-
Hepatitis B virus (HBV) is an incomplete double-stranded DNA virus from the genus Orthohepadnavirus and family Hepadnaviridae. The virus genome is approximately 3,200 bp, which encodes seven viral proteins (HBeAg, HBcAg, LHBs, MHBs, SHBs, polymerase, and HBx). On the surface of the virus, the pre-S1 sequence at amino acids 2–48 mediates the attachment of the virus to hepatocytes and facilitates virus entry into human cells. Therefore, Hepatitis B surface antigen (HBsAg) is used as a candidate vaccine, which has been approved for human use [1, 2].
The virus is considered a major causative agent for chronic HBV, and its therapy is based on antiviral factors that cannot eradicate infection and may cause toxicity for a longer time [3]. An effective vaccine against hepatitis B infection has been available since 1982 in the national vaccination program of numerous countries. Based on a report from World Health Organization (WHO) in 2017, Global Hepatitis Report suggested that 3.5% (257 million persons) of the world’s population were living with chronic hepatitis B in 2015, particularly in Western Pacific and Sub-Saharan African regions (68%) [4]. These statistics show that despite the national programs on HBsAg vaccination, the disease is a concern for WHO, and current vaccines must be more potent [5]. Present vaccines include HBsAg that are formulated in Alum and are not effective in some cases, which are known as non-responders [6].
Altering the HBsAg vaccine formulation may increase vaccine efficacy. Herein, as a vital component of the vaccine, an adjuvant may change vaccine efficacy in healthy subjects and complicated cases [7]. The word adjuvant is derived from the Latin word “adjuvare,” which means “help,” and this term is generally used for any substance that increases cellular and humoral immune responses against candidate vaccines [8]. Most vaccines, particularly those based on defined molecules and recombinants, trigger weak immunologic responses; thus, they must be formulated in an immunologic complex [9, 10]. Therefore, using adjuvants may induce enough immune responses for defined molecules as candidate vaccines [11]. Alum adjuvant is a strong inducer of Th2 and weak inducer of Th1, and it is often used to increase the immunogenicity of HBsAg [6]. To date, oil-based adjuvants are being presented for human use. For example, Montanide ISA-720 is a new adjuvant with natural metabolization. This adjuvant reduces the rate of antigen release, protects the antigen from proteolytic enzymes, and improves the transference of antigens to the T-cell area in the lymph nodes. Considerable research has shown that Montanide ISA-720 can increase antibody titers and specific responses of cytotoxic T lymphocytes [12].
Naloxone is commonly used as a rehabilitation medicine with no toxicity, and it is also approved by WHO and the Food and Drug Administration [13, 14]. Naloxone serves as an antagonist of opioid receptors, commonly used to reduce the side effects of drugs. Opioid plays a direct active role in modulating immunity by opioid receptors on the surface of immune cells [15]. Some studies show that NLX mixed with Alum adjuvant has a synergistic effect on the immunologic parameters [16-19], which may improve cellular and humoral immune responses in various vaccine models [18, 20-22].
In this study, we hypothesized that the mixture of NLX as an immunopotentiator with Montanide ISA-720 could increase the vaccine potency of HBsAg. Consequently, HBsAg was formulated in Montanide ISA-720 and NLX, and then cellular and humoral immune response parameters were assessed using a BALB/c mouse model.
-
Purified HBsAg and commercial HBsAg vaccine formulated in Alum adjuvant were prepared by the Department of HBsAg Purification and Formulation of Pasteur Institute of Iran (Karaj, Iran). HBsAg was formulated in Montanide ISA-720 (SEPPIC, France) adjuvant using a homogenizer in a clean room (Department of FMD Vaccine formulation at Razi Serum and Vaccine Institute of Iran, Karaj, Iran). In brief, 5 µg of HBsAg was admixed with Montanide ISA 720 (at a ratio of 30/70), followed by rapid shaking to develop a milky white suspension, and then homogenized using a homogenizer [23, 24]. Then, NLX powder (Sigma, USA) was dissolved in double-distilled water and then added to the formulation at 100 and 200 µg/doses based on our previous setup (5 and 10 mg/kg of body weight for a 20 g mouse) [24]. After vaccine formulation, each 100 µL of vaccine contained 5 µg of HBsAg and 100 and/or 200 µg of NLX. Finally, the prepared vaccines were stored at 4 °C until mouse immunization.
-
Six-to-eight-week-old female inbred Balb/c mice were purchased from the Pasteur Institute of Iran, Karaj, Iran (experimental animal license number; BALB/c P28). The mice were housed for 7 days prior to the experiments and given free access to food and water. The mice were maintained in standard conditions (light/dark cycle, 12 h/12 h, and 20 ± 2 °C). All mouse experiments were conducted in accordance with the Animal Care and Use Protocol of Pasteur Institute of Iran and also the Ethics Committee of the Islamic Republic of Iran (IR.IAU.PS.REC.1397.215).
-
Experimental groups of female Balb/c mice (N = 175) were divided into 14 groups (N = 10–15). The Balb/c mice were immunized subcutaneously with 100 µL of each formulation of vaccine (containing 5 μg of the antigen in the vaccine formulation) three times on days 0, 14, and 28 with proper control groups (Table 1). In addition, for long-lasting humoral immune response monitoring, experimental sera were obtained from mice on the 10th, 90th, 150th, and 220th days after the final vaccination.
Table 1. Experimental groups with the vaccine formulations which used for immunization
Group Vaccine formulations No. of mice Dose Route of immunization 1 HBsAg-ALUM 15 5 µg subcutaneous 2 FENDRIX 15 5 µg subcutaneous 3 HBsAg-ALUM-NLX-5 15 5 µg subcutaneous 4 HBsAg-ALUM-NLX-10 15 5 µg subcutaneous 5 HBsAg- MON720 15 5 µg subcutaneous 6 HBsAg-MON-720-NLX-5 15 5 µg subcutaneous 7 HBsAg-MON-720-NLX-10 15 5 µg subcutaneous 8 ALUM 10 − subcutaneous 9 MON720VG 10 − subcutaneous 10 ALUM+NLX-5 10 − subcutaneous 11 ALUM+NLX-10 10 − subcutaneous 12 MON720VG+NLX-5 10 − subcutaneous 13 MON720VG+NLX-10 10 − subcutaneous 14 PBS 10 − subcutaneous -
Ten days after the last immunization, the cell suspension was prepared by mechanically dissecting the spleens in cold phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) under an aseptic condition. Then, red blood cells were lysed by the addition of 5 mL of lysis buffer on cell pellets, and single-cell suspension was adjusted to 3 × 106 cell/mL in RPMI-1640 (Gibco, Germany), supplemented with 10% FBS, 4 mmol/L of L-glutamine, 25 mmol/L of 4-2-hydroxyethylpiperazine-1-2-ethanesulfonic acid, 0.1 mmol/L of non-essential amino acid, 1 mmol/L of sodium pyruvate, 100 µg/mL of streptomycin, and 100 IU/mL penicillin. Afterward, 1,000 µL of cell suspension, comprising 3 × 106 cells, was dispensed into 24-well culture plates (Nunc, Denmark) and then stimulated with 5 µg/mL of HBsAg as an antigen recall. After 60 h of cell stimulation, the culture supernatant was collected and stored at −70 °C for cytokine assay [23].
-
Ten days after the final immunization, spleen cells (seven mice in each vaccinated group and five mice in each control group) were prepared in a complete RPMI-1640 medium, adjusted to 3 × 106 cells/mL, and used for in vitro antigen recall. One milliliter of cell suspension comprising a total number of 3 × 106 spleen cells was added to each well of a 24-well plate and stimulated with 5 µg/mL of HBsAg as antigen recall [17, 23].
For each experimental mouse, two wells of a 24-well plate were stimulated with 5 µg/mL of HBsAg for 60 h at 37 °C under 5% CO2, and in other conditions, two wells were cultured without antigen stimulation. Then, culture supernatants of stimulated and un-stimulated samples were collected and assessed for IL-4, IL-2, IFN-γ, and TNF-α cytokines using commercial ELISA Kits (Mabtech, Sweden) according to the manufacturer’s manual [17, 24]. For each cytokine, standard samples were used. The quantity of each cytokine was calculated in accordance with the formula obtained from its standard curve, which was presented as pg/mL. For the absolute quantity calculation of each cytokine, the quantity of cytokine derived from the stimulated wells was subtracted from the un-stimulated wells of each individual mouse. In calculating the IL-2/IL-4 ratio of experimental mice, the quantity of each mouse was used for calculation.
-
Specific total IgG antibodies were evaluated using an optimized indirect ELISA on the sera of experimental mice, which were obtained on the 10th, 90th, 150th, and 220th day of the last injection. In brief, 100 µL of HBsAg (5 µg/mL) in PBS was coated in 96-well ELISA Maxisorp plates (Nunc, Naperville, IL), followed by overnight incubation at 4 °C. Then, the wells were washed three times with PBS, containing 0.05% tween 20 (washing buffer), and blocked for 1 h at 37 °C with PBS + 2% skimmed milk + 0.05% tween 20 (blocking buffer).
Serial dilutions of experimental sera, at 1/100 up to 1/838,860,800, were prepared in PBS with 1% of bovine serum albumin (PBS-BSA1%) + 0.05% tween 20. After washing the wells, 100 µL of each dilution was added to each well in duplicate and incubated at 37 °C for 2 h. After incubation, the wells were washed five times with washing buffer, and 100 µL of 1/10,000 dilution of anti-mouse conjugated to horseradish peroxidase (Sigma, USA) in blocking buffer was added and then incubated for 2 h at 37 °C. Afterward, the wells were washed five times with washing buffer, added with 100 µL of TMB substrate, and incubated for 30 min in a dark place. The reaction was stopped by adding 100 µL of 2N H2SO4, and then the color density was measured at A450/630 nm using an ELISA plate reader (BioTek, USA). Specific IgG1 and IgG2a subclasses on the 1/1,000 dilution of experimental serum samples after 10 days of final immunization were assessed using goat anti-mouse IgG1 and IgG2a secondary antibodies (Sigma, USA) in accordance with the company manual [22, 23].
-
The results were represented as Mean ± SD. Statistical analysis was performed by Graph pad prism V6.01 software. Mann-Whitney test at a 95% confidence level (P < 0.05) was conducted to compare the statistical significance among the experimental groups. P < 0.05 was considered a significant difference.
-
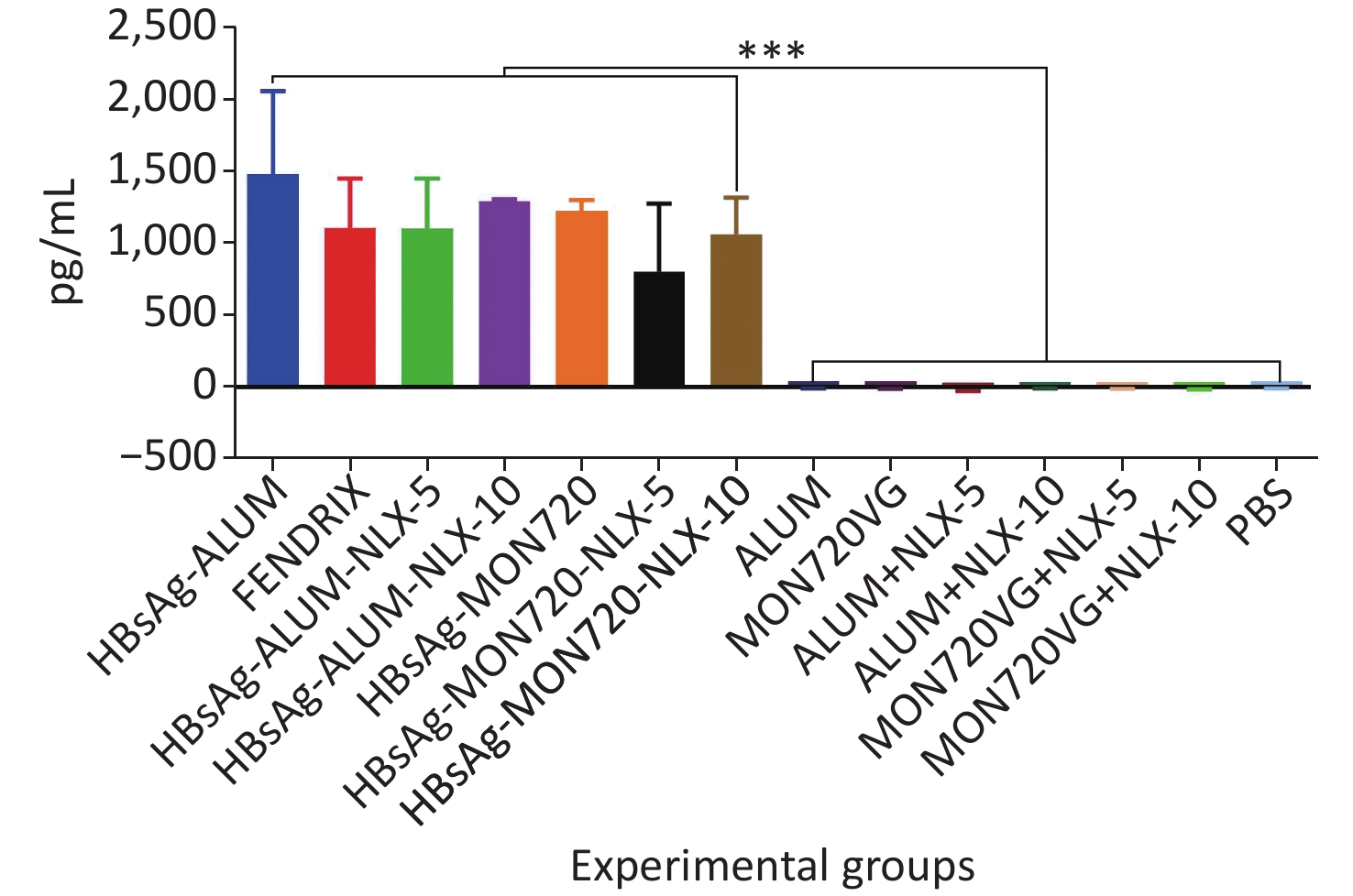
The results from the IFN-γ cytokine in all HBsAg-vaccinated groups showed a significant increase compared with the corresponding control groups (P = 0.0007). The level of IFN-γ cytokine in HBsAg-MON720, HBsAg-Alum, and Fendrix groups did not show a significant difference (P > 0.05). The application of NLX at 5 and 10 µg in HBsAg-Alum vaccine formulation did not improve the IFN-γ responses compared with the HBsAg-Alum group (P > 0.05). In addition, the formulation of HBsAg-MON720 with NLX at 5 and 10 µg did not show an evident difference compared with the HBsAg-MON720 group (P > 0.05, Figure 1).
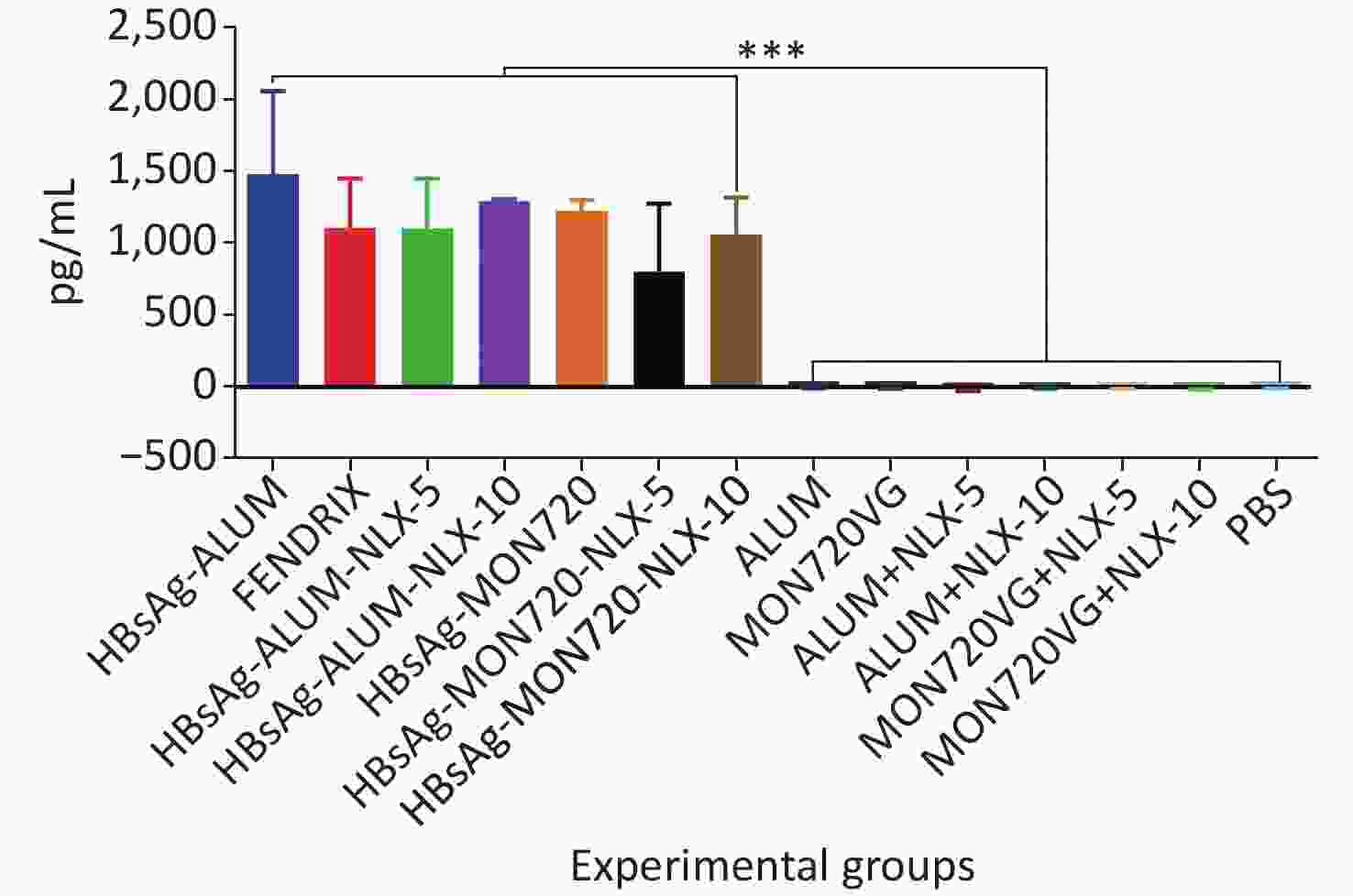
Figure 1. Results from IFN-γ cytokine response in experimental mouse groups. Experimental mice vaccinated with different formulations of Hepatitis B surface antigen (HBsAg) showed a significant increase as compared with the corresponding control groups (***P = 0.0007). The level of IFN-γ cytokine in HBsAg-Montanide ISA-720 (MON720), HBsAg-Alum, and Fendrix groups did not show significant differences among each other, and adding Naloxone (NLX) to HBsAg-MON720 and HBsAg-Alum vaccines did not show significant responses (P > 0.05).
-
Immunization with HBsAg-Alum, HBsAg-Alum-NLX-5, and HBsAg-Alum-NLX-10 exhibited a significant increase in the IL-4 cytokine release compared with their corresponding control groups (P < 0.0453). However, other vaccinated groups did not indicate any significant differences compared with their analogous control groups (P > 0.1386). Immunization with HBsAg-Alum vaccine showed a significant increase in IL-4 cytokine release compared with HBsAg-MON720 and Fendrix groups (P < 0.0004). Immunization with HBsAg-Alum-NLX-5 showed a significant decrease in IL-4 cytokine release compared with the HBsAg-Alum group (P = 0.0270), whereas HBsAg-Alum-NLX-10 showed a decrease at the borderline compared with the HBsAg-Alum group (P = 0.0695). In addition, HBsAg-MON720-NLX-5 and HBsAg-MON720-NLX-10 groups did not show significant differences in the secretion of IL-4 cytokine compared with the HBsAg-MON720 group (P > 0.9771, Figure 2).
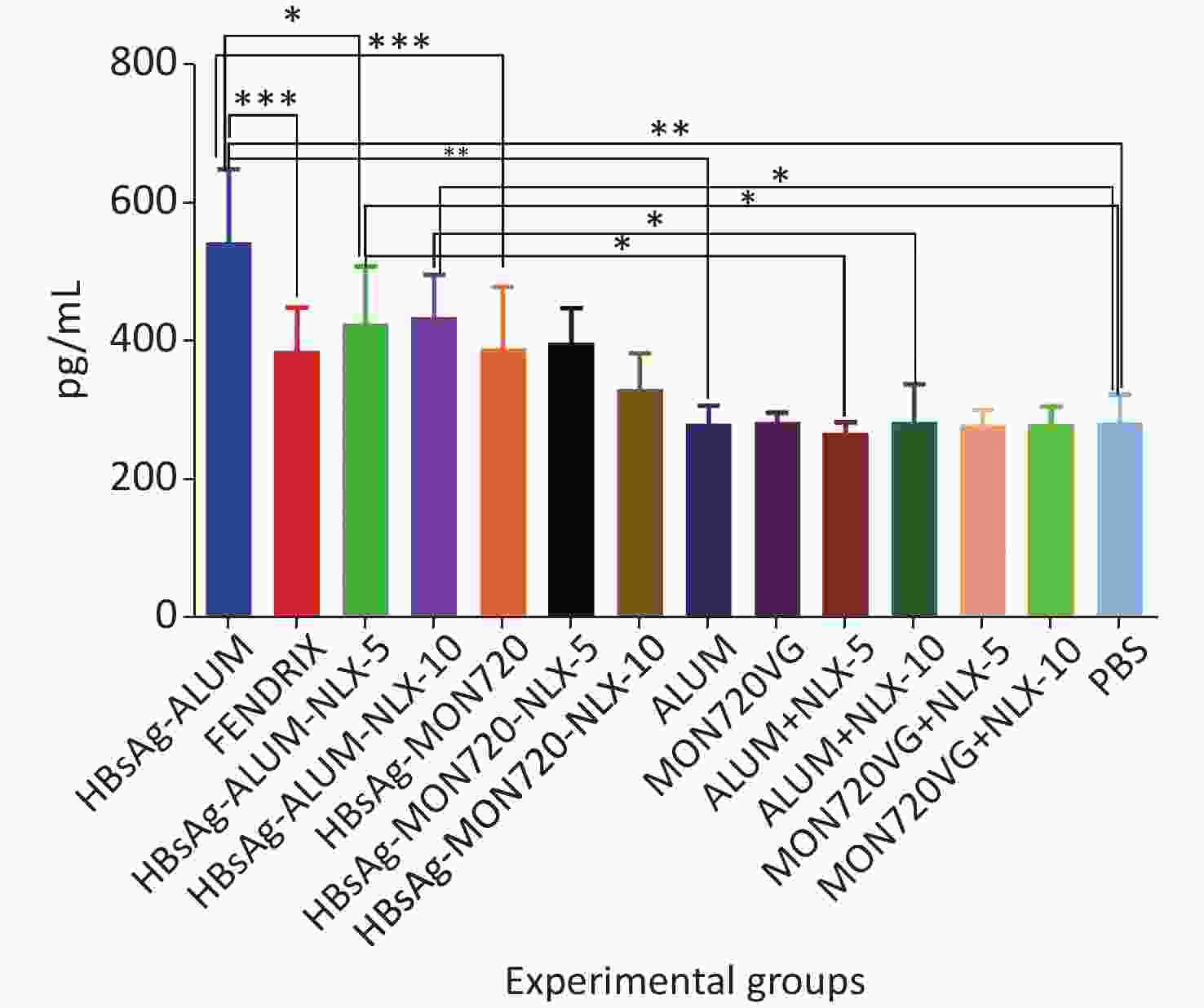
Figure 2. IL-4 cytokine response in experimental groups. Immunization with HBsAg-Alum, HBsAg-Alum-NLX-5, and HBsAg-Alum-NLX-10 showed a significant increase in IL-4 cytokine secretion compared with their corresponding control groups (P < 0.0453). In addition, the HBsAg-Alum vaccine showed a significant increase in IL-4 cytokine release compared with HBsAg-MON720 and Fendrix groups (P < 0.0004). Furthermore, the HBsAg-Alum-NLX-5 group showed a significant decrease in IL-4 cytokine release compared with the HBsAg-Alum group (P = 0.0270). *P < 0.05, **P < 0.01, ***P < 0.001.
-
The results obtained from IL-2 cytokine in all vaccine formulations showed a significant increase compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-Alum, HBsAg-MON720, and Fendrix vaccines did not result in any significant differences in IL-2 cytokine release (P > 0.1365). Immunization with HBsAg-Alum-NLX-5 and HBsAg-Alum-NLX-10 did not show a significant effect on IL-2 cytokine release compared with the HBsAg-Alum group (P > 0.9746), and immunization with HBsAg-MON720-NLX-5 and 10 did not show a significant difference in the IL-2 cytokine release compared with the HBsAg-MON720 group (P > 0.1375, Figure 3).
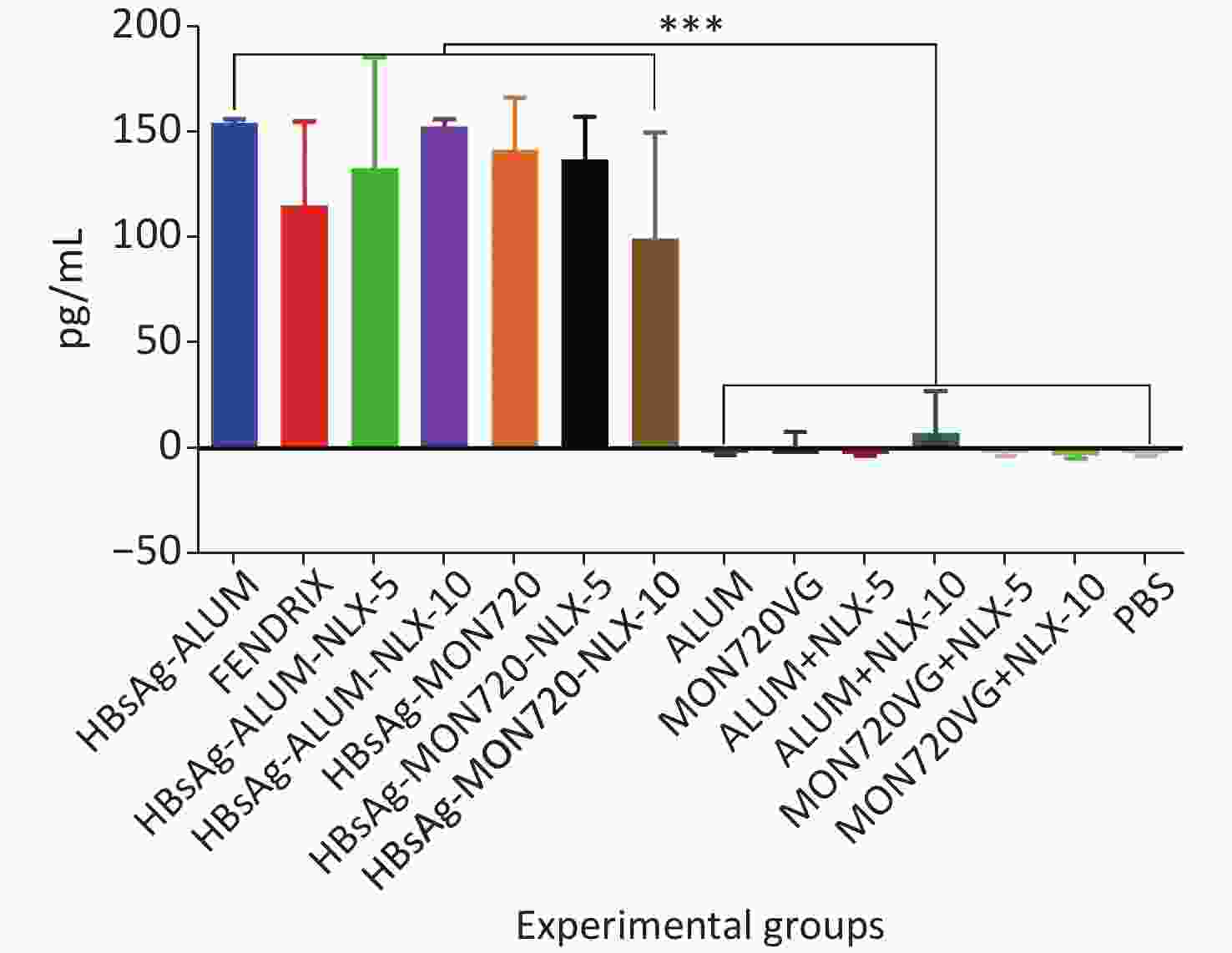
Figure 3. IL-2 cytokine assay in vaccinated mice. Results from IL-2 cytokines in all vaccine formulation groups showed a significant increase compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-Alum, HBsAg-MON720, and Fendrix vaccines did not show significant differences in IL-2 cytokine release (P > 0.1365). In addition, NLX in vaccine formulation groups did not show a positive effect on IL-2 cytokine response (P > 0.1375). ***P < 0.001.
-
The results obtained from the TNF-α cytokine showed a significant increase in all vaccine formulation groups compared with their corresponding control groups (P < 0.0001). The level of TNF-α cytokine release in HBsAg-MON720, HBsAg-Alum, and Fendrix groups did not show a significant difference (P > 0.9930). Immunization with HBsAg-Alum-NLX-5 and HBsAg-Alum-NLX-10 did not show a significant effect on TNF-α cytokine release compared with the HBsAg-Alum group (P > 0.1249), and immunization with HBsAg-MON720-NLX-5 and 10 did not show a significant difference in the TNF-α cytokine release compared with HBsAg-MON720 (P > 0.2371, Figure 4).
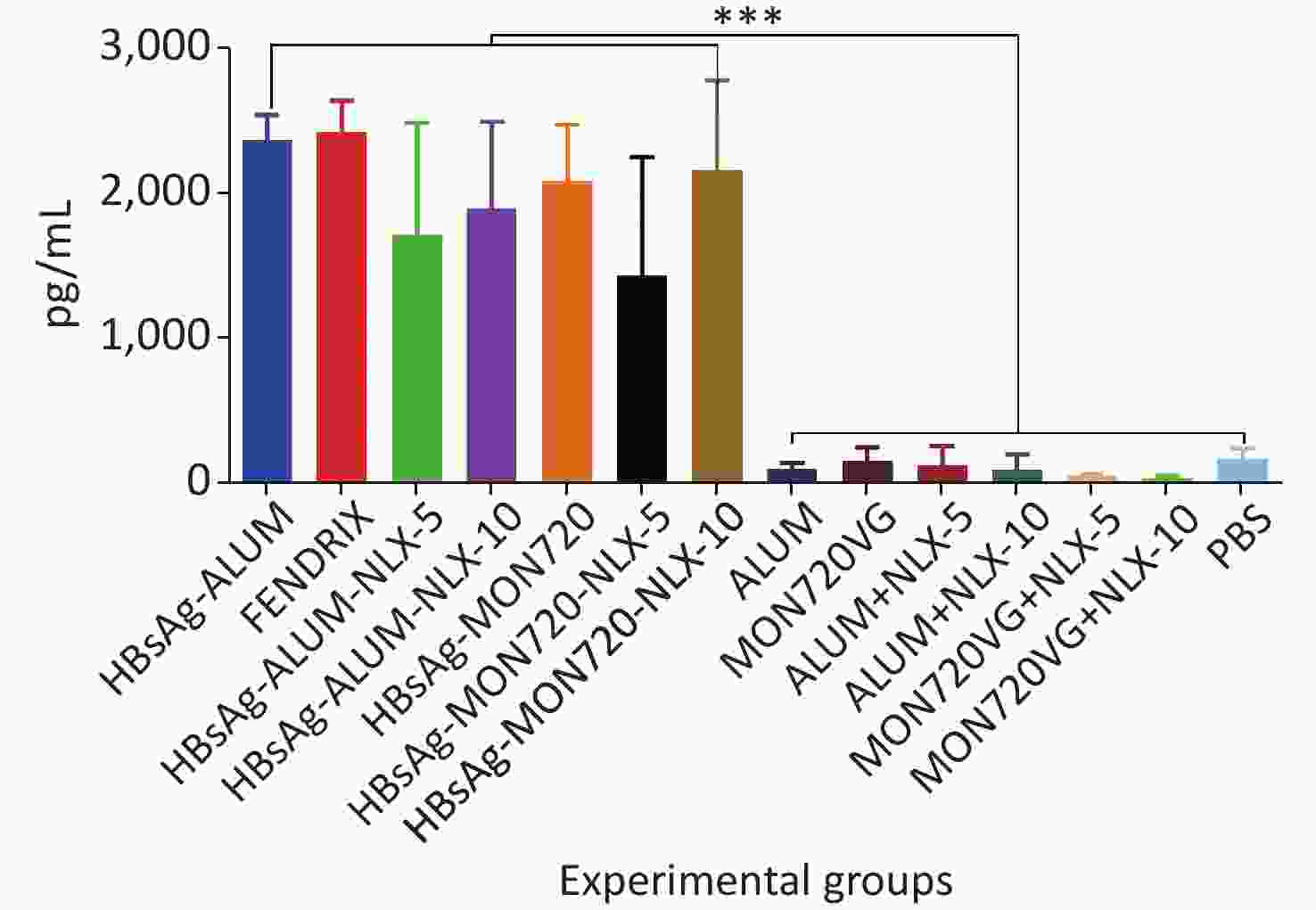
Figure 4. TNF-α cytokine response in experimental groups. Results from TNF-α cytokine showed a significant increase in all vaccine formulation groups as compared with the corresponding control groups (P < 0.0001). The level of TNF-α cytokine release in HBsAg-MON720, HBsAg-Alum, and Fendrix groups did not show significant differences (P > 0.9930). In addition, NLX in vaccine formulation groups did not show a significant response (P > 0.1249). ***P < 0.001.
-
The results obtained from the IL-2/IL-4 cytokine ratio in all vaccine formulation groups showed a significant increase as compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-MON720 showed an increase in the IL-2/IL4 ratio compared with HBsAg-Alum and Fendrix groups (P = 0.0291 and P = 0.0513, respectively), whereas no significant difference was observed among Fendrix and HBsAg-Alum groups (P = 0.9999). In addition, immunization with HBsAg-Alum-NLX-10 showed an increase in the IL-2/IL-4 ratio compared with the HBsAg-Alum group at the borderline (P = 0.0534). However, HBsAg-Alum-NLX-5 did not show a significant increase in the IL-2/IL-4 ratio compared with the HBsAg-Alum group (P > 0.05). Moreover, no significant difference in the IL-2/IL-4 ratio was observed among HBsAg-MON720-NLX-5, HBsAg-MON720-10, and HBsAg-MON720 groups (P > 0.8617, Figure 5).
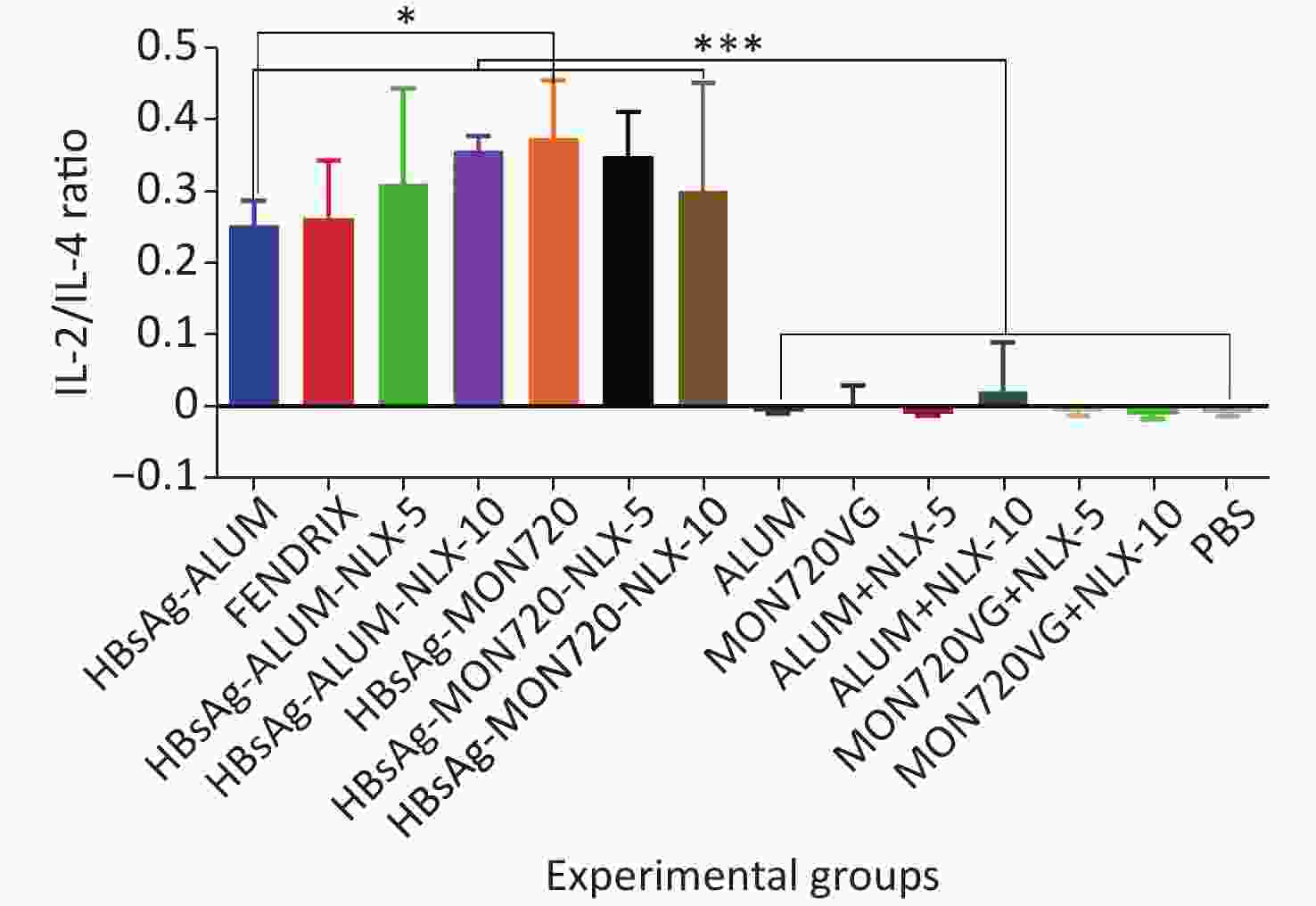
Figure 5. IL-2/IL-4 cytokine ratio in experimental groups. IL-2/IL-4 cytokine ratio in all vaccination formulation groups showed a significant increase as compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-MON720 shows an increase compared with HBsAg-Alum and Fendrix groups (P = 0.0291 and P = 0.0513, respectively). Immunization with HBsAg-Alum-NLX-10 shows an increase in the IL-2/IL-4 ratio compared with the HBsAg-Alum group at borderline (P = 0.0534). *P < 0.05, ***P < 0.001.
-
The results from specific IgG antibodies 10 days after the last immunization of Fendrix and HBsAg-Alum-NLX-10, at dilutions of 1/100 up to 1/25,600, showed a significant increase compared with their corresponding control groups (P < 0.0423). Immunization with HBsAg-MON720, HBsAg-MON720-NLX5, and HBsAg-Alum-NLX-5, at dilutions of 1/100 up to 1/51,200, showed a significant increase compared with their corresponding control groups (P < 0.0423). Meanwhile, HBsAg-MON720-NLX10 and HBsAg-Alum groups showed a significant increase compared with their corresponding control groups at dilutions of 100 up to 1/102,400 (P < 0.0359).
Immunization with HBsAg-Alum showed a significant increase in the total IgG response (at dilutions of 1/200 and 1/3,200 up to 1/51,200) compared with the Fendrix group (P < 0.0010). Immunization with HBsAg-MON720 did not show a significant difference at any dilutions in contrast with the Fendrix group (P > 0.05). In addition, immunization with HBsAg-Alum showed a significant increase in specific total IgG (at dilutions of 1/400, 1/12,800, 1/25,600, and 1/51,200) compared with HBsAg-MON720 (P < 0.0357). Immunization with HBsAg-Alum-NLX-5 (at dilutions of 1/200 to 1/800, 1/6,400 up to 1/25,600; P < 0.0001) and HBsAg-Alum-NLX-10 (at dilutions of 1/100 up to 1/102,400; P < 0.0244) significantly decreased the total IgG antibody responses compared with the HBsAg-Alum group. Moreover, immunization with HBsAg-Alum-NLX-10 showed a significant decrease in specific total IgG responses in comparison with the HBsAg-Alum-NLX-5 group (at dilutions of 1/100 up to 1/6,400, P < 0.0017). Immunization with HBsAg-MON720-NLX-10 (at dilutions of 1/100 up to 1/400, 1/1,600, 1/3,200, 1/12,800 up to 1/51,200; P < 0.0249) and HBsAg-MON720-NLX-5 (1/1,600 and 1/3,200; P < 0.0362) showed a significant increase in total IgG compared with the HBsAg-MON720 group. Furthermore, immunization with HBsAg-MON720-NLX-10 showed a significant increase in specific total IgG in comparison with the HBsAg-MON720-NLX-5 group (at dilutions of 1/200, 1/1,600 up to 1/25,600; P < 0.0007; Figure 6A).
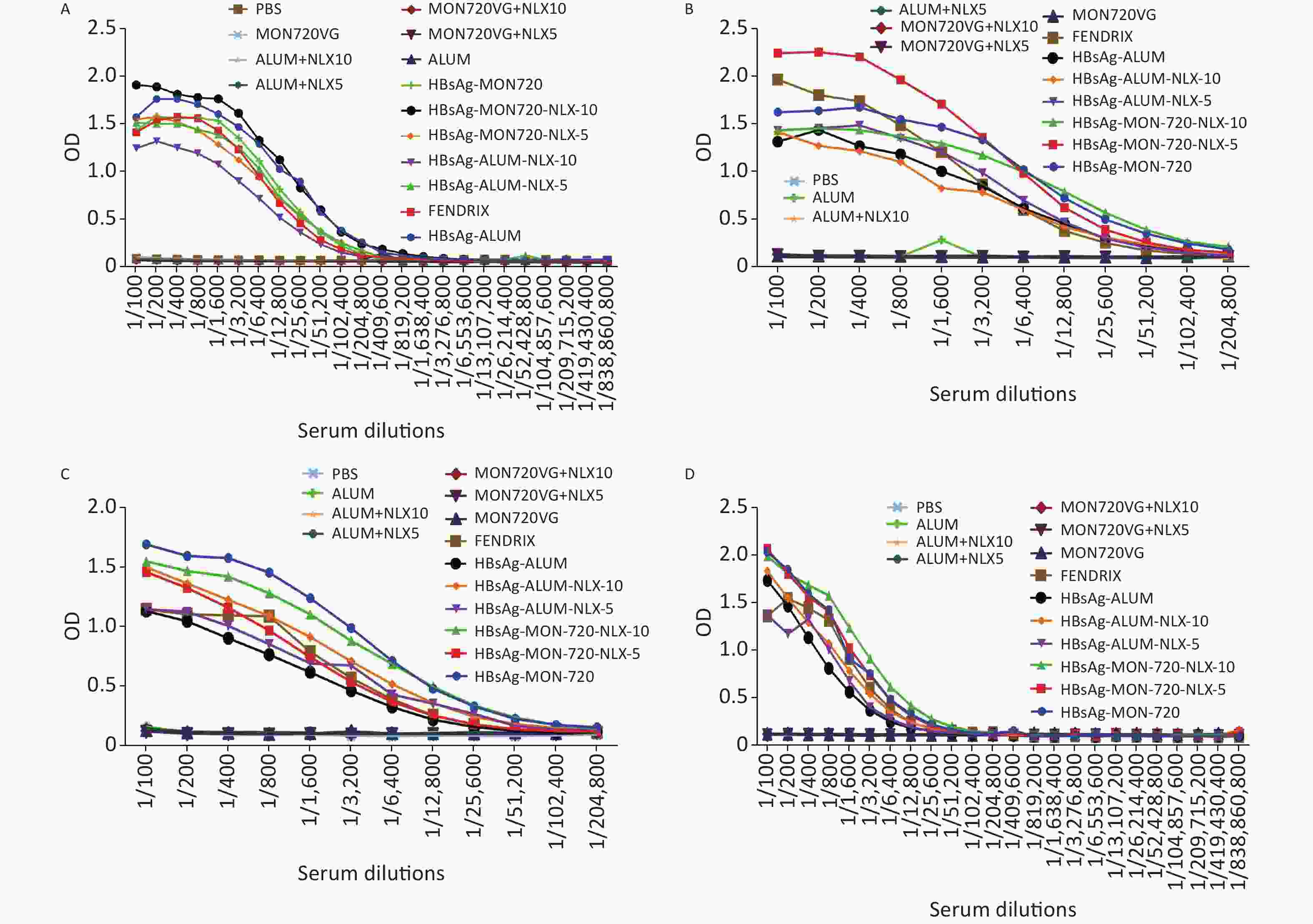
Figure 6. Humoral immune responses of experimental groups up to 220 days after the last immunization. (A) Results from IgG response on the 10th day after the last immunization. All vaccine formulation groups showed a significant increase in IgG response compared with their corresponding control groups. In addition, immunization with HBsAg-Alum showed a significant increase in total IgG response compared with Fendrix and HBsAg-MON720 groups (P < 0.0357). Immunization with HBsAg-MON720-NLX-5 and HBsAg-MON720-NLX-10 increased IgG response compared with the HBsAg-MON720 group. (B) Results of IgG response after 90 days of the last immunization. Immunization with HBsAg-MON720 shows a significant increase compared with HBsAg-Alum and Fendrix groups (P < 0.0039). Moreover, immunization with HBsAg-MON720-NLX-5 shows a significant increase in IgG responses compared with the HBsAg-MON720 group (P < 0.0032). (C) Results of IgG responses after 150 days of the last immunization. Immunization with HBsAg-MON720 showed a significant increase in total IgG compared with Fendrix and HBsAg-Alum (P < 0.0335). Furthermore, immunization with HBsAg-Alum-NLX-10 showed a significant increase in comparison with HBsAg-Alum (P < 0.0057). (D) Results of IgG response on day 220th of the final immunization. Immunization with HBsAg-MON720 showed a significant increase compared with Fendrix and HBsAg-Alum groups (P < 0.0343). In addition, immunization with HBsAg-MON720-NLX-10 showed a significant increase compared with the HBsAg-MON720 group (P = 0.0045).
-
The specific total IgG antibodies of experimental mice 90 days after the last immunization with HBsAg-Alum, HBsAg-Alum-NLX5, and HBsAg-Alum-NLX-10 at dilutions of 1/100 up to 1/6,400 indicated a significant increase compared with their corresponding control groups (P < 0.0021).
Immunization with HBsAg-MON720-NLX-5 at dilutions of 1/100 up to 1/12,800 showed a significant increase compared with the corresponding control groups (P < 0.0009). In addition, immunization with HBsAg-MON720 and HBsAg-MON720-NLX-10 at dilutions of 1/100 up to 1/25,600 showed a significant increase compared with their corresponding control groups (P < 0.0316). Immunization with Fendrix showed a significant increase (at dilutions of 1/100, 1/200, and 1/400) compared with the HBsAg-Alum group (P < 0.0170). In addition, immunization with HBsAg-MON720 showed a significant increase (at dilutions of 1/400, 1/800, 1/1,600, 1/3,200, and 1/6,400) compared with HBsAg-Alum (P < 0.0095). Immunization with HBsAg-MON720 showed a significant increase at dilutions of 1/3,200 up to 1/6,400 compared with the Fendrix group (P < 0.0039). Immunization with HBsAg-Alum-NLX-5 and HBsAg-Alum-NLX-10 did not show significant differences compared with the HBsAg-Alum group (at all experimental dilutions, P > 0.05). Moreover, immunization with HBsAg-MON720-NLX-5 (at dilutions of 1/100 up to 1/800) showed a significant increase in IgG responses compared with the HBsAg-MON720 group (P < 0.0032), whereas the HBsAg-MON720-NLX-10 group did not show a significant difference compared with the HBsAg-MON720 group at all experimental dilutions (P > 0.05). Immunization with HBsAg-MON720-NLX-5 showed a significant increase in specific total IgG compared with the HBsAg-MON720-NLX-10 group (at dilutions of 1/100, 1/200, 1/400, 1/800, 1/1,600; P < 0.0038). Furthermore, immunization with HBsAg-MON720-NLX-5 showed a significant increase compared with the Fendrix group (at dilutions of 1/200, 1/400, 1/800, 1/1,600, 1/3,200, and 1/6,400; P < 0.016; Figure 6B).
-
The results obtained from specific total IgG antibody responses 150 days after the last immunization showed that immunization with HBsAg-Alum, HBsAg-Alum-NLX-5, HBsAg-MON720-NLX-5, and Fendrix at dilutions of 1/100 up to 1/3,200 had a significant increase compared with their corresponding control groups (P < 0.0230). Immunization with HBsAg-Alum-NLX-10 at dilutions of 1/100 up to 1/6,400 showed a significant increase compared with the corresponding control groups (P < 0.0027). Similarly, immunization with HBsAg-MON720 and HBsAg-MON720-NLX-10 at dilutions of 1/100 up to 1/12,800 showed a significant increase compared with the corresponding control groups (P < 0.0142). Immunization with Fendrix showed a significant increase in total IgG at a dilution of 1/800 compared with the HBsAg-Alum group (P = 0.0014).
Immunization with HBsAg-MON720 showed a significant increase in the total IgG at dilutions of 1/100 up to 1/6,400 compared with the Fendrix group (P < 0.0014). Similarly, immunization with HBsAg-MON720 had a significant increase in the total IgG (at dilutions of 1/100 up to 1/12,800) compared with HBsAg-Alum (P < 0.0335). In addition, immunization with HBsAg-Alum-NLX-10 (at dilutions of 1/100 up to 1/1,600) significantly increased specific IgG antibody responses in comparison with HBsAg-Alum (P < 0.0057). Immunization with HBsAg-MON720-NLX-5 at dilutions of 1/200 up to 1/6,400 resulted in a significant decrease in total IgG compared with the HBsAg-MON720 group (P < 0.0215), but HBsAg-MON720-NLX-10 did not have the same result under the same condition (P > 0.05, Figure 6C).
-
The results obtained from specific total IgG antibodies 220 days after the last immunization indicated that immunization with HBsAg-Alum and HBsAg-Alum-NLX-5 (at dilutions of 1/100 up to 1/1,600) showed a significant increase compared with their corresponding control groups (P < 0.0161). Immunization with HBsAg-Alum-NLX-10 and Fendrix at dilutions of 1/100 up to 1/3,200 showed a significant increase in comparison with their corresponding control groups (P < 0.0009). Furthermore, HBsAg-MON720, HBsAg-MON720-NLX-5, and HBsAg-MON720-NLX-10 groups showed a significant increase in IgG responses (at dilutions of 1/100 up to 1/6,400) compared with their corresponding control groups (P < 0.0062). Immunization with Fendrix resulted in a significant increase in IgG responses (at dilutions of 1/400, 1/800, and 1/1,600) compared with the HBsAg-Alum group (P < 0.0223). Immunization with HBsAg-MON720 showed a significant increase compared with the Fendrix group at dilutions of 1/100 and 1/200 (P < 0.0343).
Immunization with HBsAg-MON720 showed a significant increase in total IgG responses at dilutions of 1/100 up to 1/3,200 compared with HBsAg-Alum (P < 0.0142). Immunization with HBsAg-Alum formulated in NLX-5 and NLX-10 did not show a positive effect on IgG antibody responses compared with the HBsAg-Alum group (P > 0.05). In addition, immunization with HBsAg-MON720-NLX-10 at a dilution of 1/1,600 showed a significant increase compared with the HBsAg-MON720 group (P = 0.0045) and HBsAg-MON720-NLX-5 group (P > 0.05, Figure 6D).
-
The results obtained from the specific IgG1 antibody responses showed a significant increase in all vaccine formulation groups compared with their corresponding control groups (P < 0.0009). Notably, no significant differences were observed among the vaccinated groups (P > 0.05). In addition, the results obtained from the specific IgG2a level showed a significant increase in candidate vaccine formulation groups immunized with HBsAg-Alum-NLX-5, HBsAg-MON720-NLX5, HBsAg-MON720-NLX-10, and HBsAg-MON720 compared with their corresponding control groups (P < 0.0179). All experimental vaccine formulation groups did not show any significant differences among one another (P > 0.05, Figure 7).
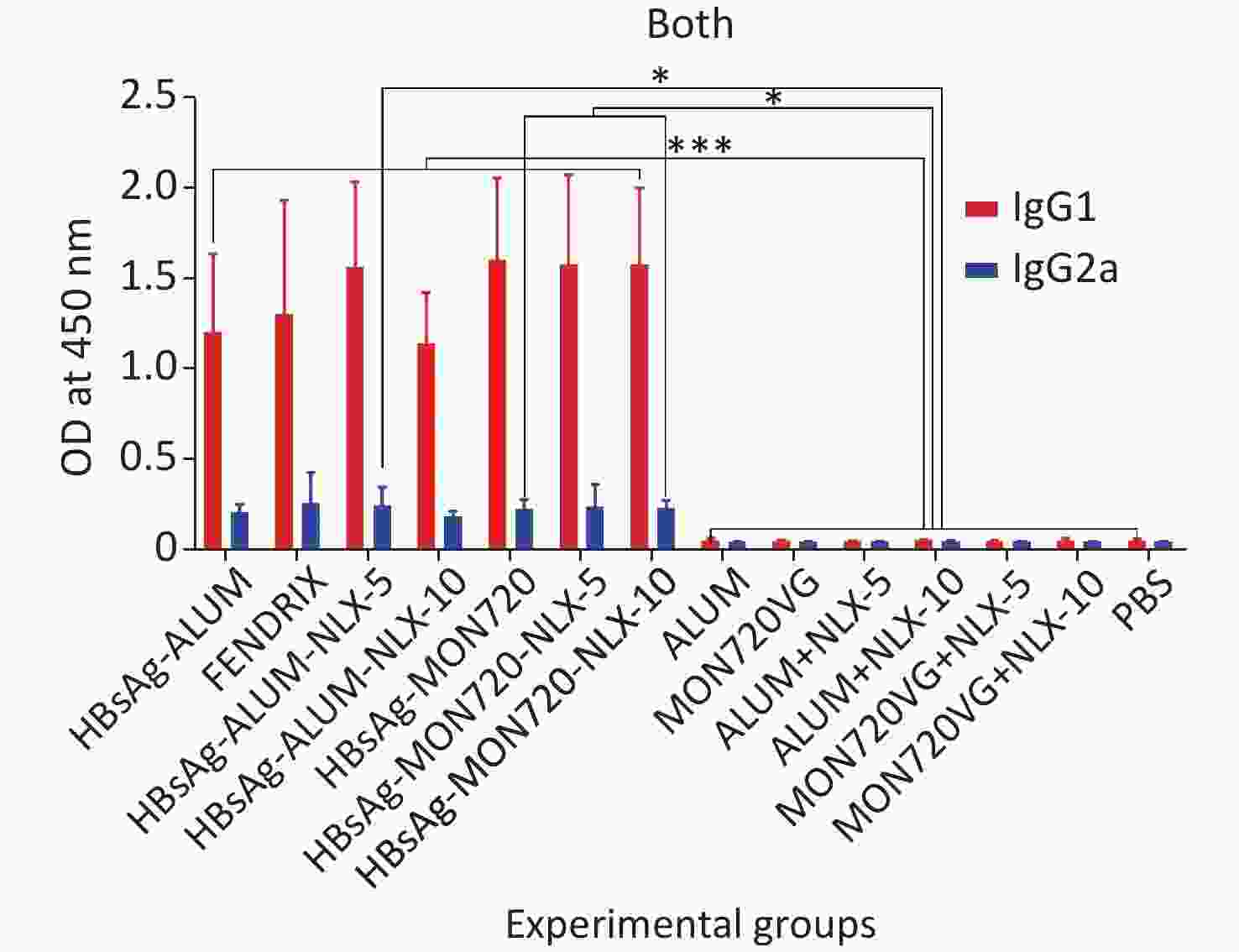
Figure 7. Specific IgG1 and IgG2a isotype responses in experimental groups. Results of IgG1 and IgG2a antibodies on different formulated vaccines demonstrated that all candidate vaccine groups induced IgG1 and IgG2a responses, which showed no significant differences among the vaccinated groups (P > 0.05). *P < 0.05, ***P < 0.001.
-
The efficacy of vaccines is highly dependent on the nature of the adjuvant used in the vaccine formulation. Recently, with the increasing number of recombinant vaccines, the need for a potent adjuvant is increasing because of their low immunogenicity [25]. In this research, complex adjuvants showed potential results in various vaccine formulations [26, 27]. Previous studies have shown that NLX has an adjuvant activity, and Alum has a synergistic effect on the induction of Th1 response [16, 17, 20, 22]. In this case, NLX combined with Montanide ISA-720 was used as a complex adjuvant. During the planning of this study, we used Alum and Montanide ISA-720 as our models because of their differences in the nature and mechanism of action, and we aimed to accurately study the combined effect of NLX on the mineral- and/or oil-based adjuvant models. The cytokine results showed no significant differences in IFN-γ, IL-2, and TNF-α cytokine release among HBsAg-MON720, Fendrix, and HBsAg-Alum groups. Furthermore, NLX in Alum- and MON720-based vaccine formulations did not exhibit a positive effect on IFN-γ, IL-2, and TNF-α cytokine responses. Similarly, previous studies have shown that the NLX/Alum mixture has synergistic effects on Th1 polarization and cytokine release in various vaccine models [18, 20, 22]. However, in this study, we did not achieve such results. In the study of Nejati et al., NLX mixed with HBsAg-Alum did not show positive effects on the IFN-γ cytokine release, which is consistent with the results of the present study [28]. However, in various studies, the NLX/Alum mixture in various vaccine models showed synergistic effects on IFN-γ and TNF-α cytokine release [18-22, 29, 30]. In studies that used HIV-1 [19, 22], HPV [18], and heat-killed Salmonella typhimurium vaccine [20] as models, a synergistic effect was observed. The major difference between our study and others is the antigen model. The antigen nature as a key player in the shifting of immune responses [31-33] may be important for the adjuvant activity of NLX. In our study and the study of Nejati et al., the same antigen in combination with Alum/NLX did not show a synergistic effect on IFN-γ response, whereas other vaccine models in combination with Alum/NLX showed a synergistic effect on IFN-γ responses [21, 34]. The cytokine IL-4 response in the HBsAg-Alum group showed the highest IL-4 cytokine release, and the addition of NLX5 and 10 to the Alum/NLX vaccine formulation suppressed IL-4 cytokine release, which suppressed Th2 response and improved the Th1 pattern. However, NLX in the HBsAg-MON720 vaccine formulation did not show a suppressive effect on the IL-4 cytokine response. The decrease of IL-4 cytokine response in the Alum/NLX group is consistent with our previous work on HBsAg vaccine model [28].
In addition, immunization with HBsAg-MON720 showed an increase in the IL-2/IL4 ratio compared with HBsAg-Alum. This finding indicates that Montanide ISA-720 is more potent than Alum in the induction of the Th1 pattern, as reported by other researchers [35]. Furthermore, the increase of the IL-2/IL4 ratio in HBsAg-Alum-NLX-10 versus HBsAg-Alum was observed, which indicates that adding NLX to Alum led to their polarization into a Th1 pattern. Thus, cellular immunity was reinforced by adding NLX10 to the Alum adjuvant, but adding NLX did not show any positive effects on the Montanide ISA 720 formulation vaccine.
The results of the cytokine assay show that NLX could reinforce Alum adjuvant activity by suppressing IL-4 release and increasing the IL-2/IL4 ratio toward a Th1 pattern. However, NLX did not show any modulatory effect on Montanide ISA-720 adjuvant in cytokine responses. The different effects of NLX on mineral- (Alum) and oil-based (Montanide ISA 720) adjuvants may indicate that the modulatory effect of NLX is highly dependent on the nature of the partner adjuvant. In addition, the interaction between NLX and partner adjuvant may play an important role in the bioactivity of NLX in the vaccine deposition site. However, the nature of antigen in the vaccine formulation should not be ignored [32]. In this study, we also focused on humoral immune responses. The results showed that NLX in HBsAg-Alum vaccine formulation does not show a positive effect, and it was even suppressed to a certain extent. Studies on the adjuvant activity of NLX on different antigen models showed an increase in humoral immune responses[18, 19, 22], but our previous study on the HBsAg vaccine model did not show positive effects on the humoral immune response, which is consistent with the results of this study [28]. Considering the improvement of humoral immune responses on other antigen models [18, 24], the adjuvant activity of NLX and the nature of the antigen may be critical in achieving a boosting effect on the humoral immune response. On the contrary, NLX5 and 10 in the HBsAg-MON720 vaccine formulation showed a significant increase in the specific total IgG response compared with the HBsAg-MON720 group. This finding indicates that apart from its antigen nature, the type of partner adjuvant active in the vaccine formulation may be another factor, making it important for the boosting effect of NLX on humoral immune responses. Herein, NLX in the Alum adjuvant was not potent in boosting the antibody response compared with HBsAg, whereas it was successful in the MON720 adjuvant formulation. In addition, the results obtained from long-lasting humoral immune responses within 220 days after the final immunization revealed that NLX in mineral- and oil-based adjuvanted vaccines could induce lasting humoral immune responses. However, the cytokine profile of NLX-formulated vaccines did not show significant differences compared with MON-720- and Alum-based vaccines, and the improvement of long-lived humoral immune responses may due to the induction of Tfh cells, which we did not assess in the present study, and/or the induction of long-lived plasma cells, which are responsible for such response. Studies show that the lasting of humoral immune response highly depends on the function of Tfh cells [23, 36]. This mechanism as a function of NLX in the vaccine formulation can be addressed in future studies.
Furthermore, the results of the long-lasting humoral immune responses showed that HBsAg-MON720 was more potent than the HBsAg-Alum vaccine in the induction of long-lasting antibody responses. Our previous report on the HBsAg-Montanide ISA 51VG vaccine showed the dominance of this vaccine over the induction of long-lasting humoral immune responses compared with the Alum-based vaccine, which may be due to the nature of the adjuvant and the mechanisms that triggered the display of antigen to the immune cells [23]. This finding indicates the advantage of oil-based vaccines in the induction of long-lasting humoral immune responses in comparison with Alum-based vaccines. The results obtained from IgG1 and IgG2a antibodies of different formulated vaccines demonstrated that all candidate vaccine groups induced both antibodies, which is inconsistent with our previous work [22, 28]. This finding can be considered an advantage in humoral immune responses.
The results of the present study show that the type of partner adjuvant and the nature of antigen are important in the adjuvant activity of NLX in the modulation and improvement of cellular and humoral immune responses. In addition, we have shown that NLX in the vaccine formulation can induce long-lasting humoral immune responses, which is important for long-term protection against infectious diseases.
-
Mina Mirzaee, Setareh Haghighat, Bahareh Golkaran, Fatemeh Asgarhalvaei, Rayhaneh Mirzaee, Mohammad Ali Savoji: Animal handling; Vaccine immunization; Serum sample preparation; Cytokines assay; IgG ELISA; Data acquisition. Morteza Taghizadeh, Behzad Esfandiari: Vaccine formulation; Statistical analysis; Drafting the manuscript. Mehdi Mahdavi: Hypothesis of the study and study planning; Interpreted data; Critical review of the manuscript.
-
The authors are grateful to Borna Zist Pazhohan Knowledge Company and their staff for technical support. We thank Dr. Akbar Khorasani for vaccine formulation from the Department of FMD of Razi Vaccine and Serum Research Institute of Iran (Karaj, Iran) and Miss Zahra Savoji for English editing of the manuscript. We also thank Dr. Louiza Chitour, Regional Sales & Marketing Manager from the Business Unit of SEPPIC Company, for providing Montanide ISA 720VG adjuvant.
doi: 10.3967/bes2022.104
Montanide ISA-720 and Naloxone in HBsAg Vaccine Formulation: Cytokine Profiling and Monitoring of Long-Lasting Humoral Immune Responses
-
Abstract:
Objective This study aimed to investigate the effects of Montanide ISA-720 and Naloxone (NLX) in Hepatitis B surface antigen (HBsAg) vaccine formulation on cytokine and long-lasting antibody responses. Methods First, the HBsAg was formulated in Montanide ISA-720 adjuvant and Naloxone at 5 and 10 mg/kg. The experimental mice were immunized three times at a 2-week interval, and then IL-4, IL-2, TNF-α, and IFN-γ cytokines; long-lasting IgG antibody responses 220 days after the last shot; and IgG1/IgG2a isotypes were assessed by ELISA. Results The HBsAg-Alum group exhibited the highest IL-4 cytokine response among the experimental groups, whereas NLX in HBsAg-MON720 vaccine formulation did not affect cytokine responses. In addition, NLX in Alum-based vaccine suppressed IL-4 cytokine response and increased the IL-2/IL-4 cytokine ratio. Moreover, HBsAg-MON720 was more potent than HBsAg-Alum in the induction of antibody responses, and NLX in Alum- and MON720-based vaccines induced long-lasting antibody responses. Conclusion NLX in Alum-based vaccine decreased IL-4 cytokine response, increased IL-2/IL-4 cytokine ratio, and improved long-lasting humoral immune responses in both vaccine formulations. Therefore, the adjuvant activity of NLX in the vaccine formulation depends on the type of adjuvant and the nature of the antigen in the vaccine formulation. -
Key words:
- HBsAg /
- Vaccine /
- Naloxone /
- Montanide ISA-720 /
- Long-lasting humoral response
-
Figure 1. Results from IFN-γ cytokine response in experimental mouse groups. Experimental mice vaccinated with different formulations of Hepatitis B surface antigen (HBsAg) showed a significant increase as compared with the corresponding control groups (***P = 0.0007). The level of IFN-γ cytokine in HBsAg-Montanide ISA-720 (MON720), HBsAg-Alum, and Fendrix groups did not show significant differences among each other, and adding Naloxone (NLX) to HBsAg-MON720 and HBsAg-Alum vaccines did not show significant responses (P > 0.05).
Figure 2. IL-4 cytokine response in experimental groups. Immunization with HBsAg-Alum, HBsAg-Alum-NLX-5, and HBsAg-Alum-NLX-10 showed a significant increase in IL-4 cytokine secretion compared with their corresponding control groups (P < 0.0453). In addition, the HBsAg-Alum vaccine showed a significant increase in IL-4 cytokine release compared with HBsAg-MON720 and Fendrix groups (P < 0.0004). Furthermore, the HBsAg-Alum-NLX-5 group showed a significant decrease in IL-4 cytokine release compared with the HBsAg-Alum group (P = 0.0270). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3. IL-2 cytokine assay in vaccinated mice. Results from IL-2 cytokines in all vaccine formulation groups showed a significant increase compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-Alum, HBsAg-MON720, and Fendrix vaccines did not show significant differences in IL-2 cytokine release (P > 0.1365). In addition, NLX in vaccine formulation groups did not show a positive effect on IL-2 cytokine response (P > 0.1375). ***P < 0.001.
Figure 4. TNF-α cytokine response in experimental groups. Results from TNF-α cytokine showed a significant increase in all vaccine formulation groups as compared with the corresponding control groups (P < 0.0001). The level of TNF-α cytokine release in HBsAg-MON720, HBsAg-Alum, and Fendrix groups did not show significant differences (P > 0.9930). In addition, NLX in vaccine formulation groups did not show a significant response (P > 0.1249). ***P < 0.001.
Figure 5. IL-2/IL-4 cytokine ratio in experimental groups. IL-2/IL-4 cytokine ratio in all vaccination formulation groups showed a significant increase as compared with their corresponding control groups (P < 0.0001). Immunization with HBsAg-MON720 shows an increase compared with HBsAg-Alum and Fendrix groups (P = 0.0291 and P = 0.0513, respectively). Immunization with HBsAg-Alum-NLX-10 shows an increase in the IL-2/IL-4 ratio compared with the HBsAg-Alum group at borderline (P = 0.0534). *P < 0.05, ***P < 0.001.
Figure 6. Humoral immune responses of experimental groups up to 220 days after the last immunization. (A) Results from IgG response on the 10th day after the last immunization. All vaccine formulation groups showed a significant increase in IgG response compared with their corresponding control groups. In addition, immunization with HBsAg-Alum showed a significant increase in total IgG response compared with Fendrix and HBsAg-MON720 groups (P < 0.0357). Immunization with HBsAg-MON720-NLX-5 and HBsAg-MON720-NLX-10 increased IgG response compared with the HBsAg-MON720 group. (B) Results of IgG response after 90 days of the last immunization. Immunization with HBsAg-MON720 shows a significant increase compared with HBsAg-Alum and Fendrix groups (P < 0.0039). Moreover, immunization with HBsAg-MON720-NLX-5 shows a significant increase in IgG responses compared with the HBsAg-MON720 group (P < 0.0032). (C) Results of IgG responses after 150 days of the last immunization. Immunization with HBsAg-MON720 showed a significant increase in total IgG compared with Fendrix and HBsAg-Alum (P < 0.0335). Furthermore, immunization with HBsAg-Alum-NLX-10 showed a significant increase in comparison with HBsAg-Alum (P < 0.0057). (D) Results of IgG response on day 220th of the final immunization. Immunization with HBsAg-MON720 showed a significant increase compared with Fendrix and HBsAg-Alum groups (P < 0.0343). In addition, immunization with HBsAg-MON720-NLX-10 showed a significant increase compared with the HBsAg-MON720 group (P = 0.0045).
Figure 7. Specific IgG1 and IgG2a isotype responses in experimental groups. Results of IgG1 and IgG2a antibodies on different formulated vaccines demonstrated that all candidate vaccine groups induced IgG1 and IgG2a responses, which showed no significant differences among the vaccinated groups (P > 0.05). *P < 0.05, ***P < 0.001.
Table 1. Experimental groups with the vaccine formulations which used for immunization
Group Vaccine formulations No. of mice Dose Route of immunization 1 HBsAg-ALUM 15 5 µg subcutaneous 2 FENDRIX 15 5 µg subcutaneous 3 HBsAg-ALUM-NLX-5 15 5 µg subcutaneous 4 HBsAg-ALUM-NLX-10 15 5 µg subcutaneous 5 HBsAg- MON720 15 5 µg subcutaneous 6 HBsAg-MON-720-NLX-5 15 5 µg subcutaneous 7 HBsAg-MON-720-NLX-10 15 5 µg subcutaneous 8 ALUM 10 − subcutaneous 9 MON720VG 10 − subcutaneous 10 ALUM+NLX-5 10 − subcutaneous 11 ALUM+NLX-10 10 − subcutaneous 12 MON720VG+NLX-5 10 − subcutaneous 13 MON720VG+NLX-10 10 − subcutaneous 14 PBS 10 − subcutaneous -
[1] Liaw YF, Chu CM. Hepatitis B virus infection. Lancet, 2009; 373, 582−92. doi: 10.1016/S0140-6736(09)60207-5 [2] Yano Y, Azuma T, Hayashi Y. Variations and mutations in the hepatitis B virus genome and their associations with clinical characteristics. World J Hepatol, 2015; 7, 583−92. doi: 10.4254/wjh.v7.i3.583 [3] McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis 2005; 25, 3−8. [4] Amponsah-Dacosta E. Hepatitis B virus infection and hepatocellular carcinoma in sub-Saharan Africa: Implications for elimination of viral hepatitis by 2030? World J Gastroenterol, 2021; 27, 6025-38. [5] Ong HT, Duraisamy G, Peng NK, et al. Genotyping of hepatitis B virus in Malaysia based on the nucleotide sequence of preS and S genes. Microbes Infect, 2005; 7, 494−500. doi: 10.1016/j.micinf.2004.12.016 [6] Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med, 2004; 350, 1118−29. doi: 10.1056/NEJMra031087 [7] Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med, 2013; 19, 1597−608. doi: 10.1038/nm.3409 [8] Mahdavi M, Ebtekar M, Khorshid HRK, et al. ELISPOT analysis of a new CTL based DNA vaccine for HIV-1 using GM-CSF in DNA prime/peptide boost strategy: GM-CSF induced long-lived memory responses. Immunol Lett, 2011; 140, 14−20. doi: 10.1016/j.imlet.2011.05.005 [9] Selvaraj G, Kaliamurthi S, Peslherbe GH, et al. Are the allergic reactions of COVID-19 vaccines caused by mRNA constructs or nanocarriers? Immunological insights. Interdiscip Sci Comput Life Sci, 2021; 13, 344−7. doi: 10.1007/s12539-021-00438-3 [10] Humayun F, Cai YT, Khan A, et al. Structure-guided design of multi-epitopes vaccine against variants of concern (VOCs) of SARS-CoV-2 and validation through In silico cloning and immune simulations. Comput Biol Med, 2022; 140, 105122. doi: 10.1016/j.compbiomed.2021.105122 [11] Harrison T. Viral hepatitis and primary liver cancer. BIOS Scientific Publishers Ltd. 1998. [12] Wang S, Liu X, Caulfield MJ. Adjuvant synergy in the response to hepatitis B vaccines. Vaccine, 2003; 21, 4297−306. doi: 10.1016/S0264-410X(03)00463-8 [13] Azami M, Ahmadi MRH, Sayehmiri K. Hepatitis B vaccination efficacy in Iranian healthcare workers: a meta-analysis study. Hepat Mon, 2017; 17, e37781. [14] Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol, 2004; 82, 488−96. doi: 10.1111/j.0818-9641.2004.01272.x [15] Newton DE. Vaccination Controversies: A Reference Handbook. ABC-CLIO. 2013. [16] Jamali A, Mahdavi M, Shahabi S, et al. Naloxone, an opioid receptor antagonist, enhances induction of protective immunity against HSV-1 infection in BALB/c mice. Microb Pathog, 2007; 43, 217−23. doi: 10.1016/j.micpath.2007.06.001 [17] Jamali A, Mahdavi M, Hassan ZM, et al. A novel adjuvant, the general opioid antagonist naloxone, elicits a robust cellular immune response for a DNA vaccine. Int Immunol, 2009; 21, 217−25. doi: 10.1093/intimm/dxn139 [18] Yasaghi M, Mahdavi M. Potentiation of human papilloma vaccine candidate using naloxone/alum mixture as an adjuvant: increasing immunogenicity of HPV-16E7d vaccine. Iran J Basic Med Sci, 2016; 19, 1003−9. [19] Fathi M, Nezamzadeh R, Mahdavi M. Studying the effects of Naloxone-Alum adjuvant mixture on cytokines in model of multi-epitope vaccine in HIV-1. J Chem Health Risks, 2014; 4, 7−14. [20] Jazani NH, Parsania S, Sohrabpour M, et al. Naloxone and alum synergistically augment adjuvant activities of each other in a mouse vaccine model of Salmonella typhimurium infection. Immunobiology, 2011; 216, 744−51. doi: 10.1016/j.imbio.2010.10.005 [21] Jazani NH, Sohrabpour M, Mazloomi E, et al. A novel adjuvant, a mixture of alum and the general opioid antagonist naloxone, elicits both humoral and cellular immune responses for heat-killed Salmonella typhimurium vaccine. FEMS Immunol Med Microbiol, 2011; 61, 54−62. doi: 10.1111/j.1574-695X.2010.00747.x [22] Farahani SV, Aghasadeghi MR, Memarnejadian A, et al. Naloxone/alum mixture a potent adjuvant for HIV-1 vaccine: induction of cellular and poly-isotypic humoral immune responses. Pathog Global Health, 2016; 110, 39−47. doi: 10.1179/2047773215Y.0000000035 [23] Savoji MA, Sereshgi MMA, Ghahari SMM, et al. Formulation of HBsAg in Montanide ISA 51VG adjuvant: Immunogenicity study and monitoring long-lived humoral immune responses. Int Immunopharmacol, 2021; 96, 107599. doi: 10.1016/j.intimp.2021.107599 [24] Fathi M, Nezamzadeh R, Abdollahpour-Alitappeh M, et al. Formulation of a recombinant HIV-1 polytope candidate vaccine with naloxone/alum mixture: induction of multi-cytokine responses with a higher regulatory mechanism. APMIS, 2021; 129, 480−8. doi: 10.1111/apm.13122 [25] Pulendran B, Arunachalam PS, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov, 2021; 20, 454−75. doi: 10.1038/s41573-021-00163-y [26] Wang H, Huang W, Gao H, et al. NY-ESO-1 protein vaccine combining alum, CpG ODN, and HH2 complex adjuvant induces protective and therapeutic anti-tumor responses in murine multiple myeloma. OncoTargets Ther, 2020; 13, 8069−77. doi: 10.2147/OTT.S255713 [27] Tifrea DF, He W, Pal S, et al. Induction of protection in mice against a Chlamydia muridarum respiratory challenge by a vaccine formulated with the major outer membrane protein in nanolipoprotein particles. Vaccines, 2021; 9, 755. doi: 10.3390/vaccines9070755 [28] Nejati S, Mirzaee S, Nouri HR, et al. Immune responses of mice immunized with HBsAg formulated in naloxone/alum mixture: comparison to fendrix vaccine. Hepat Mon, 2017; 17, e44536. [29] Shahabi S, Hajipirloo HM, Keramati A, et al. Evaluation of the adjuvant activity of propranolol, a Beta-adrenergic receptor antagonist, on efficacy of a malaria vaccine model in BALB/c mice. Iran J Allergy Asthma Immunol, 2014; 13, 307−16. [30] Khorshidvand Z, Shahabi S, Mohamadzade H, et al. Mixture of Alum-Naloxone and Alum-Naltrexone as a novel adjuvant elicits immune responses for Toxoplasma gondii lysate antigen in BALB/c mice. Exp Parasitol, 2016; 162, 28−34. doi: 10.1016/j.exppara.2016.01.001 [31] Maynard CL, Elson CO, Hatton RD, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature, 2012; 489, 231−41. doi: 10.1038/nature11551 [32] Guy B, Krell T, Sanchez V, et al. Do Th1 or Th2 sequence motifs exist in proteins?: Identification of amphipatic immunomodulatory domains in Helicobacter pylori catalase. Immunol Lett, 2005; 96, 261−75. doi: 10.1016/j.imlet.2004.09.011 [33] Bielekova B, Martin R. Antigen-specific immunomodulation via altered peptide ligands. J Mol Med, 2001; 79, 552−65. doi: 10.1007/s001090100259 [34] Motaharinia Y, Rezaee MA, Rashidi A, et al. Induction of protective immunity against brucellosis in mice by vaccination with a combination of naloxone, alum, and heat-killed Brucella melitensis 16 M. J Microbiol Immunol Infect, 2013; 46, 253−8. doi: 10.1016/j.jmii.2012.03.011 [35] Ghadimipour R, Ghorbanpoor M, Gharibi D, et al. Effects of selected adjuvants on immunogenicity and protectivity of Pasteurella multocida bacterin vaccine in chickens. Arch Razi Inst, 2020; 76, 741−9. [36] Suan D, Nguyen A, Moran I, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity, 2015; 42, 704−18. doi: 10.1016/j.immuni.2015.03.002 -




 下载:
下载:



 Quick Links
Quick Links