-
Prion disease, or transmissible spongiform encephalopathy, affects a variety of animals and humans. In humans, the related diseases include Creutzfeldt-Jakob disease (CJD), Fatal Familial Insomnia (FFI), Gerstmann-Sträussler-Scheinker syndrome (GSS), and Kuru. Approximately 85% of all CJD cases are sporadic (sporadic CJD, sCJD), 10%–15% cases are inherited (genetic CJD, gCJD), and less than 1% are infectious (iatrogenic CJD)[1, 2]. The major animal prion diseases include scrapie in goat and sheep, bovine spongiform encephalopathy in cattle, and chronic wasting disease in Cervidae[3].
PrP is a relatively well-conserved cellular membrane protein that is mainly expressed in the central nervous system[4, 5]. Under the influence of various factors, the normal and physiological conformation of PrP (PrPC) converts into the abnormal and pathological isoform PrPSc, which is believed to be the etiology for prion diseases[6]. Although the peptide sequences of PrPC and PrPSc are identical, PrPSc possesses unique biochemical features on account of its conformational change, including partial resistance to proteinase K digestion and partial tolerance to GdnCl treatment; these features are used to distinguish between the two isoforms of the protein.
Considering the high conservation of PrP, eliciting specific antibodies against PrP in wild-type animals is fairly difficult. Most of the available PrP monoclonal antibodies (mAbs) are generated by immunization of PRNP-knockout mice with PrP antigen[7]. In the present report, we prepare a PrP-specific polyclonal antibody (pAb) in PRNP-knockout mice via immunization with recombinant human PrP protein. We then verify that the prepared pAb shows reliable reactivity toward PrPC and PrPSc via Western blot, immunohistochemistry (IHC), and immunofluorescent (IFA) assays. Our results reveal pAb reactivities comparable with those of commonly used commercial anti-PrP mAbs.
-
The construction of recombinant plasmid p-Hu-PrP23-231 expressing the full-length human PrP protein from aa 23 to 231 (rHuPrP23-231) and purification of the expressed rHuPrP23-231 in Escherichia coli were carried out as described previously[8].
-
Transgenic mice (Tg-PrP0/0) featuring native PRNP knockout[9] were housed in the SPF laboratory. Then, 50 μg of the purified rHuPrP23-231 was injected into the hind leg muscles of the mice for immunization. Approximately 15 d later, the same quantity of rHuPrP23-231 was administrated intramuscularly for boosting. Tail blood samples of the immunized mice were collected 15 d later, and antibody titers were measured via an established PrP-coated ELISA technique. The mice were immunized once more if the antibody titers obtained were less than 1:20,480. The mice were sacrificed under anesthesia, and their blood was collected and aliquoted.
-
An indirect ELISA protocol was established to test the specificity and sensitivity of the prepared antibody. Briefly, rHuPrP23-231 was diluted to a final concentration of 1 μg/mL in phosphate buffered saline (PBS) and coated on the wells of 96-well microtiter plates overnight at 4 °C. After washing with PBST (phosphate buffered saline, pH 7.6, containing 0.05% Tween-20), the plates were blocked with 2% BSA in PBST at 37 °C for 2 h and then incubated with various dilutions of the serum samples. After copious rinsing with PBST, the captured antibodies were further reacted with horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibody (Jackson, USA) diluted to 1:10,000 at 37 °C for 1 h. The color of the solution was developed with 3,3’,5,5’-tetramethylbenzidine (Sigma, USA) at 37 °C for 30 min. Absorbance was measured at 450 nm (A450 nm) after quenching the wells by addition of 2 mol/L H2SO4.
-
Stored brain tissues from experimental rodents infected with scrapie and humans infected with various prion diseases were prepared into homogenates to evaluate the proposed PrP antibody. Samples included the postmortem brain tissues of one sCJD case, one G114V gCJD case, and three FFI cases; brain tissues of mice infected with scrapie agents 139A, ME7 and S15 and hamsters infected with scrapie agent 263K. The clinical and neuropathological properties of the human cases and experimental rodents were described in previous work[10]. The donated brain of a person who had passed away from a car accident and the brains of age-matched normal mice and hamsters were also enrolled as controls. Brain tissues were washed in Tris-buffered saline (TBS, 10 mmol/L Tris-HCl, 133 mmol/L NaCl, pH 7.4) three times and then homogenized in lysis buffer (100 mmol/L NaCl, 10 mmol/L EDTA, 0.5% NP-40, 0.5% sodium deoxycholate, 10 mmol/L Tris, pH 7.4) containing protease inhibitor cocktail set III. The homogenates were centrifuged at 2,000 ×g for 10 min, and the supernatant fractions were aliquoted and stored at −80 °C for further experiments.
-
The cell line SMB-S15 and its normal partner cell line SMB-PS were obtained from Roslin Institute, UK. SMB-S15 was originally established by culture from the brain of a mouse clinically affected with scrapie strain Chandler; PrPSc replication was maintained in this line along with cell passaging. SMB-PS was established from SMB-S15 cells that had been completely cured of prion by pentosane sulfate treatment without detectable PrPSc. SMB cells were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (VS500T, Auabian, Australia) in a 33 °C humidified atmosphere with 5% CO2.
-
Aliquots of the brain homogenates were separated by 12% SDS-PAGE and electroblotted onto nylon membranes. The membranes were blocked with TBS containing 5% skimmed milk at room temperature (RT) for 2 h and incubated with pAb P54 (1:1,000) or the commercial PrP mAbs 6D11 (1:2,000) or 3F4 (1:5,000) at 4 °C overnight. After washing with TBS containing 0.1% Tween 20, the membranes were incubated with HRP conjugated secondary antibody (goat anti-mouse secondary-HRP-Ab, 115-035-003, 1:2,000) at RT for 1 h. The blots were developed using an enhanced chemiluminescence system (ECL, PerkinElmer, NEL103E001EA) and visualized on autoradiography films (General Electric). Images were captured by ChemiDocTM XRS + Imager and quantified by Image J software.
The brain homogenates were digested with a final concentration of 50 μg/mL for rodents and 20 μg/mL for human and cell lines proteinase K at 37 °C for 1 h prior to Western blot assay to detect the presence of proteinase K-resistant PrPSc.
-
Brain tissues were fixed in 10% buffered formalin solution, and paraffin sections (thickness, 5 μm) were routinely prepared[11]. Sections were quenched for endogenous peroxidases with 3% H2O2 in methanol for 10 min and then blocked with 5% BSA for 15 min at RT. The sections were incubated with either pAb P54 (1:100) or commercial anti-PrP mAb (1:200) at 4 °C overnight. Afterward, the sections were incubated with HRP-conjugated secondary antibody (ZSGB-BIO, PV-6002) at 37 °C for 1 h and visualized by incubation with 3,3-diaminobenzidine tetrahydrochloride (Boster, AR 1000). The slices were counterstained with hematoxylin (Boster, AR 0005), dehydrated, and mounted in Permount. Finally, the brain slices were exposed to buffer containing 4 mol/L GdnCl at RT for 1 h and then subjected to routine IHC assay to detect PrPSc.
-
IFA tests were conducted according to a previously described protocol[11]. Briefly, brain slices were treated with 0.3% Triton-X100 for 30 min, blocked with 5% BSA for 1 h at RT, and then incubated with the prepared pAb P54 (1:100) or commercial anti-PrP antibody (6D11, 1:200, Santa Cruz, Sc-58581) at 4 °C overnight. Subsequently, the sections were incubated with Alexa Fluor® 568-conjugated goat anti-mouse antibody (1:200, Thermo, A-11004) at 37 °C for 1 h. After staining with 1 μg/mL 4’6-diamidino-2-phenylindole (Beyotime, China) for 30 min, the slices were mounted in Permount and viewed using an Operetta (PerkinElmer) or Olympus FV1000 confocal microscope.
-
All procedures adopted for the immunization of experimental mice (Tg-PrP0/0) with rHuPrP23-231, preparation of the PrP-specific antibody, and usage of the stored brain specimens of human prion diseases and experimental rodents in this study were approved by the Ethical Committee of the National Institute for Viral Disease Prevention and Control, China CDC, under protocol number 2009ZX10004-101. In addition, all animal housing and experimental protocols were in accordance with the Chinese Regulations for the Administration of Affairs Concerning Experimental Animals.
-
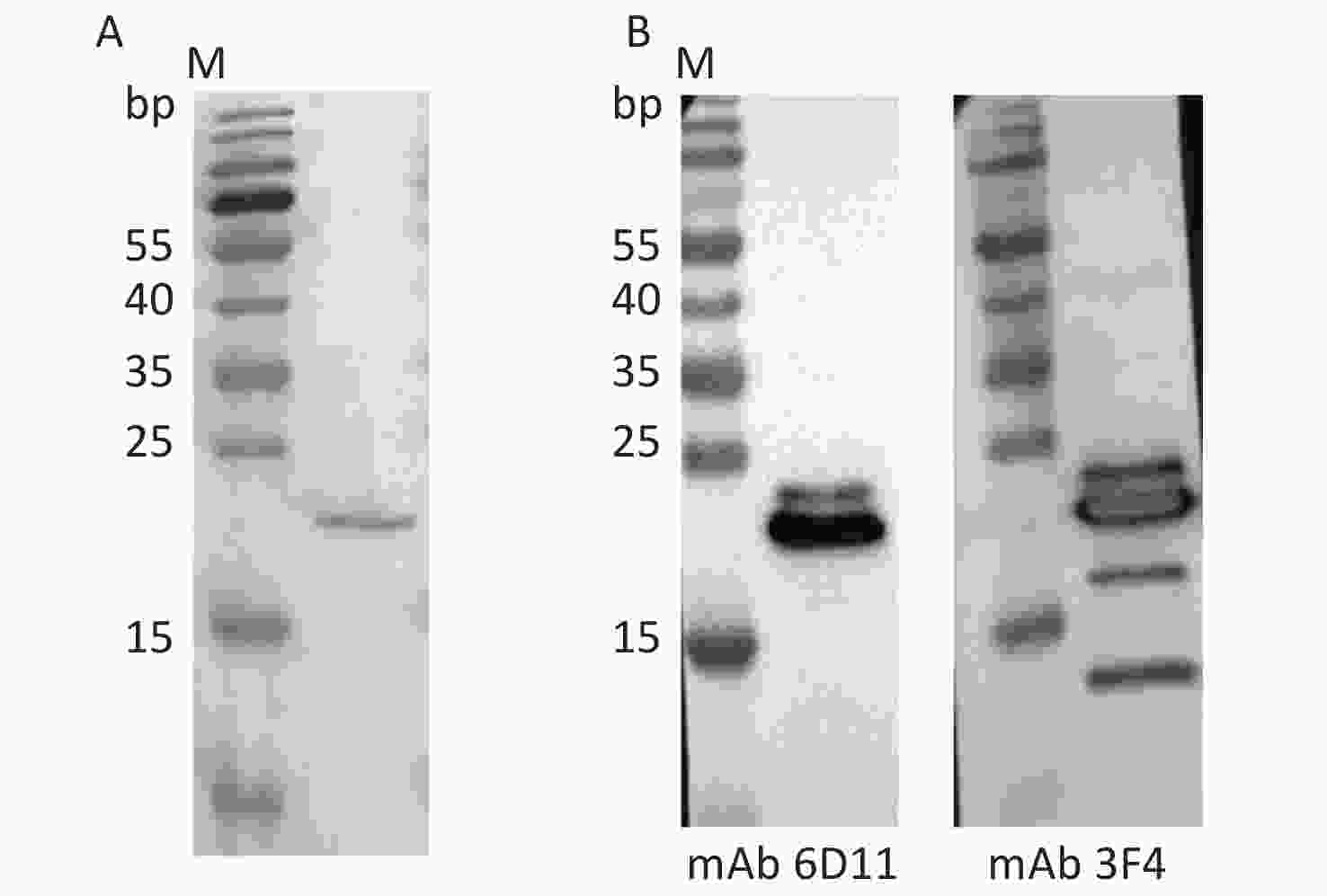
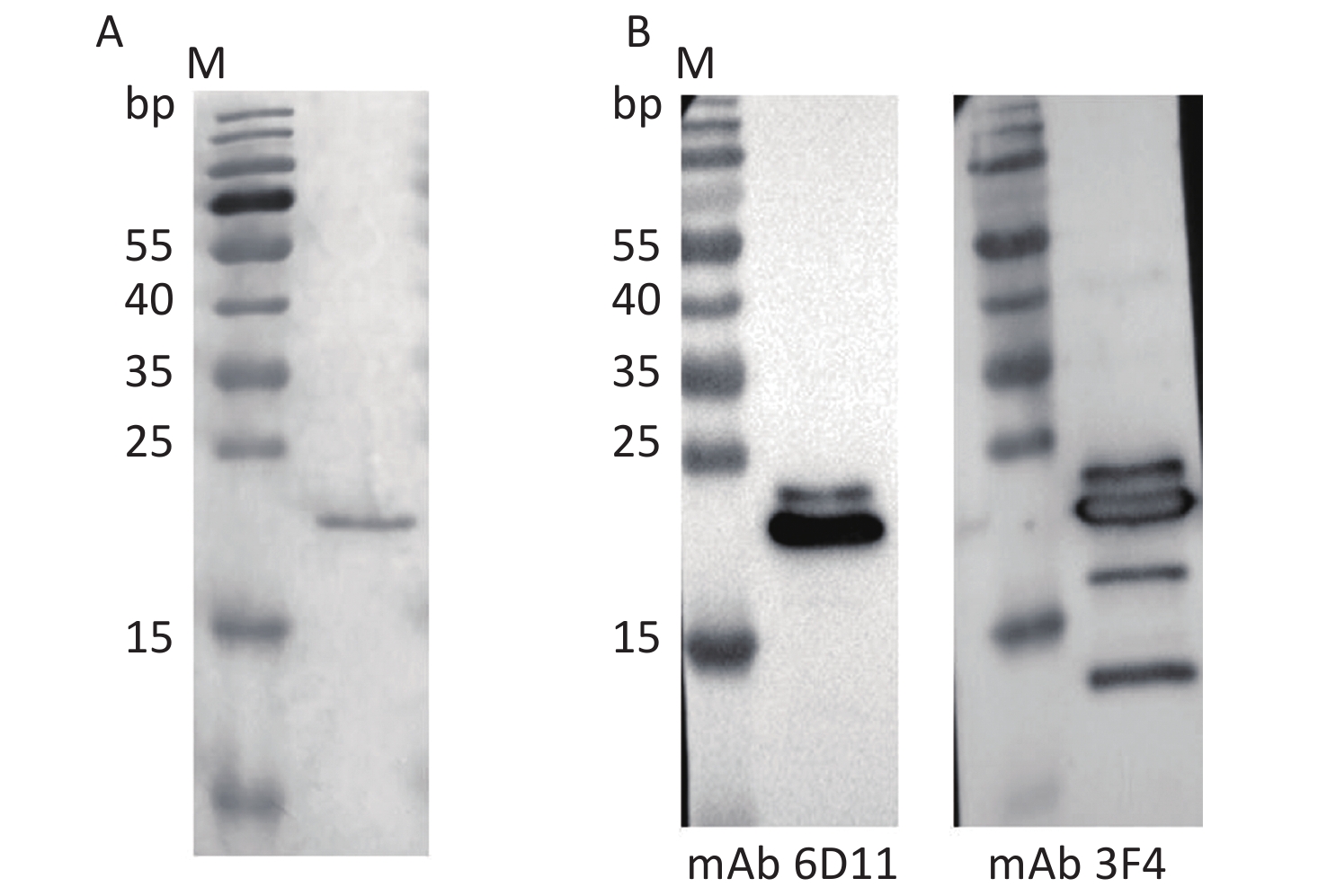
A full-length recombinant human PrP protein at aa 23–231 (rHuPrP23-231) was expressed and purified from E. coli. SDS-PAGE revealed a band of approximately 21 kD that positively immunoreacted with PrP-specific mAbs 6D11 and 3F4 in Western blot assays (Figure 1). 50 μg of the purified rHuPrP23-231 was intramuscularly immunized into 4–8-week-old Tg-PrP0/0 mice and boosted every 2 weeks until the serum titer determined by PrP-coated ELISA exceeded 1:20,480. The mouse blood was subsequently collected, and the serum was separated and pooled as pAb P54.
-
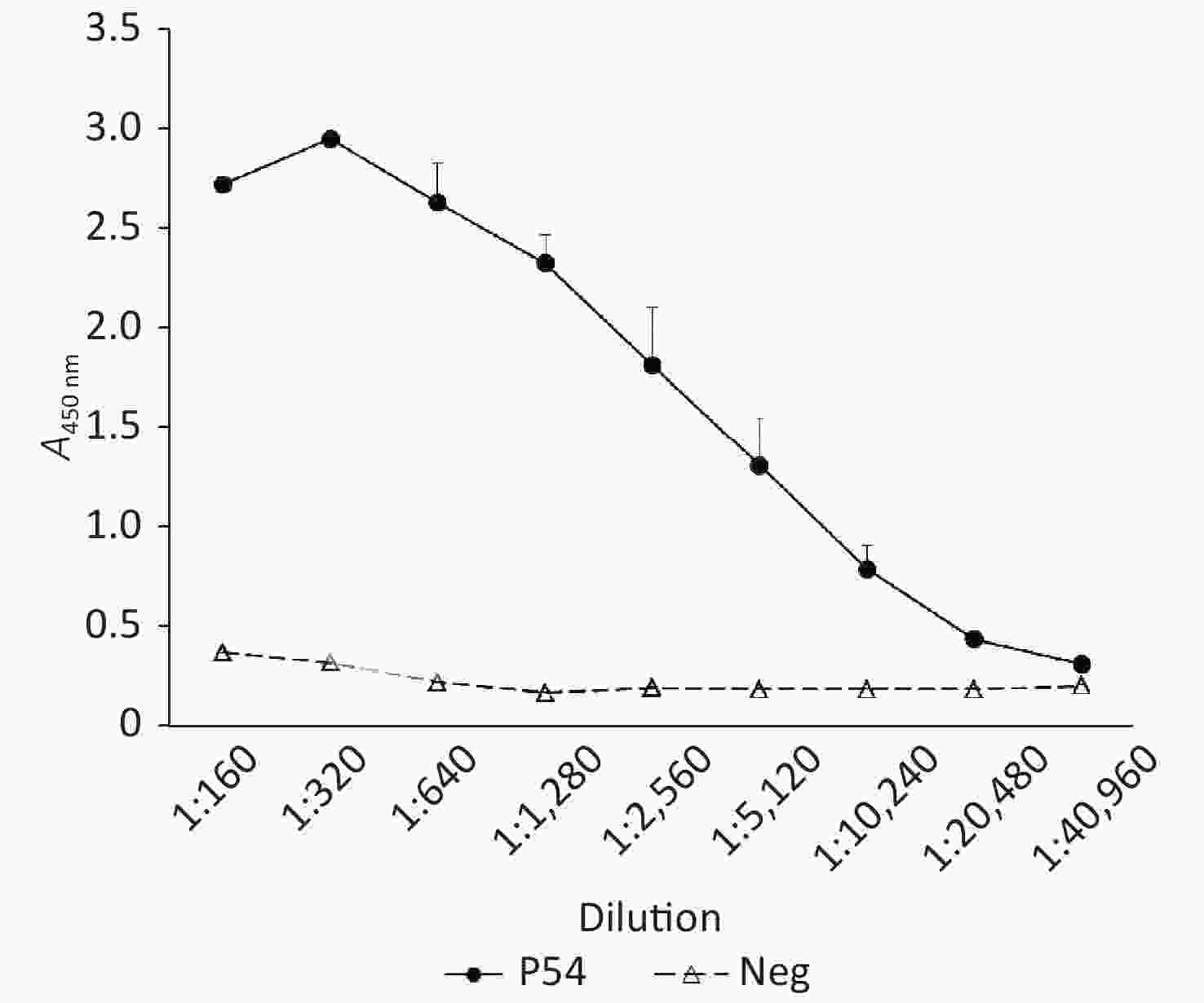
Aliquots of the pooled serum of pAb P54 were subjected to indirect ELISA by using plates coated with rHuPrP23-231 to assess the reactive titer of the prepared antibody. Serum collected from the mice before immunization was used as a negative control. As shown in Figure 2, the A450 nm of the negative serum was 0.368 in the dilution of 1:100 and gradually decreased to less than 0.2 in the dilution of 1:1,280. By comparison, the A450 nm of pAb P54 was 2.758 in the dilution of 1:100, peaked at 2.945 in the dilution of 1:200, and declined to 0.416 in the dilution of 1:20,480. Considering that an A450 nm-test sample/A450 nm-negative control ratio ≥ 2.1 is considered a positive reaction, the reactive titer of pAb P54 determined by ELISA was estimated to be 1:20,480.
-
Homogenates of mouse, hamster, and human brains were prepared separately and assayed by Western blot to evaluate the reactivity of the prepared pAb P54 with native PrP in normal brain tissues. Three typical PrP bands located at approximately 25–30 kD and representing di-, mono-, and aglycosylated PrPC were detected in the brain homogenates after reaction with pAb P54 (Figure 3A). The blots also showed patterns similar to those of homogenates reacted with PrP-specific mAb 6D11 (Figure 3B) and 3F4 (Figure 3C). mAb 6D11 reacted to PrPC in the brain homogenates of mouse and hamster but elicited very weak signals in the homogenate of human brain. By comparison, mAb 3F4 did not recognize PrPC in mouse but reacted well with those of hamster and human. In addition, no PrP band was observed in the brain homogenates of Tg-PrP0/0 mice treated with pAb P54 or mAbs 6D11 or 3F4.
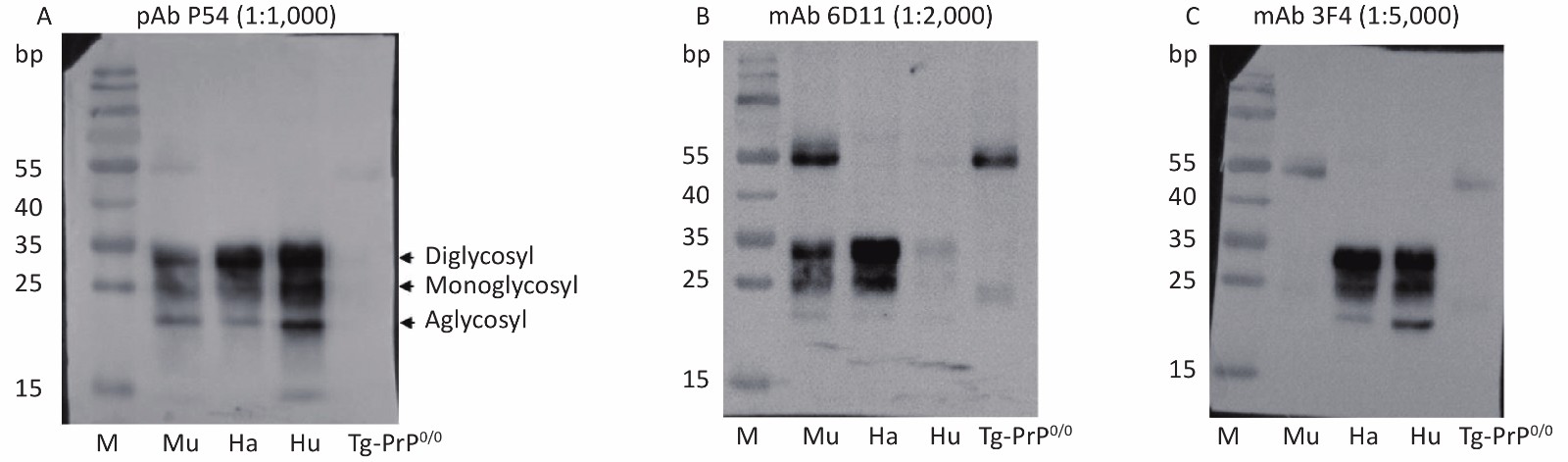
Figure 3. Western blot determination of the reactivity of pAb P54 with normal brain PrPC from mouse (Mu), hamster (Ha), and human (Hu). Approximately 4 μL of each 10% brain homogenate was applied in the tests. The brain lysate from mouse Tg-PrP0/0 was used as a control
Brain homogenates of mice and hamsters infected with various scrapie agents, as well as those of humans infected with different types of prion diseases, were prepared to test the ability of pAb P54 to recognize abnormal PrPSc. After digestion with proteinase K, three main PrP bands migrating to positions 21–30 kD were detected in the blots of brain homogenates of mice infected with scrapie agents 139A, ME7, and S15 treated with pAb P54 (Figure 4A, left) and mAb 6D11 (right). By contrast, no PrP signal was detected in the blot of the control homogenate. PK-resistant PrP bands similar in profile to those observed in mAb 3F4 (Figure 4B, right), left were also detected in the Western blot of the brain homogenates of scrapie strain 263K-infected hamsters treated with pAb P54 (Figure 4B, left). These results strongly indicate that the prepared pAb P54 can recognize pathological PrPSc in the brains of scrapie-infected experimental rodents.
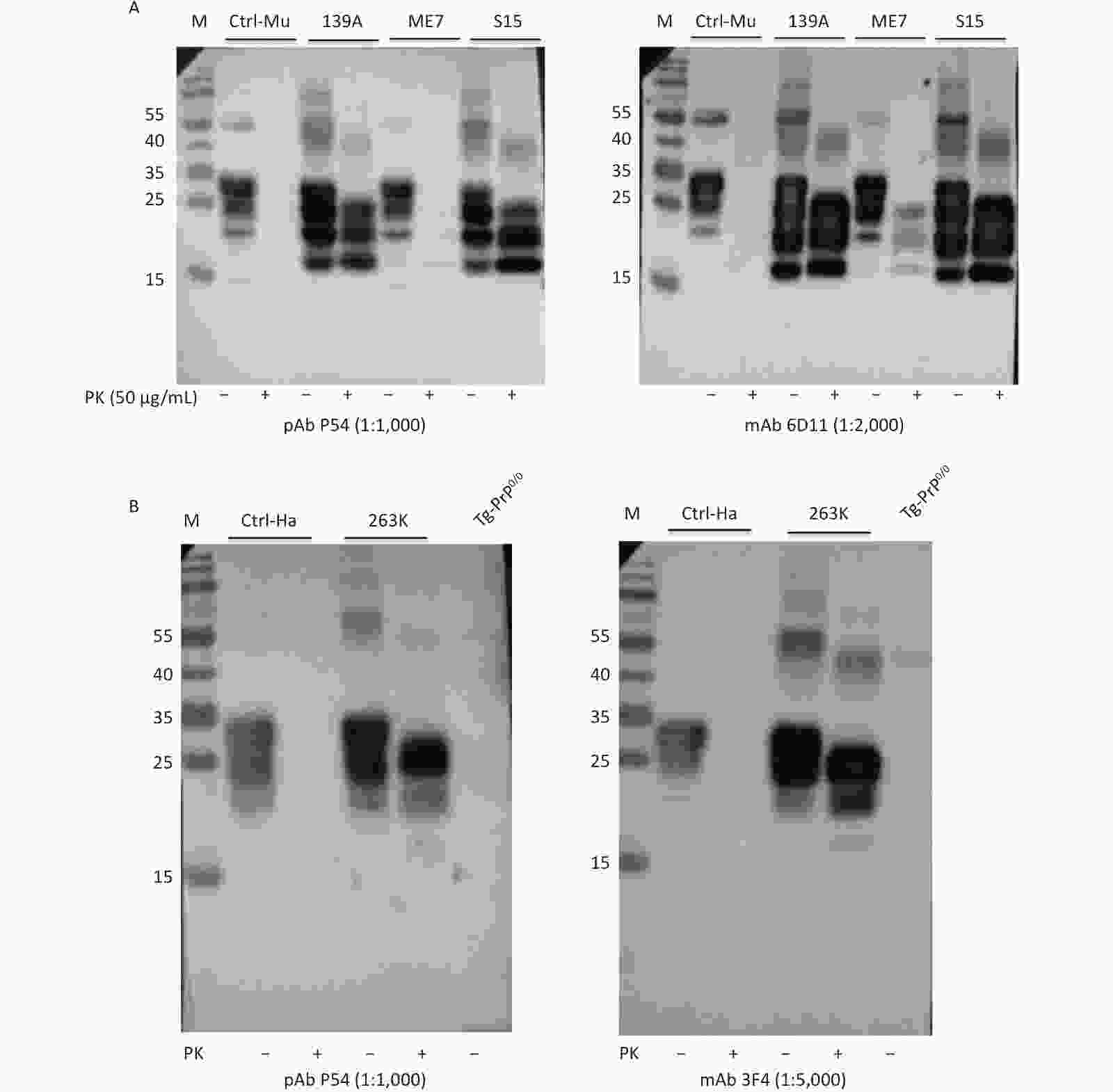
Figure 4. Western blot determination of the reactivity of pAb P54 with PrPSc molecules in the brains of scrapie-infected rodents. (A) 139A-, ME7-, and S15-infected mice. (B) 263K-infected hamster. Approximately 4 μL of each 10% brain homogenate was applied in the tests. Brain homogenates from normal animals were used as a control. Prior to SDS-PAGE, the brain lysates were digested with 50 μg/mL PK. +: with PK; -: without PK.
Brain homogenates of different human prion disease infections, including one sCJD, one G114V gCJD, and three FFI cases were exposed to pAb P54 and assayed by Western blot. Similar to the reaction with mAb 3F4 (Figure 5, bottom panel), the Western blot of pAb P54 (upper panel) revealed PK-resistant bands in the brains of sCJD and G114V gCJD, as well as weak PK-resistant signals in the brains of FFI-3. These findings suggest that the prepared pAb P54 is able to detect PrPSc in various types of human prion diseases.
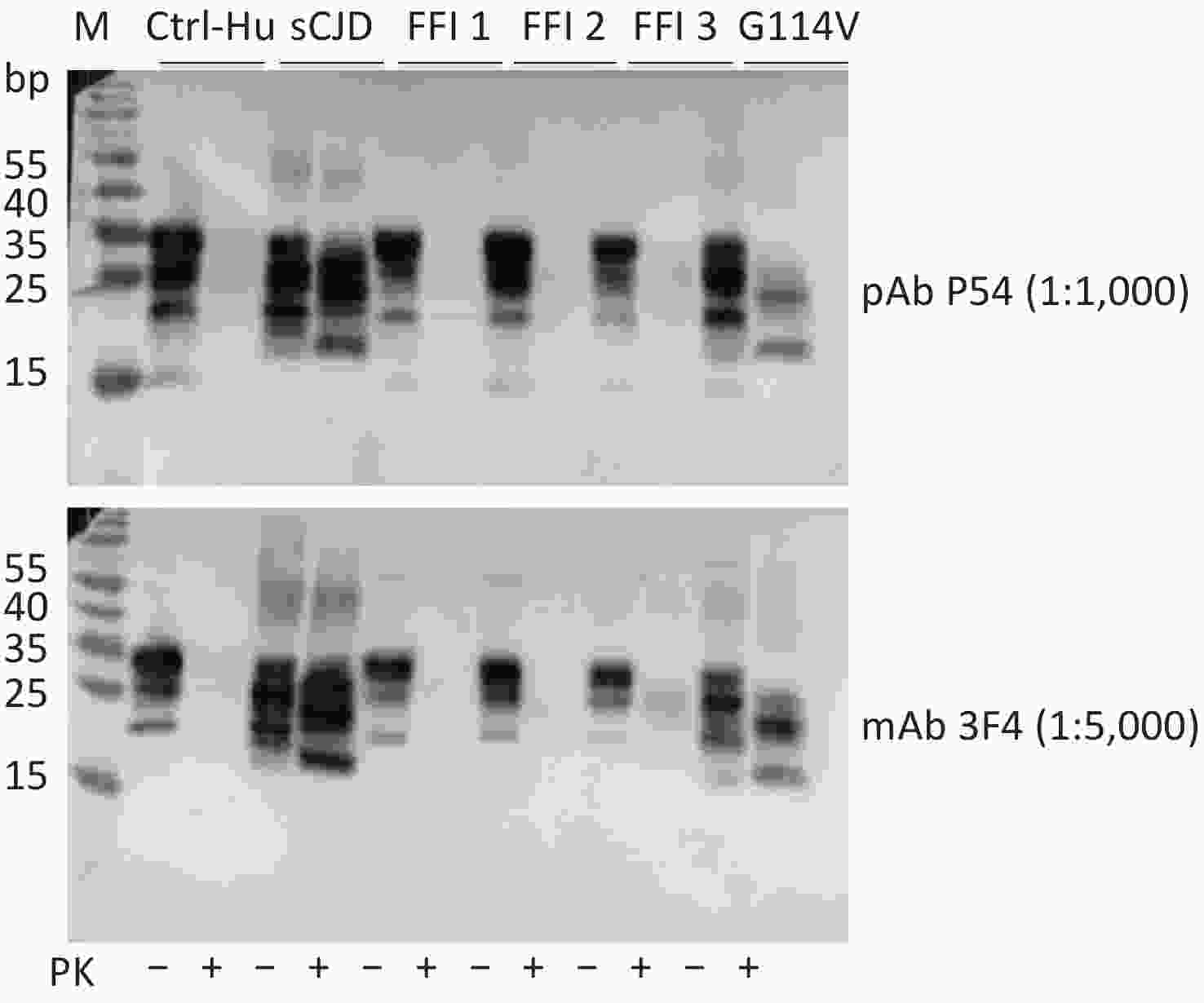
Figure 5. Western blot determination of the reactivity of pAb P54 with PrPSc molecules in the brains of prion disease-infected humans. The brain samples included one sCJD case, three FFI cases, and one G114V gCJD case. Approximately 4 μL of each 10% brain homogenate was applied in the tests. The brain homogenate of a person had passed away from a car accident was used as a control. The brain lysates were digested with 20 μg/mL PK. +: with PK; -: without PK.
-
The brain slices of normal and 139A-infected mice, as well as those of normal and 263K-infected hamsters, were prepared and subjected to PrP-specific IHC assays to test the reactivity of pAb P54; here, mAb 6D11 was used as the control. The brain slices were treated with 4 mol/L GdnCl prior to incubation with the individual PrP-specific antibodies to remove PrPC in the brain tissues. Brown PrP signals of different intensities, indicating positive reactivity, were distributed within the interior and exterior of cells of the brain tissues of 263K-infected hamsters (Figure 6A) and 139A- and ME7-infected mice (Figure 6B); these signals were accompanied with vacuoles of different sizes. By contrast, no clear brown signal was detected in the brain slices of normal animals. The image of PrPSc deposits produced by pAb P54 are similar as those produced by mAb 6D11 in mouse and hamster brains.
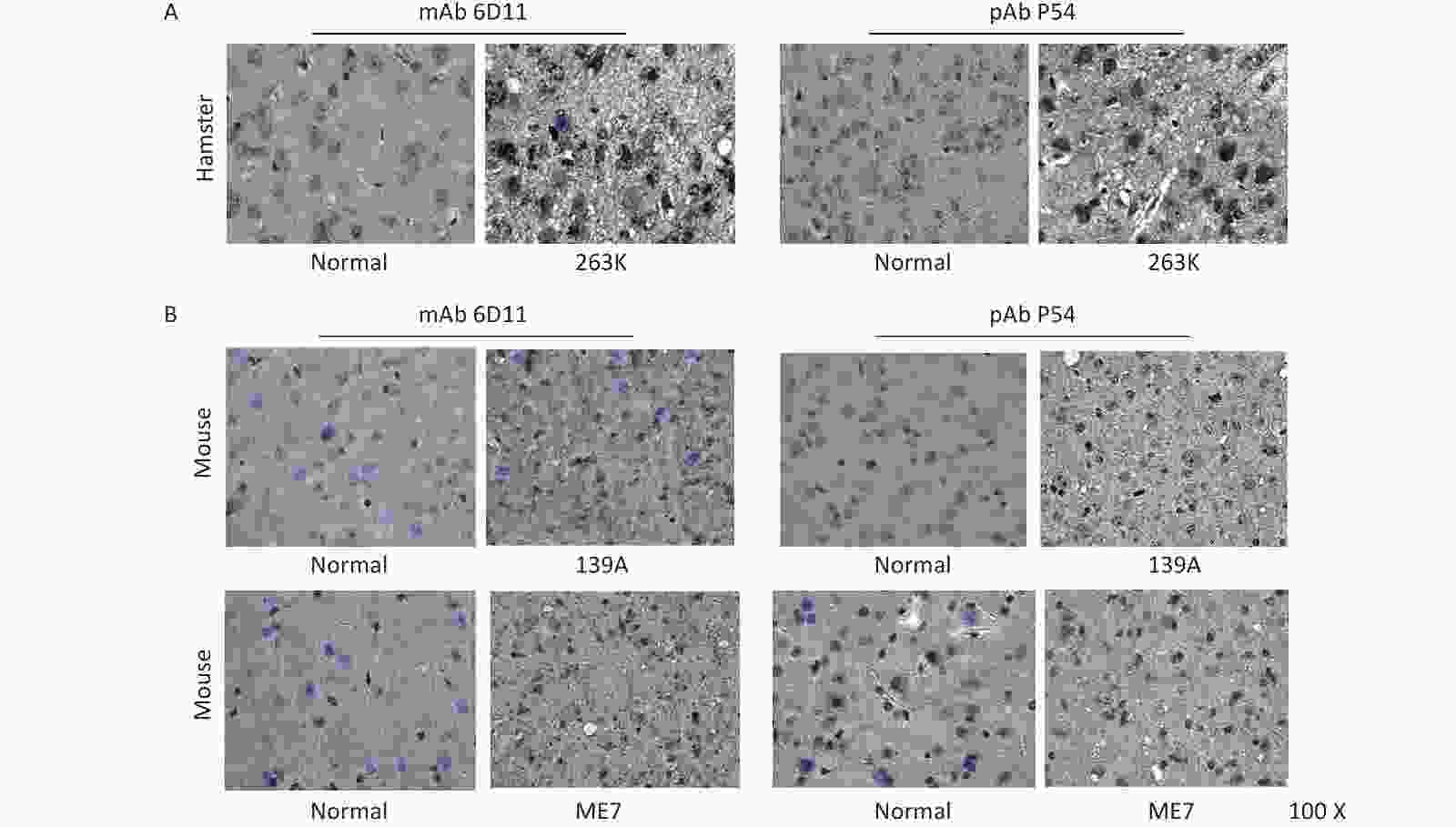
Figure 6. Immunohistochemical determination of the reactivity of pAb P54 with PrPSc deposits in the brains of scrapie-infected rodents. (A) 263K-infected hamster. mAb 3F4 was used as a control. (B) 139A- and ME7-infected mice. mAb 6D11 was used as a control. The brain slices were exposed to 4 mol/L GdnCl and then reacted with the individual PrP-specific antibodies.
-
The feasibility of the prepared pAb P54 was tested by IFA. Bright green signals were noted in SMB-S15 and SMB-PS cells treated with pAb P54 (Figure 7A). Compared with the green signals elicited by mAb 6D11, which were mainly located on the cell surface, the signals produced by pAb P54 were distributed in the cytoplasm and surfaces of the cells. When the brain slices of normal mice and hamsters were assayed, brilliant green signals surrounding blue-stained cell nuclei were observed in slices reacted with pAb P54. Moreover, pAb p54 produced morphological patterns similar to those of mAb 6D11 in mouse slices (Figure 7B) and mAb 3F4 in hamster slices (Figure 7C).
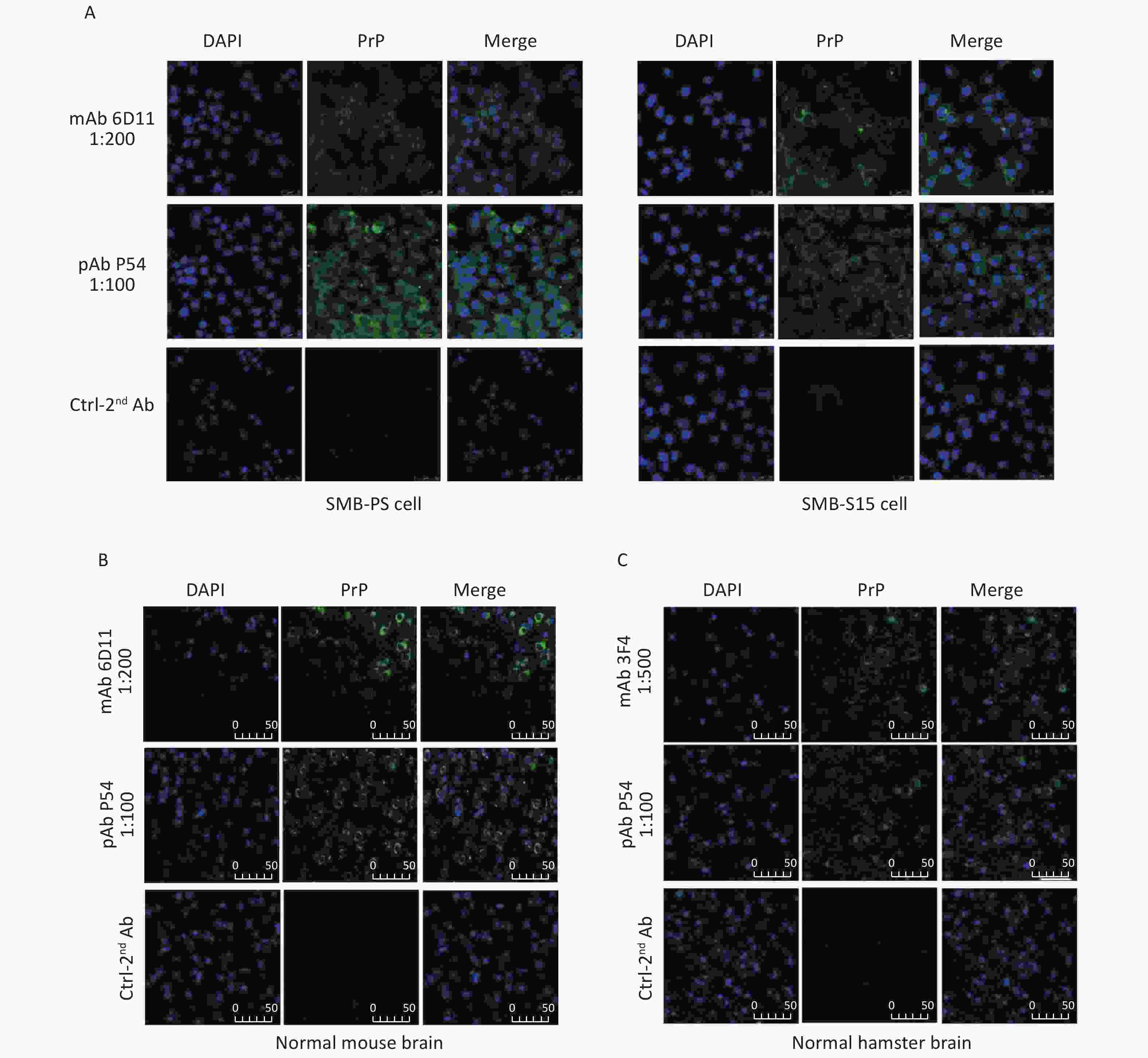
Figure 7. Immunofluorescent determination of the reactivity of pAb P54 with PrP signals in the SMB cells and brains of normal rodents. (A) SMB-S15 and SMB-PS cells. mAb 6D11 was used as a control. (B) Normal mouse. mAb 6D11 was used as a control. (C) Normal hamster. mAb 3F4 was used as a control. PrP (green), DAPI (blue) are indicated above. The images represent the results of at least three independent experiments. The bar: 50 μm.
-
In this report, we prepared PrP-specific pAb P54 by immunizing PRNP-knockout mice with recombinant full-length human PrP protein. Data obtained through ELISA, Western blot, and IHC verified that the newly prepared antibody could react with recombinant PrP protein, normal brain PrPC from healthy rodents and humans, and pathological PrPSc in the brains of experimental rodents infected with various scrapie agents, including 139A, ME7, S15, and 263K, and humans infected with different types of prion diseases, including sCJD, G114V gCJD, and FFI. The bands of three glycosylated forms of PrP, regardless of PrPC or PrPSc, were clearly displayed in the Western blots of pAb P54, and these bands showed electrophoretic patterns similar to those produced by commonly used commercial PrP mAbs. This result reveals that pAb P54 possesses not only good PrP specificity but also broad-spectrum recognition of various PrP molecules.
Our pAb P54 shows wide reactivity with PrP molecules from different species, including humans and experimental rodents; by comparison, mAb 3F4 only slightly recognizes mouse-derived PrP and mAb 6D11 reacts minimally with the human-derived protein. Although PrP protein is fairly well conserved among different species of animals, some PrP-specific mAbs reveal species limitations[12, 13]. Many pAbs exhibit broad reactivity with relevant antigens from different origins and with different epitopes[14], which sometimes makes it better in the usage for capturing more relevant antigen in the tested samples. Determination of the presence of PK-resistant PrPSc in brain lysates by Western blot and PrPSc deposits in brain slices by PK- or GdnCl-treated IHC assay are the main criteria for a definite diagnosis of human prion disease[15, 16]. The excellent performance of the prepared pAb P54 in Western blot and IHC demonstrated in the present study reflects its potential application in identifying PrPSc in the brains of human and experimental rodents infected with prion diseases.
PrP protein, as the product of a host housekeeping gene, is generally conserved among mammalian species[17, 18]. Thus, eliciting the perfect immune response simply via administration of PrP protein into wild-type animals is usually difficult. PRNP-knockout animals are an ideal basis for preparing PrP-specific pAb. In this study, we used a strain of PRNP-knockout mice without the detectable expression of PrPC in their brains to prepare the pAb; this strategy represents an approach that could overcome the difficulty described above. The prepared pAb P54 showed good specificity and acceptable sensitivity in recognizing native PrPC and PrPSc in brain tissues. Another limitation in the preparation of pAb-immunized PRNP-knockout mice is the limited amount of blood present in the animals, which means a large number of mice are necessary to obtain adequate amounts of the antibody. Individual differences in the immunized mice are inevitable. To solve this problem, we separately evaluated the serum reactive titers of individual mice at the second immunization by ELISA and sacrificed only those mice with reactive titers greater than 1:20,480. The prepared pAb P54 is simply the serum of immunized mice without further purification. Although the reactive titer of pAbs is relatively lower than that of mAbs, the simple preparation procedure presented in this work renders pAb P54 an economical alternative to its monoclonal counterpart. Further purification and concentration of pAb P54 will help increase its immunoreactive titer.
doi: 10.3967/bes2020.066
Preparation of PrP-specific Polyclonal Antibody via Immunization of PRNP-knockout Mice with Recombinant Human PrP Protein
-
Abstract:
Objective The definite diagnosis of human and animal prion diseases depends on the examination of special pathological changes and/or detection of PrPSc in the brain tissues of suspected cases. Thus, developing methods to obtain PrP antibody with good specificity and sensitivity is fundamental for prion identification. Methods We prepared a PrP-specific polyclonal antibody (pAb P54) in a PRNP-knockout mouse model via immunization with recombinant full-length human PrP protein residues 23–231. Thereafter, we verified that pAb in Western blot, immunohistochemistry (IHC), and immunofluorescent (IFA) assays. Results Western blot illustrated that the newly prepared pAb P54 could react with recombinant PrP protein, normal brain PrPC from healthy rodents and humans, and pathological PrPSc in the brains of experimental rodents infected with scrapie and humans infected with different types of prion diseases. The electrophoretic patterns of brain PrPC and PrPSc observed after their reaction with pAb P54 were nearly identical to those produced by commercial PrP monoclonal antibodies. Three glycosylated PrP molecules in the brain homogenates were clearly demonstrated in the reactions of these molecules with pAb P54. IHC assay revealed apparent PrP deposits in the GdnCl-treated brain slices of 139A-infected mice and 263K-infected hamsters. IFA tests with pAb P54 also showed clear green signals surrounding blue-stained cell nuclei. Conclusion The newly prepared pAb P54 demonstrated reliable specificity and sensitivity and, thus, may have potential applications not only in studies of prion biology but also in the diagnosis of human and experimental rodent prion diseases. -
Key words:
- Prion disease /
- PrP /
- Antibody /
- PRNP-knockout mouse
-
Figure 4. Western blot determination of the reactivity of pAb P54 with PrPSc molecules in the brains of scrapie-infected rodents. (A) 139A-, ME7-, and S15-infected mice. (B) 263K-infected hamster. Approximately 4 μL of each 10% brain homogenate was applied in the tests. Brain homogenates from normal animals were used as a control. Prior to SDS-PAGE, the brain lysates were digested with 50 μg/mL PK. +: with PK; -: without PK.
Figure 5. Western blot determination of the reactivity of pAb P54 with PrPSc molecules in the brains of prion disease-infected humans. The brain samples included one sCJD case, three FFI cases, and one G114V gCJD case. Approximately 4 μL of each 10% brain homogenate was applied in the tests. The brain homogenate of a person had passed away from a car accident was used as a control. The brain lysates were digested with 20 μg/mL PK. +: with PK; -: without PK.
Figure 6. Immunohistochemical determination of the reactivity of pAb P54 with PrPSc deposits in the brains of scrapie-infected rodents. (A) 263K-infected hamster. mAb 3F4 was used as a control. (B) 139A- and ME7-infected mice. mAb 6D11 was used as a control. The brain slices were exposed to 4 mol/L GdnCl and then reacted with the individual PrP-specific antibodies.
Figure 7. Immunofluorescent determination of the reactivity of pAb P54 with PrP signals in the SMB cells and brains of normal rodents. (A) SMB-S15 and SMB-PS cells. mAb 6D11 was used as a control. (B) Normal mouse. mAb 6D11 was used as a control. (C) Normal hamster. mAb 3F4 was used as a control. PrP (green), DAPI (blue) are indicated above. The images represent the results of at least three independent experiments. The bar: 50 μm.
-
[1] Robakis NK, Devine-Gage EA, Jenkins EC, et al. Localization of a human gene homologous to the PrP gene on the p ARM of chromosome 20 and detection of PrP-related antigens in normal human brain. Biochem Biophys Res Commun, 1986; 140, 758−65. doi: 10.1016/0006-291X(86)90796-5 [2] Brown P, Cervenáková L, Goldfarb LG, et al. Iatrogenic Creutzfeldt-Jakob disease: an example of the interplay between ancient genes and modern medicine. Neurol, 1994; 44, 291−3. doi: 10.1212/WNL.44.2.291 [3] Dormont D. Prion diseases: pathogenesis and public health concerns. Febs Letters, 2002; 529, 17−21. doi: 10.1016/S0014-5793(02)03268-4 [4] Isabelle LP, Caroline F, Luc R, et al. Developmental changes in cellular prion protein in primate visual cortex. J Comp Neurol, 2010; 504, 646−58. [5] Yasuro A, Naotaka I. Distribution of the cellular prion protein in the central nervous system of the chicken. J Chem Neuroanat, 2009; 38, 292−301. doi: 10.1016/j.jchemneu.2009.09.001 [6] Gill AC, Castle AR. The cellular and pathologic prion protein. Handbook of clinical neurology, 2018; 153. [7] Krasemann S, Jürgens T, Bodemer W. Generation of monoclonal antibodies against prion proteins with an unconventional nucleic acid-based immunization strategy. J Biotechnol, 1999; 73, 119−29. doi: 10.1016/S0168-1656(99)00115-7 [8] Ostapchenko VG, Makarava N, Savtchenko R, et al. The polybasic N-terminal region of the prion protein controls the physical properties of both the cellular and fibrillar forms of PrP. J Mol Biol, 2008; 383, 1210−24. doi: 10.1016/j.jmb.2008.08.073 [9] Büeler H, Fischer M, Lang Y, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature, 1992; 356, 577−82. doi: 10.1038/356577a0 [10] Goldman JS. Prion disease. Genetic counseling for adult neurogenetic disease, 2015; 121−9. [11] Yue M, Gao L, Kang X, et al. Alteration of NF-κB(p65) in brain tissue of scrapie-139A infected mice. Dis Surveill, 2018; 33, 32−6. [12] Sadowski MJ, Pankiewicz J, Prelli F, et al. Anti-PrP Mab 6D11 suppresses PrP replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo. Neurobiol Dis, 2009; 34, 267−78. doi: 10.1016/j.nbd.2009.01.013 [13] Takeshi Y, Akio S, Rie H, et al. Comparison of the anti-prion mechanism of four different anti-prion compounds, anti-PrP monoclonal antibody 44B1, pentosan polysulfate, chlorpromazine, and U18666A, in prion-infected mouse neuroblastoma cells. PLoS One, 2014; 9, e106516. doi: 10.1371/journal.pone.0106516 [14] Zhao X, Dong X, Zhou W. Preparation of polyclonal antibody to human prion protein using the expressed GST-PrP fusion protein as antigen. Chin J Experi Clin Virol, 2000; 14, 131−3. [15] Lu Y, Li ZW, Yang LF, et al. The overview of methods for detecting prion disease. China Animal Husbandry & Veterinary Medicine, 2011; 38, 166−9. [16] Wadsworth J DF, Adamson G, Joiner S, et al. Methods for molecular diagnosis of human Prion Dis. Methods Mol Biol, 2017; 311−46. [17] Hasegawa K, Mohri S, Yokoyama T. Comparison of the local structural stabilities of mammalian prion protein (PrP) by fragment molecular orbital calculations. Prion, 2013; 7, 185−91. doi: 10.4161/pri.23122 [18] Scheckel C, Aguzzi A. Prions, prionoids and protein misfolding disorders. Nature Rev Gene, 2018; 19, 405−18. doi: 10.1038/s41576-018-0011-4 -




 下载:
下载:



 Quick Links
Quick Links