-
Enterovirus 71 (EV71) is one of the major causative pathogens for hand-foot- and-mouth disease (HFMD). HFMD is a very contagious illness and usually affects children under the age of five. HFMD spreads quickly, and has led to outbreaks worldwide in the past few decades. It particularly occurs in many Asian countries, including China, Singapore, Malaysia and Vietnam, et al.[1]. For exemple in 2017, HFMD occured in China with 1, 952, 435 cases and 98 deaths reported[2]. Its incidence and mortality rates have been ranked at the top of the National Notifiable Infectious Diseases for the past ten years in China[3]. HFMD caused by EV71, a neurotropic virus, tends to be associated with severe syndromes of the central nervous system, respiratory system, and cardiovascular system including aseptic meningitis, acute myelitis, pulmonary edema and myocarditis[2, 4]. EV71 is a symmetric non-enveloped icosahedral virion with a diameter of about 20-30 nm. It is a single-stranded, positive-sense RNA virus belonging to the enterovirus A group (HEV-A) of the Picornaviridae family[5-7]. The structural proteins VP1 to VP3 are exposed on the surface of the capsid. It has been known that two linear neutralizing epitopes from the VP1 (SP70 and SP55) are capable of eliciting neutralizing antibodies. In particully, the SP70 is identical among all EV71 genotypes[8]. As the single SP70 peptide is poorly immunogenic, it is prudent to enhance the B-cell response through either combinating the antigen with an adjuvant or developing effective epitope presenting system[9].
As one kind of nanoparticles found in nature, virus-like particles (VLPs) can present external epitopes repetitively on their surface to be recognized by antigen presenting cells to stimulate the immune response from B cells, Th cells and cytotoxic T lymphocytes (CTL)[10]. They are widely used in vaccine development[11-14]. The core antigen (HBcAg) of the hepatitis B virus (HBV) is one of the excellent candidates for creating VLPs. HBcAg's α- helix structure allows external peptides to be inserted into the main immunogenic region (MIR), amino-terminal and carboxyl-terminal while maintaining, even enhancing, the immunogenicity of externally expressed epitopes. In addition, it can fold and assemble into VLPs in prokaryotic and eukaryotic cell expression system without providing toxicity to human cells[15-16]. It is the ideal carrier for delivering external epitopes in vivo.
Although the commercialized inactivated EV71 virus vaccine has been used in preventing HFMD, there may be some issues associated with it. For one, it can possibly induce a high titer of autoantibodies which leads to potential autoimmunity because VP1 shares a common epitope with a human brain tissue protein[17]. Therefore research and development of subunit vaccines based on gene recombination technology is necessary. In this study, we utilized truncated HBcAg as a carrier to construct the fusion proteins without His-tagged which would be expressed and purified as soluble chimeric VLPs displaying the epitope SP70 of EV71. To avoid side effects of aluminum adjuvant[18], the specific immune responses induced in mice by HBc-SP70 VLPs without aluminum adjuvant were evaluated and compared to the response of immunization with VLPs supplemented by the adjuvant. In addition to traditionally testing the protective effect of specific humoral immunity, the subtypes of the specific neutralizing antibodies and cellular immune response elicited by chimeric HBc-SP70 VLPs were also evaluated by ELISA and ELIPOT assays.
-
The human EV71 FY-15 strain (C4 genogroup, isolated in Fu Yang, Anhui, China, 2008) was utilized for determining the neutralizing antibody titer after immunization. The highly mouse-adapted virulent EV71 strain (C4 genogroup) from the National Vaccine and Serum Institute (Beijing, China) was used for the lethal challenge test. Both strains were cultured and propagated in rhabdomyosarcoma (RD) cells. The viral titer was evaluated using RD cells by micro-titration assay and expressed as the 50% tissue culture-infective dose (TCID50). Meanwhile, the purified inactivated virus was made from the FY-15 strain as described previously[19].
-
The amino acid sequence of SP70 (YPTFGEHKQEKDLEY) was inserted between the amino acid residues 78 and 79 of the truncated HBcAg (aa1-144), which is the location of HBcAg's major immunodominant region (MIR). After codon optimization of both the fusion protein coding sequence as well as that of the truncated HBc gene, the restriction enzyme sites NdeI and XhoI were introduced onto the two ends respectively. The recombinant gene fragments were synthesized by Sangon Biotech (Shanghai, China).
-
After digestion with the restriction enzymes NdeI and XhoI, the synthesized gene fragments were ligated into pET-43.1a followed by transformation into competent E.coli B21 cells. By restriction enzyme identification and DNA sequence analysis, the recombinant plasmids pHBc-SP70 and pHBc were confirmed being successfully constructed. The plasmids were transformed into E.coli BL21 cells and cultured in Luria-Bertani (LB) medium with 50 μg/mL Ampicillin, shaking overnight at 30 ℃. An equal volume of fresh LB medium was then added to the culture for continuous shaking for 1 hour at 37 ℃ followed by addition of IPTG (isopropyl β-D-thiogalactoside) at a final concentration of 1 mmol/L and cultured for 4 more hours in a 32 ℃ shaker. The bacteria were collected by spinning and resuspended in 10 mmol/L Tris-HCl buffer with 0.01% Triton X-100, pH 8.0. After lysed by sonication and centrifuged, the resulting supernatant was loaded into DEAE-Sepharose fast flow ion exchange chromatography columns. The flow-through from unbound exchange media was collected for 10% cesium chloride (CsCl) cushion centrifugation at 30, 000 rpm for 5 hours. The precipitation was then resuspended and further separated by 0-60% continuous CsCl gradient centrifugation at 28, 000 rpm overnight. The resulting fractions were collected accordingly and then subject to 15% SDS-PAGE analysis to target the fractions containing pure proteins of HBc-SP70 and HBcAg followed by dialysis against PBS overnight.
-
The purified HBcAg, chimeric HBc-SP70 VLPs and inactivated EV71 were negatively stained with phosphotungstic acid and examined by transmission electron microscope. Western blotting were carried out as described previously[20] with the following modifications: the membranes were probed with a mouse anti-HBc (Abcam Cat No: ab8638) and anti-SP70 (AbMax Biotechnology Co., Ltd., Beijing, China Cat No: Clone 22A12) monoclonal antibodies, followed by incubation with horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (ABclonal Biotechnology co., Ltd Cat No: AS003).
-
Forty 6-week-old female ICR mice (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were randomly assigned to 4 groups. Each group of 10 mice received one of four different intramuscularly injected solutions as follows: two were 10 μg of purified HBc-SP70 and HBc VLPs with aluminum adjuvant, the other was 10 μg of purified HBc-SP70 VLPs alone without aluminum, and the last was purely aluminum adjuvant alone in PBS. The mice were inoculated three times at week 0, week 2 and week 4. The serum samples were collected prior to the immunization and then at serial points: 4, 6, 8, 10, and 12 weeks after the initial immunization. Four mice from each group were euthanatized at week 6 after immunization to collect splenocytes for ELISPOT assay. The remaining 6 female mice from each group were maintained for mating and breeding. All animal experimental procedures were conducted in accordance with the guidelines of the Animal Care and Welfare Committee at the National Institute for Viral Disease Control and Prevention, China Center for Disease Control and Prevention.
-
The specific IgG titers in mice sera and the subclasses of the IgG were measured by indirect enzyme-linked immunosorbent assay (ELISA). The 96-well microtiter plates were separately coated with 200 ng HBcAg, SP70 peptide (Sangon Biotech) and inactivated EV71 and then incubated overnight at 4 ℃. After the wells were blocked with PBS containing 5% nonfat milk and 0.05% Tween 20, 50 μL of sera samples were added to HBcAg or SP70 peptide coated plates per well at 1:100 dilution in PBS while the inactivated EV71 coated plate was applied with 50 μL per well of two-fold serially diluted (22-217) sera samples for 2 hours at 37 ℃. After washing 5 times by PBST, HRP goat anti-mouse IgG (1:10, 000 dilution, ABclonal Biotechnology co., Ltd, Cat No: AS003) was applied to the plates for 30 min followed by color emerging. The absorbance at 450 nm was recorded using an ELISA plate reader (Thermo Scientific MultiskanTM GO; Thermo Fisher Scientific Oy, Vantaa, Finland, FI-01621). The subtypes of the anti-EV71 IgG were determined by SBA Clonotyping System-HRP kit (Southern Biotech, USA, Cat No: 5300-05). The 96-well plate was first coated with inactive EV71 followed by incubation with sera samples at 1:100 dilution according to the manufacturer's instruction.
-
The titer of neutralizing antibodies against EV71 was determined in RD cells by Microneutralization assays[19]. Two weeks after the three boosters, 50 μL of mice sera from twofold serial dilution (23-214) were mixed with equal volume of EV71 at a concentration of 2000TCID50/mL in the 96-well plate. When the 2 hours incubation at 37 ℃ was finished, each well was supplied with 1 × 104 RD cells for continuous culture for 7 days, and then checked under the microscope. The neutralizing titer was determined as the highest dilution that had no cellular cytopathic effects (CPE).
-
The 96-well plates from BDTM Mouse IFN-γ ELISPOT Kit and BDTM Mouse IL-4 ELISPOT Kit were pre-coated with IFN-γ and IL-4 capture antibodies. 2 × 105 murine splenocytes and 20 μL of 500 μg/mL inactive EV71 were mixed in each well and incubated at 37 ℃ for 20 hours. The liquid in wells was discarded and ELISPOT assay was performed according to the manufacturer's instructions. The positive control used ConA (5 μg/well, Sigma) as a stimulus. Finally, spot-forming cells (SFC) were counted using an automated ELISpot reader (Cellular Technology Ltd, Shaker Heights, OH, USA).
-
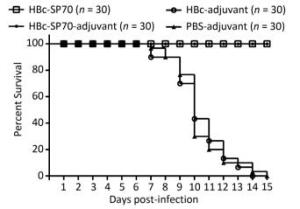
The immunized female mice were allowed to mate at week 2 after the last booster injection. Thirty neonatal mice of each group were challenged at 72 hours after birth via intracranial inoculation with 10 μL of 7.9 × 109 TCID50/mL of highly mouse-adapted virulent EV71 strain. The mortalities of each group were monitored daily for 17 days.
-
All data obtained were analyzed via SPSS software and P < 0.05 were considered to indicate a statistically significant difference between values. All figures were analyzed and plotted by GraphPad Prism 6 software.
-
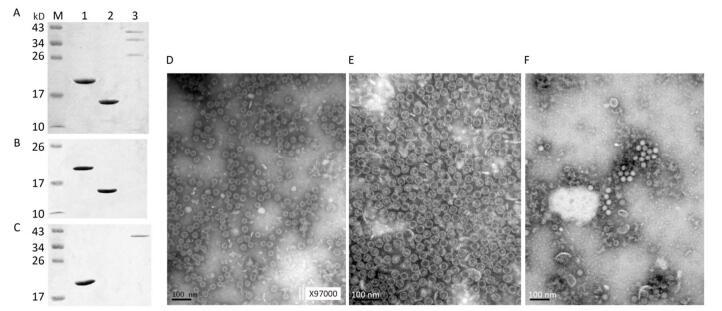
The recombinant plasmids, pHBc-SP70 and pHBc, were sequenced to confirm that the inserted HBc and SP70 coding sequences are in frame and without mutations. In the gradient fraction between 10%-20% CsCl, the concentrated milk-like protein layer, which was purified HBcAg or HBc-SP70 VLPs verified by SDS-PAGE (Figure 1A), could be clearly seen. The purified inactivated EV71 proteins were also verified by SDS-PAGE (Figure 1A). The western blot assay showed expected results: both the HBcAg and HBc-SP70 VLPs reacted with the anti-HBc monoclonal antibody (Figure 1B) while the anti-SP70 monoclonal antibody just recognized HBc-SP70 protein and VP1 of inactivated EV71 (Figure 1C), which demonstrated that the chimeric VLPs possess excellent antigenicity. Under electron microscope, both the purified fusion protein HBc-SP70 and HBc can be seen to have spontaneously folded and assembled into empty icosahedral virus-like particles which are about 28nm in diameter (Figure 1D and 1E) while the inactivated EV71 virus presented as solid icosahedral particles (Figure 1F).
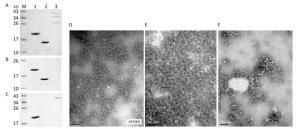
Figure 1. Antigenicity and transmission electron microscopy images of purified fusion proteins HBc-SP70, HBcAg and inactivated EV71 proteins. (A) Purified HBc-SP70, HBcAg and inactivated EV71 proteins were confirmed by 15% SDS-PAGE after density gradient ultracentrifugation. (B) Western blot result using anti-HBc monoclonal antibody. (C) Western blot result using anti-SP70 monoclonal antibody. Lane 1: HBc-SP70; Lane 2: HBcAg; Lane 3: inactivated EV71 proteins. Transmission electron microscopy images of purified chimeric HBc-SP70 VLPs (D) HBcAg (E) and inactivated EV71 FY-15 strain (F). Scale bar, 100 nm.
-
Except for the PBS-adjuvant group, ELISA results demonstrated that the sera from the other three groups of mice status post three immunizations had anti-HBc antibody which reacted with the HBcAg coated on the 96 well plates (Figure 2A). The sera collected from the two groups immunized by fusion HBc-SP70 VLPs regardless of the presence of adjuvant had detectable anti-SP70 antibody (Figure 2B). ELISA was used to detect the kinetics of anti-EV71 antibody production in the sera at twofold serial dilutions (22-217) which were collected before inoculation, and then at 4, 6, 18, and 12 weeks after that initial inoculation. As demonstrated in Figure 2C, all of the mice from both groups treated with HBc-SP70 alone and HBc-SP70-adjuvant were inducing anti-EV71 IgG from week 4 to week 12. And there was no significant difference for the titer of detectable antibody in these two groups (P > 0.05), but both of them were significantly higher than the titer of antibody detected in the HBc-adjuvant group (P < 0.001). In particular, the EV1-specific antibody titer reached a peak of 216 on the second week after the third immunization (week 6), and the antibody level was maintained at 210 for up to at least 8 weeks after immunization. Although the titer at 10 and 12 weeks post-immunization were slightly lower than they had been at 6 weeks, there was no significant difference detected (P > 0.05). The sera collected from week 6 were further measured to determine which subtypes of anti-EV71 IgG were present (Figure 2D). The sera from both groups immunized by HBc-SP70-adjuvant and HBc-SP70 had significantly higher levels of IgG1 and IgG2b compared with levels from the HBc-adjuvant group (P < 0.05) while no significant difference was observed for IgG2a and IgG3 (P > 0.05). This result implies that HBc-SP70 VLPs can elicit a mixed Th1/Th2 immune response.
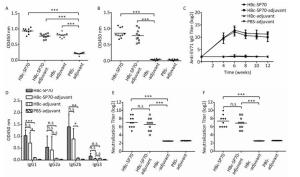
Figure 2. Humoral immune response in mice immunized with chimeric HBc-SP70 VLPs. Sera from the ten mice in each group were collected at 2 weeks after the three immunizations. The sera were diluted at 1:100 and used in ELISA to detect HBc- specific antibody (A) and SP70-specific antibody (B). The geometric mean titers (log2) of the total anti-EV71 IgG in the sera at weeks 4, 6, 8, 10, and 12 after the first immunization (C). The sera collected at 2 weeks after the third immunization were diluted at 1:100 to determine the subtypes of anti-EV71 IgG (D). Neutralization antibody titer against EV71 FY-15 strain (E) and the highly mouse-adapted virulent EV71 strain (F) were measured by using the sera from all 10 mice of each group collected at 2 weeks after the third immunization at twofold serial dilutions (23-214). Each symbol represents an individual mouse, and the line indicates the geometric mean value of the group. Statistical significance was determined by the Student t test and is indicated as follows: n.s, P > 0.05; *P < 0.05; **P < 0.01; or ***P < 0.001.
The neutralization assay revealed that the specific antibodies induced by the mice immunized with chimeric HBc-SP70 VLPs both in the presence or absence of adjuvant could successfully neutralize the EV71 FY-15 strain (Figure 2E) and highly mouse- adapted virulent EV71 strain after three immunizations (Figure 2F). The neutralization titer can reach as high as 210 with the average level being above 24 while the control groups had no specific neutralization antibody induced.
-
Compared with the splenocytes from mice treated as control, splenocytes from both groups immunized by HBc-SP70 VLPs with or without aluminum produced high level of IFN-γ-secreting T cells and high level of IL-4-secreting T cells after stimulation by inactivated EV71. This means that chimeric HBc-SP70 VLPs were capable of inducing EV71-specific cellular immune response in mice (Figure 3).
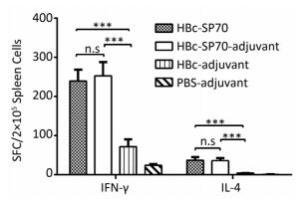
Figure 3. Enzyme-linked immunospot detection of EV71-specific IFN-γ -secreting T cells and IL-4-secreting T cells in spleen lymphocytes from immunized mice. Statistical significance was determined by the Student t test and is indicated as follows: n.s, P > 0.05; ***P < 0.001. SFC stands for spot-forming cells.
-
The neutralization assay had confirmed that mice immunized with chimeric HBc-SP70 VLPs produced EV71-specific antibody to neutralize highly mouse-adapted virulent EV71 strain. In addition, the IgG antibody could be transferred transplacentally from the immunized mother to her offspring. To assess the protective efficacy of the EV71-specific neutralizing antibody from mothers immunized with HBc-SP70 VLPs, 30 neonatal mice from each group were monitored for 15 days after challenging intracerebrally with highly mouse-adapted virulent EV71 strain. All neonatal mice from the HBc-adjuvant and PBS-adjuvant control groups started gradually displaying a series of concerning clinical symptoms including weight loss, slowed growth, hunched backs, reduced mobility and paralysis from the 3rd or 4th day and began to die from the 7th day after the lethal challenge. Eventually, they were all dead within 15 days with the highest death rate at day 10. By contrast, all of the neonatal mice born of female mice immunized with HBc-SP70 and HBc-SP70-adjuvant grew normally without manifesting any of the above symptoms or suffering from excess mortality (Figure 4). These results demonstrate that the newborn mice could be protected passively from EV71 infection by accepting the EV71-specific antibody from their HBc-SP70 VLP-immunized mothers.
-
Unlike previous study[21], a truncated sequence of HBcAg (1-144 aa) was used in this study for the purpose of creating empty VLPs. Therefore, the fusion proteins HBc-SP70 and HBcAg were visualized as empty VLPs (Figure 1D and 1E) under electron microscope while the inactivated EV71 viral particles were solid due to the presence of nucleic acids (Figure 1F). To avoid the damage to cells and potential neurotoxicity caused by the aluminum adjuvant[18], we evaluated the difference between the immunogenicity of HBc-SP70 VLPs with and without aluminum adjuvant. The results confirmed that both of them were able to induce similar level of EV71-specific antibody in mice and have sufficient neutralizing effect on the EV71 FY-15 strain and the highly mouse-adapted virulent EV71 strain (Figure 2C, 2E, and 2F). The neutralizing antibody against the EV71 FY-15 stain elicited by chimeric HBc-SP70 VLPs in the absence of aluminum adjuvant was at a titer range from 64 to 512, with an average value at 128, which is much higher than the neutralizing antibody titer in mice stimulated by either a single synthesized SP70 peptide with Freund's adjuvant or a complex of SP70 conjugated to diphtheria toxoid with Freund's adjuvant (16 to 32)[8]. The neutralization titer in this study is also slightly higher than the titer of neutralizing antibody elicited by the HBc-SP70 VLPs with aluminum adjuvant (64 to 128) reported in Ye's research[21]. It might be because we optimized the process of VLPs expression and purification. The lower temprature (32 ℃) and the longer expression time (4 hours) in our study were better for inducing high-level expression of the soluble fusion proteins without the phase of forming inclusion bodies and renaturation. Also constructed without His-tagged, these were all quite ideal for VLPs assembling[22]. At the same time, it prevented mice from producing anti-His antibody which might react with hemoglobin and normal tissues[23], interfering with later vaccine evaluation and further animal trials. Through all these results, we conclude that the HBc-SP70 VLPs we produced can elicit specific immune response effectively even without adjuvant.
The ability of the HBc-SP70 VLPs to stimulate the cellular immune system was further assessed by measuring the amount of secretion of IL4-secreting and IFN-γ-secreting T cells in splenocytes of immunized mice. The Th1 immune responses manifested primarily as production of IL2, TNF-β, and IFN-γ cytokines in mice. These cytokines are crucial for cell-mediated immune response to produce antibodies of IgG2a, IgG2b, and IgG3 subtypes. The Th2 immune response primarily produces a high level of IgG1 antibodies and cytokines IL-4 and IL-10, which are important in the humoral immune response by stimulating the maturation and differentiation of B cells[24-25]. Mice immunized with HBc-SP70 VLPs produced high levels of IFN-γ and IL-4, revealing that immunization with chimeric VLPs generated both EV71-specific cellular and humoral immunological memory via a mixed Th1/Th2 response. This conclusion was further confirmed by detecting that the main subtypes of the formed anti-EV71 IgG were IgG1 and IgG2, with a small amount of IgG2a. Additionally, the levels of IgG1 and IgG2b from HBc-SP70 group were slightly lower than that from the HBc-SP70-adjuvant group (Figure 2D), which is likely because the aluminum adjuvant stimulates the immune response more towards the Th2 response[26]. Since there is an absence of IgG3 subtype, antibody-dependent enhancement (a phenomenon commonly associated with IgG3 presence) can be prevented[27].
Previous studies have confirmed that HBc-specific cytotoxic T lymphocytes (CTL) and HBc antigen-antibody complexes can interfere with cccDNA of HBV biosynthesis from de novo infection and intracellular amplification and prevent liver damage. The chimeric HBc-SP70 VLPs in our study can evoke not only effective cellular and humora immune response to protect mice from EV71, but also high titers of anti-HBc antibodies and HBc-specific CTLs. It suggests that the chimeric HBc-SP70 VLPs have potential to prevent HBV infection and cure chronic hepatitis B[28-29]. Moreover, the method of expression and purification of the fusion protein described in this study has broad application prospect in the field of developing genetically engineered subunit vaccines. If the chimeric VLPs could displaying tandem-linked multiple epitopes of EV71 such as the recently recognized neutralizing epitope VP2-28 of EV71 structural protein VP2[30], the immune efficacy might be comparable to that induced by EV71 inactivated vaccine, or even better because they can avoid cross-reactivity with human brain tissue and elicit both Th1 and Th2 immune responses without aluminum adjuvant.
doi: 10.3967/bes2018.045
Efficient Humoral and Cellular Immune Responses Induced by a Chimeric Virus-like Particle Displaying the Epitope of EV71 without Adjuvant
-
Abstract:
Objective To eliminate the side effects of aluminum adjuvant and His-tag, we constructed chimeric VLPs displaying the epitope of EV71 (SP70) without His-tagged. Then evaluating whether the VLPs could efficiently evoke not only humoral but also cellular immune responses against EV71 without adjuvant. Methods The fusion protein was constructed by inserting SP70 into the MIR of truncated HBcAg sequence, expressed in E. Coli, and purified through ion exchange chromatography and density gradient centrifugation. Mice were immunized with the VLPs and sera were collected afterwards. The specific antibody titers, IgG subtypes and neutralizing efficacy were detected by ELISA, neutralization assay, and EV71 lethal challenge. IFN-γ and IL-4 secreted by splenocytes were tested by ELISPOT assay. Results HBc-SP70 proteins can self-assemble into empty VLPs. After immunization with HBc-SP70 VLPs, the detectable anti-EV71 antibodies were effective in neutralizing EV71 and protected newborn mice from EV71 lethal challenge. There was no significant difference for the immune efficacy whether the aluminum adjuvant was added or not. The specific IgG subtypes were mainly IgG1 and IgG2b and splenocytes from the mice immunized produced high levels of IFN-γ and IL-4. Conclusion The fusion proteins without His-tagged was expressed and purified as soluble chimeric HBc-SP70 VLPs without renaturation. In the absence of adjuvant, they were efficient to elicit high levels of Th1/Th2 mixed immune response as well as assisted by aluminum adjuvant. Furthermore, the chimeric VLPs have potential to prevent HBV and EV71 infection simultaneously. -
Key words:
- Virus-like particles /
- Enterovirus 71 /
- Neutralizing antibody /
- Humoral and cellular immunity /
- Adjuvant /
- Vaccine
-
Figure 1. Antigenicity and transmission electron microscopy images of purified fusion proteins HBc-SP70, HBcAg and inactivated EV71 proteins. (A) Purified HBc-SP70, HBcAg and inactivated EV71 proteins were confirmed by 15% SDS-PAGE after density gradient ultracentrifugation. (B) Western blot result using anti-HBc monoclonal antibody. (C) Western blot result using anti-SP70 monoclonal antibody. Lane 1: HBc-SP70; Lane 2: HBcAg; Lane 3: inactivated EV71 proteins. Transmission electron microscopy images of purified chimeric HBc-SP70 VLPs (D) HBcAg (E) and inactivated EV71 FY-15 strain (F). Scale bar, 100 nm.
Figure 2. Humoral immune response in mice immunized with chimeric HBc-SP70 VLPs. Sera from the ten mice in each group were collected at 2 weeks after the three immunizations. The sera were diluted at 1:100 and used in ELISA to detect HBc- specific antibody (A) and SP70-specific antibody (B). The geometric mean titers (log2) of the total anti-EV71 IgG in the sera at weeks 4, 6, 8, 10, and 12 after the first immunization (C). The sera collected at 2 weeks after the third immunization were diluted at 1:100 to determine the subtypes of anti-EV71 IgG (D). Neutralization antibody titer against EV71 FY-15 strain (E) and the highly mouse-adapted virulent EV71 strain (F) were measured by using the sera from all 10 mice of each group collected at 2 weeks after the third immunization at twofold serial dilutions (23-214). Each symbol represents an individual mouse, and the line indicates the geometric mean value of the group. Statistical significance was determined by the Student t test and is indicated as follows: n.s, P > 0.05; *P < 0.05; **P < 0.01; or ***P < 0.001.
Figure 3. Enzyme-linked immunospot detection of EV71-specific IFN-γ -secreting T cells and IL-4-secreting T cells in spleen lymphocytes from immunized mice. Statistical significance was determined by the Student t test and is indicated as follows: n.s, P > 0.05; ***P < 0.001. SFC stands for spot-forming cells.
-
[1] Yi EJ, Shin YJ, Kim JH, et al. Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res, 2017; 6, 4-14. doi: 10.7774/cevr.2017.6.1.4 [2] National Health and Family Planning Commision of the PRC. The epidemic situation of national notifiable infectious diseases. http://www.nhfpc.gov.cn/jkj/s3578/new_list.shtml. [2018-01-11] [3] Shigui Y, Jie W, Cheng D, et al. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak:an observational trend study. Lancet Infect Dis, 2017; 17, 716-25. doi: 10.1016/S1473-3099(17)30227-X [4] Yang F, Yuan J, Wang X, et al. Severe hand, foot, and mouth disease and coxsackievirus A6-Shenzhen, China. Clin Infect Dis, 2014; 59, 1504-5. doi: 10.1093/cid/ciu624 [5] Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis, 2010; 10, 778-90. doi: 10.1016/S1473-3099(10)70194-8 [6] Wang X, Peng W, Ren J, et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol, 2012; 19, 424-9. doi: 10.1038/nsmb.2255 [7] Huang SW, Cheng HL, Hsieh HY, et al. Mutations in the non-structural protein region contribute to intra-genotypic evolution of enterovirus 71. J Biomed Sci, 2014; 21, 33. doi: 10.1186/1423-0127-21-33 [8] Foo DG, Alonso S, Phoon MC, et al. Identification of neutralizing linear epitopes from the VP1 capsid protein of enterovirus 71 using synthetic peptides. Virus Res, 2007; 125, 61-8. doi: 10.1016/j.virusres.2006.12.005 [9] Dudek NL, Perlmutter P, Aguilar MI, et al. Epitope discovery and their use in peptide based vaccines. Curr Pharm Des, 2010; 16, 3149 -57. doi: 10.2174/138161210793292447 [10] Bachmann MF, Jennings GT. Vaccine Delivery:A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat Rev Immunol, 2010; 10, 787-96. doi: 10.1038/nri2868 [11] Brune KD, Leneghan DB, Brian IJ, et al. Plug-and-Display:decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci Rep, 2016; 6, 19234. doi: 10.1038/srep19234 [12] Thrane S, Janitzek CM, Agerbæk M, et al. A Novel Virus-Like Particle Based Vaccine Platform Displaying the Placental Malaria Antigen VAR2CSA. PLoS One, 2015; 10, e0143071. doi: 10.1371/journal.pone.0143071 [13] Koho T, Ihalainen TO, Stark M, et al. His-tagged norovirus-like particles:A versatile platform for cellular delivery and surface display. Eur J Pharm Biopharm, 2015; 96, 22-31. doi: 10.1016/j.ejpb.2015.07.002 [14] Frietze KM, Peabody DS, Chackerian B. Engineering virus-like particles as vaccine platforms. Curr Opin Virol, 2016; 18, 44-9. doi: 10.1016/j.coviro.2016.03.001 [15] Plummer EM, Manchester M. Viral Nanoparticles and Virus-Like Particles:Platforms for Contemporary Vaccine Design. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2011; 3, 174-96. doi: 10.1002/wnan.119 [16] Pumpens P, Grens E. HBV Core Particles as a Carrier for B Cell/T Cell Epitopes. Intervirology, 2011; 44, 98-114. https://www.researchgate.net/profile/Paul_Pumpens/publication/11835266_HBV_Core_Particles_as_a_Carrier_for_B_CellT_Cell_Epitopes/links/54e4a48c0cf29865c334b33b.pdf?origin=publication_list [17] Fan P, Li X, Sun S, et al. Identification of a Common Epitope between Enterovirus 71 and Human MED25 Proteins Which May Explain Virus-Associated Neurological Disease. Viruses, 2015; 7, 1558-77. doi: 10.3390/v7041558 [18] Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS):toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res, 2013; 56, 304-16. doi: 10.1007/s12026-013-8403-1 [19] Cao L, Mao F, Pang Z, et al. Protective effect of enterovirus-71 (EV71) virus-like particle vaccine against lethal EV71 infection in a neonatal mouse model. Mol Med Rep, 2015; 12, 2473-80. doi: 10.3892/mmr.2015.3680 [20] Ku Z, Ye X, Huang X, et al. Neutralizing antibodies induced by recombinant virus-like particles of enterovirus 71 genotype C4 inhibit infection at pre-and post-attachment steps. PLoS One, 2013; 8, e57601. doi: 10.1371/journal.pone.0057601 [21] Ye X, Ku Z, Liu Q, et al. Chimeric Virus-Like Particle Vaccines Displaying Conserved Enterovirus 71 Epitopes Elicit Protective Neutralizing Antibodies in Mice through Divergent Mechanisms. J Virol, 2014; 88, 72-81. doi: 10.1128/JVI.01848-13 [22] Majorek KA, Kuhn ML, Chruszcz M, et al. Double trouble-buffer selection and His-tag presence may be responsible for nonreproducibility of biomedical experiments. Protein Sci, 2014; 23, 1359-68. doi: 10.1002/pro.v23.10 [23] Zhao X, Zhang H, Liu Y, et al. Preparation of monoclonal antibodies against His-tag and epitope analysis of cross antigens. Chin J Cell Mol Immunol, 2016; 32, 683-7. (In Chinese) http://europepmc.org/abstract/med/27126949 [24] Sedlik C. Thl and Th2 subsets of T lymphocytes:Characteristics, physiological role and regulation. Bull Inst Pasteur, 1996; 94, 173-200. doi: 10.1016/S0020-2452(97)86016-2 [25] Mosmann TR, Sad S. The expanding universe of T-cells subsets:Thl, Th2 and more. Immunol Today, 1996; 17, 138-46. doi: 10.1016/0167-5699(96)80606-2 [26] Debin A, Kravtzoff R, Santiago JV, et al. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine, 2002; 20, 2752-63. doi: 10.1016/S0264-410X(02)00191-3 [27] Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus Type 71:neutralization versus antibody dependent enhancement of infection. PLoS One, 2013; 8, e64024. doi: 10.1371/journal.pone.0064024 [28] Guo F, Zhao Q, Sheraz M, et al. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. Plos Pathogens, 2017; 13, e1006658. doi: 10.1371/journal.ppat.1006658 [29] Akbar SM, Chen S, Almahtab M, et al. Strong and multi-antigen specific immunity by hepatitis B core antigen (HBcAg)-based vaccines in a murine model of chronic hepatitis B:HBcAg is a candidate for a therapeutic vaccine against hepatitis B virus. Antiviral Res, 2012; 96, 59-64. doi: 10.1016/j.antiviral.2012.07.011 [30] Xu L, He D, Yang L, et al. A broadly cross-protective vaccine presenting the neighboring epitopes within the VP1 GH loop and VP2 EF loop of enterovirus 71. Sci Rep, 2015; 5, 12973. doi: 10.1038/srep12973 -




 下载:
下载:



 Quick Links
Quick Links