-
Osteoporosis (OP) is a serious public health concern that continues to rise as the global population ages. This condition weakens bone strength and increases the risk of fractures, particularly in the lumbar spine, hip, and other skeletal areas[1]. Sedentary lifestyles and prolonged periods of inactivity are well-known risk factors for decreased bone mass, whereas mechanical loading through exercise has been found to increase it[2,3]. Previous studies have established a clear association between a sedentary lifestyle and an increased likelihood of fractures[4,5]. Physical activity (PA) can mitigate the onset of OP and reduce fracture risk through two primary mechanisms. First, PA may enhance bone strength by optimizing bone mineral density (BMD) and improving bone quality. Second, PA has the potential to reduce the risk of falling. More robust evidence exists concerning its impact on BMD in the lumbar spine than in the hip region. Programs with higher intensity, multiple exercises, and resistance training have emerged as being more effective[6]. The 2020 guidelines on PA and sedentary behavior from the World Health Organization (WHO) offer comprehensive recommendations on the appropriate amount of PA for different age groups. These guidelines also specify the recommended type of PA for pregnant and postpartum women, as well as for individuals with chronic conditions or disabilities[7].
In China, nationwide surveys have indicated that the average overall prevalence of OP ranges from 6.6% to 19.3%. However, its prevalence varies greatly across studies, regions, sexes, and bone sites. Surprisingly, no significant difference was observed between urban and rural areas[8,9]. A recent meta-analysis revealed that OP has a global prevalence of 18.3%. Women had a significantly higher prevalence (23.1%) than men (11.7%). Africa has the highest prevalence of OP, at 39.5%[10]. OP imposes a considerable economic burden, as a Bone Health and Osteoporosis Foundation report indicates. This report emphasizes the substantial financial and societal impact of bone fractures associated with OP. This further suggests that even a modest reduction in secondary fractures can result in considerable savings in Medicare Fee-for-Service. Moreover, the increased implementation of interventions targeting OP can reduce fractures and generate substantial cost savings[11]. OP has the potential to inflict serious harm on individuals, manifesting as fractures, chronic pain, and disability. Specifically, the disease increases the likelihood of fractures, particularly in the hip, spine, and wrist[1]. The pathogenesis of OP is multifactorial and involves intricate interactions between genetic, hormonal, and environmental factors. The underlying molecular mechanisms of OP are thought to be attributable to the heightened activity of osteoclasts, reduced activity of osteoblasts, or both, resulting in an imbalance in the bone remodeling process characterized by increased bone resorption and diminished bone formation[12]. However, old age, sex, steroid deficiency, lipid oxidation, decreased PA, use of glucocorticoids, and a propensity to fall are the most critical determinants of increased fracture risk.
PA is encouraged to promote bone growth and maintain bone mass, particularly during healthy aging. Based on the well-established benefits of PA, numerous interventions have been developed and evaluated for preventing and treating OP[13,14]. Guidelines from various disciplines recommend incorporating PA into OP management, focusing on older individuals[6]. This has led to the release of the PA and Sedentary Behavior Guidelines for Chinese People in 2021, addressing the lack of exercise in the population and providing strategies to promote PA. However, adherence to these guidelines remains low, with only 22.4% of adults complying with the WHO guidelines[15].
Several studies have found that resistance training is associated with higher BMD compared with those who do not engage in such training[16]. A randomized controlled trial (RCT) meta-analysis further demonstrated that physical fitness programs can help prevent or reverse bone loss in pre- and postmenopausal women, specifically in the lumbar spine and femoral neck[17]. Exercise training has also been shown to reduce the risk and frequency of falls[18,19].
RCTs of PA interventions in adults aged > 40 years have demonstrated that increased PA, especially during leisure-time or moderate-to-vigorous activity, is linked to a 1% to 40% decrease in the risk of hip and overall fractures[20]. Researchers have investigated the efficacy of exercise interventions in reducing falls among older adults living in communities. The study evaluated the impact of various types of exercise as standalone interventions on fall prevention in individuals aged 60 years and older. The results revealed a significant 23% decrease in the rate of falls (rate ratio: 0.77, 95% confidence interval: 0.71 to 0.83) based on high-certainty evidence from including 12,981 participants across 59 studies[21].
These findings underscore the importance of regular exercise for maintaining bone density and combating OP. However, when investigating the correlation between PA and OP outcomes, researchers should consider factors that might not have been measured, such as blood biochemistry, BMD, and quality of life.
In light of the limited research on the correlation between physical exercise and OP, we conducted a prospective cohort study in China to investigate the relationship between PA and OP outcomes. We established the PA in OP Outcomes (PAOPO) study, which involved a substantial number of sedentary and moderately to very active men and women. The primary focus of this study was to examine the association between PA and the risk of OP while also considering the impact of other contributing factors on OP outcomes.
-
The PAOPO study is a large community-based, prospective, long-term, observational cohort study conducted in a Chinese population. The aim is to assess the relationship between PA, either on its own or in combination with other contributing factors, and OP outcomes. The study was conducted according to the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Staff Hospital of Jidong Oil Field, China National Petroleum Corporation (Jidong, China). All participants provided written informed consent before participation.
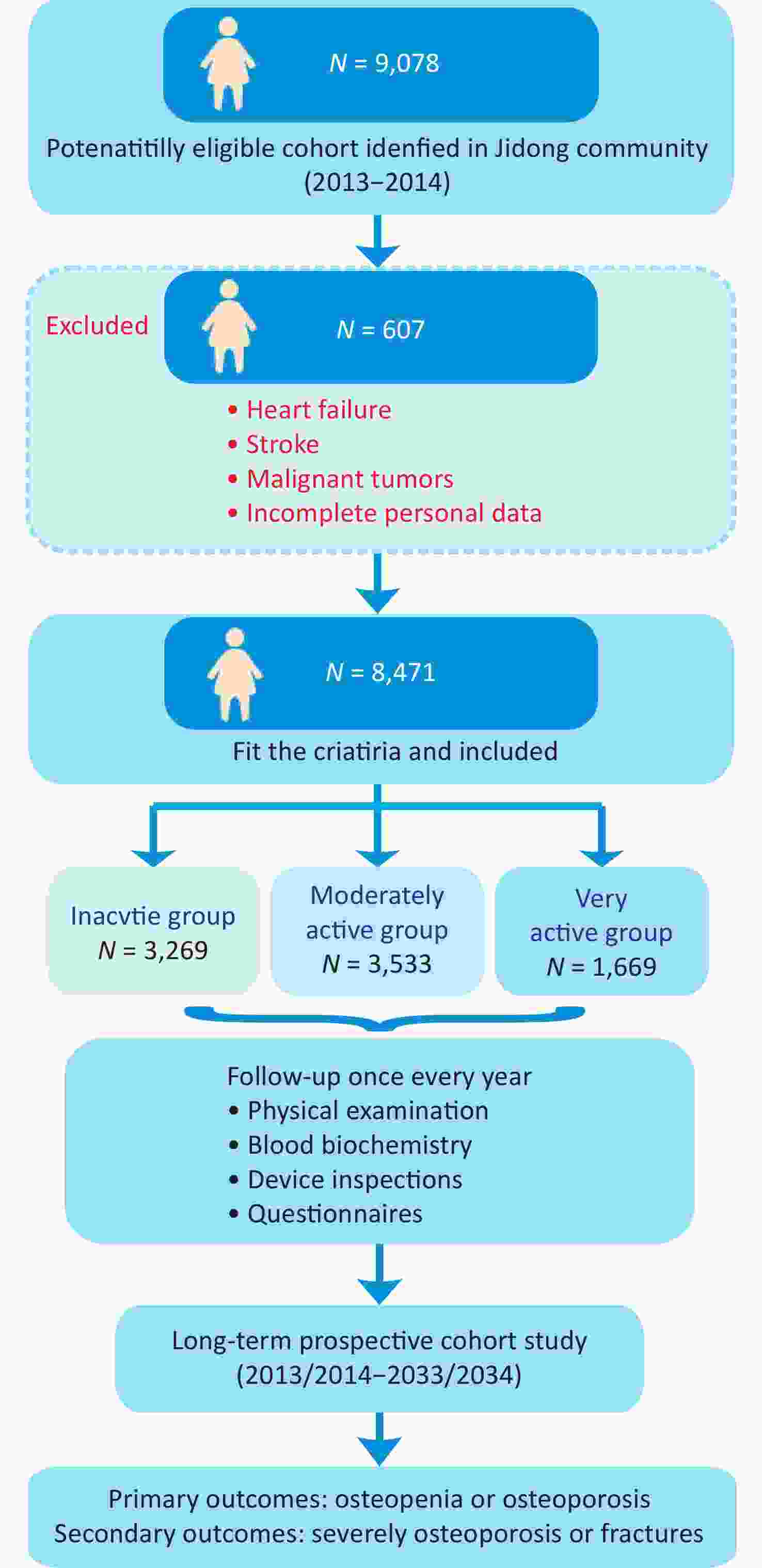
Inclusion criteria: adults ≥ 18 years of age, male or female, who agreed to provide written informed consent were enrolled. The exclusion criteria were as follows: (1) individuals with stroke, transient ischemic attack, or coronary disease, as determined by a validated questionnaire at the inception of the study; (2) those with any observable neurological impairments related to stroke, as evaluated by seasoned medical professionals; (3) patients diagnosed with malignant tumors; and (4) participants with insufficient data available at the outset of the study. A flow chart of the cohort participant recruitment process is shown in Figure 1.
-
A structured questionnaire was used to collect data on various aspects, including sociodemographic characteristics, menopausal age, hormone replacement therapy, medical background, dietary habits, previous PA levels, reproductive and menstrual history, and psychological state. The accuracy and consistency of the questionnaire were assessed before its implementation. Proficient examiners conducted in-person interviews with participants using a duly authenticated questionnaire. Throughout these interviews, the participants recollected their dietary behaviors throughout the preceding year with comprehensive visual aids to appraise portion sizes. To evaluate dietary nutrient intake, including energy, calcium, protein, vitamin D, and assorted micronutrients, researchers utilized a three-day dietary recall method. Standardized procedures were followed for the acquisition of physical measurements. Individual height was measured barefoot, with a precision of up to 0.1 cm, while weight was measured wearing light attire, with a precision of up to 0.1 kg. The average of three repeated measurements was calculated and recorded. Body mass index (BMI) was determined by dividing the weight (kg) by the square of the height (m).
-
During enrollment, comprehensive demographic information was gathered from each participant, including age, sex, BMI, educational level, occupational exertion, tobacco use, and alcohol consumption. Additionally, the presence of underlying conditions such as hypertension, diabetes, and hyperlipidemia was meticulously documented at baseline. Labor intensity was ascertained through participants’ self-reported assessments, elucidating the level of physical exertion encountered during their daily occupational activities. The classification of hypertension involved identifying a medical history characterized by arterial hypertension, concurrent use of antihypertensive therapies, or manifestation of systolic blood pressure equal to or greater than 140 mmHg or diastolic blood pressure equal to or greater than 90 mmHg at the time of data collection. The characterization of diabetes encompassed the stipulation of a prior medical diagnosis of diabetes, use of insulin or oral antidiabetic agents, or fasting blood glucose levels equal to or exceeding 126 mg/dL at the time of data collection. The categorization of hyperlipidemia entailed the establishment of a prior medical diagnosis of hyperlipidemia, ongoing administration of lipid-lowering agents, and total cholesterol levels equal to or exceeding 220 mg/dL, triglyceride levels equal to or exceeding 150 mg/dL, or low-density lipoprotein cholesterol levels equal to or exceeding 160 mg/dL at the time of data collection.
-
The investigation involved meticulously evaluating the participants’ PA levels, employing the International PA Questionnaire Short Form facilitated by adept researchers. The PA levels were classified into vigorous and moderate categories, as shown in Table 1. Only instances of PA lasting 10 minutes or more were considered in the analysis. To assess the changes at baseline, the participants were stratified into three distinct cohorts based on the overall weekly duration of their PA. The inactive (IA) group was characterized by minimal or no physical exertion or brief durations of less than 10 minutes for each activity; the moderately active (MA) group engaged in 80 minutes or less of PA per week; and the very active (VA) group surpassed 80 minutes per week of energetic engagement[22,23].
Table 1. Physical activity assessment questionnaire
No. Content F1 How much time do you spend on physical activity every week?
(Only count physical activity that durated ≥ 10 consecutive minutes each time)F2 Vigorous intensity physical activity e.g. run, swim, football, basketball, badminton hrs min per day on average;
days/week hrs min per day on average;
days/week hrs min/week.F3 Moderate intensity physical activity e.g. jog, brisk walk, cycle, Tai Chi, dance F4 Brake up periods of inactivity: e.g. watch TV, use computer, read, write, eat, play chess -
Blood samples were collected from the antecubital vein after an overnight fast for various blood biochemistry tests. These tests involved analyzing blood composition and measuring blood glucose levels, including fasting blood glucose and glycosylated hemoglobin levels. The levels of different fats in the blood, including triglycerides, total cholesterol, low-density, and high-density lipoprotein cholesterol, were also evaluated. Furthermore, indicators of liver function, such as albumin, alanine transaminase, and aspartate transaminase, and markers of kidney function, such as uric acid, blood urea nitrogen, creatinine, creatine kinase, and creatine kinase-MB were examined. Blood samples were analyzed at the central laboratory of the Staff Hospital of the Jidong Oilfield, which is owned by the China National Petroleum Corporation.
-
BMD is crucial for diagnosing OP, and the WHO has established guidelines for using dual-energy X-ray absorptiometry (DXA) measurements of the hip or spine for diagnosis[24]. DXA measures BMD as an areal density and reports it as a T-score. Peripheral DXA techniques accurately analyze BMD at the distal radius and calcaneus while minimizing radiation exposure[25]. In clinical practice, OP is diagnosed if the T-score is equal to or below −2.5 at certain sites using BMD testing. Osteopenia (ON) occurs when BMD is lower than normal but not low enough to be classified as OP[26]. In this study, a T-score of −2.5 or less was considered OP, between −1.0 and −2.5 was classified as ON, and a T-score within the normal range was considered normal.
-
Study participants will undergo annual follow-ups through in-person interviews during routine medical examinations until 2034 or the manifestation of primary outcome events. Annually, hemoglobin samples, device inspections, and surveys will be gathered and scrutinized for all participants. The focal endpoints of significance encompass cardiovascular incidents, such as myocardial infarction, acute or chronic ischemic heart disease, arterial/ventricular fibrillation, heart failure, spontaneous (cardiac) death, death caused by congestive heart failure, or overall mortality. Ancillary outcomes include the onset of hypertension, diabetes, or hyperlipidemia. The follow-up period will terminate upon the occurrence of cardiovascular incidents. Nevertheless, individuals who manifest secondary outcomes will continue being monitored until the occurrence of cardiovascular events. Moreover, individuals who do not encounter cardiovascular events within five years of diagnosing secondary outcomes shall be designated as cases subjected to censoring. The diagnoses of all ailments shall be subject to independent scrutiny by two medical practitioners. This scrutiny will be further validated by an impartial End Points Committee, which obtained approval from the Project Executive Committee prior to the commencement of the study.
-
Data analysis was performed using SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) and SAS software (version 9.1; SAS Institute, Cary, NC, USA). Continuous variables adhering to a normal distribution were elucidated as mean ± standard deviation, whereas variables exhibiting skewed distribution were articulated as median with interquartile range. Categorical variables were expressed as counts with corresponding percentages. The requisite statistical analyses, comprising independent-sample t-tests, Mann–Whitney U tests, and chi-square (χ2) tests, were employed to juxtapose male and female cohorts, as well as different groups based on PA levels. To perform a longitudinal analysis, logistic and linear regressions will be employed to discern multivariate associations among the diverse parameters. Cox multivariate regression analyses will be undertaken to evaluate the likelihood of encountering singular or amalgamated endpoint outcome(s) throughout follow-up. Furthermore, survival analysis will be employed to compare the cumulative survival proportions among distinct PA cohorts. A statistical significance threshold of P < 0.05 will be applied.
-
Between 2013 and 2014, 9,078 participants aged 18 years and older who willingly consented to follow-up were enrolled from the Jidong community in the Caofeidian district of Tangshan City, Hebei Province, China. Among them, 607 individuals were excluded because of prior medical conditions such as coronary heart disease, heart failure, stroke, or malignant tumors, as well as insufficient baseline data. As a result, 8,471 participants were considered eligible and included in the PAOPO study.
The demographic characteristics of the participants are presented in Table 2. The mean age of the participants was calculated to be 42.4 years. Significant differences emerged between men (n = 4,368, 39.7%) and women (n = 4,103, 41.9%), as women were slightly older (P < 0.01) and had a lower BMI (P < 0.001) than the men. Most individuals (96.3%) had educational qualifications at the junior high school, high school, university, or higher level.
Table 2. Demographic data of participants in the PAOPO study
Characteristics Total (N = 8,471) Male (N = 43,68) Female (N = 4,103) P value* Age, years (range) 42.4 (18.11−82.06) 39.7 (18.1−79.9) 41.9 (18.0−82.06) 0.017 BMI, n (%) < 0.001 < 18.5 280 (3.3) 59 (0.7) 221 (2.6) 18.5–23.9 3,650 (43.1) 1,351 (15.9) 2,299 (27.2) 24.0–27.9 3,128 (36.9) 2,011 (23.7) 1,117 (13.2) ≥ 28.0 1,413 (16.7) 947 (11.2) 466 (5.5) Education, n (%) < 0.001 Illiteracy 56 (0.6) 16 (0.2) 40 (0.4) Primary school 262 (3.1) 99 (1.2) 163 (1.9) Junior high school 1,114 (13.2) 524 (6.2) 590 (7.0) High school 1,888 (22.3) 874 (10.3) 1,014 (12.0) University or above 5,151 (60.8) 2,855 (33.7) 2,296 (27.1) Income, RMB (n, %) < 0.001 < 1,000 83 (1.0) 37 (0.5) 46 (0.5) 1,000–3,000 3,129 (36.9) 1,445 (17.1) 1,684 (19.8) 3,001–5,000 4,503 (53.2) 2,437 (28.8) 2,066 (24.4) > 5,000 756 (8.9) 449 (5.3) 307 (3.6) Sleeping time, hours (n, %) < 0.001 < 6 771 (9.1) 351 (4.1) 420 (5.0) 6–8 4,409 (52.0) 2,408 (28.4) 2,001 (23.6) 9–12 3,291 (38.9) 1,609 (19.0) 1,682 (19.9) Labor intensity, n (%) < 0.001 Very light 609 (7.2) 244 (2.9) 365 (4.3) Light 5,954 (70.3) 2,856 (33.7) 3,098 (36.6) Moderate 1,577 (18.6) 1,001 (11.8) 576 (6.8) Heavy 331 (3.9) 267 (3.2) 64 (0.7) Smoking, n (%) < 0.001 Never 6,008 (70.9) 1,972 (23.3) 4,036 (47.6) Current 2,152 (25.4) 2,090 (24.7) 62 (0.7) Former (quit smoking for less than 12 months) 250 (3.0) 245 (2.9) 5 (0.1) Former (quit smoking for more than 12 months) 61 (0.7) 61 (0.7) 0 (0.0) Alcohol consumption, n (%) < 0.001 Never 5,677 (67.0) 1,776 (21.0) 3,901 (46.0) Less than one standard drink 1,247 (14.7) 1,144 (13.5) 103 (1.2) More than or equal to one standard drink 1,547 (18.3) 1,448 (17.1) 99 (1.2) Disease at baseline, n (%) Hypertension 1,173 (52.4) 725 (32.4) 448 (20.0) < 0.001 Diabetes 389 (17.4) 247 (11.0) 142 (6.4) < 0.001 Hyperlipidemia 675 (30.2) 410 (18.3) 265 (11.9) < 0.001 Physical activity, n (%) < 0.001 Inactive 3,269 (38.6) 1,666 (19.7) 1,603 (18.9) Moderately active 3,533 (41.7) 1,804 (21.3) 1,729 (20.4) Very active 1,669 (19.7) 898 (10.6) 771 (9.1) Note. * Mann–Whitney U tests; BMI, body mass index. Concerning income, 1.0% of individuals had a low-income level below 1,000 RMB, while 36.9% had an income ranging from 1,000 RMB to 3,000 RMB, 53.2% had an income between 3,000 RMB and 5,000 RMB, and 8.9% had an income above 5,000 RMB. Notably, income below 3,000 RMB was higher for women than men, whereas income above 3,000 RMB was higher for men than women. Regarding sleeping patterns, 9.1% of individuals slept less than 6 hours per day, 52.0% slept 6 to 8 hours per day, and 38.9% slept approximately 8 to 12 hours per day. Men slept longer than women, both below 6 hours and in the 6 to 8 hours range. Within the participant cohort, 7.2% self-reported engaging in labor tasks of low intensity, whereas 88.9% indicated involvement in labor tasks of mild-to-moderate intensity. Conversely, only 3.9% disclosed engaging in demanding labor tasks. Regarding lifestyle variables, a significant proportion of individuals (29.1%) had a history of tobacco use, either as current or former smokers. In addition, 33% of the participants acknowledged the consumption of alcoholic beverages. Men exhibited higher levels of labor intensity as well as higher rates of smoking and alcohol consumption compared with women. These differences were statistically significant (P < 0.001). Additionally, men had higher incidence rates of hypertension, diabetes, and hyperlipidemia compared with women (P < 0.001). Regarding PA, 38.6% of the participants were classified as IA, 41.7% as MA, and 19.7% as VA. Moreover, men were found to be more active compared with women (P < 0.001). Table 3 presents the distribution of different factors among the participants based on varying levels of PA. The PA group included an IA group of 3,269 individuals (38.6% of the total), an MA group of 3,533 individuals (41.7% of the total), and a VA group of 1669 individuals (19.7% of the total). Baseline comparisons across the different PA levels revealed significant differences in age, sex, BMI, BMD, labor intensity, smoking habits, alcohol consumption, and prevalence of hypertension, diabetes, and hyperlipidemia.
Table 3. Factor distributions in participants under different physical activity
Characteristics Total Inactive Moderately active Very active P value* Age, years (mesn ± SD) 42.39 ± 13.12 42.22 ± 12.95 43.02 ± 13.30 41.41 ± 13.03 < 0.001 Gender, n (%) < 0.001 Male 4,368 (51.6) 1,666 (19.7) 1,804 (21.3) 898 (10.6) Female 4,103 (48.4) 1,603 (18.9) 1,729 (20.4) 771 (9.1) BMI, kg/m2, n (%) < 0.001 < 18.5 288 (3.4) 120 (1.4) 105 (1.2) 63 (0.8) 18.5–23.9 3,651 (43.1) 1,420 (16.8) 1,503 (17.7) 728 (8.6) 24.0–27.9 3,137 (37.0) 1,214 (14.3) 1,333 (15.7) 590 (7.0) ≥ 28.0 1,395 (16.5) 515 (6.1) 592 (7.0) 288 (3.4) BMD, n (%) < 0.001 T value ≥ −1 1,883 (78.8) 583 (24.4) 934 (39.1) 366 (15.3) −2.5 < T value < −1.0 427 (17.9) 127(5.3) 239 (10.0) 61 (2.6) T value ≤ −2.5 77 (3.2) 27 (1.1) 39 (1.6) 11 (0.5) Labor intensity, n (%) < 0.001 Very light 609 (7.2) 220 (2.6) 274 (3.2) 115 (1.4) Light 5,954 (70.3) 2,344 (27.7) 2,458 (29.0) 1,152 (13.6) Moderate 1,577 (18.6) 580 (6.8) 670 (7.9) 327 (3.9) Heavy 331 (3.9) 125 (1.5) 131 (1.5) 75 (0.9) Smoking, n (%) < 0.001 Never 6,008 (70.9) 2,319 (27.4) 2,516 (29.7) 1,173 (13.8) Current 2,152 (25.4) 819 (9.7) 890 (10.5) 443 (5.2) Former (quit smoking for less than 12 months) 250 (3.0) 102 (1.2) 105 (1.3) 43 (0.5) Former (quit smoking for more than 12 months) 61 (0.7) 29 (0.3) 22 (0.3) 10 (0.1) Alcohol consumption, n (%) < 0.001 Never 5,677 (67.0) 2,226 (26.3) 2,372 (28.0) 1,079 (12.7) Less than one standard drink 1,247 (14.7) 455 (5.4) 520 (6.1) 272 (3.2) More than or equal to one standard drink 1,547 (18.3) 588 (6.9) 641 (7.6) 318 (3.8) Disease at baseline, n (%) < 0.001 Hypertension 1,173 (52.4) 447 (20.0) 501 (22.4) 225 (10.0) Diabetes 389 (17.4) 136 (6.1) 183 (8.2) 70 (3.1) Hyperlipidemia 675 (30.2) 246 (11.0) 295 (13.2) 134 (6.0) Note. *Mann–Whitney U tests; BMD, bone mineral density. Based on a global prevalence analysis of OP among older individuals, the prevalence of OP is particularly high among older women[10]. Therefore, we conducted further investigations on the relationship between sex, age, BMD, and PA. We analyzed the general changes in BMD in the three PA groups (IA, MA, and VA) using column comparisons. A significant increase in BMD was observed in the VA group (Figure 2A), whereas no differences were observed between the IA and MA groups. Next, we compared the BMD among the three PA groups separately for women and men. Interestingly, only the VA group showed a significant increase in BMD among women (Figure 2B), whereas no significant differences were observed among men in the three groups (Figure 2C). These findings suggest that being VA may significantly improve BMD in this population, particularly in women. Furthermore, we analyzed sex differences and discovered significant increases in BMD in men compared with women across all three PA groups (Figure 2D, 2E, and 2F). We observed a significantly higher BMD in men compared with women, which is consistent with previous studies[8,27].
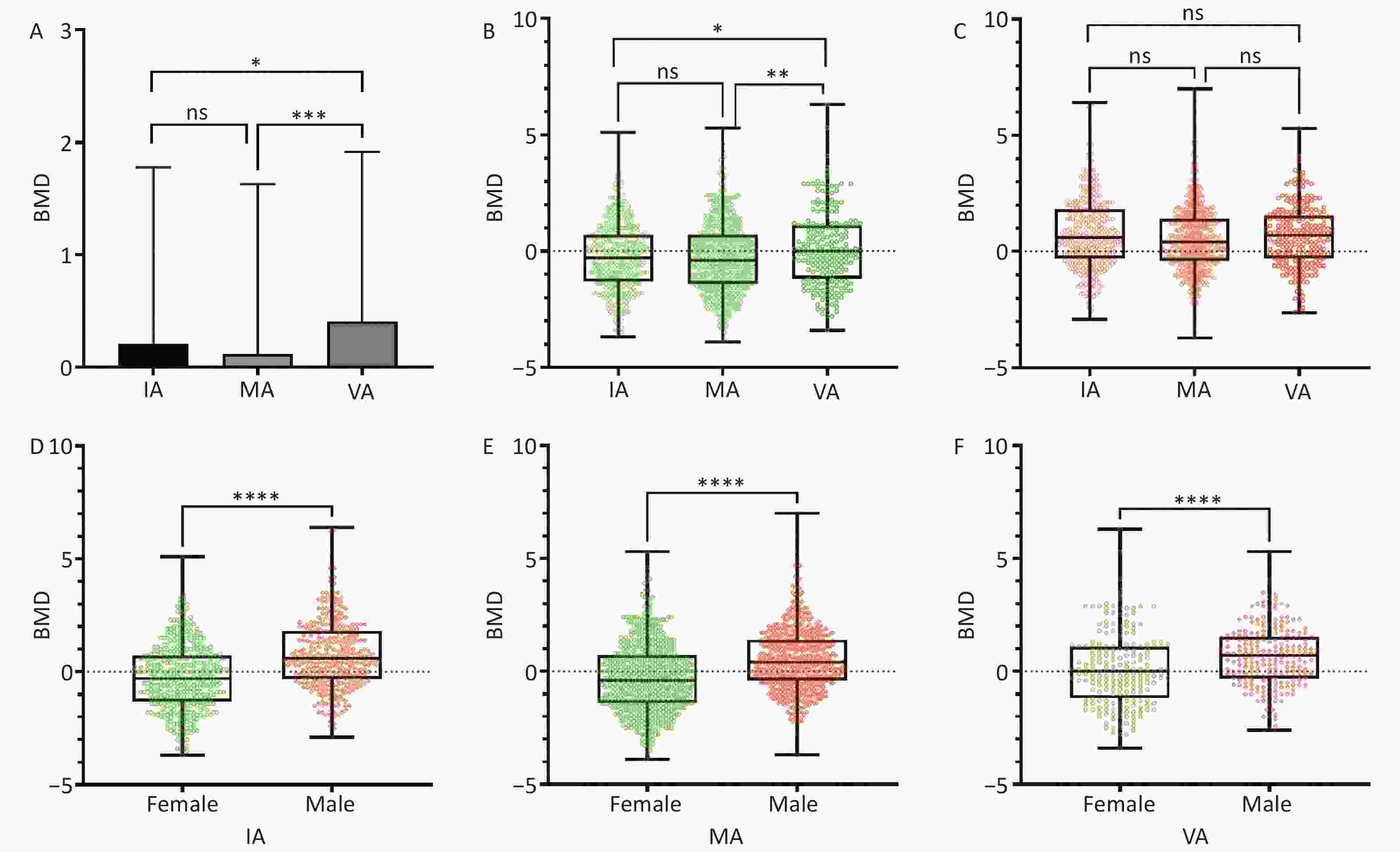
Figure 2. Distribution of box plots among three physical activity groups. (A) Comparison of BMD among individuals in the IA, MA, and VA groups. (B) Comparison of BMD among women in the IA, MA, and VA groups. (C) Comparison of BMD among men in the IA, MA, and VA groups. (D) Sex comparison of BMD in the IA group. (E) Sex comparison of BMD in the MA group. (F) Sex comparison of BMD in the VA group. BMD, bone mineral density; IA, inactive; MA, moderately active; VA, very active. ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
The study cohort was categorized into three distinct age groups: [18, 50] years, [50, 60] years, and [60, 80] years. The choice to use these three age groups was informed by specific biological rationales: 1) individuals between the ages of 18 and 50 typically maintain stable bone density levels; 2) individuals over the age of 50 undergo heightened bone breakdown in comparison to formation, resulting in accelerated bone loss; and 3) women aged 60 years or older experience a substantial transition post-menopause. The distribution of individuals with IA, MA, and VA PA levels and their BMD classifications in the normal, ON, and OP groups are shown in Figure 3. Notably, no individuals with OP were in the age range of [18 y, 50 y]. The relationships among PA, age, and BMD in individuals with normal BMD and ON are presented in Table 4. The lowest occurrence of ON (3.1%) was observed in the VA group in the age range of [18, 50] years, whereas the highest occurrence (31.2%) was observed in the MA group in the age range of [60, 80] years. The relative risk (RR) of 2.3 for the age groups [18, 50] years and [50, 60] years suggests that inactivity may increase the risk of ON, whereas moderate activity increases the risk of ON in the [60, 80] years age group. The relationships between PA, age, and BMD in individuals with normal BMD and OP are presented in Table 5. The lowest occurrence of OP (2.4%) was observed in the MA group in the age range of [50 y, 60 y], whereas the highest occurrence (11.0%) was observed in the MA group in the age range of [60 y, 80 y]. The RR of 2.1 suggests that inactivity increased the risk in the age group of [50 y, 60 y], while the RR of 2.2 for moderate activity and RR of 1.7 for the VA increased the risk in the age groups of [60 y, 80 y].
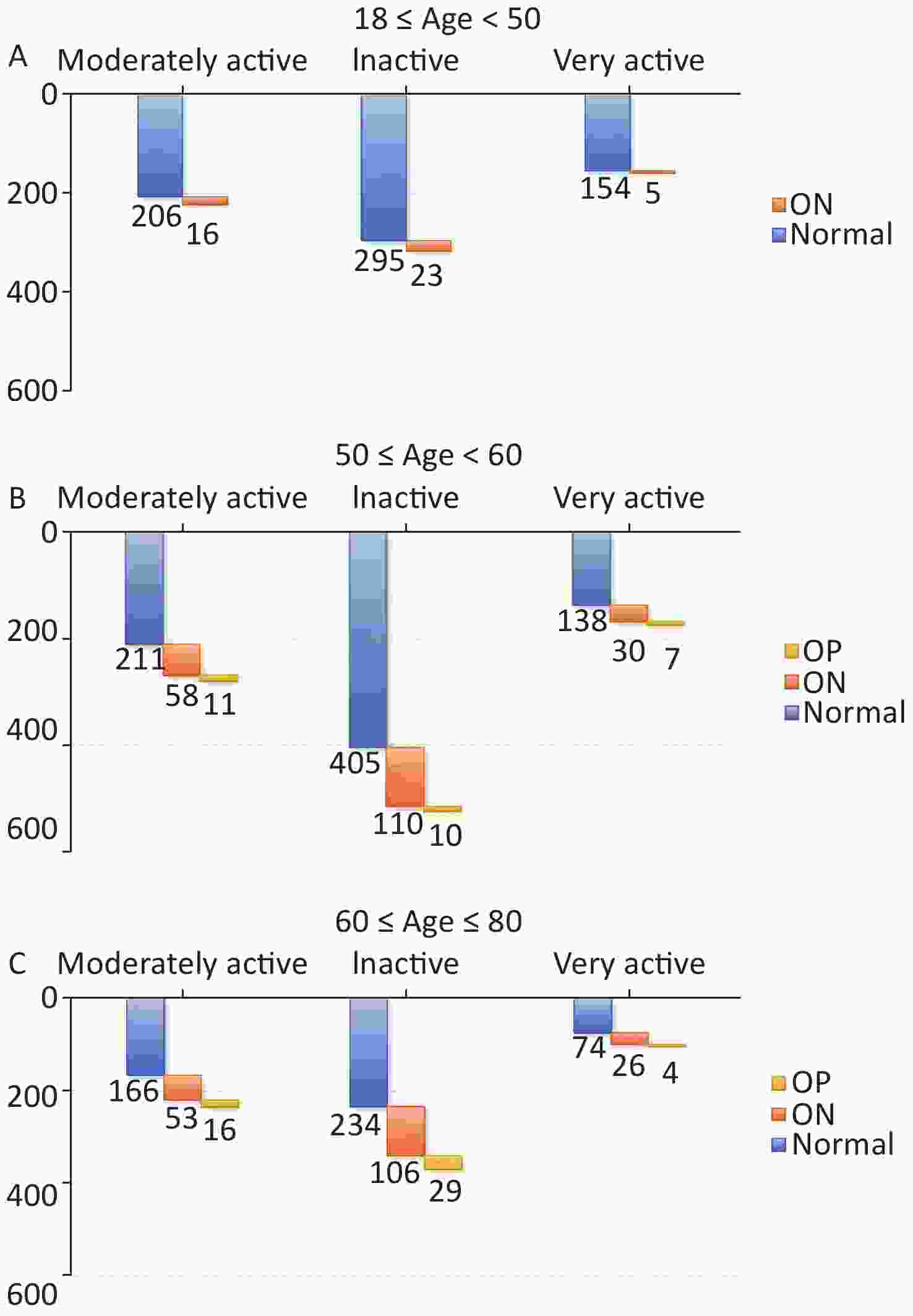
Figure 3. Distribution of three age ranges among the inactive (IA) group, moderately active (MA) group, and very active (VA) group. OP, osteoporosis; ON, osteopenia.
Table 4. The association between PA, age, and BMD in individuals classified as having normal BMD and those in ON group
Age, years PA classification ON Normal Occurrence (%) Relative risk (I/II) Relative risk (II/III) Relative risk (I/III) 18−49 Inactive (I) 16 206 7.2 1.0 Moderately active (II) 23 295 7.2 2.3 Very active (III) 5 154 3.1 2.3 50−59 Inactive (I) 58 211 21.6 1.0 Moderately active (II) 110 405 21.4 1.2 Very active (III) 30 138 17.9 1.2 60−80 Inactive (I) 53 166 24.2 0.8 Moderately active (II) 106 234 31.2 1.2 Very active (III) 26 74 26.0 0.9 Note. PA, physical activity; BMD, bone mineral density; ON, osteopenia. Table 5. The association between PA, age, and BMD in individuals classified as having normal BMD and those in OP group
Age, years PA classification OP Normal Occurrence(%) Relative risk (I/II) Relative risk (II/III) Relative risk (I/III) 18−49 Inactive (I) n.a 1 206 n.a 1 n.a 1 n.a1 n.a1 Moderately active (II) 295 Very active (III) 154 50−59 Inactive (I) 11 211 5.0 2.1 Moderately active (II) 10 405 2.4 0.5 Very active (III) 7 138 4.8 1.0 60−80 Inactive (I) 16 166 8.8 0.8 Moderately active (II) 29 234 11.0 2.2 Very active (III) 4 74 5.1 1.7 Note. 1n.a: not available; PA, physical activity; BMD, bone mineral density; OP, osteoporosis. -
OP is a common musculoskeletal disorder affected by various biological, behavioral, and environmental factors. Biological determinants such as genetics, sex, age, and hormonal changes contribute to its development. On the other hand, modifiable factors like calcium intake, PA, lifestyle choices, smoking, outdoor activities, and alcohol consumption also play an important role[28]. Regular weight-bearing and impact exercises are essential to prevent bone demineralization, and ample evidence supports their effectiveness. Resistance training has been shown to increase BMD, and high-impact sports athletes have higher BMD than low-impact athletes[29]. Moreover, according to a meta-analysis of RCTs, exercise training has been shown to effectively prevent or reverse bone loss in the lumbar spine and femoral neck regions, regardless of the menopausal status in women. Additionally, exercise training reduces the risk and frequency of falls[30-32]. Numerous longitudinal studies have investigated the impact of exercise training on skeletal health across various age groups, including children, adolescents, young adults, individuals in their prime, and older adults[33,34].
Among the three PA groups, more than half of the individuals engaged in moderate-to-vigorous physical exercise, whereas less than 40% were IA. Importantly, this corresponds to the percentage of individuals who slept between 8 to 12 hours. The evaluation of BMD across the three PA groups revealed that vigorous activity significantly enhanced the BMD of the population, as shown in Figure 2A. Consistent with the findings from scientific reviews and meta-analyses[20,35], higher levels of PA during leisure time or engaging in moderate-to-vigorous PA are consistently linked to a reduced risk of hip and overall fractures, which can be attributed to a lower BMD. Additionally, our initial assessment indicated a significant improvement in BMD among women who actively engaged in physical exercise, as depicted in Figures 2B and 2C. Furthermore, the average BMD of women in the three PA groups was significantly lower than that of men. This discrepancy can be attributed to women experiencing a faster loss of bone mass as they age, mainly because of hormonal changes and other factors[36].
Analysis of age-related BMD in the three PA groups revealed some interesting findings. No cases of OP were observed among individuals aged 18 to 50. The average occurrence of ON was 6% across all three PA groups, with the lowest incidence observed in the VA group (3.1%). For individuals aged 50 to 60, the average occurrence of ON was 20%, whereas that of OP was 3% across all three PA groups. The lowest incidence of ON (1.9%) was observed in the MA group. Among individuals aged 60 to 80, the average occurrence of ON was 25%, with an OP occurrence of 6% across all three PA groups. The VA group had the lowest incidence of ON (3.8%). These findings indicate that OP is an age-related issue and that individuals above the age of 50 years are at a higher risk of developing ON and OP. Engaging in moderate to vigorous exercise can significantly enhance bone strength in this population. Therefore, regular participation in PA significantly prevents a decline in BMD and developing OP. Moreover, the benefits of participating in PA outweigh any potential hazards, particularly among individuals aged 50 years and older. The Bone Health and Osteoporosis Foundation promotes PA for overall well-being and fracture prevention across all life stages. Weight-bearing and high-impact exercises during childhood and adolescence are essential for optimal bone mass[37].
In addition, the PA of the three activity groups indicated men are more active than women during physical exercise. These findings, along with differences in living habits and baseline risk factors such as hypertension, diabetes, and hyperlipidemia, may contribute to the risk of OP. In our baseline data, it was observed that men had a higher rate of smoking and alcohol consumption than women, as well as a higher rate of hypertension, diabetes, and hyperlipidemia. Tobacco use negatively affects musculoskeletal integrity and overall health. Smoking cessation should be encouraged to prevent OP. Limiting alcohol intake is vital for preventing skeletal health issues[38-40]. Clinicians should be aware of individuals with chronic alcohol abuse, as they may need additional assessment and intervention[41,42]. The PAOPO study aimed to investigate the association between PA and OP outcomes, including OP-related clinical outcomes and osteoporotic fractures. We administered a PA questionnaire to each participant and will be able to conduct the investigation annually during the follow-up period.
Moreover, the International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine recommend using serum procollagen type I N propeptide (measures bone formation) and serum C-terminal cross-linking telopeptide of type I collagen (measures bone resorption) to predict fracture risk[43]. Both markers were significantly associated with the risk of fractures, particularly hip fractures. Ongoing work involves standardizing bone turnover markers for improved prediction[44-46]. The PAOPO study will conduct annual blood biochemistry assessments and BMD measurements for participants over a long-term follow-up period.
In summary, the PAOPO study is a large-scale, community-centered cohort investigation conducted in China. The main objective is to explore the possible association between PA levels and OP risk and to identify potential risks. Additionally, this study aims to examine the combined effects of PA and other relevant variables to better understand their collective impact on OP outcomes. Through its comprehensive methodology, the PAOPO study seeks to provide valuable insights into the significant role of PA in the prevention and prediction of OP.
Nevertheless, our study has several limitations that must be acknowledged. First, the participants were volunteers from the Jidong community and were not randomly selected, potentially introducing selection bias. Furthermore, individuals already undergoing anti-osteoporosis treatment were not excluded, which might have led to an underestimation of the actual prevalence of osteoporosis. Additionally, because of time and practical limitations, we did not gather data on dietary intake, which could have impacted BMD values. Moreover, BMD testing was not conducted in all participants, and factors such as tobacco use, alcohol consumption, and other lifestyle variables were not fully considered. In future analyses, it will be valuable to include bone turnover biomarkers for the timely diagnosis and early prevention of OP alongside BMD measurements.
doi: 10.3967/bes2024.117
Prospective Cohort Investigation on Physical Activity of Osteoporosis Outcomes (PAOPO) in Jidong: Objectives, Study Design, and Baseline Characteristics
-
Abstract:
Objective The aim of this study was to investigate the prospective association between physical activity (PA), independently or in conjunction with other contributing factors, and osteoporosis (OP) outcomes. Methods The Physical Activity in Osteoporosis Outcomes (PAOPO) study was a community-based cohort investigation. A structured questionnaire was used to gather the participants’ sociodemographic characteristics. Bone mineral density (BMD) measurements were performed to assess OP outcomes, and the relationship between BMD and OP was evaluated within this cohort. Results From 2013 to 2014, 8,471 participants aged 18 years and older were recruited from Tangshan, China’s Jidong community. Based on their PA level, participants were categorized as inactive, moderately active, or very active. Men showed higher physical exercise levels than women across the activity groups. BMD was significantly higher in the very active group than in the moderately active and inactive groups. Individuals aged > 50 years are at a higher risk of developing OP and osteopenia. Conclusion The PAOPO study offers promising insights into the relationship between PA and OP outcomes, encouraging the implementation of PA in preventing and managing OP. -
Key words:
- Osteoporosis /
- Physical activity /
- Risk factors /
- Cohort study
The authors declare no competing interests.
&These authors contributed equally to this work.
注释:1) AUTHOR CONTRIBUTIONS: 2) DECLARATION OF INTERESTS: -
Figure 2. Distribution of box plots among three physical activity groups. (A) Comparison of BMD among individuals in the IA, MA, and VA groups. (B) Comparison of BMD among women in the IA, MA, and VA groups. (C) Comparison of BMD among men in the IA, MA, and VA groups. (D) Sex comparison of BMD in the IA group. (E) Sex comparison of BMD in the MA group. (F) Sex comparison of BMD in the VA group. BMD, bone mineral density; IA, inactive; MA, moderately active; VA, very active. ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Table 1. Physical activity assessment questionnaire
No. Content F1 How much time do you spend on physical activity every week?
(Only count physical activity that durated ≥ 10 consecutive minutes each time)F2 Vigorous intensity physical activity e.g. run, swim, football, basketball, badminton hrs min per day on average;
days/week hrs min per day on average;
days/week hrs min/week.F3 Moderate intensity physical activity e.g. jog, brisk walk, cycle, Tai Chi, dance F4 Brake up periods of inactivity: e.g. watch TV, use computer, read, write, eat, play chess Table 2. Demographic data of participants in the PAOPO study
Characteristics Total (N = 8,471) Male (N = 43,68) Female (N = 4,103) P value* Age, years (range) 42.4 (18.11−82.06) 39.7 (18.1−79.9) 41.9 (18.0−82.06) 0.017 BMI, n (%) < 0.001 < 18.5 280 (3.3) 59 (0.7) 221 (2.6) 18.5–23.9 3,650 (43.1) 1,351 (15.9) 2,299 (27.2) 24.0–27.9 3,128 (36.9) 2,011 (23.7) 1,117 (13.2) ≥ 28.0 1,413 (16.7) 947 (11.2) 466 (5.5) Education, n (%) < 0.001 Illiteracy 56 (0.6) 16 (0.2) 40 (0.4) Primary school 262 (3.1) 99 (1.2) 163 (1.9) Junior high school 1,114 (13.2) 524 (6.2) 590 (7.0) High school 1,888 (22.3) 874 (10.3) 1,014 (12.0) University or above 5,151 (60.8) 2,855 (33.7) 2,296 (27.1) Income, RMB (n, %) < 0.001 < 1,000 83 (1.0) 37 (0.5) 46 (0.5) 1,000–3,000 3,129 (36.9) 1,445 (17.1) 1,684 (19.8) 3,001–5,000 4,503 (53.2) 2,437 (28.8) 2,066 (24.4) > 5,000 756 (8.9) 449 (5.3) 307 (3.6) Sleeping time, hours (n, %) < 0.001 < 6 771 (9.1) 351 (4.1) 420 (5.0) 6–8 4,409 (52.0) 2,408 (28.4) 2,001 (23.6) 9–12 3,291 (38.9) 1,609 (19.0) 1,682 (19.9) Labor intensity, n (%) < 0.001 Very light 609 (7.2) 244 (2.9) 365 (4.3) Light 5,954 (70.3) 2,856 (33.7) 3,098 (36.6) Moderate 1,577 (18.6) 1,001 (11.8) 576 (6.8) Heavy 331 (3.9) 267 (3.2) 64 (0.7) Smoking, n (%) < 0.001 Never 6,008 (70.9) 1,972 (23.3) 4,036 (47.6) Current 2,152 (25.4) 2,090 (24.7) 62 (0.7) Former (quit smoking for less than 12 months) 250 (3.0) 245 (2.9) 5 (0.1) Former (quit smoking for more than 12 months) 61 (0.7) 61 (0.7) 0 (0.0) Alcohol consumption, n (%) < 0.001 Never 5,677 (67.0) 1,776 (21.0) 3,901 (46.0) Less than one standard drink 1,247 (14.7) 1,144 (13.5) 103 (1.2) More than or equal to one standard drink 1,547 (18.3) 1,448 (17.1) 99 (1.2) Disease at baseline, n (%) Hypertension 1,173 (52.4) 725 (32.4) 448 (20.0) < 0.001 Diabetes 389 (17.4) 247 (11.0) 142 (6.4) < 0.001 Hyperlipidemia 675 (30.2) 410 (18.3) 265 (11.9) < 0.001 Physical activity, n (%) < 0.001 Inactive 3,269 (38.6) 1,666 (19.7) 1,603 (18.9) Moderately active 3,533 (41.7) 1,804 (21.3) 1,729 (20.4) Very active 1,669 (19.7) 898 (10.6) 771 (9.1) Note. * Mann–Whitney U tests; BMI, body mass index. Table 3. Factor distributions in participants under different physical activity
Characteristics Total Inactive Moderately active Very active P value* Age, years (mesn ± SD) 42.39 ± 13.12 42.22 ± 12.95 43.02 ± 13.30 41.41 ± 13.03 < 0.001 Gender, n (%) < 0.001 Male 4,368 (51.6) 1,666 (19.7) 1,804 (21.3) 898 (10.6) Female 4,103 (48.4) 1,603 (18.9) 1,729 (20.4) 771 (9.1) BMI, kg/m2, n (%) < 0.001 < 18.5 288 (3.4) 120 (1.4) 105 (1.2) 63 (0.8) 18.5–23.9 3,651 (43.1) 1,420 (16.8) 1,503 (17.7) 728 (8.6) 24.0–27.9 3,137 (37.0) 1,214 (14.3) 1,333 (15.7) 590 (7.0) ≥ 28.0 1,395 (16.5) 515 (6.1) 592 (7.0) 288 (3.4) BMD, n (%) < 0.001 T value ≥ −1 1,883 (78.8) 583 (24.4) 934 (39.1) 366 (15.3) −2.5 < T value < −1.0 427 (17.9) 127(5.3) 239 (10.0) 61 (2.6) T value ≤ −2.5 77 (3.2) 27 (1.1) 39 (1.6) 11 (0.5) Labor intensity, n (%) < 0.001 Very light 609 (7.2) 220 (2.6) 274 (3.2) 115 (1.4) Light 5,954 (70.3) 2,344 (27.7) 2,458 (29.0) 1,152 (13.6) Moderate 1,577 (18.6) 580 (6.8) 670 (7.9) 327 (3.9) Heavy 331 (3.9) 125 (1.5) 131 (1.5) 75 (0.9) Smoking, n (%) < 0.001 Never 6,008 (70.9) 2,319 (27.4) 2,516 (29.7) 1,173 (13.8) Current 2,152 (25.4) 819 (9.7) 890 (10.5) 443 (5.2) Former (quit smoking for less than 12 months) 250 (3.0) 102 (1.2) 105 (1.3) 43 (0.5) Former (quit smoking for more than 12 months) 61 (0.7) 29 (0.3) 22 (0.3) 10 (0.1) Alcohol consumption, n (%) < 0.001 Never 5,677 (67.0) 2,226 (26.3) 2,372 (28.0) 1,079 (12.7) Less than one standard drink 1,247 (14.7) 455 (5.4) 520 (6.1) 272 (3.2) More than or equal to one standard drink 1,547 (18.3) 588 (6.9) 641 (7.6) 318 (3.8) Disease at baseline, n (%) < 0.001 Hypertension 1,173 (52.4) 447 (20.0) 501 (22.4) 225 (10.0) Diabetes 389 (17.4) 136 (6.1) 183 (8.2) 70 (3.1) Hyperlipidemia 675 (30.2) 246 (11.0) 295 (13.2) 134 (6.0) Note. *Mann–Whitney U tests; BMD, bone mineral density. Table 4. The association between PA, age, and BMD in individuals classified as having normal BMD and those in ON group
Age, years PA classification ON Normal Occurrence (%) Relative risk (I/II) Relative risk (II/III) Relative risk (I/III) 18−49 Inactive (I) 16 206 7.2 1.0 Moderately active (II) 23 295 7.2 2.3 Very active (III) 5 154 3.1 2.3 50−59 Inactive (I) 58 211 21.6 1.0 Moderately active (II) 110 405 21.4 1.2 Very active (III) 30 138 17.9 1.2 60−80 Inactive (I) 53 166 24.2 0.8 Moderately active (II) 106 234 31.2 1.2 Very active (III) 26 74 26.0 0.9 Note. PA, physical activity; BMD, bone mineral density; ON, osteopenia. Table 5. The association between PA, age, and BMD in individuals classified as having normal BMD and those in OP group
Age, years PA classification OP Normal Occurrence(%) Relative risk (I/II) Relative risk (II/III) Relative risk (I/III) 18−49 Inactive (I) n.a 1 206 n.a 1 n.a 1 n.a1 n.a1 Moderately active (II) 295 Very active (III) 154 50−59 Inactive (I) 11 211 5.0 2.1 Moderately active (II) 10 405 2.4 0.5 Very active (III) 7 138 4.8 1.0 60−80 Inactive (I) 16 166 8.8 0.8 Moderately active (II) 29 234 11.0 2.2 Very active (III) 4 74 5.1 1.7 Note. 1n.a: not available; PA, physical activity; BMD, bone mineral density; OP, osteoporosis. -
[1] Clynes MA, Harvey NC, Curtis EM, et al. The epidemiology of osteoporosis. Br Med Bull, 2020; 133, 105−17. [2] Huang CF, Chen JF, Reid IR, et al. Asia-pacific consensus on osteoporotic fracture prevention in postmenopausal women with low bone mass or osteoporosis but no fragility fractures. J Formos Med Assoc, 2023; 122 Suppl 1, S14-20. [3] Khosla S, Wright NC, Elderkin AL, et al. Osteoporosis in the USA: prevention and unmet needs. Lancet Diabetes Endocrinol, 2023; 11, 19−20. doi: 10.1016/S2213-8587(22)00322-9 [4] Schumm AK, Craige EA, Arora NK, et al. Does adding exercise or physical activity to pharmacological osteoporosis therapy in patients with increased fracture risk improve bone mineral density and lower fracture risk? A systematic review and meta-analysis. Osteoporos Int, 2023; 34, 1867−80. doi: 10.1007/s00198-023-06829-0 [5] Alsop T, Woodforde J, Rosbergen I, et al. Perspectives of health professionals on physical activity and sedentary behaviour in hospitalised adults: A systematic review and thematic synthesis. Clin Rehabil, 2023; 37, 1386−405. doi: 10.1177/02692155231170451 [6] Pinheiro MB, Oliveira J, Bauman A, et al. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Act, 2020; 17, 150. doi: 10.1186/s12966-020-01040-4 [7] Ding D, Mutrie N, Bauman A, et al. Physical activity guidelines 2020: comprehensive and inclusive recommendations to activate populations. Lancet, 2020; 396, 1780−2. doi: 10.1016/S0140-6736(20)32229-7 [8] Wang J, Shu B, Tang DZ, et al. The prevalence of osteoporosis in China, a community based cohort study of osteoporosis. Front Public Health, 2023; 11, 1084005. doi: 10.3389/fpubh.2023.1084005 [9] Xiao PL, Cui AY, Hsu CJ, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int, 2022; 33, 2137−53. doi: 10.1007/s00198-022-06454-3 [10] Salari N, Ghasemi H, Mohammadi L, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res, 2021; 16, 609. doi: 10.1186/s13018-021-02772-0 [11] Nayak S, Singer A, Greenspan SL. Cost-effectiveness of secondary fracture prevention intervention for Medicare beneficiaries. J Am Geriatr Soc, 2021; 69, 3435−44. doi: 10.1111/jgs.17381 [12] Liang B, Burley G, Lin S, et al. Osteoporosis pathogenesis and treatment: existing and emerging avenues. Cell Mol Biol Lett, 2022; 27, 72. doi: 10.1186/s11658-022-00371-3 [13] Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol, 2021; 6, 55. doi: 10.3390/jfmk6020055 [14] Proia P, Amato A, Drid P, et al. The impact of diet and physical activity on bone health in children and adolescents. Front Endocrinol, 2021; 12, 704647. doi: 10.3389/fendo.2021.704647 [15] Chen L, Zhang ZX, Long Y. Association between leisure-time physical activity and the built environment in China: Empirical evidence from an accelerometer and GPS-based fitness app. PLoS One, 2021; 16, e0260570. doi: 10.1371/journal.pone.0260570 [16] D'Onofrio G, Kirschner J, Prather H, et al. Musculoskeletal exercise: its role in promoting health and longevity. Prog Cardiovasc Dis, 2023; 77, 25−36. doi: 10.1016/j.pcad.2023.02.006 [17] Mohebbi R, Shojaa M, Kohl M, et al. Exercise training and bone mineral density in postmenopausal women: an updated systematic review and meta-analysis of intervention studies with emphasis on potential moderators. Osteoporos Int, 2023; 34, 1145−78. doi: 10.1007/s00198-023-06682-1 [18] Ganz DA, Latham NK. Prevention of falls in community-dwelling older adults. N Engl J Med, 2020; 382, 734−43. doi: 10.1056/NEJMcp1903252 [19] Wiedenmann T, Held S, Rappelt L, et al. Exercise based reduction of falls in communitydwelling older adults: a network meta-analysis. Eur Rev Aging Phys Act, 2023; 20, 1. doi: 10.1186/s11556-023-00311-w [20] Cauley JA, Giangregorio L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol, 2020; 8, 150−62. doi: 10.1016/S2213-8587(19)30351-1 [21] Sherrington C, Fairhall N, Wallbank G, et al. Exercise for preventing falls in older people living in the community: an abridged Cochrane systematic review. Br J Sports Med, 2020; 54, 885−91. doi: 10.1136/bjsports-2019-101512 [22] Bei YH, Peng WJ, Zhao J, et al. Protocol of a prospective cohort study of physical activity in cardiovascular outcomes (PACVO) in China: objective, design, and baseline characteristics. J Cardiovasc Transl Res, 2022; 15, 918−25. doi: 10.1007/s12265-021-10194-w [23] Zhou Y, Li Y, Xu L, et al. Asymptomatic polyvascular abnormalities in community (APAC) study in China: objectives, design and baseline characteristics. PLoS One, 2013; 8, e84685. doi: 10.1371/journal.pone.0084685 [24] Bartl R. Dual-energy X-ray Absorptiometry (DXA) and other technologies. In: Bartl R. Osteoporosis in Clinical Practice. Springer. 2023, 51−62. [25] Wong AKO, Manske SL. A comparison of peripheral imaging technologies for bone and muscle quantification: a review of segmentation techniques. J Clin Densitom, 2020; 23, 92−107. doi: 10.1016/j.jocd.2018.04.001 [26] Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, et al. Current status of the diagnosis and management of osteoporosis. Int J Mol Sci, 2022; 23, 9465. doi: 10.3390/ijms23169465 [27] Vescini F, Chiodini I, Falchetti A, et al. Management of osteoporosis in men: a narrative review. Int J Mol Sci, 2021; 22, 13640. doi: 10.3390/ijms222413640 [28] Tayyem R, Abuhijleh H, Al-Khammash A. Lifestyle and dietary patterns as risk factors for osteoporosis: a literature review. Curr Nutr Food Sci, 2023; 19, 806−16. doi: 10.2174/1573401319666221020150214 [29] Rocha-Rangel J, Liang MTC, Tsai AHT, et al. Bone bending strength and BMD of female athletes in volleyball, soccer, and long-distance running. Eur J Appl Physiol, 2023; 123, 2213−23. doi: 10.1007/s00421-023-05231-2 [30] Rodrigues F, Monteiro AM, Forte P, et al. Effects of muscle strength, agility, and fear of falling on risk of falling in older adults. Int J Environ Res Public Health, 2023; 20, 4945. doi: 10.3390/ijerph20064945 [31] Huang TY, Chou MY, Liang CK, et al. Physical activity plays a crucial role in multidomain intervention for frailty prevention. Aging Clin Exp Res, 2023; 35, 1283−92. doi: 10.1007/s40520-023-02412-z [32] Dent E, Daly RM, Hoogendijk EO, et al. Exercise to prevent and manage frailty and fragility fractures. Curr Osteoporos Rep, 2023; 21, 205−15. doi: 10.1007/s11914-023-00777-8 [33] Torres-Costoso A, López-Muñoz P, Martínez-Vizcaíno V, et al. Association between muscular strength and bone health from children to young adults: a systematic review and meta-analysis. Sports Med, 2020; 50, 1163−90. doi: 10.1007/s40279-020-01267-y [34] Sfeir JG, Drake MT, Khosla S, et al. Skeletal aging. Mayo Clin Proc, 2022; 97, 1194−208. doi: 10.1016/j.mayocp.2022.03.011 [35] Brooke-Wavell K, Skelton DA, Barker KL, et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med, 2022; 56, 837−46. doi: 10.1136/bjsports-2021-104634 [36] Cheng CH, Chen LR, Chen KH. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int J Mol Sci, 2022; 23, 1376. doi: 10.3390/ijms23031376 [37] Ng CA, Gandham A, Mesinovic J, et al. Effects of moderate‐ to high‐impact exercise training on bone structure across the lifespan: a systematic review and meta‐analysis of randomized controlled trials. J Bone Miner Res, 2023; 38, 1612−34. doi: 10.1002/jbmr.4899 [38] Dai Y, Huang JC, Hu QH, et al. Association of cigarette smoking with risk of chronic musculoskeletal pain: a meta-analysis. Pain Physician, 2021; 24, 495−506. [39] Wieczorek M, Gwinnutt JM, Ransay-Colle M, et al. Smoking, alcohol consumption and disease-specific outcomes in rheumatic and musculoskeletal diseases (RMDs): systematic reviews informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open, 2022; 8, e002170. doi: 10.1136/rmdopen-2021-002170 [40] Fernández-Torres J, Zamudio-Cuevas Y, Martínez-Nava GA, et al. Impact of cadmium mediated by tobacco use in musculoskeletal diseases. Biol Trace Elem Res, 2022; 200, 2008−15. doi: 10.1007/s12011-021-02814-y [41] Godos J, Giampieri F, Chisari E, et al. Alcohol consumption, bone mineral density, and risk of osteoporotic fractures: a dose-response meta-analysis. Int J Environ Res Public Health, 2022; 19, 1515. doi: 10.3390/ijerph19031515 [42] Hsu CY, Chen LR, Chen KH. Osteoporosis in patients with chronic kidney diseases: a systemic review. Int J Mol Sci, 2020; 21, 6846. doi: 10.3390/ijms21186846 [43] Schini M, Vilaca T, Gossiel F, et al. Bone turnover markers: basic biology to clinical applications. Endocr Rev, 2023; 44, 417−73. doi: 10.1210/endrev/bnac031 [44] Ladang A, Rauch F, Delvin E, et al. Bone turnover markers in children: from laboratory challenges to clinical interpretation. Calcif Tissue Int, 2023; 112, 218−32. [45] Zhang XW, Krishnamoorthy S, Tang CTL, et al. Association of bone mineral density and bone turnover markers with the risk of diabetes: Hong Kong osteoporosis study and Mendelian randomization. J Bone Miner Res, 2023; 38, 1782−90. doi: 10.1002/jbmr.4924 [46] Vasikaran SD, Miura M, Pikner R, et al. Practical considerations for the clinical application of bone turnover markers in osteoporosis. Calcif Tissue Int, 2023; 112, 148−57. -




 下载:
下载:



 Quick Links
Quick Links