-
Rotavirus (RV) is the most common cause of viral gastroenteritis among children younger than 5-year-old worldwide. RV has nine groups (Group A to I) and Group A (RVA) is the main cause of severe gastroenteritis disease in children. Human adenovirus (HAdV) consists of 7 species (HAdV-A through HAdV-G) including over 70 serotypes, and group F serotypes 40 and 41 are related to gastroenteritis [1]. There were reports that revealed co-infection of AdV with RVA and Norovirus[2]. Here we report an epidemiological and clinical analysis of RVA and AdV infection through a single-centered retrospective case-control study.
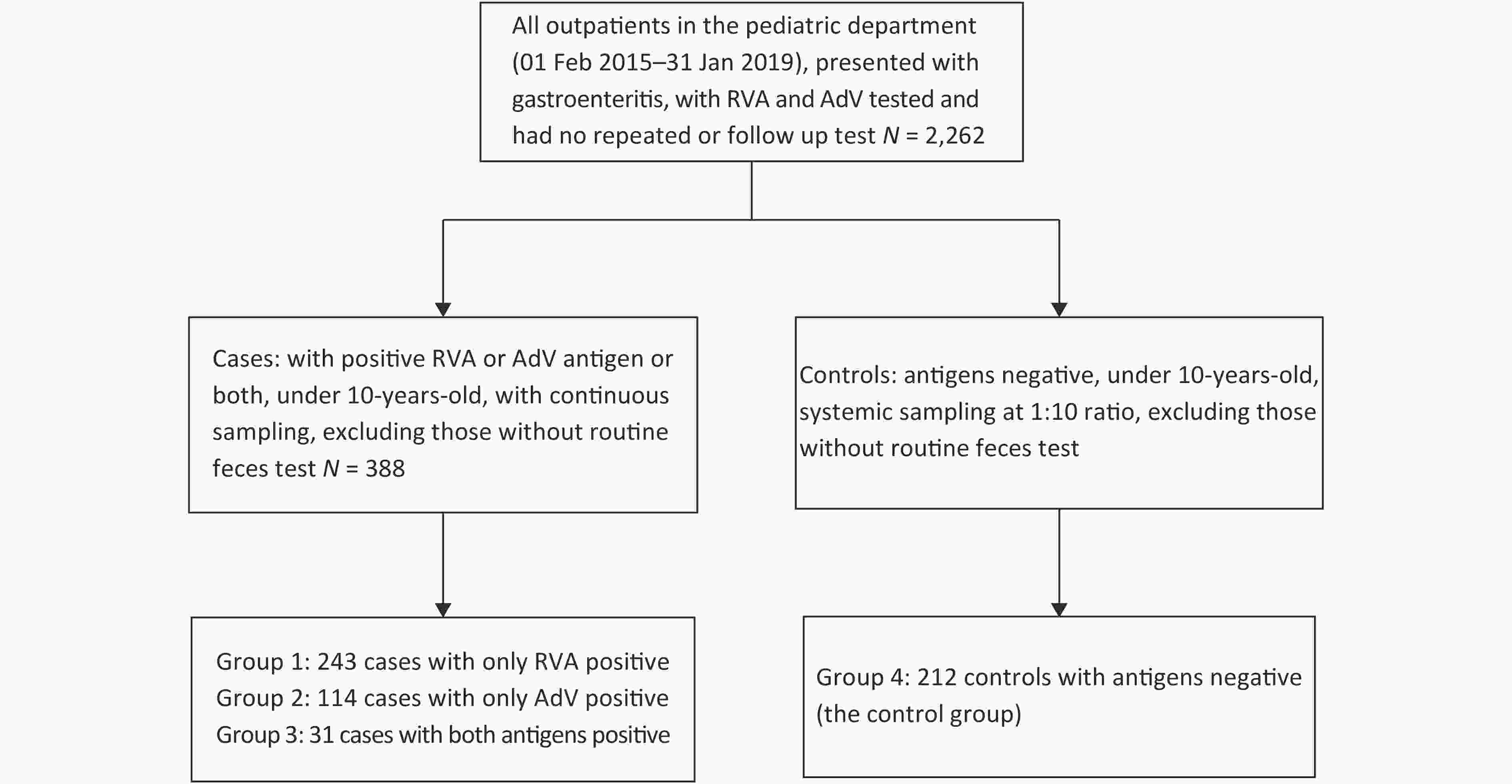
Outpatients in the Pediatric Department of Beijing Tsinghua Changgung Hospital who presented with gastroenteritis from 01 Feb 2015 to 31 Jan 2019 were included in the study. Inclusion criteria were: 1) aged 0–10 years-old, 2) diagnosed with acute gastroenteritis, 3) positive for RVA antigen, AdV antigen, or both, and 4) with routine feces test and fecal occult blood (FOB) test on the same day. Patients with repeated or follow-up tests were excluded. A total of 388 cases met the criteria and were included in the analysis, including 243 patients with RVA antigen positive (i.e., Group 1), 114 patients with AdV antigen positive (i.e., Group 2), and 31 patients with both antigens positive (i.e., Group 3). A total of 212 patients who had proved negative antigen results for RVA and AdV but met other inclusion criteria, with systemic sampling at 1:10 ratio in a chronological manner to ensure that the sampling represented the time distribution of the overall patient population, were selected as the control (Group 4). (Supplementary Figure S1, available in www.besjournal.com).
RVA and AdV antigens were tested simultaneously using a RVA and AdV diagnostic kit based on the immune colloidal gold labeling method (Genfocus Bio Engineering Tech Co., Ltd., Beijing, China), which targeted antigens of RV Group A and AdV Group F serotypes 40 and 41. FOB was tested using occult blood diagnostic kit based on the immune colloidal gold labeling method (Chemtron Biotech Co., Ltd., Shanghai, China). Routine feces test was performed by experienced laboratory technicians.
Data analysis was performed using the Statistical Product and Service Solutions (SPSS) Statistics 22nd edition. The age was described by median (interquartile range). Comparison of the indicators’ rates were analyzed by cross-tab χ2-test. Risk factors were analyzed by multifactorial binary logistic regression. All statistical analyses were two-tailed with a significance level (P value) of < 0.05.
During the 4-year study period, RVAs were found positive in 243 (10.7%) from the 2,262 qualified patients, and the positive rate did not vary much over the years, with 12.2% (24/197) in 2015, 10.1% (53/527) in 2016, 12.0% (91/757) in 2017, and 10.7% (75/702) in 2018. AdVs were positive in 114 (5.0%) of the 2,262 cases, with 4.1% (8/197) in 2015, 7.0% (37/527) in 2016, 4.2% (32/757) in 2017, and 4.7% (33/702) in 2018. The incidence of RVA infection was similar with a study in Shanghai (138/1,479, 9.3%) [3] based on PCR, but was lower than the results of another study in Gansu (66/229, 28.8%) and in Beijing (138/481, 28.7%)[4] based on PCR. The incidence of AdV infection was lower not only than another study in Beijing during 2011–2012 (219/2,233, 9.8%) but also lower than that reported in Tanzania (34/439, 7.7%) [5] and in Bangladesh (93/871, 10.7%) [6] based on PCR. Data collected in the four years were analyzed by month and by season. A tendency in 12 consecutive months and in four seasons was detected (Figure 1). The number of patients of the RVA-/AdV- group increased in summer (χ2 = 8.566, P = 0.036) compared with the other three seasons. We attributed this to bacterial infection or non-infectious gastroenteritis caused by improper diet during summer. The number of patients in the RVA+/AdV- group were mostly distributed in winter (176/243, 72.4%) and spring (48/243, 19.7%), and only 1.6% (4/243) were found in summer (χ2 = 308.786, P = 0.001). RVA-/AdV+ group showed mildly but insignificantly higher numbers of cases during the summer (χ2 = 7.965, P = 0.085). The number of patients in the RVA+/AdV+ group was significantly increased in winter (15/31, 48.3%) and spring (8/31, 25.8%) (χ2 = 10.419, P = 0.015), showing the same distribution as the RVA+/AdV- group. The seasonality of RVA infection was obvious based on these results. Relative humidity and temperature was linked to RVA infection, with most RV genotypes favoring low temperature and low humidity [7]. Beijing has a latitude of 39.5N and belongs to the temperate monsoon climate with distinct seasons, which favors the periodical spreading of RVA. A large-scale study in China indicated that the occurrence of diarrhea peaked in summer in northern China, no matter whether the children were younger or older than 5-year-old[7]. It is necessary to find the seasons that are related to RVA infection in certain regions to control the disease in advance. In our study, children was in a weak tendency to be AdV antigen positive in summer. In another study, the seasonality of AdV infection was also not evident[8]. Research showed that both enteric HAdV-F and non-enteric HAdV-A, B, C, and D can cause gastroenteritis among children [6,9]. We could not fully eliminate the possibility of other AdV-related gastroenteritis other than those caused by HAdV-40 and 41.
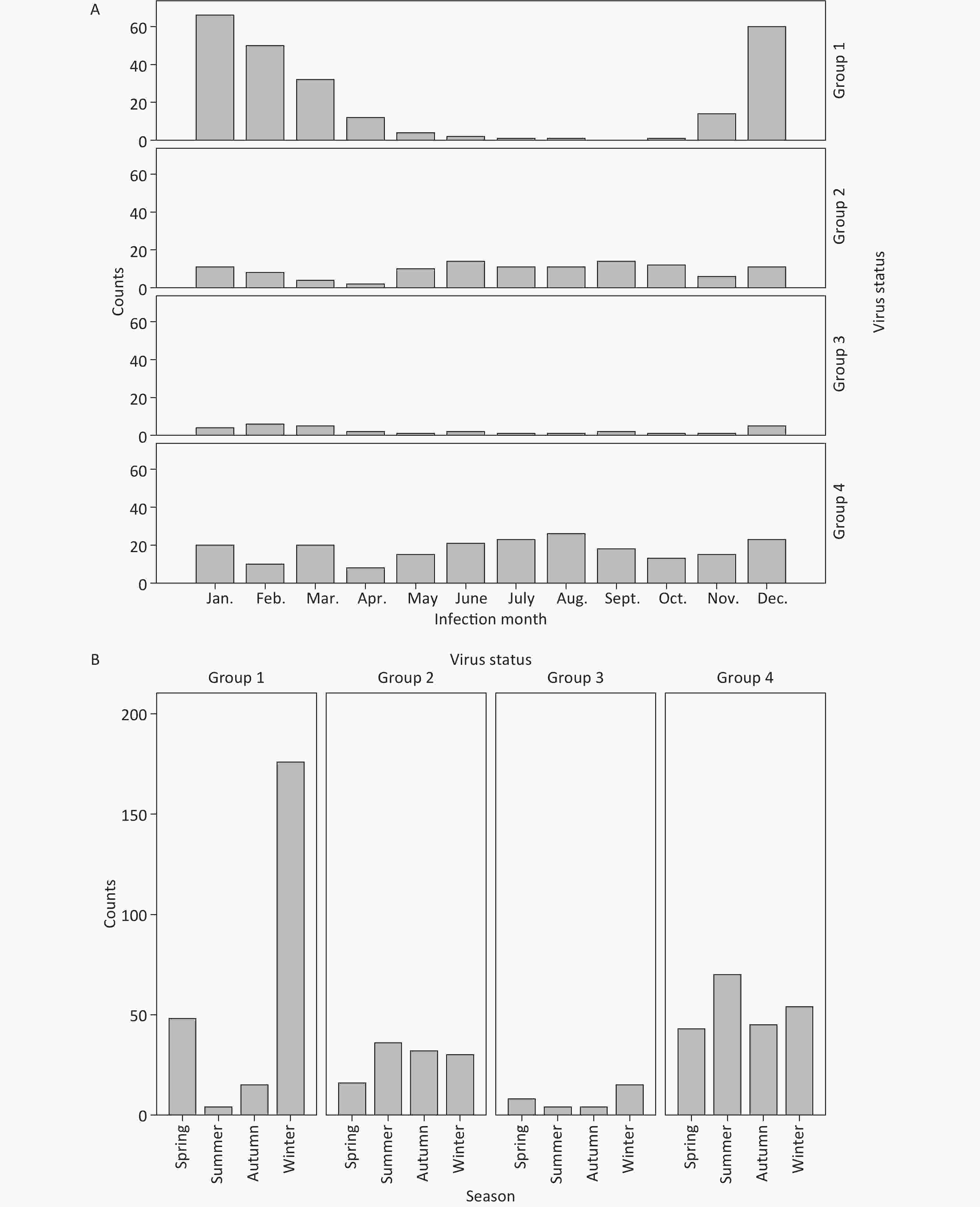
Figure 1. (A) Monthly distribution of RVA and AdV infection. Each histogram represents the cumulative case number in the month during 2015–2018. Group 1, RVA+/AdV-; Group 2, RVA-/AdV+; Group 3, RVA+/AdV+; Group 4, RVA-/AdV-. (B) Seasonality of RVA and AdV infection. March, April, and May were defined as spring; June, July, and August as summer; September, October, and November as autumn; and December, January, and February as winter. Each histogram represents the cumulative cases in this season during 2015–2018.
Most of the 388 patients with pathogens detected and the 212 patients without pathogen detected were under 5 years of age when they sought medical intervention (Table 1). The average age of the RVA+/AdV- group [16.0 (12.0, 25.0)] was older than that of the RVA-/AdV+ group [15.0 (9.0, 24.0)] and the RVA+/AdV+ group [12.0 (6.0, 19.0)]. Both RVA and AdV antigens were predominantly detected in children younger than 24 months of age, with the predisposing age for RVA infection was 12−23 months (130/243, 47.4%). It has been suggested that patients younger than 12 months or older than 24 months are relatively immune to RVA infection, which was supported by our findings. We attributed this to maternal antibodies or more limited contact with the outside environment for children younger than 12 months, while acquired immune system is established after 24 months. For the higher proportion of patients aged under 12 months in the RVA-/AdV- group, we believe it may be attributed to feeding factors (i.e., how the child was fed), and most importantly, the age distribution was also affected by the fact that parents’ decision to consult a doctor tended to be determined by the severity of the child's symptoms, and the age of the child.
Table 1. Demographics and symptoms of the patients
Clinical characteristics Group 1 (RVA+/AdV-)
(n = 243)Group 2
(RVA-/AdV+)
(n = 114)Group 3
(RVA+/AdV+)
(n = 31)Group 4
(RVA-/AdV-)
(n = 212)P1 value (Group 1 vs. Group 2) P2 value (Group 1 vs. Group 3) P3 value (Group 1 vs. Group 4) Median age (months) [IQ] 16.0 (12.0–25.0) 15.0 (9.0–24.0) 12.0 (6.0–19.0) 12.0 (6.0–18.7) 0.025* 0.002* < 0.0001* Months, n (%) < 12 74 (27.0) 40 (35.1) 15 (48.4) 101 (47.6) NS 0.045* 0.003* 12–23 130 (47.4) 45 (39.5) 14 (45.2) 74 (34.9) 0.013* NS < 0.0001* 24–35 35 (12.8) 19 (16.7) 1 (3.2) 24 (11.8) NS NS NS 36–59 33 (12.0) 9 (8.8) 2 (6.4) 12 (5.7) NS NS NS 60–120 2 (0.7) 1 (0.9) 1 (3.2) 2 (0.9) NS NS NS Gender, n (%) Female 101 (41.6) 52 (45.6) 15 (48.4) 91 (42.9) NS NS NS Male 142 (58.4) 62 (54.4) 16 (51.6) 121 (57.1) NS NS NS Fever, n (%) Yes 142 (58.2) 26 (22.8) 14 (45.1) 39 (18.4) < 0.0001* NS < 0.0001* No 102 (41.7) 88 (77.2) 17 (54.8) 172 (81.5) Vomiting, n (%) Yes 133 (54.5) 25 (21.9) 12 (38.7) 27 (12.7) < 0.0001* NS < 0.0001* No 111 (45.5) 89 (78.1) 19 (61.3) 185 (87.2) Note. *Significant (P < 0.05). IQ, interquartile range; NS, not statistically significant. The proportions of children presenting fever (142/243, 58.2%) and vomiting (133/243, 54.5%) in the RVA+/AdV- group were significantly higher than those in the RVA-/AdV+ group (26/114, 22.8% for fever; 25/114, 21.9% for vomiting) and the RVA-/AdV- group (39/212, 18.4% for fever; 27/212, 12.7% for vomiting) (P < 0.0001) (Table 1). There was no significant difference between the RVA+/AdV- group and the RVA+/AdV+ group in the proportions of fever and vomiting (P > 0.05). This indicated that RVA infection was more likely to cause fever and vomiting than AdV infection, as release of serotonin (5-HT) from human enterochromaffin cells and the activation of brain structures involved in nausea and vomiting involved in pathogenesis of RVA infection [10].
Routine feces test included the consistency of the feces specimen, fecal leukocytes counts, and FOB. In general practice, the consistency of feces specimen was classified into the following categories: 1) soft or mushy feces; 2) watery feces; 3) feces bearing a resemblance to egg drop soup, and 4) mucus feces. In infants and young children, soft and mushy feces were considered to be normal. Among the 600 gastroenteritis cases included in this study, only 202 (33.7%) cases were proven to be watery feces, with 34.1% (83/243), 39.4% (45/114), 35.4% (11/31), and 29.7% (63/212), respectively, in the four groups (Table 2). The results indicated that watery feces were not typical in RVA and AdV infection. We attributed this situation to the sample collection methods, such as feces collected from diapers, and also to medical interventions before the patients coming to the hospital. This suggested that it is necessary to take RVA and AdV tests for young children with diarrhea or feces with normal consistency.
Table 2. RVA and AdV infection and routine feces test
Clinical characteristics Group 1
(RVA+/AdV-)
(n = 243)Group 2
(RVA-/AdV+)
(n = 114)Group 3
(RVA+/AdV+)
(n = 31)Group 4
(RVA-/AdV-)
(n = 212)P1 value (Group 1 vs. Group 2) P2 value (Group 1 vs. Group 3) P3 value (Group 1 vs. Group 4) Watery 83 (34.1) 45 (39.4) 11 (35.4) 63 (29.7) NS NS NS Bearing a resemblance to egg drop soup 15 (6.1) 11 (9.6) 7 (22.5) 21 (9.9) NS 0.002* NS Mucus 1 (0.4) 8 (7.0) 4 (12.9) 17 (8.0) < 0.0001* < 0.0001* 0.007* Soft or mushy 144 (59.2) 54 (47.3) 9 (29.0) 111 (52.3) 0.035* 0.001* NS FOB-positive 55 (22.6) 46 (40.4) 18 (58.1) 71 (33.4) 0.001* 0.000* NS Feces leukocyte-positive 8 (3.3) 25 (22.0) 15 (48.4) 64 (30.2) < 0.001* < 0.001* < 0.001* Note. *Significant (P < 0.05). NS, not statistically significant. Compared with the RVA+/AdV- group, the incidence of leukocyte-positive (more than 1 cell/HPF) and FOB positive feces was significantly higher in the RVA-/AdV+ group (P < 0.05). This indicated that AdV infection was more likely to cause bloody feces than RVA infection. AdV can cause cell damage through immunopathological mechanisms and cause mild inflammation of the intestine. Some studies showed that the infection of AdV was significantly correlated with hemorrhagic cystitis in immune compromised patients. The level of leukocytes in feces was negatively correlated with RVA infection, which was consistent with the pathogenic mechanism that RVA mainly causes secretory and absorptive diarrhea rather than local inflammation. Compared with both the RVA+/AdV- group and the RVA-/AdV+ group, the percentages of feces bearing a resemblance to egg drop soup (7/31, 22.5%), feces being leukocytes positive (15/31, 48.4%), and FOB-positive feces (18/31, 58.1%) in the RVA+/AdV+ group were significantly higher (P < 0.05) (Table 2). It may imply that the symptoms were more severe in the co-infection group, but more cases of AdV+/RVA- are required to substantiate this conclusion.
Multifactorial binary logistic regression was employed to analyze the risk factors of RVA and AdV infections (Supplementary Table S1, available in www.besjournal.com). In order to increase data uniformity and statistic efficacy, leukocyte count was converted into four categories as follows: 1) 0–1 cell/HPF was defined as 0 point; 2) 2–10 cells/HPF was defined as 1 point; 3) 11–30 cells/HPF was defined as 2 points; and 4) more than 30 cells/HPF was defined as 3 points. Using multi-factor binomial regression, the independent variables entering the equation for RVA infection were leukocytes in feces, season, fever, and vomiting. RVA infection had a significant positive correlation with seasons [OR = 1.576, 95% CI (1.336–1.859), P < 0.001], fever [OR = 3.778, 95% CI (2.482–5.794), P < 0.0001], vomiting (OR = 3.110, 95% CI (2.019–4.790), P < 0.0001] and a significant negative correlation with feces leukocyte-positive [OR = 0.569, 95% CI (0.389–0.833), P = 0.004]. The independent variables entering the equation for AdV infection were FOB and fever. AdV infection had a significant positive correlation with FOB [OR = 1.850, 95% CI (1.238–2.764), P = 0.003] and a significant negative correlation with fever [OR = 0.577, 95% CI (0.380–0.876), P = 0.010].
Table S1. Risk factors of RVA and AdV infection
Factors B S.E. Wald P Exp(B) 95% CI Lower Upper RVA Leukocyte −0.564 0.194 8.410 0.004 0.569 0.389 0.833 Season 0.455 0.084 29.121 < 0.001 1.576 1.336 1.859 Fever 1.329 0.214 38.496 < 0.001 3.778 2.482 5.794 Vomiting 1.135 0.220 26.514 < 0.001 3.110 2.019 4.790 Constant −2.257 0.291 60.169 < 0.001 0.105 AdV FOB 0.615 0.205 9.015 0.003 1.850 1.238 2.764 Fever −0.550 0.213 6.651 0.010 0.577 0.380 0.876 Soft and mushy −0.385 0.200 3.706 0.054 0.680 0.460 1.007 Constant −0.605 0.292 4.284 0.038 0.546 Note. B, regression coefficient; CI, confidence interval; Exp(B), odds ratio; FOB, fecal occult blood; SE, standard error; Sig, significant. In summary, the study provided more information for the diagnosis of gastroenteritis in pediatrics. But there were some limitations. Firstly, it is possible that other pathogens might contribute to the gastroenteritis while the test results were negative. Secondly, all the data included in this study were from one hospital. Therefore, it is necessary to perform a large scale study about RVA and AdV related gastroenteritis in China.
DONG Jing Xiao contributed to data collection and organization, data statistics, and article writing; LI Ao Fei contributed to data collection and data analysis; LI Run Qing contributed to data statistics; CHAO Shuang and YANG Song contributed to sample collection and detection. ZHAO Xiu Ying contributed to the design of the study, data analysis and the revision of the article. All authors reviewed and approved the final version of the article for publication.
The authors declare that they have no conflict of interest.
doi: 10.3967/bes2020.027
Epidemiological and Clinical Features of Rotavirus and Adenovirus Related Gastroenteritis in Beijing: A Retrospective Case-control Study in Pediatric Patients
-
-
Figure 1. (A) Monthly distribution of RVA and AdV infection. Each histogram represents the cumulative case number in the month during 2015–2018. Group 1, RVA+/AdV-; Group 2, RVA-/AdV+; Group 3, RVA+/AdV+; Group 4, RVA-/AdV-. (B) Seasonality of RVA and AdV infection. March, April, and May were defined as spring; June, July, and August as summer; September, October, and November as autumn; and December, January, and February as winter. Each histogram represents the cumulative cases in this season during 2015–2018.
Table 1. Demographics and symptoms of the patients
Clinical characteristics Group 1 (RVA+/AdV-)
(n = 243)Group 2
(RVA-/AdV+)
(n = 114)Group 3
(RVA+/AdV+)
(n = 31)Group 4
(RVA-/AdV-)
(n = 212)P1 value (Group 1 vs. Group 2) P2 value (Group 1 vs. Group 3) P3 value (Group 1 vs. Group 4) Median age (months) [IQ] 16.0 (12.0–25.0) 15.0 (9.0–24.0) 12.0 (6.0–19.0) 12.0 (6.0–18.7) 0.025* 0.002* < 0.0001* Months, n (%) < 12 74 (27.0) 40 (35.1) 15 (48.4) 101 (47.6) NS 0.045* 0.003* 12–23 130 (47.4) 45 (39.5) 14 (45.2) 74 (34.9) 0.013* NS < 0.0001* 24–35 35 (12.8) 19 (16.7) 1 (3.2) 24 (11.8) NS NS NS 36–59 33 (12.0) 9 (8.8) 2 (6.4) 12 (5.7) NS NS NS 60–120 2 (0.7) 1 (0.9) 1 (3.2) 2 (0.9) NS NS NS Gender, n (%) Female 101 (41.6) 52 (45.6) 15 (48.4) 91 (42.9) NS NS NS Male 142 (58.4) 62 (54.4) 16 (51.6) 121 (57.1) NS NS NS Fever, n (%) Yes 142 (58.2) 26 (22.8) 14 (45.1) 39 (18.4) < 0.0001* NS < 0.0001* No 102 (41.7) 88 (77.2) 17 (54.8) 172 (81.5) Vomiting, n (%) Yes 133 (54.5) 25 (21.9) 12 (38.7) 27 (12.7) < 0.0001* NS < 0.0001* No 111 (45.5) 89 (78.1) 19 (61.3) 185 (87.2) Note. *Significant (P < 0.05). IQ, interquartile range; NS, not statistically significant. Table 2. RVA and AdV infection and routine feces test
Clinical characteristics Group 1
(RVA+/AdV-)
(n = 243)Group 2
(RVA-/AdV+)
(n = 114)Group 3
(RVA+/AdV+)
(n = 31)Group 4
(RVA-/AdV-)
(n = 212)P1 value (Group 1 vs. Group 2) P2 value (Group 1 vs. Group 3) P3 value (Group 1 vs. Group 4) Watery 83 (34.1) 45 (39.4) 11 (35.4) 63 (29.7) NS NS NS Bearing a resemblance to egg drop soup 15 (6.1) 11 (9.6) 7 (22.5) 21 (9.9) NS 0.002* NS Mucus 1 (0.4) 8 (7.0) 4 (12.9) 17 (8.0) < 0.0001* < 0.0001* 0.007* Soft or mushy 144 (59.2) 54 (47.3) 9 (29.0) 111 (52.3) 0.035* 0.001* NS FOB-positive 55 (22.6) 46 (40.4) 18 (58.1) 71 (33.4) 0.001* 0.000* NS Feces leukocyte-positive 8 (3.3) 25 (22.0) 15 (48.4) 64 (30.2) < 0.001* < 0.001* < 0.001* Note. *Significant (P < 0.05). NS, not statistically significant. S1. Risk factors of RVA and AdV infection
Factors B S.E. Wald P Exp(B) 95% CI Lower Upper RVA Leukocyte −0.564 0.194 8.410 0.004 0.569 0.389 0.833 Season 0.455 0.084 29.121 < 0.001 1.576 1.336 1.859 Fever 1.329 0.214 38.496 < 0.001 3.778 2.482 5.794 Vomiting 1.135 0.220 26.514 < 0.001 3.110 2.019 4.790 Constant −2.257 0.291 60.169 < 0.001 0.105 AdV FOB 0.615 0.205 9.015 0.003 1.850 1.238 2.764 Fever −0.550 0.213 6.651 0.010 0.577 0.380 0.876 Soft and mushy −0.385 0.200 3.706 0.054 0.680 0.460 1.007 Constant −0.605 0.292 4.284 0.038 0.546 Note. B, regression coefficient; CI, confidence interval; Exp(B), odds ratio; FOB, fecal occult blood; SE, standard error; Sig, significant. -
[1] B. Ghebremedhin. Human adenovirus: viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp), 2014; 4, 26−33. doi: 10.1556/EuJMI.4.2014.1.2 [2] Chen SY, Chang YC, Lee YS, et al. Molecular epidemiology and clinical manifestations of viral gastroenteritis in hospitalized pediatric patients in Northern Taiwan. J Clin Microbiol, 2007; 45, 2054−57. doi: 10.1128/JCM.01519-06 [3] Shen Z, Wang G, Zhang W, et al. RV infection and its genetic characterization in non-hospitalized adults with acute gastroenteritis in Shanghai, China. Arch Virol, 2013; 158, 1671−7. doi: 10.1007/s00705-013-1663-1 [4] Zhang J, Liu H, Jia L, et al. Active, population-based surveillance for RV gastroenteritis in Chinese children: Beijing municipality and Gansu province, China. J Pediat Inf Dis Soc, 2014; 34, 40−46. [5] Verma H, Chitambar SD, Varanasi G. Identification and characterization of enteric AdVes in infants and children hospitalized for acute gastroenteritis. J Med Virol, 2009; 81, 60−4. doi: 10.1002/jmv.21331 [6] Afrad MH, Avzun T, Haque J, et al. Detection of enteric- and non-enteric AdVes in gastroenteritis patients, Bangladesh, 2012-2015. J Med Virol, 2018; 90, 677−84. doi: 10.1002/jmv.25008 [7] Xu Z, Hu W, Zhang Y, et al. Exploration of diarrhoea seasonality and its drivers in China. Sci Rep-UK, 2015; 5, 8241. doi: 10.1038/srep08241 [8] Liu L, Qian Y, Zhang Y, et al. Adenoviruses associated with acute diarrhea in children in Beijing, China. PLoS One, 2014; 9, e88791. doi: 10.1371/journal.pone.0088791 [9] Moyo S, Hanevik K, Blomberg B, et al. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect Dis, 2014; 14, 666. doi: 10.1186/s12879-014-0666-1 [10] Hagbom M, Istrate C, Engblom D, et al. Stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in Nausea and Vomiting. PLoS Pathogens, 2011; 7, e1002115. doi: 10.1371/journal.ppat.1002115 -




 下载:
下载:



 Quick Links
Quick Links