P = 0.119), where nulliparous women had the highest prevalence of 11.7%. Overall, an elevated fasting glucose level was the least prevalent component in our study cohort, and abdominal obesity was the most prevalent component.
-
As depicted in Figure 3, there was no association between parity and low HDL cholesterol levels. Regarding the other four components and MetS, there was an increasing trend of ORs
among the parity groups. Compared with women with parity of one, nulliparous women had significantly decreased ORs in all models. Women with parity of two had statistically significant odds of abdominal obesity (OR = 1.45, 95% CI: 1.17–1.81, P < 0.001), high blood pressure (OR = 1.26, 95% CI: 1.03–1.54, P = 0.025), elevated fasting glucose levels (OR = 1.36, 95% CI: 1.03–1.79, P = 0.029), and MetS (OR = 1.39, 95% CI: 1.13–1.73, P = 0.002), and those with parity of three or more had statistically significant odds of elevated triglyceride levels (OR = 1.42, 95% CI: 1.04–1.94, P = 0.027) and MetS (OR = 1.50, 95% CI: 1.10–2.05, P = 0.011) after complete adjustment for age, postmenopausal status, marital status, current smoking, alcohol use, oral contraceptive use, physical activity, education level, and income (Supplementary Table S1 available in www.besjournal.com). 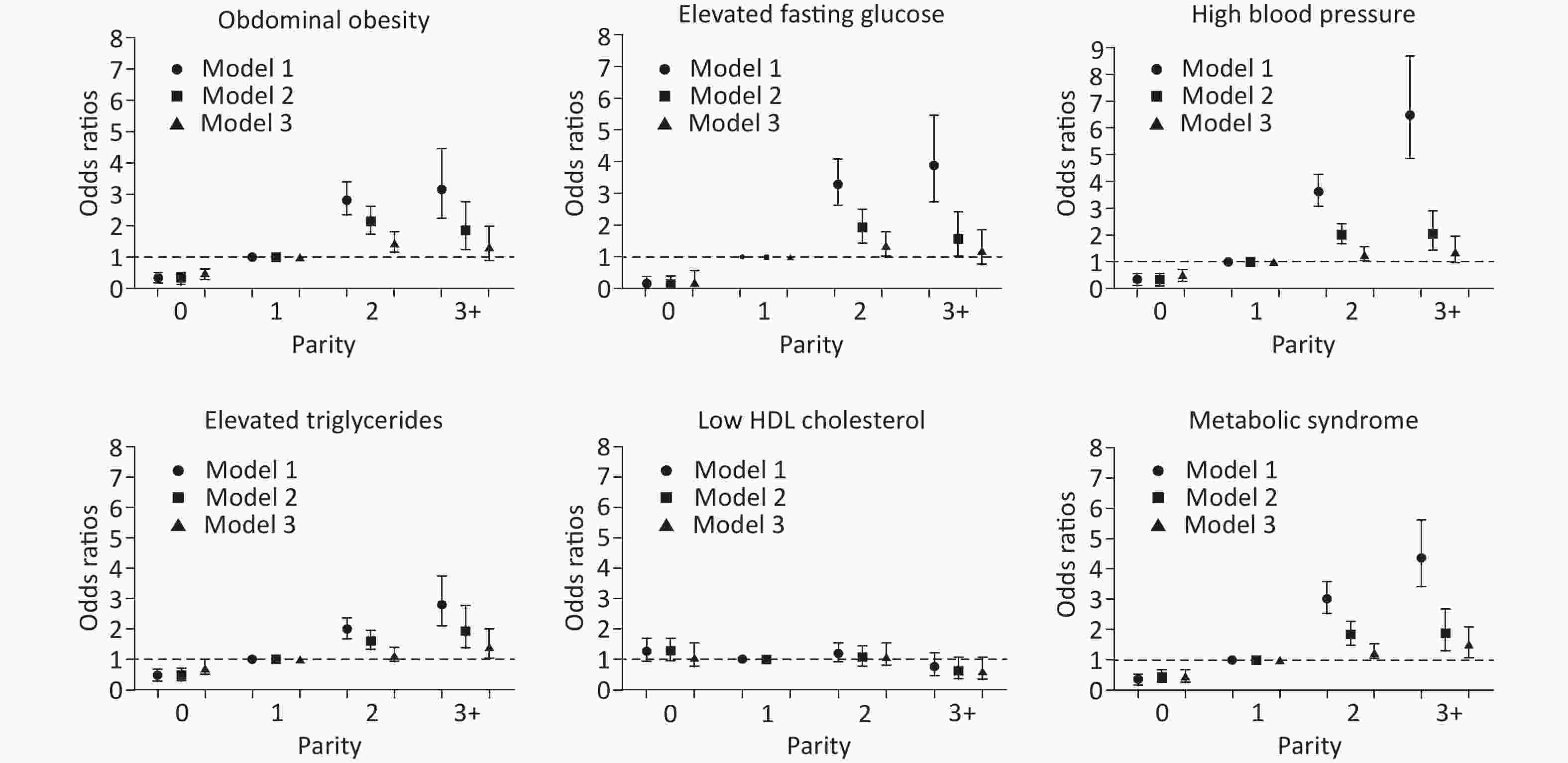
Figure 3. The associations between parity and metabolic syndrome and its components. Model 1: unadjusted. Model 2: adjusted for age. Model 3: adjusted for age, postmenopausal status, marital status, current smoking, alcohol use, oral contraceptive use, physical activity, education level, and income.
Table S1. Adjusted ORs for the associations between parity and the metabolic syndrome and its components
Parity Abdominal obesity Elevated triglycerides Elevated fasting glucose Low HDL cholesterol High blood pressure Metabolic syndrome OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P Model 1 0 0.34
(0.28—0.41)< 0.001 0.47
(0.36—0.63)< 0.001 0.16
(0.07—0.37)< 0.001 1.27
(0.95—1.69)0.109 0.33
(0.25—0.43)< 0.001 0.35
(0.24—0.51)< 0.001 1 1 1 1 1 1 1 2 2.82
(2.34—3.39)< 0.001 2.00
(1.69—2.37)< 0.001 3.27
(2.63—4.08)< 0.001 1.19
(0.93—1.54)0.171 3.61
(3.07—4.25)< 0.001 3.01
(2.53—3.58)< 0.001 ≥ 3 3.16
(2.34—4.27)< 0.001 2.81
(2.19—3.59)< 0.001 3.80
(2.79—5.17)< 0.001 0.76
(0.48—1.21)0.246 6.47
(4.90—8.54)< 0.001 4.37
(3.41—5.61)< 0.001 Model 2 0 0.34
(0.28—0.42)< 0.001 0.48
(0.36—0.64)< 0.001 0.17
(0.08—0.39)< 0.001 1.28
(0.96—1.71)0.097 0.35
(0.27—0.45)< 0.001 0.37
(0.25—0.54)< 0.001 1 1 1 1 1 1 1 2 2.14
(1.74—2.63)< 0.001 1.62
(1.33—1.98)< 0.001 1.91
(1.45—2.51)< 0.001 1.07
(0.80—1.44)0.634 2.01
(1.67—2.42)< 0.001 1.84
(1.49—2.27)< 0.001 ≥ 3 1.83
(1.29—2.61)< 0.001 1.94
(1.43—2.63)< 0.001 1.57
(1.06—2.32)0.023 0.64
(0.37—1.08)0.093 2.03
(1.45—2.82)< 0.001 1.89
(1.38—2.56)< 0.001 Model 3 0 0.50
(0.40—0.63)< 0.001 0.72
(0.53—1.00)0.048 0.24
(0.10—0.57)0.001 1.09
(0.78—1.53)0.619 0.52
(0.38—0.70)< 0.001 0.45
(0.30—0.68)< 0.001 1 1 1 1 1 1 1 2 1.45
(1.17—1.81)< 0.001 1.15
(0.93—1.41)0.196 1.36
(1.03—1.79)0.029 1.11
(0.81—1.53)0.507 1.26
(1.03—1.54)0.025 1.39
(1.13—1.73)0.002 ≥ 3 1.30
(0.90—1.87)0.167 1.42
(1.04—1.94)0.027 1.20
(0.81—1.77)0.362 0.62
(0.36—1.07)0.084 1.37
(0.97—1.94)0.071 1.50
(1.10—2.05)0.011 Note. Model 1: unadjusted. Model 2: adjusted for age. Model 3: adjusted for age, postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income. -
As shown in Table 2, subgroup analyses for the associations between parity and MetS and its components were performed according to BMI and age. BMI was subdivided by 25, age was subdivided by the median, and parity was included as a categorical variable.
Table 2. Subgroup analyses for the association between parity and five components and metabolic syndrome according to BMI and age
Variables BMI* Age# ≤ 25 kg/m2 > 25 kg/m2 P-interaction ≤ 45 > 45 P-interaction Abdominal obesity 0.152 0.442 0 0.48 (0.37–0.64) 1.10 (0.45–2.70) 0.53 (0.41–0.69) 0.86 (0.30–2.46) 1 1 1 1 1 2 1.32 (1.02–1.72) 2.41 (1.19–4.89) 1.03 (0.61–1.75) 1.58 (1.26–1.99) ≥ 3 0.88 (0.56–1.38) 2.59 (0.97–6.92) 1.63 (0.27–9.88) 1.50 (1.07–2.09) Elevated triglycerides < 0.001 0.155 0 0.58 (0.37–0.91) 1.44 (0.85–2.44) 0.87 (0.60–1.26) 1.10 (0.34–3.51) 1 1 1 1 1 2 1.33 (0.98–1.81) 0.93 (0.70–1.25) 2.10 (1.15–3.83) 1.10 (0.90–1.34) ≥ 3 1.43 (0.88–2.32) 1.23 (0.81–1.87) 3.84 (0.63–23.45) 1.45 (1.15–1.91) Elevated fasting glucose 0.595 0.991 0 NA 0.64 (0.25–1.61) 0.46 (0.18–1.21) NA 1 1 1 1 1 2 1.23 (0.78–1.93) 1.34 (0.94–1.91) 1.82 (0.61–5.38) 1.08 (0.81–1.43) ≥ 3 0.91 (0.47–1.78) 1.23 (0.76–2.01) NA 0.73 (0.48–1.12) Low HDL cholesterol 0.034 0.359 0 1.29 (0.85–1.94) 1.19 (0.64–2.21) 1.01 (0.70–1.47) 2.69 (0.74–9.82) 1 1 1 1 1 2 1.35 (0.85–2.14) 0.89 (0.57–1.38) 0.88 (0.37–2.09) 1.34 (0.98–1.85) ≥ 3 0.54 (0.22–1.35) 0.60 (0.30–1.19) NA 0.81 (0.49–1.35) High blood pressure 0.078 0.523 0 0.48 (0.33–0.71) 0.81 (0.48–1.37) 0.69 (0.48–0.98) 1.13 (0.40–3.16) 1 1 1 1 1 2 1.22 (0.92–1.60) 1.21 (0.88–1.68) 1.33 (0.71–2.47) 1.66 (1.37–2.02) ≥ 3 0.96 (0.60–1.53) 1.73 (1.01–3.00) 5.88 (0.96–36.06) 2.54 (1.88–3.44) Metabolic syndrome < 0.001 0.497 0 0.35 (0.16–0.79) 0.89 (0.51–1.55) 0.65 (0.39–1.08) 1.01 (0.28–3.65) 1 1 1 1 1 2 1.28 (0.89–1.83) 1.17 (0.88–1.57) 1.93 (0.92–4.05) 1.45 (1.19–1.78) ≥ 3 0.90 (0.52–1.54) 1.53 (1.01–2.31) 6.48 (1.06–39.73) 1.90 (1.44–2.51) Note. *Adjusted for age, postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income.
#Adjusted for postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income. NA: not available.After comparison of all the associations depicted between Figure 3 and Table 2, it appeared that BMI subgroups had no effects on the associations between parity and low HDL cholesterol levels, which showed no significant differences. BMI subgroups partially modified the associations between parity and other four components and MetS. Regarding MetS (Table 2), there were no more associations among women with parity of zero and BMI > 25 kg/m2 and among those with parity of two or with parity of three or more and BMI ≤ 25 kg/m2.
Similar to the above-described results, age subgroups had no effects on the associations between parity and low HDL cholesterol levels, whereas they partially modified the associations between parity and other four components and MetS. Regarding MetS (Table 2), the associations remained significant only among women with parity of three or more and among those with parity of two and age > 45 years.
-
In this study, we evaluated the prevalence and the associations between parity and MetS and its components among women in Northern China. We also explored the effects of BMI and age on these associations in subgroup analyzes. We found that there was a trend of increased prevalence of MetS and its four components with an increase in parity. Moreover, higher parity showed a positive association with MetS and select components, including abdominal obesity, high blood pressure, elevated fasting glucose levels, and elevated triglyceride levels. Furthermore, BMI and age subgroups modified the associations between parity and MetS.
Several cross-sectional studies have investigated the association between parity and MetS, with the majority of them reporting that multiparity was associated with an increased risk of developing MetS in different racial populations[9,19,20]. The longitudinal CARDIA (the Coronary Artery Risk Development in Young Adults) study also found that future development of MetS was associated with increasing parity and was independent of prior obesity and pregnancy-related weight gain[21]. In this study, we also found positive associations between high parity and MetS, which is consistent with past studies[9,19-21]. It is worth noting that the majority of subjects in our cohort were women with parity of one (72.9%), which is due to the specific one-child policy in China. Different birth control policies among countries would result in various sociodemographic confounders.
Regarding the associations between parity and MetS components, the most consistent finding across studies was the positive association between parity and abdominal obesity[9,19]. Some studies reported a linear association between increased parity and elevated triglyceride levels[19,20], which was similar to our results. Some studies found a significant association between increased parity and low HDL cholesterol levels[19,20], but another research found no association[22], and our findings were consistent with the latter. Although some studies have reported no associations between parity and high blood pressure[19,20], we found a positive association between parity and high blood pressure. Overall, results of the previous studies on the associations between parity and MetS components have been inconsistent. Several reasons might explain these discrepancies, including different ethnicities, components defined using different diagnostic criteria, women enrolled at different age groups, different adjusted confounders, and the proportion of nulliparous, parous, and multiparous women[9,21,23,24].
BMI, an important modifiable risk factor for a number of cardiovascular diseases, primarily represents the body weight. To our knowledge, this is the first study to perform an intensive assessment of the effect of BMI subgroups on the associations between parity and MetS and its components. We found that BMI subgroups partially modified the associations between parity and MetS and its four components, including abdominal obesity, high blood pressure, elevated fasting glucose levels, and elevated triglyceride levels, indicating that it might be important for women to control body weight to avoid the risk of MetS and the associated factors. Furthermore, there could be other potential factors mediating the development of MetS, such as the percentage of body fat and fat distribution pattern[9,25]. A study on a US Hispanic/Latina population showed that further adjustment for the percentage of body fat significantly influenced the relationships between parity and metabolic syndrome[9]. Moreover, age subgroups partially modified these associations similar to BMI subgroups, whereas age was an unmodifiable risk factor.
The mechanisms underlying the associations between parity and MetS remain unknown. However, previous studies have proposed a few possible biological mechanisms to understand these associations. First, pregnancy is associated with chronic metabolic alterations, including increase of lipid metabolism, insulin resistance, dysglycemia, obesity, and folate deficiency[26,27]. These effects can accumulate, especially for multiparous women. Second, the levels of sex hormones are modified during pregnancy, resulting in relatively lower protection by estrogen[28]. Reduced exposure to estrogen may promote the metabolic dysregulation of its components, including high blood pressure, hyperlipidemia, and hyperglycemia[29]. Furthermore, women have to go through a great deal of physiological, psychological, and social stress during childbearing and childrearing, which results in long-term variations in metabolic profiles[30,31]. All these biological alterations and lifestyle factors can facilitate the prevalence of MetS and its components.
Finally, it is necessary to consider several limitations of the present study. First, women in this cohort came from Northern China and had specific sociodemographic characteristics; thus, this could hinder the generalization of results to other provinces or other countries. In addition, there were particular family planning policies in China such as the one-child policy, so that the composition of the study population might be different from other cohorts. Moreover, this study did not assess reproductive factors, including age at gestation, miscarriage, abortions, and lactation, and perinatal complications such as gestational hypertension and gestational diabetes mellitus. Finally, we did not perform examinations for male individuals in this population, as their information could provide further insights into the role of childrearing.
In conclusion, this study suggested that multiparity was positively associated with MetS and select components in Northern Chinese women. BMI played a vital role in the associations between parity and MetS and its components. Regarding the high prevalence of MetS and its components in Chinese women, it is important to perform regular examinations, especially for multiparous women. Early detection of high-risk individuals can help in the primary prevention of MetS and cardiovascular diseases[32]. Further studies are required to identify whether there are causal relationships between parity and MetS and the underlying mechanisms.
-
We thank all the enrolled participants and their family members.
doi: 10.3967/bes2020.002
Effect of Body Mass Index on the Associations between Parity and Metabolic Syndrome and its Components among Northern Chinese Women
-
Abstract:
Objectives The aims of this study were to assess the associations between parity and metabolic syndrome (MetS) and its components and to evaluate the effects of body mass index (BMI) on these associations. Methods A total of 5,674 women were enrolled from Jidong and Kailuan communities (Tangshan, Hebei) in Northern China. All participants completed standardized questionnaires, physical examination, and biochemical measurements. Logistic regression analysis was used to test the associations. Results Compared with women with parity of one, nulliparous women had decreased odds ratios (ORs ); those with parity of two had odds of abdominal obesity [OR = 1.45, 95% confidence interval (CI) 1.17–1.81, P < 0.001], high blood pressure (OR = 1.26, 95% CI: 1.03–1.54, P = 0.025), elevated fasting glucose levels (OR = 1.36, 95% CI: 1.03–1.79, P = 0.029), and MetS (OR = 1.39, 95% CI: 1.13–1.73, P = 0.002); and those with parity of three or more had increased odds of elevated triglyceride levels (OR = 1.42, 95% CI: 1.04–1.94, P = 0.027) and MetS (OR = 1.50, 95% CI: 1.10–2.05, P = 0.011) after complete adjustment for confounders. Furthermore, BMI and age subgroups partially modified the associations between parity and MetS and its components. Conclusions Parity is positively associated with MetS and select components in women. BMI is an important modifier involved in the associations between parity and MetS. -
Key words:
- Parity /
- Metabolic syndrome /
- BMI /
- Risk factor /
- Association
-
Figure 3. The associations between parity and metabolic syndrome and its components. Model 1: unadjusted. Model 2: adjusted for age. Model 3: adjusted for age, postmenopausal status, marital status, current smoking, alcohol use, oral contraceptive use, physical activity, education level, and income.
Table 1. Baseline characteristics of participants stratified by parity
Characteristics Overall Parity group P value 0 1 2 ≥ 3 Number, n (%) 5,674 513 (9.1) 4,138 (72.9) 739 (13.0) 284 (5.0) Age, year 45.4 ± 12.7 28.2 ± 6.2 43.7 ± 9.7 58.5 ± 8.8 67.3 ± 8.0 < 0.001 Age at menopause, year 49.8 ± 4.0 44.4 ± 7.3 49.5 ± 4.0 50.3 ± 3.7 50.0 ± 4.3 < 0.001 Postmenopause, n (%) 1,844 (32.5) 5 (1.0) 1,024 (24.8) 564 (76.3) 251 (88.4) < 0.001 Married, n (%) 5,311 (93.6) 292 (56.9) 4,042 (97.7) 714 (96.6) 263 (92.6) < 0.001 Current smoking, n (%) 120 (2.1) 5 (1.0) 75 (1.8) 24 (3.3) 16 (5.6) < 0.001 Alcohol use, n (%) 203 (3.6) 29 (5.7) 154 (3.7) 19 (2.6) 1 (0.4) < 0.001 Antihypertensive medication, n (%) 659 (11.6) 2 (0.4) 336 (8.1) 214 (29.0) 107 (37.7) < 0.001 Insulin or oral hypoglycemic drug, n (%) 206 (3.6) 1 (0.2) 109 (2.6) 70 (9.5) 26 (9.2) < 0.001 Antilipemic agent, n (%) 81 (1.4) 0 45 (1.1) 19 (2.6) 17 (6.0) < 0.001 Oral contraceptives, n (%) 148 (2.6) 4 (0.8) 91 (2.2) 33 (4.5) 20 (7.0) < 0.001 Estrogen replacement therapy, n (%) 35 (0.6) 1 (0.2) 27 (0.7) 5 (0.7) 2 (0.7) 0.647 Physical activity, n (%) < 0.001 Inactive 2,017 (35.6) 178 (34.7) 1,513 (36.6) 238 (32.2) 88 (31.0) Moderate active 1,062 (18.7) 103 (20.1) 829 (20.0) 86 (11.6) 44 (15.5) Active 2,595 (45.7) 232 (45.2) 1,796 (43.4) 415 (56.2) 152 (53.5) Education level, n (%) < 0.001 Illiteracy/primary school 319 (5.6) 1 (0.2) 86 (2.1) 148 (20.0) 84 (29.6) Middle/high school 2,705 (47.7) 73 (14.2) 1,960 (47.4) 501 (67.8) 171 (60.2) College or above 2,650 (46.7) 439 (85.6) 2,092 (50.5) 90 (12.2) 29 (10.2) Income, ¥/month, n (%) < 0.001 ≤ 3,000 3,194 (56.3) 159 (31.0) 2,221 (53.7) 584 (79.0) 230 (81.0) 3,001–5,000 2,153 (38.0) 304 (59.3) 1,667 (40.3) 134 (18.1) 48 (16.9) > 5,000 327 (5.7) 50 (9.7) 250 (6.0) 21 (2.9) 6 (2.1) S1. Adjusted ORs for the associations between parity and the metabolic syndrome and its components
Parity Abdominal obesity Elevated triglycerides Elevated fasting glucose Low HDL cholesterol High blood pressure Metabolic syndrome OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P OR
(95% CI)P Model 1 0 0.34
(0.28—0.41)< 0.001 0.47
(0.36—0.63)< 0.001 0.16
(0.07—0.37)< 0.001 1.27
(0.95—1.69)0.109 0.33
(0.25—0.43)< 0.001 0.35
(0.24—0.51)< 0.001 1 1 1 1 1 1 1 2 2.82
(2.34—3.39)< 0.001 2.00
(1.69—2.37)< 0.001 3.27
(2.63—4.08)< 0.001 1.19
(0.93—1.54)0.171 3.61
(3.07—4.25)< 0.001 3.01
(2.53—3.58)< 0.001 ≥ 3 3.16
(2.34—4.27)< 0.001 2.81
(2.19—3.59)< 0.001 3.80
(2.79—5.17)< 0.001 0.76
(0.48—1.21)0.246 6.47
(4.90—8.54)< 0.001 4.37
(3.41—5.61)< 0.001 Model 2 0 0.34
(0.28—0.42)< 0.001 0.48
(0.36—0.64)< 0.001 0.17
(0.08—0.39)< 0.001 1.28
(0.96—1.71)0.097 0.35
(0.27—0.45)< 0.001 0.37
(0.25—0.54)< 0.001 1 1 1 1 1 1 1 2 2.14
(1.74—2.63)< 0.001 1.62
(1.33—1.98)< 0.001 1.91
(1.45—2.51)< 0.001 1.07
(0.80—1.44)0.634 2.01
(1.67—2.42)< 0.001 1.84
(1.49—2.27)< 0.001 ≥ 3 1.83
(1.29—2.61)< 0.001 1.94
(1.43—2.63)< 0.001 1.57
(1.06—2.32)0.023 0.64
(0.37—1.08)0.093 2.03
(1.45—2.82)< 0.001 1.89
(1.38—2.56)< 0.001 Model 3 0 0.50
(0.40—0.63)< 0.001 0.72
(0.53—1.00)0.048 0.24
(0.10—0.57)0.001 1.09
(0.78—1.53)0.619 0.52
(0.38—0.70)< 0.001 0.45
(0.30—0.68)< 0.001 1 1 1 1 1 1 1 2 1.45
(1.17—1.81)< 0.001 1.15
(0.93—1.41)0.196 1.36
(1.03—1.79)0.029 1.11
(0.81—1.53)0.507 1.26
(1.03—1.54)0.025 1.39
(1.13—1.73)0.002 ≥ 3 1.30
(0.90—1.87)0.167 1.42
(1.04—1.94)0.027 1.20
(0.81—1.77)0.362 0.62
(0.36—1.07)0.084 1.37
(0.97—1.94)0.071 1.50
(1.10—2.05)0.011 Note. Model 1: unadjusted. Model 2: adjusted for age. Model 3: adjusted for age, postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income. Table 2. Subgroup analyses for the association between parity and five components and metabolic syndrome according to BMI and age
Variables BMI* Age# ≤ 25 kg/m2 > 25 kg/m2 P-interaction ≤ 45 > 45 P-interaction Abdominal obesity 0.152 0.442 0 0.48 (0.37–0.64) 1.10 (0.45–2.70) 0.53 (0.41–0.69) 0.86 (0.30–2.46) 1 1 1 1 1 2 1.32 (1.02–1.72) 2.41 (1.19–4.89) 1.03 (0.61–1.75) 1.58 (1.26–1.99) ≥ 3 0.88 (0.56–1.38) 2.59 (0.97–6.92) 1.63 (0.27–9.88) 1.50 (1.07–2.09) Elevated triglycerides < 0.001 0.155 0 0.58 (0.37–0.91) 1.44 (0.85–2.44) 0.87 (0.60–1.26) 1.10 (0.34–3.51) 1 1 1 1 1 2 1.33 (0.98–1.81) 0.93 (0.70–1.25) 2.10 (1.15–3.83) 1.10 (0.90–1.34) ≥ 3 1.43 (0.88–2.32) 1.23 (0.81–1.87) 3.84 (0.63–23.45) 1.45 (1.15–1.91) Elevated fasting glucose 0.595 0.991 0 NA 0.64 (0.25–1.61) 0.46 (0.18–1.21) NA 1 1 1 1 1 2 1.23 (0.78–1.93) 1.34 (0.94–1.91) 1.82 (0.61–5.38) 1.08 (0.81–1.43) ≥ 3 0.91 (0.47–1.78) 1.23 (0.76–2.01) NA 0.73 (0.48–1.12) Low HDL cholesterol 0.034 0.359 0 1.29 (0.85–1.94) 1.19 (0.64–2.21) 1.01 (0.70–1.47) 2.69 (0.74–9.82) 1 1 1 1 1 2 1.35 (0.85–2.14) 0.89 (0.57–1.38) 0.88 (0.37–2.09) 1.34 (0.98–1.85) ≥ 3 0.54 (0.22–1.35) 0.60 (0.30–1.19) NA 0.81 (0.49–1.35) High blood pressure 0.078 0.523 0 0.48 (0.33–0.71) 0.81 (0.48–1.37) 0.69 (0.48–0.98) 1.13 (0.40–3.16) 1 1 1 1 1 2 1.22 (0.92–1.60) 1.21 (0.88–1.68) 1.33 (0.71–2.47) 1.66 (1.37–2.02) ≥ 3 0.96 (0.60–1.53) 1.73 (1.01–3.00) 5.88 (0.96–36.06) 2.54 (1.88–3.44) Metabolic syndrome < 0.001 0.497 0 0.35 (0.16–0.79) 0.89 (0.51–1.55) 0.65 (0.39–1.08) 1.01 (0.28–3.65) 1 1 1 1 1 2 1.28 (0.89–1.83) 1.17 (0.88–1.57) 1.93 (0.92–4.05) 1.45 (1.19–1.78) ≥ 3 0.90 (0.52–1.54) 1.53 (1.01–2.31) 6.48 (1.06–39.73) 1.90 (1.44–2.51) Note. *Adjusted for age, postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income.
#Adjusted for postmenopause, marital status, current smoking, alcohol use, oral contraceptives, physical activity, education level and income. NA: not available. -
[1] Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009; 120, 1640−5. doi: 10.1161/CIRCULATIONAHA.109.192644 [2] Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J Am Coll Cardiol, 2010; 56, 1113−32. doi: 10.1016/j.jacc.2010.05.034 [3] McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care, 2005; 28, 385−90. doi: 10.2337/diacare.28.2.385 [4] Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation, 2004; 110, 1245−50. doi: 10.1161/01.CIR.0000140677.20606.0E [5] Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet, 2005; 365, 1398−405. doi: 10.1016/S0140-6736(05)66375-1 [6] Skilton MR, Sérusclat A, Begg LM, et al. Parity and carotid atherosclerosis in men and women insights into the roles of childbearing and child-Rearing. Stroke, 2009; 40, 1152−7. doi: 10.1161/STROKEAHA.108.535807 [7] Skilton MR, Bonnet F, Begg LM, et al. Childbearing, child-Rearing, cardiovascular risk factors, and progression of carotid intima-media thickness. Stroke, 2010; 41, 1332−7. doi: 10.1161/STROKEAHA.110.579219 [8] Dior UP, Hochner H, Friedlander Y, et al. Association between number of children and mortality of mothers: Results of a 37-year follow-up study. Ann Epidemiol, 2013; 23, 13−8. doi: 10.1016/j.annepidem.2012.10.005 [9] Vladutiu CJ, Siega-Riz AM, Sotres-Alvarez D, et al. Parity and components of the metabolic syndrome among US Hispanics/Latina women: results from the Hispanic community health study/study of Latinos. Circ Cardiovasc Qual Outcomes, 2016; 9, S62−9. doi: 10.1161/CIRCOUTCOMES.115.002464 [10] Lee Y, Lee HN, Kim SJ, et al. Higher parity and risk of metabolic syndrome in Korean postmenopausal women: Korea National Health and Nutrition Examination Survey 2010-2012. J Obstet Gynaecol Res, 2018; 44, 2045−52. doi: 10.1111/jog.13766 [11] Chen S, Li W, Jin C, et al. Resting heart rate trajectory pattern predicts arterial stiffness in a community-based Chinese cohort. Arterioscler Thromb Vasc Biol, 2017; 37, 359−64. doi: 10.1161/ATVBAHA.116.308674 [12] Hao Z, Zhang Y, Li Y, et al. The Association between Ideal Cardiovascular Health Metrics and Extracranial Carotid Artery Stenosis in a Northern Chinese Population: A Cross-Sectional Study. Sci Rep, 2016; 6, 31720. doi: 10.1038/srep31720 [13] Qiu MS, Wang XS, Yao Y, et al. Protocol of Jidong Women Health Cohort Study: Rationale, Design and Baseline Characteristics. Biomed Environ Sci, 2019; 32, 144−52. [14] Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser, 2000; 894, 1−253. [15] Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. National Institutes of Health. Obes Res, 1998; 6(suppl 2), 51S−209S. [16] Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA, 2001; 285, 2486-97. [17] He J, Gu D, Reynolds K, et al. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation, 2004; 110, 405−11. doi: 10.1161/01.CIR.0000136583.52681.0D [18] Lv H, Yang X, Zhou Y, et al. Parity and serum lipid levels: a cross-sectional study in Chinese female adults. Sci Rep, 2016; 6, 33831. doi: 10.1038/srep33831 [19] Cohen A, Pieper CF, Brown AJ, et al. Number of children and risk of metabolic syndrome in women. J Womens Health (Larchmt), 2006; 15, 763−73. doi: 10.1089/jwh.2006.15.763 [20] Mousavi E, Gharipour M, Tavassoli A, et al. Multiparity and risk of metabolic syndrome: Isfahan Healthy Heart Program. Metab Syndr Relat Disord, 2009; 7, 519−24. doi: 10.1089/met.2008.0076 [21] Gunderson EP, Jacobs DR Jr, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol, 2009; 201, 177.e1−9. doi: 10.1016/j.ajog.2009.03.031 [22] Akter S, Jesmin S, Rahman MM, et al. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS One, 2013; 8, e68319. doi: 10.1371/journal.pone.0068319 [23] Moradi S, Zamani F, Pishgar F, et al. Parity, duration of lactation and prevalence of maternal metabolic syndrome: a cross-sectional study. Eur J Obstet Gynecol Reprod Biol, 2016; 201, 70−4. doi: 10.1016/j.ejogrb.2016.03.038 [24] Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol, 2010; 20, 629−41. doi: 10.1016/j.annepidem.2010.03.015 [25] Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet, 2005; 365, 1415−28. doi: 10.1016/S0140-6736(05)66378-7 [26] Chandra A, Neeland IJ, Berry JD, et al. The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. J Am Coll Cardiol, 2014; 64, 997−1002. doi: 10.1016/j.jacc.2014.05.057 [27] Smith DE, Lewis CE, Caveny JL, et al. Longitudinal changes in adiposity associated with pregnancy: The CARDIA study. JAMA, 1994; 271, 1747−51. doi: 10.1001/jama.1994.03510460039030 [28] Hill M, Pašková A, Kančeva R, et al. Steroid profiling in pregnancy: a focus on the human fetus. J Steroid Biochem Mol Biol, 2014; 139, 201−22. doi: 10.1016/j.jsbmb.2013.03.008 [29] Arnold AP, Cassis LA, Eghbali M, et al. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol, 2017; 37, 746−56. doi: 10.1161/ATVBAHA.116.307301 [30] Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women’s Heart and Health Study and the British Regional Heart Study. Circulation, 2003; 107, 1260−4. doi: 10.1161/01.CIR.0000053441.43495.1A [31] Raikkonen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: A comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care, 2007; 30, 872−7. doi: 10.2337/dc06-1857 [32] Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. J Am Coll Cardiol, 2011; 57, 1404−23. doi: 10.1016/j.jacc.2011.02.005 -




 下载:
下载:



 Quick Links
Quick Links