-
Pneumonia is a leading cause of death worldwide [1]. Community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP) are highly prevalent in the elderly, and the incidence of pneumonia increases with age [2]. Staphylococcus aureus is one of the most common pathogens [3], and its strain, methicillin-resistant Staphylococcus aureus (MRSA), is a multidrug resistant “super bacterium” resistant to penicillin, cephalosporins, chloramphenicol, lincomycin, aminoglycosides, tetracyclines, macrolides, quinophthalones, sulfonamides, and rifampicin; this increased resistance poses a difficult problem in clinical treatment [4]. Vancomycin has long been considered the first-line antibiotic for the treatment of severe MRSA infections, and serum trough concentrations of 15–20 mg/L are recommended by the Infectious Diseases Society of America (IDSA) [5].
The dosage of vancomycin must be adjusted by therapeutic drug monitoring because of its narrow therapeutic concentration window [6], as higher vancomycin trough levels are associated with a greater risk for vancomycin-associated acute kidney injury (VA-AKI) [7,8]. Our previous investigation revealed a low (47.1%) proportion of elderly patients with vancomycin serum trough concentrations reaching the target concentrations (≥ 15 mg/L) [9]. In addition, adjusting the vancomycin regimen to reach the target trough concentration took a considerable amount of time, which might have affected the efficacy.
Vancomycin serum trough concentrations correlate with various factors,especially creatinine clearance rate (CCR) [9,10]. Our previous study indicated that CCR and daily dosage of vancomycin significantly correlated with vancomycin serum trough concentrations in elderly patients, and a model for predicting vancomycin trough concentrations was established as:
serum trough concentration (mg/L) = 15.942 − 0.101 × CCR (mL/min) + 0.347 × vancomycin daily dose [(mg/(kg∙d)] [9].
An initial vancomycin regimen based on this model may help improve the proportion of elderly patients with serum trough concentrations reaching the target values, but it is yet to be tested. The present study aimed to evaluate the clinical benefits of individualized vancomycin therapy in elderly patients with severe pneumonia.
-
This study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee (approval number M2019017). Written informed consent to participate in the study was obtained from all patients or their legally-authorized representatives. This study is registered at ClinicalTrials.gov (number NCT04088305).
-
This was a prospective single-center, open-label, randomized controlled trial conducted in the medical intensive care unit (ICU) of Peking University Third Hospital.
-
Hospitalized patients with severe CAP [11], HAP, or VAP [12] were eligible for the study if they met the following criteria: 1) ≥ 60 years of age; 2) Suspected or confirmed MRSA pneumonia requiring vancomycin therapy.
Severe pneumonia was defined as meeting one major criteria, or three or more minor criteria [11]. The criteria are as follows: 1) Septic shock with need for vasopressors; 2) Respiratory failure requiring mechanical ventilation; 3) Respiratory rate > 30 breaths/min; 4) Ratio of the partial pressure of oxygen in arterial blood to the fraction of inspired oxygen (PaO2/FiO2) < 250; 5) Multilobar infiltrates; 6) Confusion/disorientation; 7) Uremia [blood urea nitrogen level (BUN) > 20 mg/dL]; 8) Leukopenia (white blood cell count < 4,000 cells/μL) due to infection; 9) Thrombocytopenia (platelet count < 100,000/μL); 10) Hypothermia (core temperature < 36 ℃); 11) Hypotension requiring aggressive fluid resuscitation.
Patients were excluded if they met one of the following criteria: 1) Had documented hypersensitivity to vancomycin; 2) Accepted blood purification therapy; 3) Had antibodies to the human immunodeficiency virus; 4) Had known/suspected tuberculosis, viral, or fungal infection at baseline.
Specimens from the lower respiratory tract included sputum (with < 10 squamous epithelial cells and > 25 polymorphonuclear cells per ×10 magnification field), tracheal aspirates, and bronchoalveolar lavage fluid. Isolation and identification of the causative bacteria were done by following standard procedures.
Baseline data of the patients were analyzed, including sex, age, weight, CAP or HAP/VAP, Acute Physiology and Chronic Health Evaluation (APACHE) II score, complete blood count, procalcitonin, PaO2/FiO2, creatinine concentration in serum (Cr), BUN, as well as the concentrations of alanine aminotransferase, aspartate aminotransferase, and albumin (ALB). CCR was calculated using the Cockcroft–Gault method [13].
-
Patients were assigned to the study or control group based on a computer-generated randomization schedule.
Patients in the control group received vancomycin with a regimen decided by the attending physician. The specific empirical regimens were 1,000 mg of vancomycin every 8 or 12 hours if CCR was ≥ 130 mL/min, 1,000 mg every 12 hours if CCR was between 70 and 130 mL/min, 500 mg every 8 hours if CCR was between 50 and 70 mL/min, 500 mg every 12 hours if CCR was between 30 and 50 mL/min, and 500 mg every 24 hours if CCR was < 30 mL/min.
Patients in the study group received vancomycin with a regimen based on a serum trough concentration model [9], using the minimum daily dosage that estimated the serum trough concentration could reach 15 mg/L.
Patients were intermittently infused with 500 or 1,000 mg of vancomycin every 8, 12, or 24 hours with the duration of the infusion being one hour.
The doses of vancomycin were adjusted according to the serum trough concentrations: the daily dose was increased by 500–1,000 mg when the trough concentration was < 15 mg/L, left unchanged when the trough concentration was between 15 and 20 mg/L, and decreased by 500–1,000 mg when the trough concentration was > 20 mg/L. The next dose was canceled when the trough concentration exceeded 30 mg/L.
Serum vancomycin concentrations were measured using a chemiluminescent microparticle immunoassay on an automated Abbott Architect i2000 (Abbott Laboratories). Blood sampling was performed before the fourth or fifth dose of vancomycin (trough). Serum was separated by centrifugation immediately after sampling.
All other treatments were at the discretion of the attending physician. If gram-negative bacilli co-infection was suspected or confirmed, broad-spectrum antibiotics were administered in accordance with the standard clinical practice.
-
The primary endpoint was the proportion of patients with trough concentrations reaching the target value (≥ 15 mg/L). The secondary endpoints were clinical response, vancomycin treatment duration, and VA-AKI.
Clinical response was categorized as “clinical success” (cure or improvement of all signs and symptoms caused by the infection and no requirement for additional antibacterial therapy) or “clinical failure” (persistence or worsening of any sign or symptom, development of any new signs or symptoms of infection, or requirement for other systemic antimicrobial therapy at the end of vancomycin therapy). Clinical response was assessed one week after vancomycin withdrawal.
Bacteriological response was categorized as “bacteriologic success” (eradication and presumed eradication) or “bacteriologic failure” (persistence and presumed persistence). The bacteriologic response was assessed by cultures of the sputum or tracheal aspirates obtained on the day after treatment cessation.
VA-AKI was defined as an increase in serum Cr by 0.3 mg/dL (26.5 μmol/L) or more within 48 hours, or 1.5 times or more increase in Cr from baseline, or a urine volume less than 0.5 mL/(kg∙h) for 6 hours [14].
-
Continuous variables were expressed as medians (interquartile range) and categorical variables as frequencies (%). Clinical data, as well as the baseline data and outcomes, were compared between the two patient groups. Continuous variables were compared using the non-parametric Mann-Whitney U test, and categorical variables were compared using the χ2 test.
Based on our previous study on an individualized administration model of vancomycin in elderly patients with sepsis [9], the proportion of patients with trough concentrations reaching the target values (≥ 15 mg/L) was estimated to be 80% in the study group and 50% in the control group. Therefore, we ascertained that 33 patients in each group would provide 80% power to detect a significant difference in the proportion of patients with trough concentrations reaching target values with P < 0.05 (two-sided) denoting significance.
All the analyses were conducted using SPSS, version 22.0 (IBM). A P < 0.05 was considered statistically significant.
-
From October 2019 to June 2022, 66 patients with severe pneumonia were enrolled and assigned randomly to the study (33 cases) or control group (33 cases) (Figure 1). The median age of the patients (39 men, 27 women) was 81 years. The median APACHE II score of the patients was 20, and CAP accounted for 43.9% (29 cases) of all cases. Baseline characteristics of the two groups were similar (Table 1).
Figure 1. Flowchart of patients who were included in and excluded from the study. MRSA, methicillin-resistant Staphylococcus aureus.
Table 1. Comparison of the demographic and clinical data of elderly patients with severe pneumonia between the study group and control group (n = 66)
Characteristics All patients (n = 66) Study group (n = 33) Control group (n = 33) P-value Age (years) 81 (69−85) 81 (69−84) 81 (69−86) 0.973 Men 39 (59.1) 18 (54.5) 21 (63.6) 0.453 Body weight (kg) 60.0 (52.2−72.0) 62.5 (52.7−75.0) 59.0 (51.0−66.4) 0.284 Comorbidities COPD 5 (7.6) 2 (6.1) 3 (9.1) 1.000 Diabetes mellitus 21 (31.8) 8 (24.2) 13 (39.4) 0.186 Hypertension 29 (43.9) 14 (42.4) 15 (45.5) 0.804 Cerebrovascular disease 24 (36.4) 12 (36.4) 12 (36.4) 1.000 Neoplasm 6 (9.1) 3 (9.1) 3 (9.1) 1.000 Liver disease 2 (3.0) 0 2 (6.1) 0.492 Heart failure 18 (27.3) 12 (36.4) 6 (18.2) 0.097 Renal disease 8 (12.1) 3 (9.1) 5 (15.2) 0.708 Community−acquired pneumonia 29 (43.9) 18 (54.5) 11 (33.3) 0.083 WBC (× 109/L) 10.5 (7.7−16.9) 10.4 (6.1−16.9) 10.6 (8.1−17.8) 0.320 NEU (%) 85.2 (75.3−92.0) 83.0 (73.8−88.7) 87.6 (77.9−93.5) 0.121 HGB (g/L) 105.5 (92.0−120.5) 109.0 (91.0−125.5) 100.0 (93.5−116.0) 0.281 PLT (× 109/L) 171.5 (102.3−282.3) 158.0 (96.5−268.0) 172.0 (118.0−284.0) 0.681 PCT (ng/mL) 0.4 (0.1−2.9) 0.3 (0.1−3.0) 0.4 (0.2−2.4) 0.323 BUN (mmol/L) 9.9 (6.4−15.4) 8.4 (5.8−14.9) 10.0 (6.5−16.1) 0.696 Serum creatinine (µmol/L) 74.5 (51.0−106.8) 76.0 (52.5−99.0) 73.0 (50.0−140.5) 0893 CCR (mL/min) 58.8 (40.6−81.5) 60.5 (47.2−75.0) 54.8 (30.0−95.5) 0.906 ALT (U/L) 23.0 (16.8−39.5) 23.0 (17.5−38.0) 25.0 (13.0−57.5) 1.000 AST (U/L) 35.0 (22.0−65.5) 37.0 (24.0−63.0) 34.0 (20.0−80.5) 0.778 Serum albumin (g/L) 32.1 (28.0−37.3) 30.8 (28.6−36.7) 33.1 (28.0−37.3) 0.852 PaO2/FiO2 220.0 (164.5−314.0) 231.5 (195.2−330.3) 219.0 (149.5−313.5) 0.627 APACHEII score 20.0 (13.0−26.0) 18.0 (12.0−23.0) 21.5 (15.0−27.0) 0.136 Note. Data are presented as n (%) or medians (interquartile range). COPD: Chronic obstructive pulmonary disease; WBC: White blood cell; NEU: Neutrophil; HGB: Hemoglobin; PLT: Platelet; PCT: Procalcitonin; BUN: Blood urea nitrogen; CCR: Creatinine clearance; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; APACHE: Acute Physiology and Chronic Health Evaluation. Prevalence of serum trough concentration ≥ 15 mg/L with the initial vancomycin regimen was significantly higher in the study group than that in the control group (75.8% vs. 42.4%, P = 0.006) (Figure 2). The proportion of patients with trough concentrations reaching target values of 15–20 mg/L was also significantly higher in the study group (24.2% reached less than 15 mg/L, 48.5% between 15–20 mg/L, 27.3% more than 20 mg/L) compared to the control group (57.6% less than 15 mg/L, 15.2% between 15–20 mg/L, 27.3% more than 20 mg/L), P = 0.006 (Figure 3).
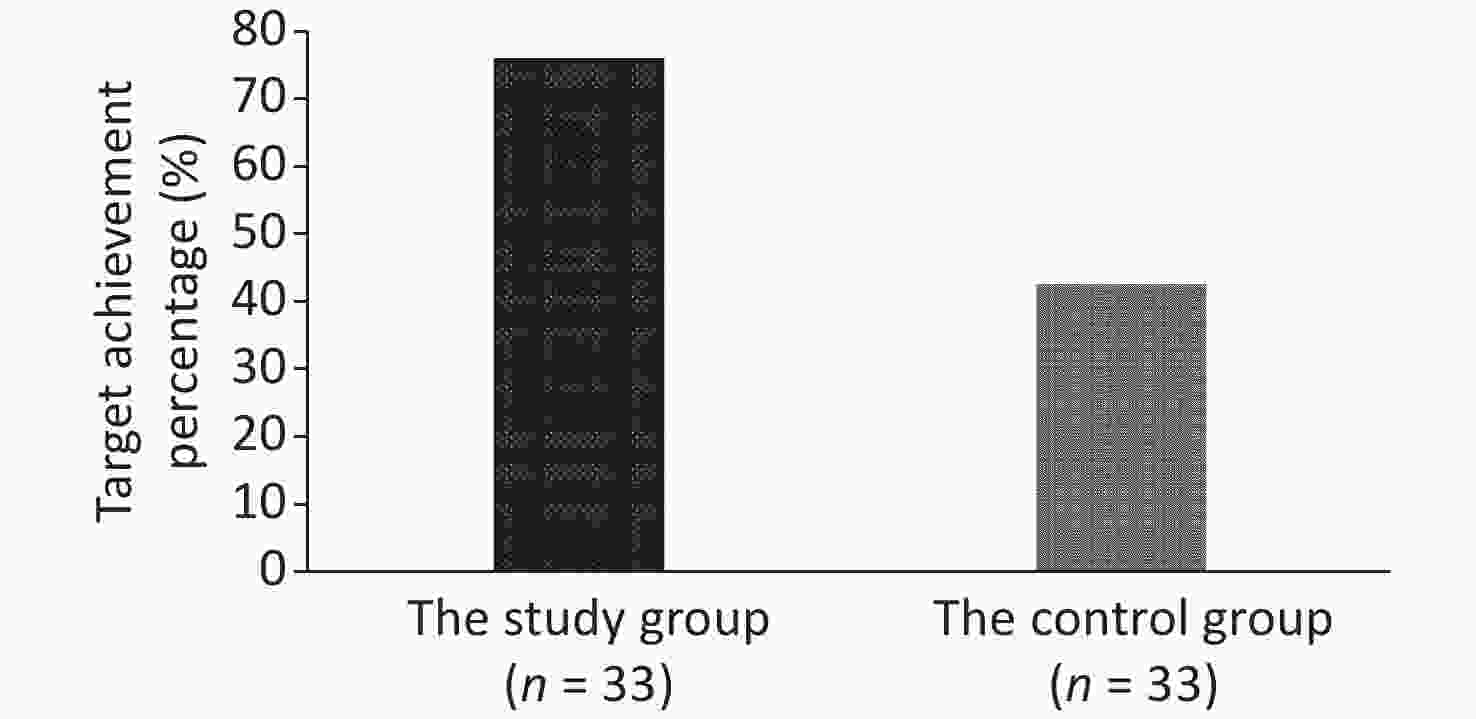
Figure 2. Proportion of patients with target serum trough concentration achievement (≥ 15 mg/L). The initial vancomycin regimen was significantly higher in the study group compared to the control group (75.8% vs. 42.4%, P = 0.006).
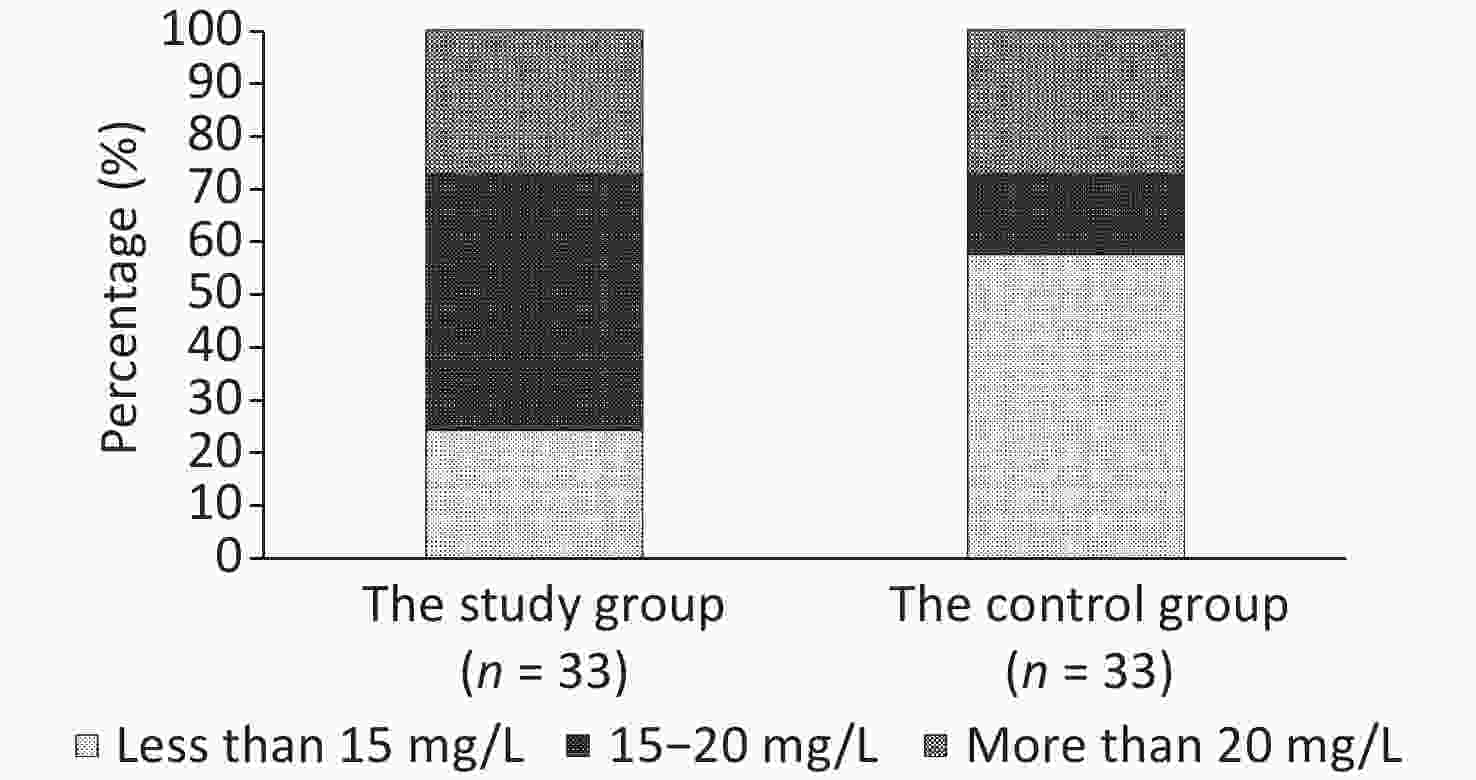
Figure 3. Proportion of patients with trough concentrations reaching the target range of 15–20 mg/L was significantly higher in the study group compared to the control group. In the study group:24.2% patients achieved less than 15 mg/L, 48.5% between 15–20 mg/L, and 27.3% more than 20 mg/L; in the control group: 57.6% patients achieved less than 15 mg/L, 15.2% between 15–20 mg/L, and 27.3% more than 20 mg/L; P = 0.006.
Forty-five patients (68.2%) achieved clinical success, the median duration of vancomycin therapy was ten days, VA-AKI occurred in eight patients (12.1%), and 18 patients (27.3%) accounted for in-hospital mortality. However, there were no significant differences in these parameters between the two groups (Table 2).
Table 2. Comparison of the clinical response and side effects of vancomycin therapy between the study group and control group (n = 66)
Characteristics All patients (n = 66) Study group (n = 33) Control group (n = 33) P-value Clinical response to vancomycin therapy 0.792 Clinical success 45 (68.2) 23 (69.7) 22 (66.7) Clinical failure 21 (31.8) 10 (30.3) 11 (33.3) Side effects of vancomycin therapy VA-AKI 8 (12.1) 2 (6.1) 6 (18.2) 0.258 Rashes 2 (3.0) 2 (6.1) 0 0.492 ALT/AST increase 4 (6.1) 1 (3.0) 3 (9.1) 0.613 Daily dosage of initial vancomycin regimen (g) 1.0 (1.0–1.5) 1.0 (1.0–1.5) 1.0 (0.8–1.5) 0.709 Duration of vancomycin therapy (day) 10.0 (7.0–14.0) 10.0 (7.0–14.0) 10.0 (7.0–14.0) 0.918 In-hospital mortality 18 (27.3) 8 (24.2) 10 (30.3) 0.580 Note. Data are presented as n (%) or medians (interquartile range). VA-AKI: Vancomycin associated acute kidney injury; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase. Gram-positive cocci were isolated from 34 patients, including 11 strains of Staphylococcus aureus, of which 45.5% were methicillin-resistant; 21 strains of Coagulase-negative staphylococci, with methicillin-resistant strains accounting for 51.7%; and two strains of Enterococcus. Pathogens of severe pneumonia in the two groups were similar, and minimum inhibitory concentration (MIC) values of vancomycin were significantly higher in the study group compared to the control group (Table 3).
Table 3. Comparison of the bacteriological response to vancomycin therapy between the study group and control group (n = 34)
Characteristics All patients (n = 34) Study group (n = 18) Control group (n = 16) P-value Bacteriological response to vancomycin therapy 1.000 Bacteriologic success 27 (79.4) 14 (77.8) 13 (81.3) Bacteriologic failure 7 (20.6) 4 (22.2) 3 (18.8) Isolated gram-positive cocci 0.068 Staphylococcus aureus 11 (32.4) 8 (44.4) 3 (18.8) Coagulase-negative staphylococci 21 (61.8) 8 (44.4) 13 (81.3) Enterococcus 2 (5.9) 2 (11.1) 0 MIC values for vancomycin (mg/L) 1.0 (0.5–1.0) 1.0 (1.0–2.0) 0.5 (0.5–1.0) 0.003 Note. Data are presented as n (%) or medians (interquartile range). MIC: Minimum inhibitory concentration. The model for predicting vancomycin trough concentrations used in the present study was established by evaluating 87 elderly patients in our previous study [9]. After optimization using the data of 66 patients in the present study, the model for predicting vancomycin trough concentrations was upgraded to:
serum trough concentration (mg/L) = 17.194 – 0.104 × CCR (mL/min) + 0.313 × vancomycin daily dose [mg/(kg∙d)] (Table 4).
Table 4. The upgraded model for predicting vancomycin trough concentrations
Parameter B Std. Error t P-value 95% confidence interval Lower Bound Upper Bound Constant 17.194 1.822 9.437 < 0.001 13.593 20.794 Creatinine clearance (mL/min) −0.104 0.019 −0.459 < 0.001 −0.141 −0.067 Vancomycin daily dose [mg/(kg∙d)] 0.313 0.079 3.960 < 0.001 0.157 0.468 -
In the present study, a vancomycin dosage strategy based on a serum trough concentration model was evaluated in elderly patients with severe pneumonia. We found that the number of patients with trough concentrations reaching the target values in the individualized vancomycin therapy group was significantly elevated compared to the control group.
Vancomycin is a glycopeptide antibiotic and is the first-line treatment for infections caused by MRSA and other multidrug-resistant gram-positive cocci. The area under the concentration-time curve to MIC ratio is the most useful pharmacodynamic parameter to predict vancomycin effectiveness, which suggests a target ratio of 400 or greater to eradicate Staphylococcus aureus [15,16]. The corresponding serum trough concentrations are 15–20 mg/L when MIC ≤ 1.0 mg/L [17]. Therefore, vancomycin serum trough concentrations of 15–20 mg/L are recommended for severe infections by the IDSA. However, VA-AKI often limits its use, and higher vancomycin trough concentrations of > 20 mg/L are independently associated with VA-AKI [7]. Patients aged ≥ 80 years with vancomycin trough concentration ≥ 20 mg/L appear to have a worse prognosis than that of patients with lower vancomycin trough concentrations [18]. Therefore, the initial vancomycin regimen should be individualized to improve outcomes and patient safety, especially in elderly patients.
The incidence of subtherapeutic trough concentrations of vancomycin is high in patients receiving vancomycin treatment. The Defining Antibiotic Levels in Intensive Care study demonstrated a large interindividual variability in vancomycin pharmacokinetic and pharmacodynamic target attainment among ICU patients, while the target trough concentrations were achieved in only 39% and 71% of patients receiving intermittent and continuous infusion, respectively [19]. Molina et al.[20] reported a 46% incidence of subtherapeutic trough concentrations of vancomycin in 119 trauma patients. Furthermore, subtherapeutic concentrations (< 10 mg/L) have been observed in 56.3% of morbidly obese patients [21]. Our previous study showed that the incidence of subtherapeutic trough concentrations of vancomycin in elderly patients was 52.9% [9], which was consistent with the results of previous studies. In addition, the majority of patients had subtherapeutic vancomycin concentrations, which increases the risk of treatment failures and resistance.
The initial trough concentration is important for achieving better outcomes with vancomycin treatment, and individualized vancomycin treatment is helpful in achieving target trough concentrations. Kondo et al. [22] reported that a higher vancomycin concentration tended to be associated with a shorter time to achieve a 50% decline in C-reactive protein levels. Furthermore, the time to resolution of fever in patients with a higher initial trough level was significantly lower than that in patients with a lower trough level, suggesting that the initial trough concentration was important for achieving better outcomes with vancomycin treatment. Truong et al. [23] retrospectively compared the time to attain a target trough level and incidence of VA-AKI in ICU patients whose vancomycin was dosed by a pharmacy pharmacokinetic (PK) dosing and monitoring service to the standard of care. Fifty patients were included in each group. The proportions of patients attaining goal trough concentrations in the PK and traditional groups were 84.4% and 29.4% by 48 hours (P = 0.0001), 88.4% and 60.7% by 72 hours (P = 0.009), and 92.9% and 77.8% by 96 hours (P = 0.100), respectively. Our present study evaluated elderly patients, and the vancomycin dosage strategy based on a serum trough concentration model significantly improved the achievement of target trough concentration from 42.4% to 75.8% (P = 0.006).
Many factors can influence the trough concentration, such as renal function [24-26], age [27], ALB blood level [28], sepsis duration [29], and concomitant diuretic use [30]. Nevertheless, in elderly patients, renal function is the most important variable for vancomycin trough concentration. In our previous study conducted on 78 elderly patients who were at least 60 years old, multivariate linear regression showed that the CCR and daily dosage of vancomycin were independent predictors of vancomycin serum trough concentrations, yielding the model for predicting vancomycin trough concentration [9].
After optimization using data from 66 patients in the present study, the model for predicting vancomycin trough concentrations was upgraded to:
serum trough concentration (mg/L) = 17.194 − 0.104 × CCR (mL/min) + 0.313 × vancomycin daily dose [mg/(kg∙d)].
This model can improve the accuracy of prediction and decrease the number of patients with subtherapeutic or supratherapeutic trough concentrations.
Our study has two main limitations. First, our previous model for predicting vancomycin trough concentrations was developed for patients aged ≥ 60 years; therefore, the present study was limited to elderly patients. Second, we determined the CCR using the Cockcroft-Gault formula, which may not be accurate in critically ill patients. However, the Cockcroft-Gault equation is widely used by clinicians because it is convenient as well as the most timely indicator to determine the initial vancomycin regimen.
-
A strategy for vancomycin dosage based on a serum trough concentration model can improve the proportion of patients with trough concentrations approaching the target values among elderly patients with severe pneumonia. The model for predicting vancomycin trough concentrations was upgraded to: serum trough concentration (mg/L) = 17.194 – 0.104 × CCR (mL/min) + 0.313 × vancomycin daily dose [mg/(kg∙d)]. Our findings have potential value for the development of individualized antibiotic regimens.
-
ZHOU Qing Tao designed and coordinated the study. YAN Wei and SUN Xiao Yan were responsible for patient screening, enrollment, and follow-up. WANG Meng and ZHAO Fei Fan collected and analyzed the data. YAN Wei and SUN Xiao Yan drafted the manuscript. ZHOU Qing Tao critically revised the manuscript. All authors had full access to all study data, read and approved the final version of the manuscript.
-
The authors have no conflicts of interest that are directly relevant to the content of this study.
doi: 10.3967/bes2023.049
Clinical Evaluation of a Vancomycin Dosage Strategy Based on a Serum Trough Concentration Model in Elderly Patients with Severe Pneumonia
-
Abstract:
Objective This study aimed to evaluate the clinical benefits of a vancomycin dosage strategy based on a serum trough concentration model in elderly patients. Methods This prospective single-center, open-label, randomized controlled trial categorized 66 elderly patients with severe pneumonia into study and control groups. The control group received vancomycin using a regimen decided by the attending physician. Meanwhile, the study group received individualized vancomycin therapy with a dosing strategy based on a serum trough concentration model. The primary endpoint was the proportion of patients with serum trough concentrations reaching the target values. The secondary endpoints were clinical response, vancomycin treatment duration, and vancomycin-associated acute kidney injury (VA-AKI) occurrence. Results All patients were at least 60 years old (median age = 81 years). The proportion of patients with target trough concentration achievement (≥ 15 mg/L) with the initial vancomycin regimen was significantly higher in the study group compared to the control group (75.8% vs. 42.4%, P = 0.006). Forty-five patients (68.2%) achieved clinical success, the median duration of vancomycin therapy was 10.0 days, and VA-AKI occurred in eight patients (12.1%). However, there were no significant differences in these parameters between the two groups. The model for predicting vancomycin trough concentrations was upgraded to: serum trough concentration (mg/L) = 17.194 − 0.104 × creatinine clearance rate (mL/min) + 0.313 × vancomycin daily dose [(mg/(kg∙d)]. Conclusion A vancomycin dosage strategy based on a serum trough concentration model can improve the proportion of patients achieving target trough concentrations in elderly patients with severe pneumonia. -
Key words:
- Pneumonia /
- Clinical trials /
- Vancomycin /
- Trough concentration /
- Elderly patients
注释: -
Figure 3. Proportion of patients with trough concentrations reaching the target range of 15–20 mg/L was significantly higher in the study group compared to the control group. In the study group:24.2% patients achieved less than 15 mg/L, 48.5% between 15–20 mg/L, and 27.3% more than 20 mg/L; in the control group: 57.6% patients achieved less than 15 mg/L, 15.2% between 15–20 mg/L, and 27.3% more than 20 mg/L; P = 0.006.
Table 1. Comparison of the demographic and clinical data of elderly patients with severe pneumonia between the study group and control group (n = 66)
Characteristics All patients (n = 66) Study group (n = 33) Control group (n = 33) P-value Age (years) 81 (69−85) 81 (69−84) 81 (69−86) 0.973 Men 39 (59.1) 18 (54.5) 21 (63.6) 0.453 Body weight (kg) 60.0 (52.2−72.0) 62.5 (52.7−75.0) 59.0 (51.0−66.4) 0.284 Comorbidities COPD 5 (7.6) 2 (6.1) 3 (9.1) 1.000 Diabetes mellitus 21 (31.8) 8 (24.2) 13 (39.4) 0.186 Hypertension 29 (43.9) 14 (42.4) 15 (45.5) 0.804 Cerebrovascular disease 24 (36.4) 12 (36.4) 12 (36.4) 1.000 Neoplasm 6 (9.1) 3 (9.1) 3 (9.1) 1.000 Liver disease 2 (3.0) 0 2 (6.1) 0.492 Heart failure 18 (27.3) 12 (36.4) 6 (18.2) 0.097 Renal disease 8 (12.1) 3 (9.1) 5 (15.2) 0.708 Community−acquired pneumonia 29 (43.9) 18 (54.5) 11 (33.3) 0.083 WBC (× 109/L) 10.5 (7.7−16.9) 10.4 (6.1−16.9) 10.6 (8.1−17.8) 0.320 NEU (%) 85.2 (75.3−92.0) 83.0 (73.8−88.7) 87.6 (77.9−93.5) 0.121 HGB (g/L) 105.5 (92.0−120.5) 109.0 (91.0−125.5) 100.0 (93.5−116.0) 0.281 PLT (× 109/L) 171.5 (102.3−282.3) 158.0 (96.5−268.0) 172.0 (118.0−284.0) 0.681 PCT (ng/mL) 0.4 (0.1−2.9) 0.3 (0.1−3.0) 0.4 (0.2−2.4) 0.323 BUN (mmol/L) 9.9 (6.4−15.4) 8.4 (5.8−14.9) 10.0 (6.5−16.1) 0.696 Serum creatinine (µmol/L) 74.5 (51.0−106.8) 76.0 (52.5−99.0) 73.0 (50.0−140.5) 0893 CCR (mL/min) 58.8 (40.6−81.5) 60.5 (47.2−75.0) 54.8 (30.0−95.5) 0.906 ALT (U/L) 23.0 (16.8−39.5) 23.0 (17.5−38.0) 25.0 (13.0−57.5) 1.000 AST (U/L) 35.0 (22.0−65.5) 37.0 (24.0−63.0) 34.0 (20.0−80.5) 0.778 Serum albumin (g/L) 32.1 (28.0−37.3) 30.8 (28.6−36.7) 33.1 (28.0−37.3) 0.852 PaO2/FiO2 220.0 (164.5−314.0) 231.5 (195.2−330.3) 219.0 (149.5−313.5) 0.627 APACHEII score 20.0 (13.0−26.0) 18.0 (12.0−23.0) 21.5 (15.0−27.0) 0.136 Note. Data are presented as n (%) or medians (interquartile range). COPD: Chronic obstructive pulmonary disease; WBC: White blood cell; NEU: Neutrophil; HGB: Hemoglobin; PLT: Platelet; PCT: Procalcitonin; BUN: Blood urea nitrogen; CCR: Creatinine clearance; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; APACHE: Acute Physiology and Chronic Health Evaluation. Table 2. Comparison of the clinical response and side effects of vancomycin therapy between the study group and control group (n = 66)
Characteristics All patients (n = 66) Study group (n = 33) Control group (n = 33) P-value Clinical response to vancomycin therapy 0.792 Clinical success 45 (68.2) 23 (69.7) 22 (66.7) Clinical failure 21 (31.8) 10 (30.3) 11 (33.3) Side effects of vancomycin therapy VA-AKI 8 (12.1) 2 (6.1) 6 (18.2) 0.258 Rashes 2 (3.0) 2 (6.1) 0 0.492 ALT/AST increase 4 (6.1) 1 (3.0) 3 (9.1) 0.613 Daily dosage of initial vancomycin regimen (g) 1.0 (1.0–1.5) 1.0 (1.0–1.5) 1.0 (0.8–1.5) 0.709 Duration of vancomycin therapy (day) 10.0 (7.0–14.0) 10.0 (7.0–14.0) 10.0 (7.0–14.0) 0.918 In-hospital mortality 18 (27.3) 8 (24.2) 10 (30.3) 0.580 Note. Data are presented as n (%) or medians (interquartile range). VA-AKI: Vancomycin associated acute kidney injury; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase. Table 3. Comparison of the bacteriological response to vancomycin therapy between the study group and control group (n = 34)
Characteristics All patients (n = 34) Study group (n = 18) Control group (n = 16) P-value Bacteriological response to vancomycin therapy 1.000 Bacteriologic success 27 (79.4) 14 (77.8) 13 (81.3) Bacteriologic failure 7 (20.6) 4 (22.2) 3 (18.8) Isolated gram-positive cocci 0.068 Staphylococcus aureus 11 (32.4) 8 (44.4) 3 (18.8) Coagulase-negative staphylococci 21 (61.8) 8 (44.4) 13 (81.3) Enterococcus 2 (5.9) 2 (11.1) 0 MIC values for vancomycin (mg/L) 1.0 (0.5–1.0) 1.0 (1.0–2.0) 0.5 (0.5–1.0) 0.003 Note. Data are presented as n (%) or medians (interquartile range). MIC: Minimum inhibitory concentration. Table 4. The upgraded model for predicting vancomycin trough concentrations
Parameter B Std. Error t P-value 95% confidence interval Lower Bound Upper Bound Constant 17.194 1.822 9.437 < 0.001 13.593 20.794 Creatinine clearance (mL/min) −0.104 0.019 −0.459 < 0.001 −0.141 −0.067 Vancomycin daily dose [mg/(kg∙d)] 0.313 0.079 3.960 < 0.001 0.157 0.468 -
[1] Corrado RE, Lee D, Lucero DE, et al. Burden of adult community-acquired, health-care-associated, hospital-acquired, and ventilator-associated Pneumonia: New York City, 2010 to 2014. Chest, 2017; 152, 930−42. doi: 10.1016/j.chest.2017.04.162 [2] Ticona JH, Zaccone VM, McFarlane IM. Community-acquired Pneumonia: A focused review. Am J Med Case Rep, 2021; 9, 45−52. [3] Yin YY, Zhao CJ, Li HN, et al. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: a 10-year prospective observational study in China. Eur J Clin Microbiol Infect Dis, 2021; 40, 683−90. doi: 10.1007/s10096-020-04046-9 [4] Guo YL, Song GH, Sun ML, et al. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol, 2020; 10, 107. doi: 10.3389/fcimb.2020.00107 [5] Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis, 2011; 52, e18−55. doi: 10.1093/cid/ciq146 [6] Ye ZK, Chen YL, Chen K, et al. Therapeutic drug monitoring of vancomycin: a guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. J Antimicrob Chemother, 2016; 71, 3020−5. doi: 10.1093/jac/dkw254 [7] Park SJ, Lim NR, Park HJ, et al. Evaluation of risk factors for vancomycin-induced nephrotoxicity. Int J Clin Pharm, 2018; 40, 1328−34. doi: 10.1007/s11096-018-0634-8 [8] Perin N, Roger C, Marin G, et al. Vancomycin Serum Concentration after 48 h of administration: A 3-years survey in an intensive care unit. Antibiotics (Basel), 2020; 9, 793. doi: 10.3390/antibiotics9110793 [9] Zhou QT, Zhao FF, Wang M. An individualized administration model of vancomycin in elderly patients with sepsis and factors influencing augmented renal clearance. J Clin Pharm Ther, 2021; 46, 447−53. doi: 10.1111/jcpt.13304 [10] Masich AM, Kalaria SN, Gonzales JP, et al. Vancomycin pharmacokinetics in obese patients with sepsis or septic shock. Pharmacotherapy, 2020; 40, 211−20. doi: 10.1002/phar.2367 [11] Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired Pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med, 2019; 200, e45−67. doi: 10.1164/rccm.201908-1581ST [12] Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis, 2016; 63, e61−e111. doi: 10.1093/cid/ciw353 [13] Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron, 1976; 16, 31−41. doi: 10.1159/000180580 [14] Ad-Hoc Working Group of ERBP, Fliser D, Laville M, et al. A European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant, 2012; 27, 4263−72. doi: 10.1093/ndt/gfs375 [15] Ueda T, Takesue Y, Nakajima K, et al. Validation of vancomycin area under the concentration-time curve estimation by the Bayesian approach using one-point samples for predicting clinical outcomes in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics (Basel), 2022; 11, 96. doi: 10.3390/antibiotics11010096 [16] Mogle BT, Steele JM, Seabury RW, et al. Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents, 2018; 52, 805−10. doi: 10.1016/j.ijantimicag.2018.08.024 [17] Álvarez R, Cortés LEL, Molina J, et al. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother, 2016; 60, 2601−9. doi: 10.1128/AAC.03147-14 [18] Wang YC, Dai N, Wei W, et al. Outcomes and nephrotoxicity associated with vancomycin treatment in patients 80 years and older. Clin Interv Aging, 2021; 16, 1023−35. doi: 10.2147/CIA.S308878 [19] Blot S, Koulenti D, Akova M, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care, 2014; 18, R99. doi: 10.1186/cc13874 [20] Molina KC, Hall ST, Barletta JF, et al. Utilization of augmented renal clearance in trauma intensive care scoring system to improve vancomycin dosing in trauma patients at risk for augmented renal clearance. Surg Infect (Larchmt), 2020; 21, 43−7. doi: 10.1089/sur.2019.026 [21] Kosmisky DE, Griffiths CL, Templin MA, et al. Evaluation of a new vancomycin dosing protocol in morbidly obese patients. Hosp Pharm, 2015; 50, 789−97. doi: 10.1310/hpj5009-789 [22] Kondo M, Nakagawa S, Orii S, et al. Association of initial trough concentrations of vancomycin with outcomes in pediatric patients with gram-positive bacterial infection. Biol Pharm Bull, 2020; 43, 1463−8. doi: 10.1248/bpb.b19-01003 [23] Truong J, Smith SR, Veillette JJ, et al. Individualized pharmacokinetic dosing of vancomycin reduces time to therapeutic trough concentrations in critically Ill patients. J Clin Pharmacol, 2018; 58, 1123−30. doi: 10.1002/jcph.1273 [24] Tang L, Ding XY, Duan LF, et al. A regression model to predict augmented renal clearance in critically Ill obstetric patients and effects on vancomycin treatment. Front Pharmacol, 2021; 12, 622948. doi: 10.3389/fphar.2021.622948 [25] Qian XD, Du GT, Weng CM, et al. Evaluation of the variability and safety of serum trough concentrations of vancomycin in patients admitted to the intensive care unit. Int J Infect Dis, 2017; 60, 17−22. doi: 10.1016/j.ijid.2017.04.018 [26] Zhang XW, Wang DH. The characteristics and impact indicator of vancomycin pharmacokinetics in cancer patients complicated with severe pneumonia. J Infect Chemother, 2020; 26, 492−7. doi: 10.1016/j.jiac.2019.12.019 [27] He J, Mao EQ, Feng J, et al. The pharmacokinetics of vancomycin in patients with severe acute pancreatitis. Eur J Clin Pharmacol, 2016; 72, 697−702. doi: 10.1007/s00228-016-2018-0 [28] Hirai T, Hanada K, Iwamoto T, et al. Involvement of the effect of renal hypoperfusion medications on vancomycin trough concentration: A secondary analysis using a retrospective observational data. Basic Clin Pharmacol Toxicol, 2021; 129, 376−84. doi: 10.1111/bcpt.13646 [29] Chuma M, Makishima M, Imai T, et al. Duration of systemic inflammatory response syndrome influences serum vancomycin concentration in patients with sepsis. Clin Ther, 2016; 38, 2598−609. doi: 10.1016/j.clinthera.2016.10.009 [30] Chen CY, Xu P, Xu T, et al. Influence of cerebrospinal fluid drainage and other variables on the plasma vancomycin trough levels in postoperative neurosurgical patients. Br J Neurosurg, 2021; 35, 133−8. doi: 10.1080/02688697.2020.1769023 -




 下载:
下载:



 Quick Links
Quick Links