-
Mammalian adipose tissues can be broadly divided into white adipose tissue (WAT), beige adipose tissue, and brown adipose tissue (BAT) [1]. The function of WAT is to store superfluous energy and is characterized by unilamellar lipid droplets. WAT, as a prominent endocrine organ, regulates feeding and satiety by producing hormones. Compared with WAT, beige adipose tissue has some smaller multilocular lipid droplets and is located in WAT depots. However, BAT contains an abundance of mitochondria, uncoupling protein-1 (UCP1), and multilocular lipid droplets[2]. BAT is an important non-shivering thermogenesis organ, with the capacity to oxidize metabolic substrates, including fatty acids and glucose, to produce heat. The main mechanism of heat production depends on UCP1. It transports protons into mitochondria, leading to the collapse of the proton gradient for oxidative phosphorylation; subsequently, cells generate heat instead of ATP. The thermogenic activity of brown adipocytes enables them to safeguard other tissues and themselves from lipid overaccumulation. Many studies have confirmed that promoting brown adipose thermogenic activity or the browning of white fat contributes to curbing obesity, diabetes, and other metabolic diseases[3-7]. Brown adipocytes are derived from Myf5+ progenitors with a high expression of PRDM16, BMP7, and PPARγ. These transcription regulators drive progenitors to develop into mature brown adipocytes[8]. Meanwhile, a development process is required for brown adipogenesis to suppress adipogenic inhibitors, including Wnt, necdin, and preadipocyte factor-1 (Pref-1). Numerous studies have confirmed that many signaling pathways promote brown adipocyte differentiation, including rhACE2, SIRT5, RGS2, STAT3, RepSox, and SENP2 (Figure 1). Tu et al.[9] reported that RepSox promoted brown preadipocyte differentiation by inhibiting TGF-β signaling. Shuai et al.[10] demonstrated that SIRT5 enhanced the expression of brown adipogenic promoters, including PPARγ and PRDM16. Klepac et al.[11] identified a crucial role for RGS2, which antagonized the inhibitory effect of Gq/Rho/ROCK signaling, in the acceleration brown adipogenesis. Cantwell et al.[12] revealed the significance of STAT3 in the early induction of primary Myf5+ brown adipogenesis through its suppression of Wnt/β-catenin signaling. Kawabe et al.[13] proved that rhACE2 increased the levels of PRDMl6 and PGC1α to boost differentiation of BAT. Recently, Liang et al.[14] demonstrated that brown adipocyte differentiation was facilitated via the SENP2-mediated deSUMOylation for necdin.
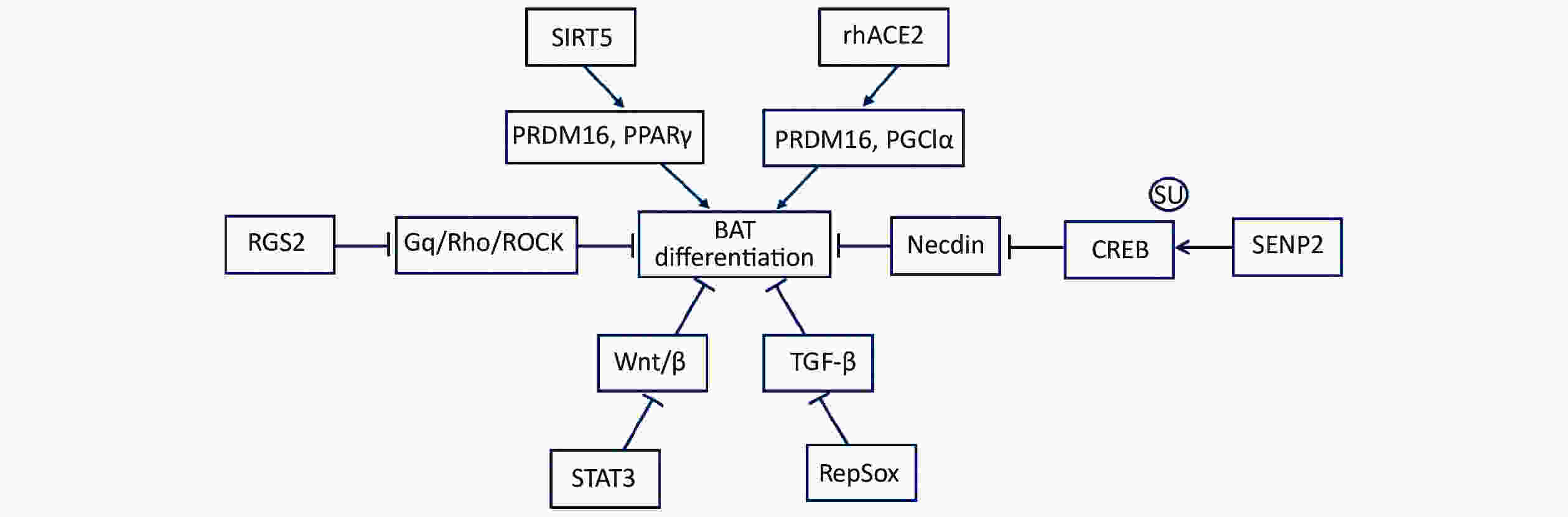
Figure 1. Numerous signaling pathways promote brown adipocyte differentiation. Numerous signal pathways promote brown adipocyte differentiation, including rhACE2, SIRT5, RGS2, STAT3, RepSox, and SENP2. rhACE2 increases the expression of both PRDMl6 and PGC1α to promote the differentiation of BAT. SIRT5 plays a crucial role in increasing the levels of PPARγ and PRDM16 during brown adipogenesis. RGS2 promotes brown adipogenesis by offsetting the inhibitory effect of Gq/Rho/ROCK signaling. STAT3 inhibits Wnt/β-catenin signal transduction during the induction of primary Myf5+ brown adipogenesis. RepSox induces brown adipogenesis via the inhibition of TGF-β signaling. SENP2 suppresses necdin expression via the de-SUMOylation of CREB, thereby facilitating brown adipose differentiation. BAT, brown adipose tissue; rhACE2, recombinant human angiotensin converting enzyme 2; PRDMl6, positive regulatory homologous domain containing 16; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1a; SIRT5, sirtuin-5; PPARγ, peroxisome proliferator-activated receptor γ; RGS2, regulator of G protein signaling 2; STAT3, signal transducer and activator of transcription 3; RepSox, a selective TGFβRI/ALK5 inhibitor; SENP2, small ubiquitin-like modifier (SUMO)-specific protease 2; necdin, neutrally differentiated embryonal carcinoma-derived protein; CREB, cAMP response element-binding protein
SUMOylation, the conjugation of a small ubiquitin-like modifier (SUMO) to a target protein, is associated with the formation of isopeptide (amide) binding between the ɛ-amino group of lysine residues within the specific target proteins and the C-terminal glycine of SUMO[15]. The process depends on the sequential action of an activating enzyme E1, an E2-conjugating enzyme, and an E3 ligase. SUMOylation is responsible for the modulation of numerous biological processes, such as nuclear transport and transcription, DNA replication and repair, cell proliferation, and signal transduction[16]. More importantly, SUMOylation plays an essential role in adipocyte differentiation. For example, the E2-SUMO ligase ubiquitin carrier protein 9 (Ubc9) negatively modulates the transactivation function of PPARγ, resulting in the suppressed expression of the BAT gene in human subcutaneous adipocytes[17,18]. Moreover, Ubc9 and miR-30a have the opposite expression in adipose tissue, as miR-30a expression accompanies a vast increase in brown fat[19]. Furthermore, Ubc9, regarded as a key regulator of the insulin sensitivity of glucose transport in adipocytes, upregulates the expression of GLUT4 in L6 myoblast cells[20]. Similarly, SUMO E3 ligase is also essential for adipocyte differentiation. It has been reported that Cbx4, as a polycomb group protein, is a SUMO E3 ligase for SUMOylation of Prdm16, which is linked to thermogenic gene expression and the browning of white fat[21].
SUMOylation is a dynamic and reversible process[22]. The key step in reversing the SUMOylation pathway is its capacity to be deSUMOylated by SUMO-specific proteases (SENPs). Seven enzymes, SENP1–3 and SENP5–8, have been identified in mammals. These enzymes differ in their subcellular locations and substrate specificities. They are involved in various biological pathways, such as ribosome biogenesis, mitochondrial dynamics, cell cycle regulation and anticancer[23]. Interestingly, SENP2, located in the nuclear pore complex, contains nuclear import and export signals, allowing it to shuttle between the cytoplasm and the nucleus[24]. Furthermore, a large body of evidence supports the important roles of SENP2 in the differentiation of various adipocytes or stem cells. In 2010, Chung et al.[25] confirmed that SENP2 was strongly related to adipogenesis. Their results showed an exceptional increase in the expression of SENP2 during adipocyte differentiation. The specific regulation mechanism is that SENP2 deSUMOylates and stabilizes C/EBPβ, which in turn increases the expression of PPARγ and C/EBPα[25]. In addition, Mitani et al.[26] revealed that theobromine, a methylxanthine originating from cacao beans, inhibited the expression of SENP2, which decreased adipocyte differentiation via facilitating C/EBPβ degradation. One year later, Zheng et al.[27] found that Senp2 could suppress SET domain bifurcated 1 function by deSUMOylation; as a result, the process suppressed the expression of lipid metabolism-related target genes, such as Pparg and Cebpa, which suppressed the capability of adipocytes for lipid storage and adipogenesis. Recently, Liang et al.[14] revealed that SENP2 suppressed neurally differentiated embryonal carcinoma-derived protein (necdin) expression in brown adipocyte differentiation.
To prove that SENP2 was involved in the brown adipocyte differentiation, Liang et al.[14] first established a conditional Senp2BKO model by crossing Senp2f/f with Myf5-Cre mice. Compared with their Senp2f/f littermates, Senp2 expression was conspicuously reduced in BAT, rather than in WAT, in Senp2BKO mice. Moreover, given the first occurrence of BAT at a later gestational stage, they separately determined BAT in embryos and newborns. The results of the study found that BATs in Senp2BKO embryos at embryonic day 18.5 or newborn mice were smaller than those in the Senp2f/f control mice. Ki67 is a nuclear marker of cell proliferation and PRDM16 is a crucial regulator in brown preadipocyte commitment. Liang et al.[14] also found a low expression of Ki67-positive cells and PRDM16 in Senp2BKO BAT than in the BAT of Senp2f/f controls. Furthermore, Liang et al.[14] also created a SENP2 knockout C3H10T1/2 cell strain, from which they determined that Senp2 deficiency could only reduce the expression of brown preadipocyte differentiation genes (PRDM16, PGC1a, and Ebf2) and not that of post-differentiation genes (PPARγ and Fabp4), in C3H10T1/2 cells. These encouraging data suggested that SENP2 modulates brown preadipocyte differentiation.
They further investigated the specific mechanism of SENP2 in the regulation of brown adipocyte differentiation. Many studies have confirmed that necdin is activated by phosphatase PP2A, which de-phosphorylates CREB[28,29]. Necdin interacts with various partners, including as E2Fs, p53, and Gα, to suppress cell growth and induce differentiation in several cell types[30], and promotes the expression of other adipogenic inhibitors, including Pref-1 and Wnt10, during the adipogenic process[31]. Therefore, the inhibition of necdin expression is a prerequisite for brown adipose differentiation. Liang et al.[14] confirmed that a higher expression of necdin was observed in Senp2 KO cells. Meanwhile, necdin KO restored the expression of the adipogenesis-related genes in Senp2 KO cells. All the above evidence indicated that SENP2 promoted brown adipocyte differentiation through the negative regulation of necdin. But how does SENP2 affect the expression of necdin? First, they confirmed that the CREB lost the ability to activate necdin transcription in the presence of CREB SUMOylation by mutating two sites: K285 and K304. Second, the SENP2-mediated de-SUMOylation interrupted CREB binding with PP2A, to phosphorylate CREB, which then suppressed necdin expression. These interesting results showed that SENP2 suppressed necdin expression via the de-SUMOylation of CREB, resulting in the facilitation of brown adipose differentiation. Notably, brown adipose tissue and skeletal muscle originate from Myf5+ progenitors. Qi et al.[32] identified the SENP2-mediated de-SUMOylation of MEF2A in the promotion of myostatin expression, which led to the inhibition of myogenesis. Hence, it is meaningful to explore how to determine the differentiation of Myf5+ progenitors into myocytes or brown adipocyte by controlling the signaling cascades of SENP2 expression.
The important role of SENP2 in brown adipose differentiation makes it a promising novel therapeutic target. SENP2 activators, such as leptin and palmitate, may have beneficial effects in brown adipocyte differentiation. Leptin is a protein hormone secreted by adipose tissue. In view of its function of promoting satiety and increasing energy expenditure, exogenous recombinant leptin has been regarded as an effective pharmacological tool for the treatment of obesity[33]. Leptin was recently reported to increase SENP2 expression through the pathway of the leptin receptor/STAT3 in C2C12 myotubes[34]. It is well known that Hedgehog (Hh) signaling pathway is composed primarily of Hh ligand, Patched (Ptch), Smoothened (Smo), and Glioma-associated oncogene homolog (Gli)[35]. Some studies have shown that the Hh signaling pathway suppresses adipogenesis in an evolutionarily conserved manner[36,37]. Surprisingly, leptin triggers white adipocyte browning via suppression of the Hh signaling pathway[38]. Thus, it is meaningful to explore whether leptin promotes brown adipose differentiation by increasing SENP2 expression or blocking the Hh signaling pathway. Unlike leptin, palmitate is a derivative of a saturated fatty acid with the ability to induce nuclear factor-κB (NF-κB) activation. When NF-κB is activated by palmitate, it results in the increase of expression of SENP2 in C2C12 myotubes[39]. In addition, palmitate promotes the differentiation of mouse embryonic stem cells into white adipocyte lineages and enhances the expression of C/EBPβ, which is the pivotal cofactor of PRDM16[8,40]. Therefore, further research should focus on whether palmitate can promote brown adipose differentiation by increasing SENP2 expression in brown preadipocytes. Collectively, given the effects of leptin and palmitate activation, they can be considered the potential drugs to target obesity, diabetes, and other metabolic diseases.
In summary, SENP2 has crucial roles in the regulation of adipose lipid storage and adipogenesis. Liang et al.[14] reported that the SENP2-mediated de-SUMOylation of CREB was associated with the suppression of necdin expression, which, in turn, induced brown preadipocyte differentiation. Hence, drugs targeting SENP2 may open a new pathway for therapeutic interventions for obesity, diabetes, and other metabolic diseases.
doi: 10.3967/bes2020.119
-
These authors contributed equally to this work
注释: -
Figure 1. Numerous signaling pathways promote brown adipocyte differentiation. Numerous signal pathways promote brown adipocyte differentiation, including rhACE2, SIRT5, RGS2, STAT3, RepSox, and SENP2. rhACE2 increases the expression of both PRDMl6 and PGC1α to promote the differentiation of BAT. SIRT5 plays a crucial role in increasing the levels of PPARγ and PRDM16 during brown adipogenesis. RGS2 promotes brown adipogenesis by offsetting the inhibitory effect of Gq/Rho/ROCK signaling. STAT3 inhibits Wnt/β-catenin signal transduction during the induction of primary Myf5+ brown adipogenesis. RepSox induces brown adipogenesis via the inhibition of TGF-β signaling. SENP2 suppresses necdin expression via the de-SUMOylation of CREB, thereby facilitating brown adipose differentiation. BAT, brown adipose tissue; rhACE2, recombinant human angiotensin converting enzyme 2; PRDMl6, positive regulatory homologous domain containing 16; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1a; SIRT5, sirtuin-5; PPARγ, peroxisome proliferator-activated receptor γ; RGS2, regulator of G protein signaling 2; STAT3, signal transducer and activator of transcription 3; RepSox, a selective TGFβRI/ALK5 inhibitor; SENP2, small ubiquitin-like modifier (SUMO)-specific protease 2; necdin, neutrally differentiated embryonal carcinoma-derived protein; CREB, cAMP response element-binding protein
-
[1] Zhu Q, Glazier BJ, Hinkel BC, et al. Neuroendocrine regulation of energy metabolism involving different types of adipose tissues. Int J Mol Sci, 2019; 20, 2707. doi: 10.3390/ijms20112707 [2] Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology, 2013; 154, 2992−3000. doi: 10.1210/en.2013-1403 [3] Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev, 2004; 84, 277−359. doi: 10.1152/physrev.00015.2003 [4] Hussain MF, Roesler A, Kazak L. Regulation of adipocyte thermogenesis: mechanisms controlling obesity. FEBS J, 2020. [5] Wankhade UD, Shen M, Yadav H, et al. Novel browning agents, mechanisms, and therapeutic potentials of brown adipose tissue. Biomed Res Int, 2016; 2016, 2365609. [6] Lee K, Lee YJ, Kim KJ, et al. Gomisin N from Schisandra chinensis ameliorates lipid accumulation and induces a brown fat-like phenotype through AMP-activated protein kinase in 3T3-L1 adipocytes. Int J Mol Sci, 2020; 21, 2153. doi: 10.3390/ijms21062153 [7] Czech MP. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab, 2020; 34, 27−42. doi: 10.1016/j.molmet.2019.12.014 [8] Zhang J, Wu H, Ma S, et al. Transcription regulators and hormones involved in the development of brown fat and white fat browning: transcriptional and hormonal control of brown/beige fat development. Physiol Res, 2018; 67, 347−62. [9] Tu WZ, Fu YB, Xie X. RepSox, a small molecule inhibitor of the TGFbeta receptor, induces brown adipogenesis and browning of white adipocytes. Acta Pharmacol Sin, 2019; 40, 1523−31. doi: 10.1038/s41401-019-0264-2 [10] Shuai L, Zhang LN, Li BH, et al. SIRT5 regulates brown adipocyte differentiation and browning of subcutaneous white adipose tissue. Diabetes, 2019; 68, 1449−61. doi: 10.2337/db18-1103 [11] Klepac K, Yang J, Hildebrand S, et al. RGS2: a multifunctional signaling hub that balances brown adipose tissue function and differentiation. Mol Metab, 2019; 30, 173−83. doi: 10.1016/j.molmet.2019.09.015 [12] Cantwell MT, Farrar JS, Lownik JC, et al. STAT3 suppresses Wnt/beta-catenin signaling during the induction phase of primary Myf5+ brown adipogenesis. Cytokine, 2018; 111, 434−44. doi: 10.1016/j.cyto.2018.05.023 [13] Kawabe Y, Mori J, Morimoto H, et al. ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. Am J Physiol Endocrinol Metab, 2019; 317, E1140−9. doi: 10.1152/ajpendo.00311.2019 [14] Liang Q, Zheng Q, Zuo Y, et al. SENP2 suppresses necdin expression to promote brown adipocyte differentiation. Cell Rep, 2019; 28, 2004−11. doi: 10.1016/j.celrep.2019.07.083 [15] Zhao H, Yao P, Fu N, et al. deSUMOylation signaling: a novel mechanism of liver CSC properties and hepatocarcinogenesis in hypoxia. Acta Biochim Biophys Sin (Shanghai), 2017; 49, 1135−7. doi: 10.1093/abbs/gmx109 [16] Zhao X. SUMO-mediated regulation of nuclear functions and signaling processes. Mol Cell, 2018; 71, 409−18. doi: 10.1016/j.molcel.2018.07.027 [17] Yamashita D, Yamaguchi T, Shimizu M, et al. The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells, 2004; 9, 1017−29. doi: 10.1111/j.1365-2443.2004.00786.x [18] Hartig SM, Bader DA, Abadie KV, et al. Ubc9 impairs activation of the brown fat energy metabolism program in human white adipocytes. Mol Endocrinol, 2015; 29, 1320−33. doi: 10.1210/me.2015-1084 [19] Koh EH, Chen Y, Bader DA, et al. Mitochondrial activity in human white adipocytes is regulated by the ubiquitin carrier protein 9/microRNA-30a axis. J Biol Chem, 2016; 291, 24747−55. doi: 10.1074/jbc.M116.749408 [20] Liu LB, Omata W, Kojima I, et al. The SUMO conjugating enzyme Ubc9 is a regulator of GLUT4 turnover and targeting to the insulin-responsive storage compartment in 3T3-L1 adipocytes. Diabetes, 2007; 56, 1977−85. doi: 10.2337/db06-1100 [21] Chen Q, Huang L, Pan D, et al. Cbx4 sumoylates prdm16 to regulate adipose tissue thermogenesis. Cell Rep, 2018; 22, 2860−72. doi: 10.1016/j.celrep.2018.02.057 [22] Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci, 2007; 32, 286−95. doi: 10.1016/j.tibs.2007.05.002 [23] Huang CJ, Wu D, Khan FA, et al. DeSUMOylation: An Important Therapeutic Target and Protein Regulatory Event. DNA Cell Biol, 2015; 34, 652−60. doi: 10.1089/dna.2015.2933 [24] Kumar A, Zhang KY. Advances in the development of SUMO specific protease (SENP) inhibitors. Comput Struct Biotechnol J, 2015; 13, 204−11. doi: 10.1016/j.csbj.2015.03.001 [25] Chung SS, Ahn BY, Kim M, et al. Control of adipogenesis by the SUMO-specific protease SENP2. Mol Cell Biol, 2010; 30, 2135−46. doi: 10.1128/MCB.00852-09 [26] Mitani T, Watanabe S, Yoshioka Y, et al. Theobromine suppresses adipogenesis through enhancement of CCAAT-enhancer-binding protein beta degradation by adenosine receptor A1. Biochim Biophys Acta Mol Cell Res, 2017; 1864, 2438−48. doi: 10.1016/j.bbamcr.2017.09.017 [27] Zheng Q, Cao Y, Chen Y, et al. Senp2 regulates adipose lipid storage by de-SUMOylation of Setdb1. J Mol Cell Biol, 2018; 10, 258−66. doi: 10.1093/jmcb/mjx055 [28] Cypess AM, Zhang H, Schulz TJ, et al. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology, 2011; 152, 3680−9. doi: 10.1210/en.2011-1229 [29] Hatting M, Rines AK, Luo C, et al. Adipose tissue CLK2 promotes energy expenditure during high-fat diet intermittent fasting. Cell Metab, 2017; 25, 428−37. doi: 10.1016/j.cmet.2016.12.007 [30] Fujimoto I, Hasegawa K, Fujiwara K, et al. Necdin controls EGFR signaling linked to astrocyte differentiation in primary cortical progenitor cells. Cell Signal, 2016; 28, 94−107. doi: 10.1016/j.cellsig.2015.11.016 [31] Tseng YH, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol, 2005; 7, 601−11. doi: 10.1038/ncb1259 [32] Qi Y, Zuo Y, Yeh ET, et al. An essential role of small ubiquitin-like modifier (SUMO)-specific protease 2 in myostatin expression and myogenesis. J Biol Chem, 2014; 289, 3288−93. doi: 10.1074/jbc.M113.518282 [33] Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA, 1999; 282, 1568−75. doi: 10.1001/jama.282.16.1568 [34] Koo YD, Lee JS, Lee SA, et al. SUMO-specific protease 2 mediates leptin-induced fatty acid oxidation in skeletal muscle. Metabolism, 2019; 95, 27−35. doi: 10.1016/j.metabol.2019.03.004 [35] Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev, 2008; 22, 2454−72. doi: 10.1101/gad.1693608 [36] Suh JM, Gao X, McKay J, et al. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab, 2006; 3, 25−34. doi: 10.1016/j.cmet.2005.11.012 [37] Lee HJ, Jo SB, Romer AI, et al. Overweight in mice and enhanced adipogenesis in vitro are associated with lack of the hedgehog coreceptor boc. Diabetes, 2015; 64, 2092−103. doi: 10.2337/db14-1017 [38] Wang J, Ge J, Cao H, et al. Leptin promotes white adipocyte browning by inhibiting the Hh signaling pathway. Cells, 2019; 8, 372. doi: 10.3390/cells8040372 [39] Koo YD, Choi JW, Kim M, et al. SUMO-specific rotease 2 (SENP2) is an important regulator of fatty acid metabolism in skeletal muscle. Diabetes, 2015; 64, 2420−31. doi: 10.2337/db15-0115 [40] Petrighi P, Lomax MA, Docherty K. Palmitate enhances the differentiation of mouse embryonic stem cells towards white adipocyte lineages. Mol Cell Endocrinol, 2012; 361, 40−50. doi: 10.1016/j.mce.2012.03.010 -




 下载:
下载:



 Quick Links
Quick Links