-
Trichloroacetic acid (TCA), a major chlorinated disinfection by-product, is a potential carcinogen in humans[1]. Although several laboratory animal studies have been conducted to evaluate the potential mechanisms through which TCA induces tumors, few reports have examined the neurotoxicity effects and possible mechanisms in vitro. Some in vivo studies have indicated that TCA causes free radical generation in the brain [2-4]. Boron (B), an essential mineral, has roles in several important biological processes [5]. The central nervous system shows improvements, and immune organs exhibit enhanced immunity after B supplementation. Animals and humans fed diets supplemented with B show effects in the metabolism of numerous enzymes and minerals, and benefits in wound healing and cancer therapy. Research results have indicated the beneficial effects of B. Oxidative stress via macrophage activation is a potential mechanism of TCA’s action. Studies have shown that macrophages are activated and become a source of reactive oxygen species (ROS), which damage surrounding tissues [6]. With increased doses of TCA, apoptosis is enhanced, thus, leading to increased neuronal death and consequently lower brain weight than that in controls. The fetal central nervous system is susceptible to the toxic effects of TCA [7]. Little evidence of neurotoxicity of TCA has been reported, except for one experimental study suggesting that TCA has neuroembryopathic effects in rats exposed during organogenesis [8]. To date, reports on TCA or B have been performed primarily in laboratory animals, whereas few studies have focused on the TCA-induced neurotoxicity and protective effects of B in vitro. This study aimed to investigate the mode of action of the neurotoxicity of TCA and the possible protective mechanisms of B.
We purchased TCA (CAS 76-03-9) of analytical grade from Sinopharm Group Chemical Reagent Co., Ltd. (Beijing, China) and borax (Na2B4O7·10H2O, CAS 1303-96-4, 99.5% purity) from Sigma-Aldrich (St. Louis, MO, USA). Insulin, L-glutamine (200 mmol/L), fetal bovine serum, antibiotic antimitotic (100×), high glucose Dulbecco’s modified Eagle’s medium, HBSS and HEPES (1 mol/L) were purchased from Sigma-Aldrich (St. Louis, MO, USA). A Cell counting kit 8 (WST-8), ROS Assay Kit, mitochondrial membrane potential (MMP) kit, Nuclear and Cytoplasmic Protein Extraction Kit, Enhanced BCA Protein Assay Kit and B1 primary antibody were purchased from Beyotime Biotechnology Co. Ltd. (Shanghai, China). ELISA kits for IL-2, IL-6, TNF-α, TNF-β and NF-κB were all purchased from Jiang Lai Biology Co. Ltd. (Shanghai, China). Primary antibodies to p53, p-p53, p38, p-p38, bax, and β-actin were all purchased from Cell Signaling Technology. A JC-1 assay kit was purchased from Beijing Co. Ltd. A 12–230 kD Wes ProteinSimple Separation Module and Detection Modules were purchased from bio-techne (MN, USA).
Mouse BV2 cells purchased from the National Infrastructure of Cell Line Resource (Beijing, China) were cultured and maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Pre-trials used different concentrations of TCA and borax ranging from 0.25 mmol/L to 1,000 mmol/L for different treatment periods (3 h and 24 h).
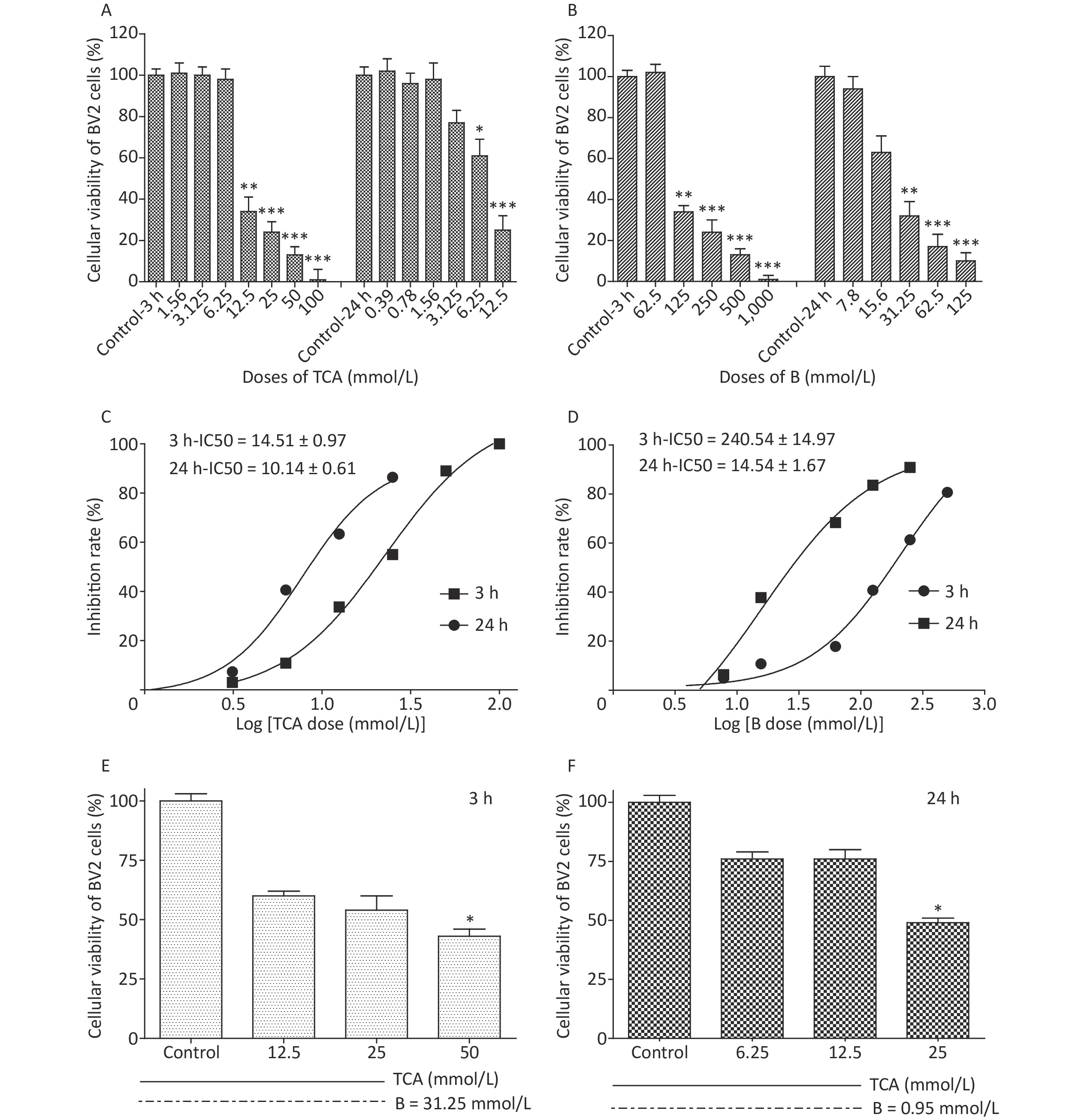
Cell viability was evaluated with a Cell counting kit 8 (WST-8). A total of 5,000–10,000 cells per well (100 μL for a 96-well plate) were treated with TCA and borax, and the absorbance was measured at 460 nm after 10 μL/well of WST-8 solution was added to each well and incubated for 2 h at 37 ℃ (with protection from light). Compared with control cells, the number of BV2 cells treated with 12.5 mmol/L and 6.25 mmol/L TCA (P < 0.05) decreased significantly, after 3 h or 24 h exposure, respectively, showing a time- and dose-dependent relationship (Figure 1A). The viability of cells decreased dramatically with 125 mmol/L and 31.25 mmol/L B treatment (Figure 1B). The IC50 of BV2 cells significantly decreased after 24 h B exposure (Figure 1D). The effects of TCA and B, or combined exposure, on cellular viability were calculated after 80% confluence was reached. Cellular viability clearly decreased after TCA treatment but was scarcely affected by B, because of the low concentrations used. After 3 h of combined exposure, the cellular viability of the TCA groups significantly increased (P < 0.01) after B supplementation (Figure 1E), in a dose-responsive relationship (Figure 1F). Microglia in the brain regulate brain development, maintenance of neuronal networks and injury repair. Microglia are brain macrophages distinct from other tissue macrophages, owing to their unique homeostatic phenotype and tight regulation by the central nervous system microenvironment [9]. Furthermore, as the primary source of proinflammatory cytokines, microglia are crucial mediators of neuroinflammation, and induce or modulate a broad spectrum of cellular responses. We chose BV2 cells because they are immortal cell lines commonly used in medical research, owing to their morphology, phenotype and various functional characteristics of primary cultured microglia. Little research has been performed to evaluate the adverse effects of TCA or B on BV2 cells. In this study, TCA and B inhibited cellular viability after a single exposure at some concentrations. The decrease in cellular viability dramatically decreased after B treatment. This protective effect might occur partially through B adjusting the pH of the culture medium and stabilizing the cellular environment to protect against TCA cytotoxicity.
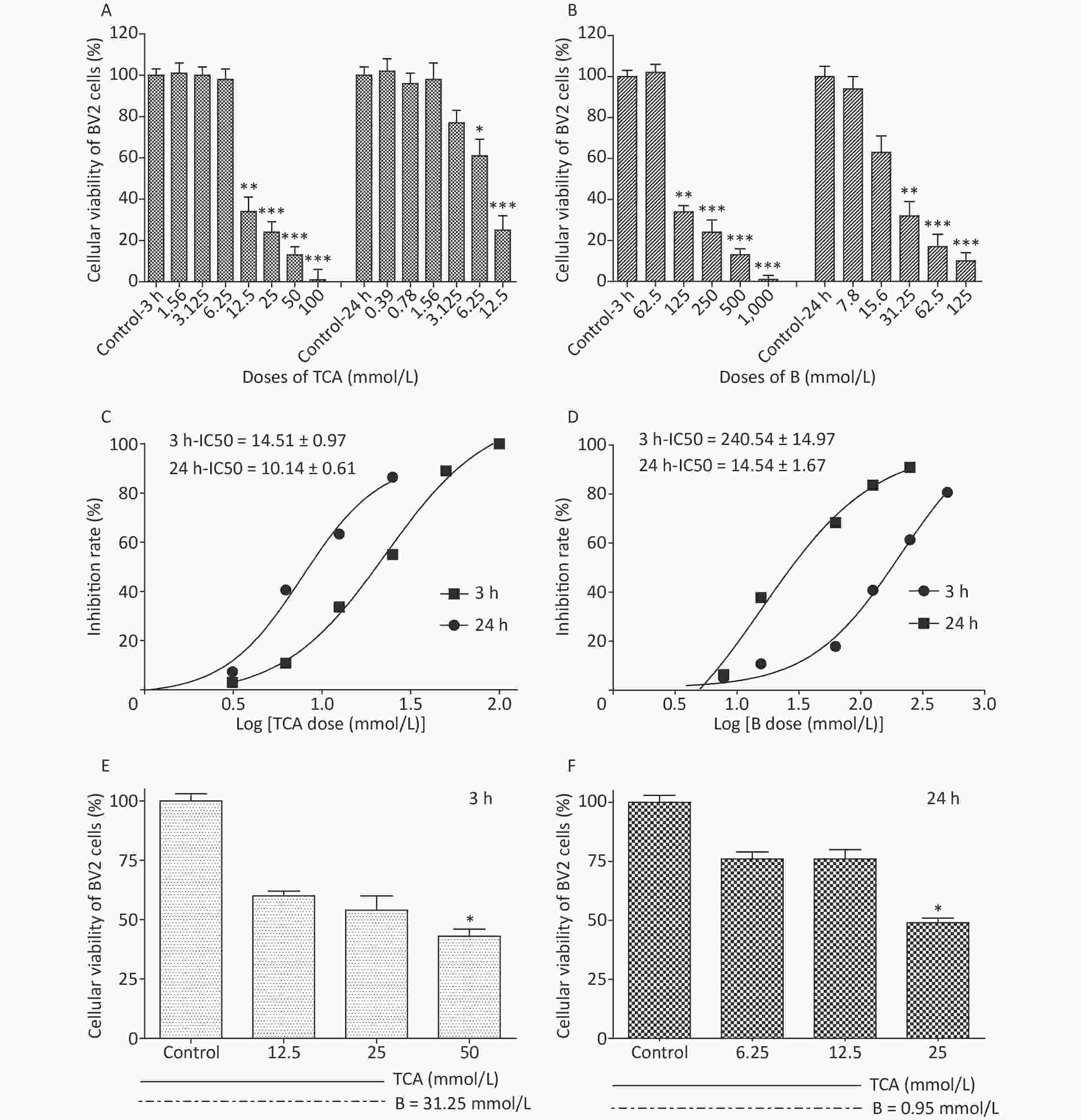
Figure 1. Cell viability and IC50 after 3 h and 24 h exposure to different concentrations of TCA and B. Cell survival rates decreased significantly and with a dose-dependent relationship by the single treatment of TCA and B (A and B). Inhibition rate of BV2 cells increased dramatically with the increase concentrations of single TCA and B (C and D). Cellular viability increased significantly after B supplement 3 h (E) and 24 h (F). The data represent the mean ± SD of ten independent experiments in quadruplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control groups respectively. B, Boron; TCA, trichloroacetic acid.
ROS and MMP determination was performed on 2 × 105/mL BV2 cells seeded directly on 12-well culture plates to ensure 80% confluence. On the day of the experiments, fresh medium and 1× ROS label (10 μmol/L DCFH-DA) were added into each well and incubated for 20 min at 37 °C in the dark. Positive controls were simultaneously treated with Rosup (50 mg/mL). Then cells were washed twice with 1× wash buffer and observed under a fluorescence microscope with standard excitation/emission filter sets compatible with fluorescein (Ex/Em = 488/525 nm) to measure ROS. MMP was determined according to the kit instructions. Briefly, JC-1 working solution was added into each well, and cells were incubated in a CO2 incubator at 37 °C for 20 min. Then BV2 cells were washed with 1× dilution buffer twice in the dark. Finally, all collected cells were plated on 96-well plates to detect the fluorescence of fluorescein (Ex/Em = 490/530 nm and Ex/Em = 525/590 nm).
The findings suggested that TCA might increase ROS, whereas the levels of ROS decreased after B supplementation (Figure 2). In addition, the ratio of red to green fluorescence for treatment groups clearly increased after B treatment (Supplementary Figure S1, available in www.besjournal.com). Oxidative stress has been postulated to be a key contributor to dopaminergic neurodegeneration, because nigrostriatal dopaminergic neurons, the main neuronal type affected in Parkinson’s disease, are uniquely vulnerable to oxidative damage. A decrease in MMP is a hallmark of early apoptosis events. Mitochondria are considered the main source of ROS in cells. B might have unfavorable effects on the central nervous system and inhibition of apoptosis in the brain [10]. Mechanisms underlying B’s action were associated with decreasing intracellular ROS levels, thus ultimately averting apoptosis. In another study by our team [11], metabolomics results from rats gavaged with borax have shown that B elevates nicotinamide metabolism; the highest boron treatment led to significant increases in nicotinamide and several nicotinamide-derived metabolites.
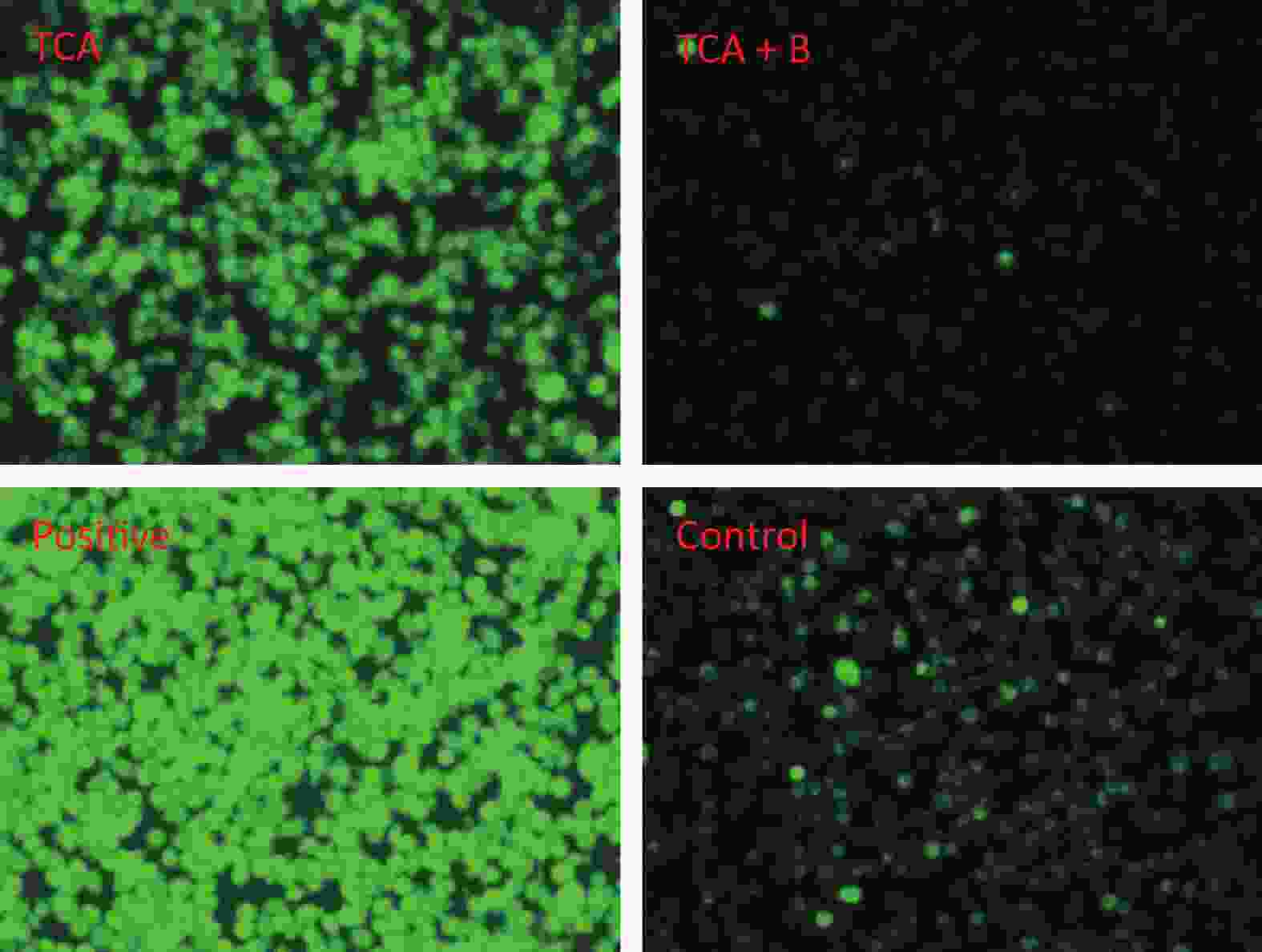
Figure 2. Cellular reactive oxygen species after 24 h TCA exposure and the effects of B treatment. T and B represent 6.25 mmol/L TCA and 0.95 mmol/L B respectively.
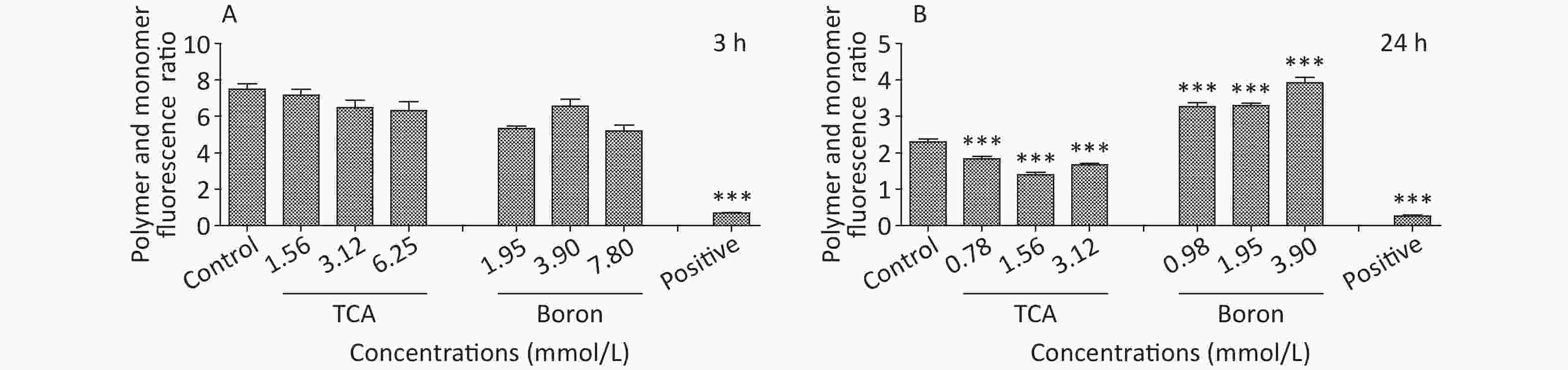
Figure S1. MMP of different treatment of TCA and B after 3 h and 24 h exposure. BV2 cells stained with JC-1 were observed. JC-1 polymer and monomer fluorescence ratio was shown below (Ex/Em = 490/530 nm for JC-1 monomer and Ex/Em = 525/590 nm for JC-1 polymer). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control groups respectively.
Proinflammatory cytokines, including IL-2, IL-6, TNF-α, TNF-β, and NF-κB were determined according to the ELISA kit instructions. Culture supernatants from the treated cells were tested. The optical density was measured spectrophotometrically at a wavelength of 450 nm. No clear changes after single exposure were observed except for a decrease in IL-6 in the B groups (Supplementary Table S1, available in www.besjournal.com). In the combined exposure groups, TNF-β was significantly up-regulated at 3.12 mmol/L TCA, while IL-6 did not change (Supplementary Table S2, available in www.besjournal.com). IL-6 did not decrease, possibly because the increase in IL-6 caused by TCA attenuated the decrease in IL-6 induced by B. TNF-β levels significantly changed after combined exposure, particularly in the highest dose group, thus, suggesting that TNF-β was easily affected by the combined exposure.
Table S1. Concentrations of IL-2, IL-6, and TNF-α after 3 h and 24 h exposure to TCA and B
Time Cytokins Control
(pg/mL)TCA
(1.56 mmol/L)TCA
(3.12 mmol/L)TCA
(6.25 mmol/L)B
(31.25 mmol/L)B
(62.5 mmol/L)B
(125 mmol/L)(pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) IL-2 108.42 ± 9.72 118.29 ± 13.75 125.22 ± 13.34 112.92 ± 14.44 129.51 ± 14.34 125.74 ± 16.22 126.23 ± 12.82 3 h IL-6 42.79 ± 2.30 36.32 ± 0.72 37.52 ± 2.90 41.08 ± 1.81 39.60 ± 2.34 35.96 ± 5.35 38.23 ± 1.56 TNF-α 881.50 ± 10.48 900.28 ± 12.49 839.92 ± 51.49 918.66 ± 60.00 905.37 ± 9.12 870.55 ± 47.94 883.13 ± 56.81
24 hIL-2 124.82 ± 10.21 131.25 ± 19.32 118.77 ± 10.02 119.78 ± 19.33 130.31 ± 13.06 132.08 ± 10.69 119.99 ± 14.61 IL-6 50.93 ± 3.30 46.12 ± 0.70 47.39 ± 1.10 46.50 ± 0.43 43.78 ± 0.39* 47.00 ± 0.44* 43.93 ± 2.62* TNF-α 1019.55 ± 37.18 969.34 ± 60.17 928.16 ± 45.06 968.37 ± 21.81 979.35 ± 30.20 946.50 ± 67.37 829.25 ± 4.25 Note. *P < 0.05 and **P < 0.01 compared with control or TCA groups respectively. Table S2. Concentrations of NF-κB, TNF-β and IL-6 and TNF-α after 24 h exposure to TCA and B or combined exposure
24 h Control
(pg/mL)TCA
(0.78 mmol/L)TCA
(1.56 mmol/L)TCA
(3.12 mmol/L)B
(0.50 mmol/L)TCA (0.78 mmol/L)
+ B (0.50 mmol/L)TCA (1.56 mmol/L)
+ B (0.50 mmol/L)TCA (3.12 mmol/L)
+ (0.50 mmol/L)(pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) NF-κB 0.64 ± 0.04 0.59 ± 0.1 0.58 ± 0.03 0.60 ± 0.02 0.58 ± 0.04 0.58 ± 0.04 0.59 ± 0.02 0.58 ± 0.03 TNF-β 76.34 ± 8.46 70.63 ± 6.11 72.80 ± 4.66 73.34 ± 8.46 73.86 ± 1.38 76.73 ± 6.88 80.79 ± 6.22 84.44 ± 1.76* IL-6 51.89 ± 1.93 48.64 ± 1.29 51.56 ± 3.21 48.01 ± 1.83 43.94 ± 1.11* 48.87 ± 7.97 48.49 ± 1.93 45.29 ± 2.37 Note. *P < 0.05 represents TCA (1.56 mmol/L) compared with TCA (1.56 mmol/L) + B (0.50 mmol/L) groups respectively. The cytoplasm and nuclei were extracted from treated BV2 cells to determine protein expression. An automated capillary electrophoresis Jess Simple Western System (ProteinSimple, San Jose, CA, USA) and Compass for SW software were used to quantify proteins according to the manufacture's protocol. The following primary antibodies were used: mouse anti-p53 (1:50), rabbit anti-p-p53 (1:50), rabbit anti-p38 (1:50), rabbit anti-p-p38 (1:50), rabbit anti-bax (1:50) and mouse anti-β-actin (1:50), all from USA Cell Signaling Technology; and mouse anti-B1 (1:50) from Beyotime in China. Both p38 and p53 were expressed in the cytoplasm and nuclei of the cells. p53 was phosphorylated in all groups except for the cytoplasm with 24 h B treatment. p38 was expressed in only the cytoplasm after short (3 h) TCA exposure, but was observed in the nucleus after long (24 h) TCA exposure. p38 was found in only the cytoplasm with 24 h B treatment. No significant change in p38 expression was observed among all groups. As shown in Figure 3A, the levels of pp53 in the cytoplasm and nuclei increased with the concentration of TCA. In the high dose group of B, pp53 was absent in the nucleus. After a single exposure to TCA or B for 24 h, the phosphorylation of p53 occurred only in the nuclei in the TCA groups. Only p53 and p38 were expressed in the cytoplasm (Figure 3B) after B 24 h treatment. These results suggested that TCA and B have different mechanisms in cells. In the combined exposure groups, pp53 clearly decreased in the cytoplasm, and Bax was down-regulated mainly in the cytoplasm. These results indicated that B decreased apoptosis by down-regulating pp53 and Bax (Figure 3C, 3D). Together, the results suggested that the anti-apoptotic effects of B might occur via the p53 pathway by decreasing Bax and the phosphorylation of p53.
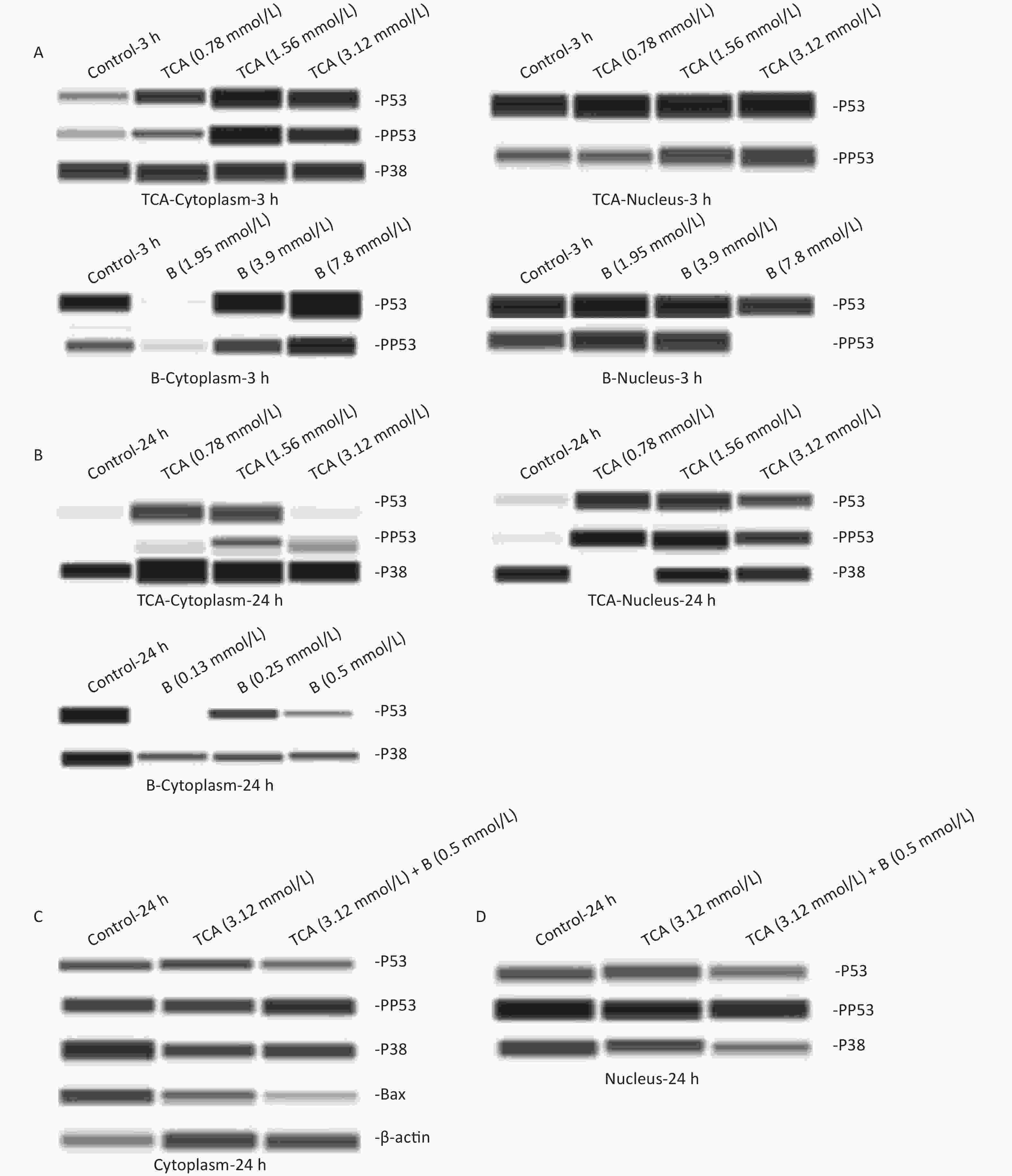
Figure 3. p53, pp53, p38, and pp38 proteins levels in the cytoplasm and the nucleus exposure to single TCA (0.78 mmol/L, 1.56 mmol/L, and 3.12 mmol/L) and B (1.95 mmol/L, 3.9 mmol/L, and 7.8 mmol/L) exposure for 3 and 24 h were determined by Western blotting (A–B). p53, pp53, p38, pp38, Bax proteins levels exposure to combined TCA (3.12 mmol/L) and B (0.5 mmol/L) for 24 h were measured (C and D).
All data were analyzed in SPSS 16.0 software (International Business Machines Corporation, USA). The experimental results are expressed as the mean values ± standard deviation. If the data followed a normal distribution, one-way ANOVA was used for comparison between groups. If the difference between groups was statistically significant, the Bonferroni method was used to compare the two groups. If a normal distribution was not found, the Kruskal-Wallis rank-sum test was used for comparisons between two groups. If a statistical difference was observed between groups, the DSCF method was used for multiple comparisons. Two-tailed P-values < 0.05 were considered significant.
In conclusion, the current research highlights the health effects of B on the roles of anti-oxidative and anti-inflammatory effects. We performed a series of tests using TCA to establish a cell damage model to explore the possible positive effects of B. As expected, B enhanced cell survival, decreased oxidative damage and affected the secretion of several pro-inflammatory cytokines. In addition, the results indicated the signaling pathway mechanisms of TCA and B. TCA induced apoptosis by up-regulating the expression of pp53, whereas B significantly decreased the expression levels of pp53 and Bax, thus having an anti-apoptotic effect. These results provide a broader investigation of the beneficial effects of B. However, the underlying mechanisms require further study.
The authors declare no conflicts of interest.
We thank our colleagues, ZHANG Shao Ping, ZHI Hong, Kong Jian, and RUAN Hong Jie from National Institute of Environmental Health, China CDC for their help in experimental implementation.
doi: 10.3967/bes2022.086
Oxidative Damage to BV2 Cells by Trichloroacetic Acid: Protective Role of Boron via the p53 Pathway
-
Abstract: This study aimed to investigate the neurotoxicity induced by trichloroacetic acid (TCA) and the possible protective mechanisms of boron (B). Mouse BV2 cells were treated with TCA (0, 0.39, 0.78, 1.56, 3.12, 6.25, or 12.5 mmol/L) and B (0, 7.8, 15.6, 31.25, 62.5, 125, 500, or 1,000 mmol/L) for 3 h and 24 h, respectively. Then, reactive oxygen species, and supernatant proinflammatory cytokine and protein levels were analyzed after 24 h of combined exposure. Beyond the dose-dependent decrease in the cellular viability, it clearly increased after B supplementation (P < 0.05). Moreover, B decreased oxidative damage, and significantly down-regulated IL-6 levels and up-regulated TNF-β production (P < 0.05). B also decreased apoptosis via the p53 pathway. The present findings indicated that TCA may induce oxidative damage, whereas B mitigates these adverse effects by decreasing cell apoptosis.
-
Key words:
- Trichloroacetic acid /
- Boron /
- Oxidative damage /
- Neurotoxicity /
- Cell apoptotic pathway
注释: -
Figure 1. Cell viability and IC50 after 3 h and 24 h exposure to different concentrations of TCA and B. Cell survival rates decreased significantly and with a dose-dependent relationship by the single treatment of TCA and B (A and B). Inhibition rate of BV2 cells increased dramatically with the increase concentrations of single TCA and B (C and D). Cellular viability increased significantly after B supplement 3 h (E) and 24 h (F). The data represent the mean ± SD of ten independent experiments in quadruplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control groups respectively. B, Boron; TCA, trichloroacetic acid.
S1. MMP of different treatment of TCA and B after 3 h and 24 h exposure. BV2 cells stained with JC-1 were observed. JC-1 polymer and monomer fluorescence ratio was shown below (Ex/Em = 490/530 nm for JC-1 monomer and Ex/Em = 525/590 nm for JC-1 polymer). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control groups respectively.
Figure 3. p53, pp53, p38, and pp38 proteins levels in the cytoplasm and the nucleus exposure to single TCA (0.78 mmol/L, 1.56 mmol/L, and 3.12 mmol/L) and B (1.95 mmol/L, 3.9 mmol/L, and 7.8 mmol/L) exposure for 3 and 24 h were determined by Western blotting (A–B). p53, pp53, p38, pp38, Bax proteins levels exposure to combined TCA (3.12 mmol/L) and B (0.5 mmol/L) for 24 h were measured (C and D).
S1. Concentrations of IL-2, IL-6, and TNF-α after 3 h and 24 h exposure to TCA and B
Time Cytokins Control
(pg/mL)TCA
(1.56 mmol/L)TCA
(3.12 mmol/L)TCA
(6.25 mmol/L)B
(31.25 mmol/L)B
(62.5 mmol/L)B
(125 mmol/L)(pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) IL-2 108.42 ± 9.72 118.29 ± 13.75 125.22 ± 13.34 112.92 ± 14.44 129.51 ± 14.34 125.74 ± 16.22 126.23 ± 12.82 3 h IL-6 42.79 ± 2.30 36.32 ± 0.72 37.52 ± 2.90 41.08 ± 1.81 39.60 ± 2.34 35.96 ± 5.35 38.23 ± 1.56 TNF-α 881.50 ± 10.48 900.28 ± 12.49 839.92 ± 51.49 918.66 ± 60.00 905.37 ± 9.12 870.55 ± 47.94 883.13 ± 56.81
24 hIL-2 124.82 ± 10.21 131.25 ± 19.32 118.77 ± 10.02 119.78 ± 19.33 130.31 ± 13.06 132.08 ± 10.69 119.99 ± 14.61 IL-6 50.93 ± 3.30 46.12 ± 0.70 47.39 ± 1.10 46.50 ± 0.43 43.78 ± 0.39* 47.00 ± 0.44* 43.93 ± 2.62* TNF-α 1019.55 ± 37.18 969.34 ± 60.17 928.16 ± 45.06 968.37 ± 21.81 979.35 ± 30.20 946.50 ± 67.37 829.25 ± 4.25 Note. *P < 0.05 and **P < 0.01 compared with control or TCA groups respectively. S2. Concentrations of NF-κB, TNF-β and IL-6 and TNF-α after 24 h exposure to TCA and B or combined exposure
24 h Control
(pg/mL)TCA
(0.78 mmol/L)TCA
(1.56 mmol/L)TCA
(3.12 mmol/L)B
(0.50 mmol/L)TCA (0.78 mmol/L)
+ B (0.50 mmol/L)TCA (1.56 mmol/L)
+ B (0.50 mmol/L)TCA (3.12 mmol/L)
+ (0.50 mmol/L)(pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) (pg/mL) NF-κB 0.64 ± 0.04 0.59 ± 0.1 0.58 ± 0.03 0.60 ± 0.02 0.58 ± 0.04 0.58 ± 0.04 0.59 ± 0.02 0.58 ± 0.03 TNF-β 76.34 ± 8.46 70.63 ± 6.11 72.80 ± 4.66 73.34 ± 8.46 73.86 ± 1.38 76.73 ± 6.88 80.79 ± 6.22 84.44 ± 1.76* IL-6 51.89 ± 1.93 48.64 ± 1.29 51.56 ± 3.21 48.01 ± 1.83 43.94 ± 1.11* 48.87 ± 7.97 48.49 ± 1.93 45.29 ± 2.37 Note. *P < 0.05 represents TCA (1.56 mmol/L) compared with TCA (1.56 mmol/L) + B (0.50 mmol/L) groups respectively. -
[1] Richardson SD, Plewa MJ, Wagner ED, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res, 2007; 636, 178−242. doi: 10.1016/j.mrrev.2007.09.001 [2] Celik I, Isik I. Determination of chemopreventive role of Foeniculum vulgare and Salvia officinalis infusion on trichloroacetic acid-induced increased serum marker enzymes lipid peroxidation and antioxidative defense systems in rats. Nat Prod Res, 2008; 22, 66−75. doi: 10.1080/14786410701590426 [3] Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol, 2009; 47, 145−9. doi: 10.1016/j.fct.2008.10.020 [4] Celik I, Tuluce Y. Elevation protective role of Camellia sinensis and Urtica dioica infusion against trichloroacetic acid-exposed in rats. Phytother Res, 2007; 21, 1039−44. doi: 10.1002/ptr.2204 [5] Melnik L, Vysotskaja O, Kornilovich B. Boron behavior during desalination of sea and underground water by electrodialysis. Desalination, 1999; 124, 125−30. doi: 10.1016/S0011-9164(99)00096-X [6] Kato-Weinstein J, Stauber AJ, Orner GA, et al. Differential effects of dihalogenated and trihalogenated acetates in the liver of B6C3F1 mice. J Appl Toxicol, 2001; 21, 81−9. doi: 10.1002/jat.717 [7] Villanueva CM, Gracia-Lavedan E, Julvez J, et al. Drinking water disinfection by-products during pregnancy and child neuropsychological development in the INMA Spanish cohort study. Environ Int, 2018; 110, 113−22. doi: 10.1016/j.envint.2017.10.017 [8] Singh R. Neuroembryopathic effect of trichloroacetic acid in rats exposed during organogenesis. Birth Defects Res B Dev Reprod Toxicol, 2006; 77, 47−52. doi: 10.1002/bdrb.20064 [9] Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol, 2017; 35, 441−68. doi: 10.1146/annurev-immunol-051116-052358 [10] Tang J, Zheng XT, Xiao K, et al. Effect of boric acid supplementation on the expression of BDNF in African ostrich chick brain. Biol Trace Elem Res, 2016; 170, 208−15. doi: 10.1007/s12011-015-0428-y [11] Wang C, Kong ZQ, Duan L, et al. Reproductive toxicity and metabolic perturbations in male rats exposed to boron. Sci Total Environ, 2021; 785, 147370. doi: 10.1016/j.scitotenv.2021.147370 -
 22065Supplementary Materials.pdf
22065Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links