-
Hepatic fibrosis is a major health problem worldwide and is considered a process of prolonged wound healing caused by various chronic injuries such as those arising from viruses, alcohol and drug use, and cholestasis[1]. Liver fibrosis is reversible but can develop into end-stage liver diseases such as cirrhosis and liver cancer if not controlled[2]. Ultimately, it can only be treated with liver transplantation. However, according to statistics, less than 10% of the transplant requirements can be met[3]. Therefore, studies focused on the treatment of liver fibrosis are urgently required.
Cholestasis is an important factor that causes liver fibrosis, with a total incidence of approximately 10.26% in patients with chronic liver disease[4]. Although the progression of cholestatic liver fibrosis can be delayed by clearing the etiology, improving cholestasis, and reducing its toxic effects, the treatment of liver fibrosis remains a key and difficult point in clinical therapeutics, and there are currently no clinically approved anti-liver fibrosis drugs.
Hepatic stellate cells (HSCs) play a central role in the development of liver fibrosis. During chronic cholestasis, HSCs convert from a quiescent to activated state, leading to excessive deposition of extracellular matrix (ECM) and thus giving rise to liver fibrosis.
Recently, cell therapy has emerged as an interesting option for the treatment of liver fibrosis and cirrhosis. Certain cell types such as primary liver cells, liver progenitor cells, endothelial progenitor cells, and mesenchymal stem cells (MSCs) have been studied for the treatment of liver diseases[5], and the use of MSCs is becoming increasingly widespread. However, many constraints affect the curative effect of MSCs such as cell number, route of administration, and targeting[6-8]. Commonly suggested routes of MSC transplantation include systemic infusion such as peripheral and local injections through the portal vein[9]. After systemic infusion, only a small proportion of MSCs migrate to damaged tissues, and the majority are entrapped in the capillary beds of the lungs[10]. This may impair their clinical efficacy. In contrast, local implantation relies on the activity of the locally transplanted MSCs at the target site. Studies have demonstrated that MSCs administered via the portal vein exhibit a better ability to attenuate liver inflammation, reduce necrosis, and promote liver regeneration than do MSCs that were transplanted via peripheral veins[11,12]. Although intravenous injections are most commonly used, various vascular administration pathways have also exhibited significant advantages. To date, few comparative studies investigating different methods for the transplantation of BMSCs for liver fibrosis are available. Therefore, exploring safe and effective routes of administration is crucial for BMSC therapy.
In this study, a mouse model of cholestatic liver fibrosis was established using common bile duct ligation (BDL). Bone mesenchymal stem cells (BMSCs) were injected into the liver tissue of BDL mice via the tail and portal veins. The therapeutic effect of BMSCs on cholestatic liver fibrosis was evaluated using two transplantation routes.
-
Four-week-old male SD rats were humanely sacrificed and soaked in a large beaker containing 75% alcohol. Neonatal rat femurs and tibiae were isolated under aseptic conditions and rinsed three times with PBS. The bone marrow was washed twice with DMEM/F12 containing 10% fetal bovine serum (FBS), and these washes were repeated three times until the bone turned white. The rinse solution was collected, filtered through a 200-mesh sieve, and centrifugated at 1,000 rpm for 5 min. The cells were cultured in DMEM/F12 (Gibco, USA) with 10% FBS (Gibco, USA) at 37 °C, 5% CO2, and 100% relative humidity (RH). The culture medium was changed on day 3 to remove non-adherent cells, and the cells were passaged when the fusion reached greater than 80%. The medium was replaced once every 3–4 days, and P3 cells were used for flow cytometry-based identification.
-
Rat BMSC markers CD29 (102215; BioLegend, San Diego, CA, USA), CD90 (202529; BioLegend), CD45 (202211; BioLegend), and CD11b (201807; BioLegend) were detected. P3 BMSCs were digested with 0.25% trypsin containing EDTA, inactivated with FBS, washed twice with PBS, and centrifuged at 1,000 rpm for 5 min to remove the supernatant. These cells were suspended in 100 µL staining buffer and incubated at 4 °C for 20 minutes with APC-conjugated anti-rat CD 29, FITC-conjugated anti-rat CD 90, Alexa Fluor-conjugated anti-rat CD 45, and PE-conjugated anti-rat CD 11b. After washing twice with staining buffer, the phenotypes of the BMSCs were tested using a spectral analyzer. The data were analyzed using FlowJo software.
-
The hepatic stellate cell line JS-1 was provided by the First Hospital of Shanxi Medical University and cultured in low-glucose DMEM (Gibco, USA) with 10% FBS. JS-1 cells were cultured in the lower layer of the transwellµ culture plate, whereas BMSCs were cultured in the upper layer. BMSCs and JS-1 cells were cocultured at a ratio of 1:1. After cell adhesion, JS-1 cells were cultured with 2% FBS as a starvation treatment for 24, 48, or 72 h, and BMSCs were cultured with 10% FBS.
-
Eight-week-old male C57BL/6 mice were acquired from the Shanxi Medical University Animal Center and maintained under specific pathogen-free conditions in a 12-h light-dark cycle. A mouse model of cholestatic liver fibrosis was established by BDL (n = 8). Mice that underwent laparotomy without BDL were used as the sham group (n = 8). After BDL and according to a previous study[13], the mice were immediately injected with BMSCs (6 × 106 cells/mouse) via the tail and portal veins (n = 8). The mice were sacrificed for analysis at 1, 2, and 4 weeks (n = 8).
According to a review by the Animal Experiment Ethics Committee of Shanxi Medical University, the experimental scheme followed animal welfare and ethical principles and met the requirements of ethical norms.
-
The trypsin-digested BMSCs were transferred to a 15 mL sterile centrifuge tube and centrifuged at 1,000 rpm for 5 min to remove the supernatant. After adding the prepared DIL staining solution (DIL 7.5 µL + enhancer 2.5 µL + DMEM / F12 medium 990 µL), BMSCs were incubated at 37 °C for 25 minutes. Subsequently, the cells were washed 1–2 times with PBS and then resuspended in PBS. After filtering with a 0.75 μm filter, the cells were counted for subsequent experiments.
BMSCs that were DIL-stained were transplanted through the tail and portal veins at 1 week before the liver tissues were harvested. Paraffin-embedded sections were observed for red fluorescence using a fluorescence microscope.
-
After homogenization, the mice serum samples were collected by centrifuging for 20 minutes at 13,000 rpm and 4 °C. Alanine aminotransferase (ALT, C009), aspartate aminotransferase (AST, C010), albumin (ALB, A028), and total bilirubin (TBIL, C019) were detected using specific assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
-
The total RNA from live tissues was extracted with TRIzol reagent (Invitrogen), and 1 μg of RNA was reverse-transcribed to cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (RR047A, Takara). Realtime qPCR was carried out using the 2 x TB Green Premix Ex Taq (RR820Q, Takara) in a 20 µL reaction system following the manufacturer’s guidelines. The target genes were measured using the 2 –ΔΔCt method, and GAPDH was used as a control. The primers used in this study are listed in Table 1.
Table 1. Primers sequences for qRT-PCR
Gene Forward primer (5'–3') Reverse primer (5'–3') α-SMA CTCCATCGTCCACCGCAAAT GGCCAGGGCTACAAGTTAAGG Collagen I GCTCCTCTTAGGGGCCACT ATTGGGGACCCTTAGGCCAT TNFα ACCCTCACACTCACAAACCA GAGGCAACCTGACCACTCTC IL-6 TAGTCCTTCCTACCCCAATTTCC TTGGTCCTTAGCCACTCCTTC IL-1β GCAACTGTTCCTGAACTCAACT ATCTTTTGGGGTCCGTCAACT GAPDH CCACTCACGGCAAATTCAAC CTCCACGACATACTCAGCAC -
Liver specimens were fixed in 10% formaldehyde solution for 24 h and sliced into 4 μm sections after they were embedded in paraffin to subsequently investigate the histopathological damage to the liver.
The group sections were dewaxed with xylene, rehydrated with ethanol solutions of different concentrations, and stained with H&E.
-
Liver tissues were embedded in paraffin and sliced at a thickness of 5 μm. The sections were stained with Sirius red for 1 h, and this was followed by alcohol hydration and xylene transparency treatment after rinsing under running water for 10 s and soaking in 0.5% acetic acid for 20 s. Fluorescence was determined using a BX43 Biological Microscope (Olympus) and quantified for five random areas using ImageJ software (version 7.0).
-
Liver tissue was paraffin-embedded and sliced at a thickness of 4 μm. The sections were incubated with rabbit anti-mouse α-SMA (1:500, ab124964, Abcam) and collagen I (1:300, ab270993, Abcam) antibodies overnight at 4 °C and then incubated with HRP-conjugated secondary antibodies (PV6000, Zhongshan Technology) for 20 minutes. Immunoreactive signals were visualized using DAB (ZSGB-BIO, Beijing) as the substrate.
-
After fixation and permeabilization, the JS-1 cells were blocked with goat serum and subsequently incubated with primary antibodies specific for α-SMA (1:200, ab7817, Abcam) at 4 °C overnight. After thorough washing, secondary antibody was added and incubated for 90 min at room temperature. Nuclei were visualized with DAPI (C1005, Beyotime, China).
-
All data are expressed as mean ± standard deviation (SD), and all calculations were performed using GraphPad Prism 8.0 (GraphPad Software, America) or SPSS 23.0 (IBM, America) statistical software. Additionally, statistical significance was evaluated using Student’s t-test or one-way analysis of variance. Statistical significance was set at P < 0.05.
-
To investigate the therapeutic effects of BMSCs on cholestatic liver fibrosis, rat BMSCs were extracted. Both P1 and P3 BMSCs exhibited adherent growth and a typical spindle-shaped morphology (Figure 1A). BMSC surface markers were identified using flow cytometry. The results demonstrated positive expression of CD29 and CD90 and negative expression of CD45 and CD11b (Figure 1B) that met the International Society for Cellular Therapy (ISCT) standards for MSC markers[14], thus indicating that BMSCs were extracted successfully. The strategy for transplantation of BMSCs via these two routes is illustrated in Figure 1C.
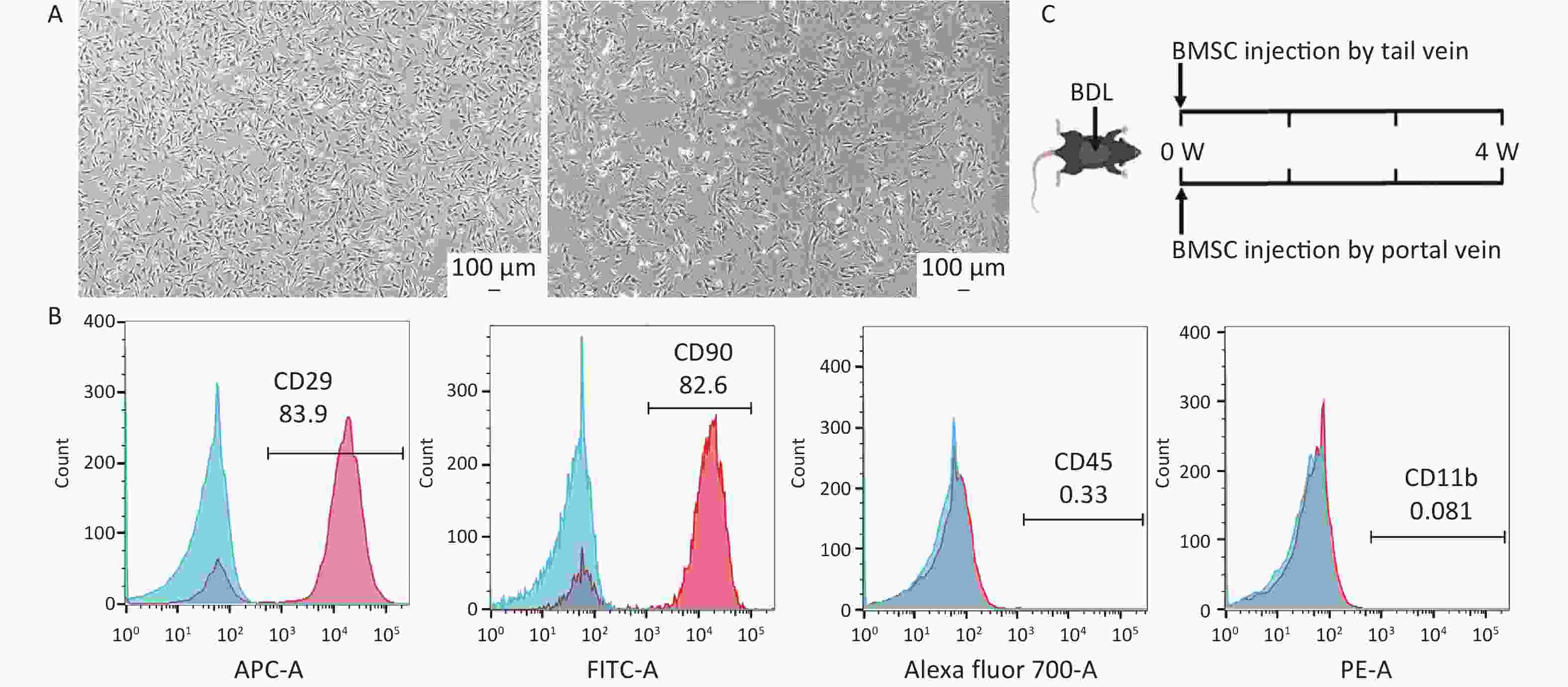
Figure 1. Characterization of mouse bone-derived stem cells. (A) Morphology of mouse bone marrowderived mesenchymal stem cells (BMSCs) (n = 3). Scale bar: 100 µm. (B) Evaluation of BMSC markers by flow cytometry (n = 3). (C) Schematic representation of the timing strategy used to evaluate BMSC transplantation via different pathways in the context of BDL-induced liver fibrosis. All data are presented as the mean ± SD.
-
HSC activation is an indispensable component in the progression of liver fibrosis[15]. Starvation is a common method of activating resting HSCs in vitro[16]. To investigate whether BMSCs could regulate the activity of HSCs in vitro, they were co-cultured with JS-1 cells activated by starvation. In the presence of BMSCs, the number of activated HSCs induced by starvation was markedly decreased, particularly at 48 h (Figure 2A), indicating that BMSCs exerted the strongest inhibition of HSC activation at 48 h. Meanwhile, as determined by immunofluorescence, the upregulation of α-SMA by starvation was decreased by BMSCs (Figure 2B). As expected, the suppression of HSC activation was also confirmed by qRT-PCR analysis that revealed that the fibrosis-related genes, including α-SMA and collagen I mRNA, were downregulated by BMSCs compared to that of the activated HSCs (Figure 2C, D). These results demonstrate that HSC activation can be inhibited by treatment with BMSCs.
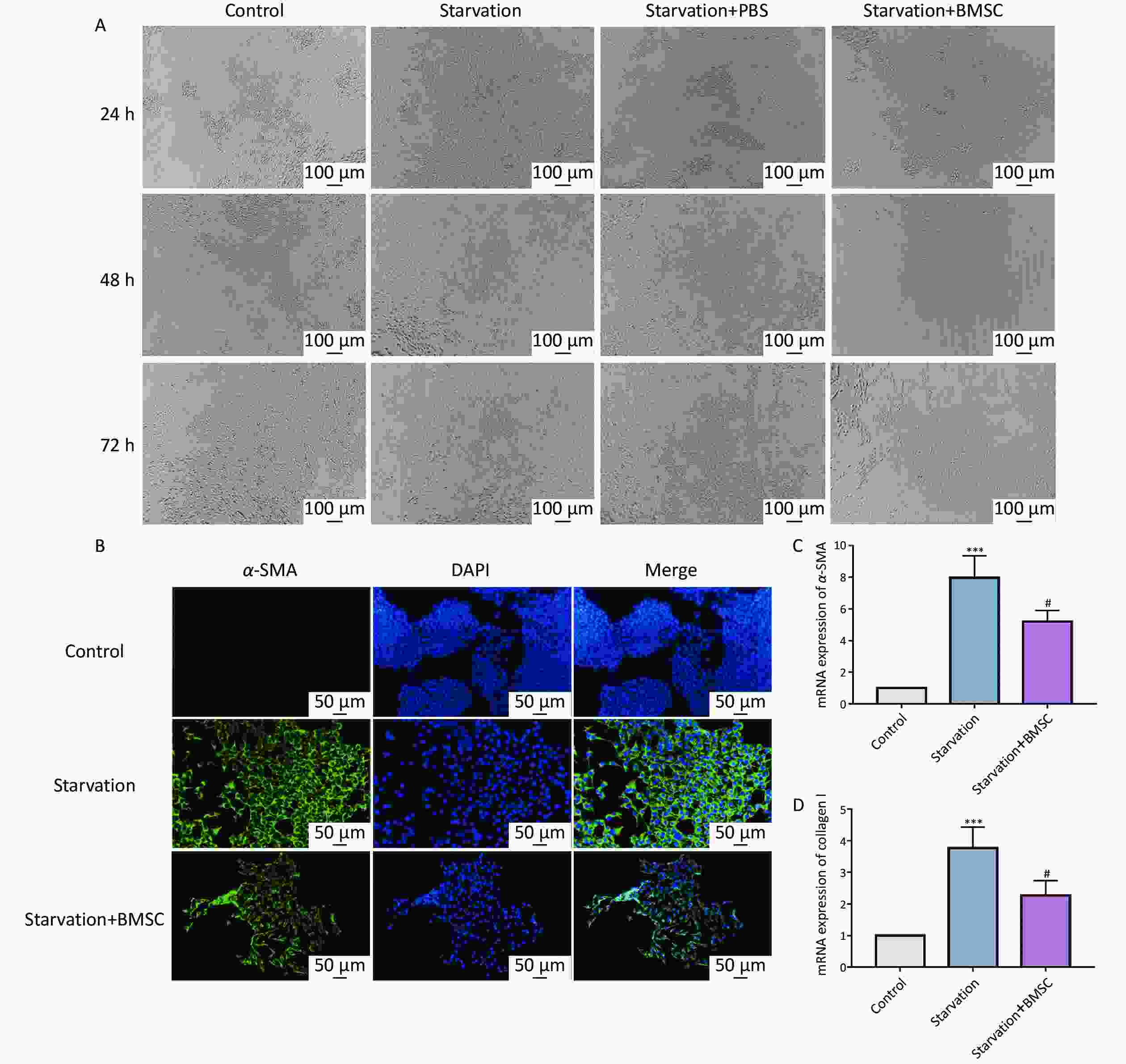
Figure 2. Bone marrowderived mesenchymal stem cells (BMSCs) attenuate hepatic stellate cells (HSCs) activation. (A) Starvation-induced JS-1 cells were observed after co-culturing with BMSCs for 24, 48, and 72 h (n = 3). Scale bar: 100 µm. (B) Immunofluorescence of α-SMA in starvation-induced JS-1 cells co-cultured with BMSCs. The cells were stained for α-SMA (green) and DAPI (blue). Scale bars: 50 µm. (C, D) mRNA levels of α-SMA and collagen I with qPCR in starvation-induced JS-1 cells co-cultured with BMSCs (n = 3). All data are presented as the mean ± SD. *P < 0.05, ***P < 0.001 vs. control group, #P < 0.05 vs. starvation group.
-
Recently, multiple studies have reported that cholestasis causes liver injury in animal models[17]. BDL in rodents has been a well-established experimental procedure for many years to model cholestatic liver diseases that are similar to the pathology of clinical primary biliary cirrhosis (PBC). Therefore, this model is suitable for designing therapeutic strategies against cholestatic liver fibrosis. In this study, we evaluated the therapeutic efficacy of BMSC treatment via two different pathways in BDL-induced liver fibrosis.
As illustrated in Figure 3A, DIL red fluorescence was markedly increased after BMSC portal vein transplantation compared to that after BMSC tail vein transplantation. Compared to the BDL group, mice in the BMSC portal vein transplantation group exhibited weight gain at 2 and 4 weeks, whereas there was no increase in the BMSC tail vein transplantation group (Figure 3B). To further clarify the effects of the two pathways of BMSCs treatment on liver injury, macroscopic inspection of the livers was performed. As presented in Figure 3C, the livers in the control and sham groups were ruddy with a soft texture, sharp edges, and a small gallbladder. Compared to the sham group, the liver in the BDL group was light yellow, with a harder texture, blunt edges, and gradual enlargement of the gallbladder. After the two routes of BMSC transplantation, the liver color became slightly reddish, with a slightly soft texture, clearer edges, and a smaller gallbladder. Importantly, this effect was even more pronounced in the BMSC portal vein group than it was in the tail vein group.
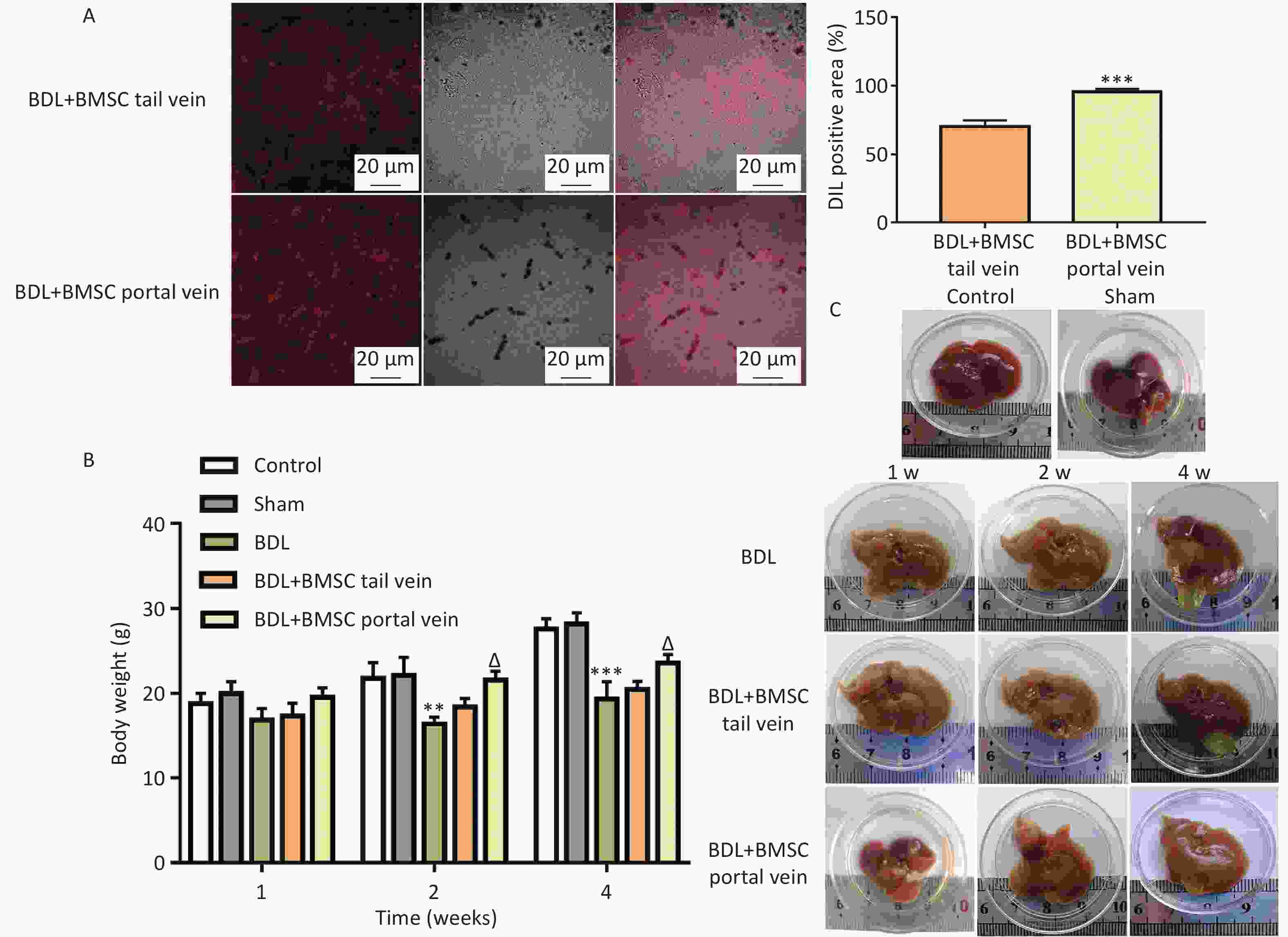
Figure 3. Bone marrowderived mesenchymal stem cells (BMSCs) treatment through the portal vein was superior to that through the tail vein for improving BDL-induced liver injury. (A) DIL staining was performed to assess BMSC localization in the liver (n = 8). Scale bar: 20 µm. ***P < 0.001 vs. BDL+BMSC tail vein group. (B) Body weights of mice in the BDL-induced mouse liver after BMSC transplantation (n = 8). **P < 0.01, ***P < 0.001 vs. sham group, ΔP < 0.05 vs. BDL group. (C) Representative images of gross liver appearance in mice (n = 3). All data are presented as the mean ± SD.
-
It has been demonstrated that the BDL model can induce systemic inflammation and elevate levels of inflammatory cytokines[18,19]. To explore the protective effects of BMSCs, we assessed biochemical indicators of inflammation. As illustrated in Figure 4A–D, serum ALT, AST, and TBIL levels were significantly downregulated in the BDL+BMSC portal vein group compared to those in the BDL+BMSC tail vein group, and ALB exhibited the opposite trend. Additionally, the up-regulation of inflammatory cytokines, including tumor necrosis factor (TNFα), IL-1β, and IL-6, have been reported to be the key features of liver inflammation. The mRNAs for TNFα, IL-1β, and IL-6 in the context of liver fibrosis were investigated. qRT-PCR demonstrated that BMSC transplantation via two routes, including tail vein and portal vein, both decreased the BDL-induced TNFα, IL-1β, and IL-6 mRNA expression, with BMSCs portal vein transplantation exhibiting a more significant reduction effect (Figure 4E–G). Similar to these results, HE staining indicated that BDL-induced liver inflammation that was characterized by hepatocyte edema, inflammatory cell infiltration, bile duct over-proliferation, and necrosis was reduced after BMSCs treatment, particularly when BMSCs were transplanted into the portal vein (Figure 5A, B). These data indicate that BMSC transplantation through the portal vein exerted a better effect on liver inflammation than did transplantation through the tail vein.
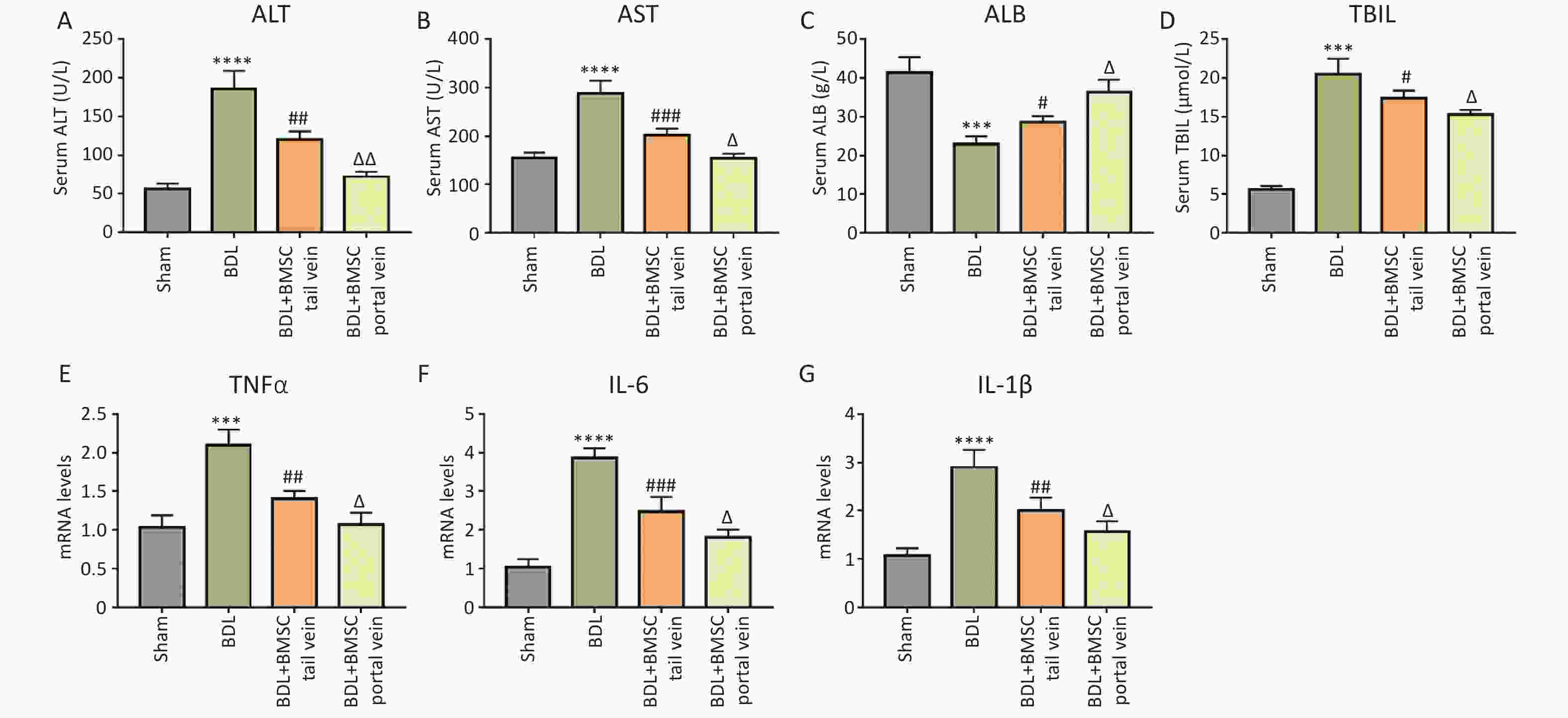
Figure 4. BMSC treatment via the portal vein was superior to that via the tail vein for alleviating BDL-induced inflammation. (A–D) Serum ALT, AST, ALB, and TBIL levels in the livers of BDL-treated mice after BMSC transplantation (n = 3). (E–G) The mRNA expression of TNFα, IL-1β, and IL-6 in BDL-induced mice liver after BMSC transplantation (n = 3). All data are presented as the mean ± SD. ***P < 0.001, ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01 vs. BDL+BMSC tail vein group. BMSC, Bone marrowderived mesenchymal stem cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; BDL, bile duct ligation.

Figure 5. Bone marrowderived mesenchymal stem cells (BMSCs) treatment via the portal vein was superior to that via the tail vein for reducing bile duct ligation (BDL)-induced collagen fiber formation. (A) Representative histological staining (hematoxylin and eosin) of liver sections from BDL-treated mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. (B) Suzuki histological score for hematoxylin and eosin staining. (C, D) Representative images of Sirius red staining in BDL-induced mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. (E, F) Representative images of Masson’s trichrome staining of the BDL-induced mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. All data are presented as the mean ± SD. ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01 vs. BDL+BMSC tail vein group.
-
After BDL, toxic bile acid activates hepatic stellate cells (HSCs) and promotes their transdifferentation into myofibroblasts that express α-smooth muscle actin (α-SMA) during liver restoration[15,20], ultimately forming collagen fibers. Sirius red staining demonstrated that BMSC transplantation via the two routes markedly alleviated BDL-triggered collagen fiber formation, with BMSC transplantation through the portal vein exerting a more pronounced effect (Figure 5C, D). Masson staining confirmed these results (Figure 5E and F).
α-SMA and collagen I signals occurred intracellularly in HSC-derived myofibroblasts. The α-SMA IHC results indicated that BMSC treatment via the portal vein reduced the activation of myofibroblasts in damaged regions of liver to a greater extent than did BMSC transplantation via the tail vein (Figure 6A, B). Consistent with these results, the collagen I protein level was the lowest in the BDL+BMSC portal vein (Figure 6C, D). These data suggest that BMSC transplantation through the portal vein resulted in a remarkable improvement in HSC activation and liver fibrosis.
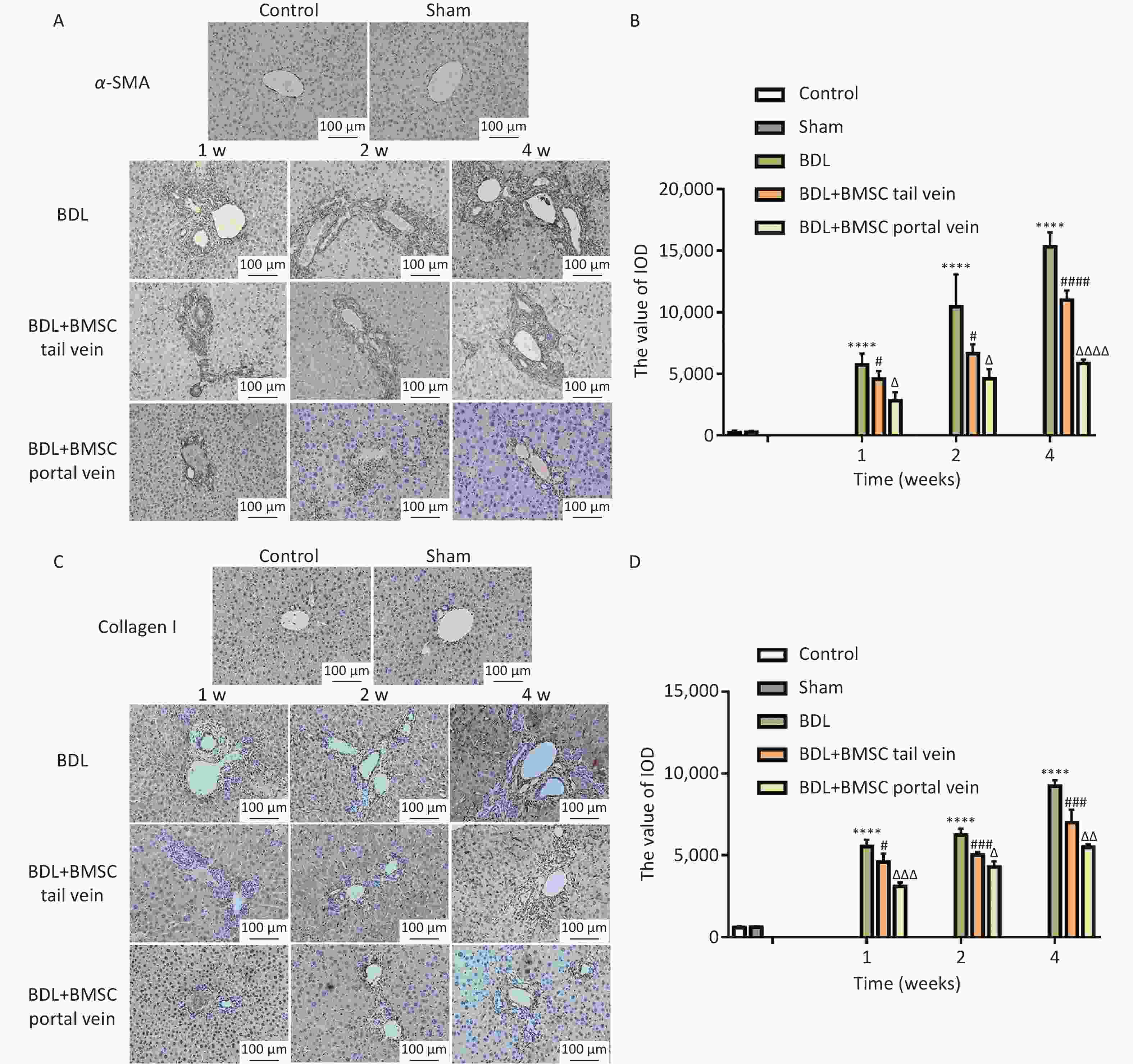
Figure 6. Portal vein injection is superior to tail vein injection for Bone marrowderived mesenchymal stem cells (BMSCs) treatment in regard to ameliorating BDL-induced hepatic stellate cells (HSCs) activation and liver fibrosis. (A, B) α-SMA was semi-quantitatively detected by immunohistochemical staining (n = 8). Scale bars: 100 µm. (C, D) Collagen I was quantitatively detected using immunohistochemical staining (n = 8). Scale bars: 100 µm. All data are presented as the mean ± SD. ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001, ΔΔΔΔP < 0.0001 vs. bile duct ligation (BDL)+BMSC tail vein group.
-
The liver exhibits the highest regenerative capacity of any organ. Various factors, including viral infections, alcohol abuse, lipid metabolism disorders, and cholestasis, can cause chronic inflammation and fibrosis, ultimately leading to irreversible liver dysfunction[21]. Despite advances in surgery and drug treatment, liver disease remains the main cause of death worldwide. Liver diseases pose a significant threat to human health, resulting in approximately 2 million deaths annually. Liver cirrhosis accounts for approximately 50% of these deaths[3]. Therefore, cell therapy for liver diseases has become a promising treatment to address the shortage of donor organs for orthotopic liver transplantation.
Reversible liver fibrosis is an early stage of liver cirrhosis that causes hepatocyte damage and irreversible scar tissue. An increasing number of studies have indicated that MSCs exert anti-liver fibrosis effects through various mechanisms such as promoting liver cell differentiation[22,23]. Given their ease of isolation and extraction from the bone marrow and their low immunogenicity, high self-renewal, and multi-differentiation ability, BMSCs are promising cells for the treatment of liver fibrosis[24]. Multiple animal experiments have demonstrated that BMSCs exert antifibrotic effects by improving symptoms and liver function[25,26]. However, the clinical application of MSCs faces many substantive issues such as transplantation pathways that directly affect their therapeutic effect[27]. Therefore, in this study, we transplanted BMSCs through the portal vein and tail vein to evaluate their therapeutic effects on BDL-induced cholestatic liver fibrosis.
In 2006, the International Society for Cell Therapy (ISCT) proposed a series of characteristic biomarkers to identify MSCs and established a universal consensus on MSCs. CD105, CD90, and CD73 are positively expressed as MSCs surface markers, while CD45, CD34, CD14, CD11b, CD19 are negatively expressed[14] and are still currently used. In this study, flow cytometry analysis revealed that BMSCs expressed CD29 on their surface but not CD45 and CD11b, and this was consistent with MSCs stemness. Combined with their spindle-like morphology, these results confirmed the successful extraction of BMSCs.
CM-DIL that is used to track various types of cells or tissues is an orange-red fluorescent dye that can directly contact the cell membrane and diffuse throughout the cell. Using the same method[28], BMSCs were labeled with CM-DIL and transplanted into BDL mice using two intravenous injection methods. Fluorescence microscopy was used to observe the homing of BMSCs to the liver. Three factors affect the homing of BMSCs to the liver: i) the type and severity of liver damage; ii) the expression of chemokines prompting the homing of BMSCs to the damaged liver; iii) the number of BMSCs in the circulation. MSCs possess an inherent chemotactic ability to migrate to sites in damaged areas[29]. The homing rates of the BMSCs transplanted via different routes also differed. Our results demonstrated that the colonization rate of BMSCs in the liver after portal vein transplantation was significantly higher than that after tail vein transplantation, indicating that the number of BMSCs at the target site may be higher and confirming the effect of portal vein injection of BMSCs for promoting BMSC homing to the injured liver. This is consistent with the observation that the therapeutic effect of MSC local transplantation reported in the literature is superior to venous transplantation[30].
Activation of HSCs is the central link in the occurrence and development of liver fibrosis. HSCs are the main source of ECM, and a strategy for regulating HSCs activation is crucial for treating liver fibrosis. Excessive extracellular matrix (ECM) production is the main pathological feature of liver fibrosis[31]. HSCs are quiescent in the normal liver, and the synthesis and degradation of the ECM is balanced. However, when HSCs are activated into myofibroblasts by inflammation or cytokines, ECM production increases, and this is accompanied by increased α-SMA and collagen fiber production[32]. Growing evidence supported the idea that MSCs could alleviate liver fibrosis via restraining HSC activation[33,34]. In this study, we demonstrated that BMSCs inhibited HSC activation in vitro, and this provides a reference for the next comparative therapy of BMSCs in vivo. After BDL, toxic bile acids initially stimulated bile duct cells, leading to inflammatory cell infiltration in the portal area and gradually spreading to liver parenchymal cells. This stimulated liver cells to secrete various inflammatory cytokines, including TNFα, IL-6 and IL-1β, further promoting HSCs activation and ultimately leading to the increased expression of α-SMA and collagen I.
One study suggested that MSC therapy could improve liver function and alleviate clinical symptoms without serious adverse events, and the effectiveness varied slightly between peripheral intravenous injections and hepatic arterial injections[35]. Another study reported that intraportal MSC transplantation improved function, inhibited apoptosis, and prolonged the survival of swine with acute liver failure[11]. Similarly, in our study, BMSC treatment improved liver function, reduced bile stasis, and decreased inflammatory factor levels. Meanwhile, the protein expression of α-SMA and collagen I were downregulated, indicating that BMSCs exert an anti-liver fibrosis effect.
Selecting an appropriate MSC transplantation route is vital for cell survival, the induction of cell differentiation, and the restoration of liver function. The route of implantation may have affected the curative effects of implantation. One study indicated that the endovascular injection of BMSCs exerted profound inhibitory effects on hepatocellular death, leading to reduced hepatocyte apoptosis, enhanced liver regeneration, and an increased number of proliferating hepatocytes[27]. In this study, we injected via two different routes. Compared to those injected via the tail vein, BMSC transplantation through the portal vein resulted in reduced inflammatory cell infiltration and collagen fiber generation in the portal area and less α-SMA and collagen I levels, indicating that BMSCs injected by portal vein exert a stronger inhibitory effect on HSC activation and that BMSCs injected through the portal vein may exert better therapeutic effect on liver fibrosis. These results indicated that the implantation route may affect the curative effect of implantation.
In animal experiments, MSC transplantation typically proceeds via portal vein, hepatic artery, peripheral vein, intraperitoneal, or local injections into the liver or spleen. In clinical applications, the principal transplantation methods include portal vein, hepatic artery, and peripheral vein injections. To address these clinical issues, BMSC treatment between the portal vein and the hepatic artery must be further explored. For therapeutic applications, it is important to understand the possible repair mechanisms of BMSCs, including their differentiation into functional hepatic cells and reduction in hepatocyte apoptosis, and this requires further investigation.
-
In summary, during the development of cholestatic liver fibrosis in mice, BMSC transplantation alleviated the inflammatory response in the mouse liver tissues, reduced collagen fiber formation, inhibited HSC activation, and improved liver fibrosis. The therapeutic effect of BMSCs on cholestatic liver fibrosis in mice through portal vein injection was superior to that of BMSCs injected through the tail vein.
doi: 10.3967/bes2024.180
Exploring the Efficacy of BMSC Transplantation via Various Pathways for Treating Cholestatic Liver Fibrosis in Mice
-
Abstract:
Objective To compare the therapeutic efficacy of portal and tail vein transplantation of bone marrow-derived mesenchymal stem cells (BMSCs) against cholestatic liver fibrosis in mice. Methods BMSCs were isolated and co-cultured with starvation-activated hepatic stellate cells (HSCs). HSC activation markers were identified using immunofluorescence and qRT-PCR. BMSCs were injected into the liver tissues of bile duct ligation (BDL) mice via the tail and portal veins. Histomorphology, liver function, inflammatory cytokines, and the expression of key proteins were all determined in the liver tissues. Results BMSCs inhibited HSC activation by reducing α-SMA and collagen I expression. Compared to tail vein injection, DIL-labeled BMSCs injected through the portal vein maintained a high homing rate in the liver. Moreover, BMSCs transplanted through the portal vein resulted in greater improvement in liver color, hardness, and gallbladder size than did those transplanted through the tail vein. Furthermore, BMSCs injected by portal vein, but not tail vein, markedly ameliorated liver function, reduced the secretion of inflammatory cytokines, including TNF-α, IL-6, and IL-1β, and decreased α-SMA + hepatic stellate cell (HSC) activation and collagen fiber formation. Conclusion The therapeutic effect of BMSCs on cholestatic liver fibrosis in mice via portal vein transplantation was superior to that of tail vein transplantation. This comparative study provides reference information for further BMSC studies focused on clinical cholestatic liver diseases. -
Key words:
- Bone marrow mesenchymal stem cells /
- Portal vein /
- Tail vein /
- Cholestatic hepatic fibrosis /
- Administration route
The authors declare no conflict of interest.
This animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Shanxi Medical University (License Key: SCXK2022-0006).
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. Characterization of mouse bone-derived stem cells. (A) Morphology of mouse bone marrowderived mesenchymal stem cells (BMSCs) (n = 3). Scale bar: 100 µm. (B) Evaluation of BMSC markers by flow cytometry (n = 3). (C) Schematic representation of the timing strategy used to evaluate BMSC transplantation via different pathways in the context of BDL-induced liver fibrosis. All data are presented as the mean ± SD.
Figure 2. Bone marrowderived mesenchymal stem cells (BMSCs) attenuate hepatic stellate cells (HSCs) activation. (A) Starvation-induced JS-1 cells were observed after co-culturing with BMSCs for 24, 48, and 72 h (n = 3). Scale bar: 100 µm. (B) Immunofluorescence of α-SMA in starvation-induced JS-1 cells co-cultured with BMSCs. The cells were stained for α-SMA (green) and DAPI (blue). Scale bars: 50 µm. (C, D) mRNA levels of α-SMA and collagen I with qPCR in starvation-induced JS-1 cells co-cultured with BMSCs (n = 3). All data are presented as the mean ± SD. *P < 0.05, ***P < 0.001 vs. control group, #P < 0.05 vs. starvation group.
Figure 3. Bone marrowderived mesenchymal stem cells (BMSCs) treatment through the portal vein was superior to that through the tail vein for improving BDL-induced liver injury. (A) DIL staining was performed to assess BMSC localization in the liver (n = 8). Scale bar: 20 µm. ***P < 0.001 vs. BDL+BMSC tail vein group. (B) Body weights of mice in the BDL-induced mouse liver after BMSC transplantation (n = 8). **P < 0.01, ***P < 0.001 vs. sham group, ΔP < 0.05 vs. BDL group. (C) Representative images of gross liver appearance in mice (n = 3). All data are presented as the mean ± SD.
Figure 4. BMSC treatment via the portal vein was superior to that via the tail vein for alleviating BDL-induced inflammation. (A–D) Serum ALT, AST, ALB, and TBIL levels in the livers of BDL-treated mice after BMSC transplantation (n = 3). (E–G) The mRNA expression of TNFα, IL-1β, and IL-6 in BDL-induced mice liver after BMSC transplantation (n = 3). All data are presented as the mean ± SD. ***P < 0.001, ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01 vs. BDL+BMSC tail vein group. BMSC, Bone marrowderived mesenchymal stem cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBIL, total bilirubin; BDL, bile duct ligation.
Figure 5. Bone marrowderived mesenchymal stem cells (BMSCs) treatment via the portal vein was superior to that via the tail vein for reducing bile duct ligation (BDL)-induced collagen fiber formation. (A) Representative histological staining (hematoxylin and eosin) of liver sections from BDL-treated mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. (B) Suzuki histological score for hematoxylin and eosin staining. (C, D) Representative images of Sirius red staining in BDL-induced mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. (E, F) Representative images of Masson’s trichrome staining of the BDL-induced mouse livers after BMSC transplantation (n = 8). Scale bar: 100 µm. All data are presented as the mean ± SD. ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01 vs. BDL+BMSC tail vein group.
Figure 6. Portal vein injection is superior to tail vein injection for Bone marrowderived mesenchymal stem cells (BMSCs) treatment in regard to ameliorating BDL-induced hepatic stellate cells (HSCs) activation and liver fibrosis. (A, B) α-SMA was semi-quantitatively detected by immunohistochemical staining (n = 8). Scale bars: 100 µm. (C, D) Collagen I was quantitatively detected using immunohistochemical staining (n = 8). Scale bars: 100 µm. All data are presented as the mean ± SD. ****P < 0.0001 vs. sham group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. BDL group. ΔP < 0.05, ΔΔP < 0.01, ΔΔΔP < 0.001, ΔΔΔΔP < 0.0001 vs. bile duct ligation (BDL)+BMSC tail vein group.
Table 1. Primers sequences for qRT-PCR
Gene Forward primer (5'–3') Reverse primer (5'–3') α-SMA CTCCATCGTCCACCGCAAAT GGCCAGGGCTACAAGTTAAGG Collagen I GCTCCTCTTAGGGGCCACT ATTGGGGACCCTTAGGCCAT TNFα ACCCTCACACTCACAAACCA GAGGCAACCTGACCACTCTC IL-6 TAGTCCTTCCTACCCCAATTTCC TTGGTCCTTAGCCACTCCTTC IL-1β GCAACTGTTCCTGAACTCAACT ATCTTTTGGGGTCCGTCAACT GAPDH CCACTCACGGCAAATTCAAC CTCCACGACATACTCAGCAC -
[1] Yuan ZH, Wang J, Zhang HR, et al. Glycocholic acid aggravates liver fibrosis by promoting the up-regulation of connective tissue growth factor in hepatocytes. Cell Signal, 2023; 101, 110508. doi: 10.1016/j.cellsig.2022.110508 [2] Hirschfield GM, Dyson JK, Alexander GJM, et al. The British society of gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut, 2018; 67, 1568−94. doi: 10.1136/gutjnl-2017-315259 [3] Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol, 2019; 70, 151−71. doi: 10.1016/j.jhep.2018.09.014 [4] Cao XX, Gao YQ, Zhang WH, et al. Cholestasis morbidity rate in first-hospitalized patients with chronic liver disease in Shanghai. Chin J Hepatol, 2015; 23, 569−73. (In Chinese) [5] Pinheiro D, Dias I, Silva KR, et al. Mechanisms underlying cell therapy in liver fibrosis: an overview. Cells, 2019; 8, 1339. doi: 10.3390/cells8111339 [6] Lee JH, Lee S, Park HJ, et al. Human liver stem cell transplantation alleviates liver fibrosis in a rat model of CCl4-induced liver fibrosis. Int J Stem Cells, 2021; 14, 475−84. doi: 10.15283/ijsc21031 [7] Sun XJ, Guo SL. Effectiveness of cell- and colony stimulating factor-based therapy for liver cirrhosis: a network meta-analysis of randomized controlled trials. Cytotherapy, 2022; 24, 516−25. doi: 10.1016/j.jcyt.2021.11.006 [8] Carvalho AB, Quintanilha LF, Dias JV, et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells, 2008; 26, 1307−14. doi: 10.1634/stemcells.2007-0941 [9] Kean TJ, Lin P, Caplan AI, et al. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int, 2013; 2013, 732742. [10] Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng, 2019; 3, 90−104. doi: 10.1038/s41551-018-0325-8 [11] Sang JF, Shi XL, Han B, et al. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatob Pancreat Dis Int, 2016; 15, 602−11. doi: 10.1016/S1499-3872(16)60141-8 [12] Cao HC, Yang JF, Yu J, et al. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med, 2012; 10, 56. doi: 10.1186/1741-7015-10-56 [13] Zhang EG, Yang Y, Chen SY, et al. Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res Ther, 2018; 9, 311. doi: 10.1186/s13287-018-1045-4 [14] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006; 8, 315−7. doi: 10.1080/14653240600855905 [15] Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol, 2017; 14, 397−411. doi: 10.1038/nrgastro.2017.38 [16] Endo S, Nakata K, Ohuchida K, et al. Autophagy is required for activation of pancreatic stellate cells, associated with pancreatic cancer progression and promotes growth of pancreatic tumors in mice. Gastroenterology, 2017; 152, 1492-1506. e24. [17] Liu RP, Li XJY, Huang ZM, et al. C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice. Hepatology, 2018; 67, 1441−57. doi: 10.1002/hep.29540 [18] Mousavi K, Niknahad H, Li HF, et al. The activation of nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling blunts cholestasis-induced liver and kidney injury. Toxicol Res (Camb), 2021; 10, 911−27. doi: 10.1093/toxres/tfab073 [19] Ommati MM, Niknahad H, Najibi A, et al. Cholestasis-associated pulmonary inflammation, oxidative stress, and tissue fibrosis: the protective role of the biogenic amine agmatine. Pharmacology, 2023; 108, 379−393. doi: 10.1159/000530307 [20] Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology, 2017; 65, 1039−43. doi: 10.1002/hep.28948 [21] Siapati EK, Roubelakis MG, Vassilopoulos G. Liver regeneration by hematopoietic stem cells: have we reached the end of the road?. Cells, 2022; 11, 2312. doi: 10.3390/cells11152312 [22] Mishra PJ, Banerjee D. Activation and differentiation of mesenchymal stem cells. Methods Mol Biol, 2017; 1154, 201−9. [23] Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell, 2011; 19, 257−72. doi: 10.1016/j.ccr.2011.01.020 [24] Duman DG, Zibandeh N, Ugurlu MU, et al. Mesenchymal stem cells suppress hepatic fibrosis accompanied by expanded intrahepatic natural killer cells in rat fibrosis model. Mol Biol Rep, 2019; 46, 2997−3008. doi: 10.1007/s11033-019-04736-4 [25] Feng Y, Li YJ, Xu MX, et al. Bone marrow mesenchymal stem cells inhibit hepatic fibrosis via the AABR07028795.2/rno-miR-667-5p axis. Stem Cell Res Ther, 2022; 13, 375. doi: 10.1186/s13287-022-03069-7 [26] Xu YN, Xu W, Zhang X, et al. BM-MSCs overexpressing the Numb enhance the therapeutic effect on cholestatic liver fibrosis by inhibiting the ductular reaction. Stem Cell Res Ther, 2023; 14, 45. doi: 10.1186/s13287-023-03276-w [27] Sun LH, Fan XT, Zhang LJ, et al. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med, 2014; 34, 987−96. doi: 10.3892/ijmm.2014.1890 [28] Huang QL, Cheng XM, Luo C, et al. Placental chorionic plate-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating macrophage polarization via secreting TSG-6. Stem Cell Res Ther, 2021; 12, 337. doi: 10.1186/s13287-021-02411-9 [29] Yuan MQ, Hu X, Yao LC, et al. Mesenchymal stem cell homing to improve therapeutic efficacy in liver disease. Stem Cell Res Ther, 2022; 13, 179. doi: 10.1186/s13287-022-02858-4 [30] Lu YB, Zhang W, Tian ZM, et al. The optimal transplantation strategy of umbilical cord mesenchymal stem cells in spinal cord injury: a systematic review and network meta-analysis based on animal studies. Stem Cell Res Ther, 2022; 13, 441. [31] Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol, 2003; 38, S38-53. [32] Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res, 2017; 352, 420−6. doi: 10.1016/j.yexcr.2017.02.038 [33] Tan YW, Huang Y, Mei R, et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis, 2022; 13, 319. doi: 10.1038/s41419-022-04764-2 [34] Lin Y, Yan MC, Bai ZT, et al. Huc-MSC-derived exosomes modified with the targeting peptide of aHSCs for liver fibrosis therapy. J Nanobiotechnol, 2022; 20, 432. doi: 10.1186/s12951-022-01636-x [35] Liu YW, Dong YT, Wu X, et al. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res Ther, 2022; 13, 204. doi: 10.1186/s13287-022-02882-4 -




 下载:
下载:




 Quick Links
Quick Links