-
Diabetes mellitus is a global public health concern affecting an estimated 537 million people, and patients with diabetes are more susceptible to developing chronic wounds[1,2]. The wound healing process is often affected by various factors, including infection, tissue hypoxia, necrosis, exudates, and excessive inflammatory cytokines[3]. Presently, there is no effective treatment for refractory wound healing, and the development of new techniques and therapeutic strategies is urgently required for patients with diabetes who have chronic wounds.
Studies have revealed that mesenchymal stem cells (MSCs) are effective for wound healing, which might be related to the cytokines secreted by MSC paracrine. The survival ability of MSCs treated with hypoxia can be enhanced in vivo[4,5], which makes them more suitable in the hypoxic microenvironment and improves their therapeutic potential. However, there are relatively few reports on hypoxic MSCs produced by employing industrial processes and their therapeutic effects on diabetic wound healing. The industrial manufacturing process required for their use as a clinical product remains insufficient[6]. In this study, we analyzed the biological functions and effects of normoxic and hypoxic MSCs prepared via industrial processes on diabetic wound healing in rats and explored the possible mechanisms using in vitro experiments. These results provide technical support for the clinical transformation of MSCs.
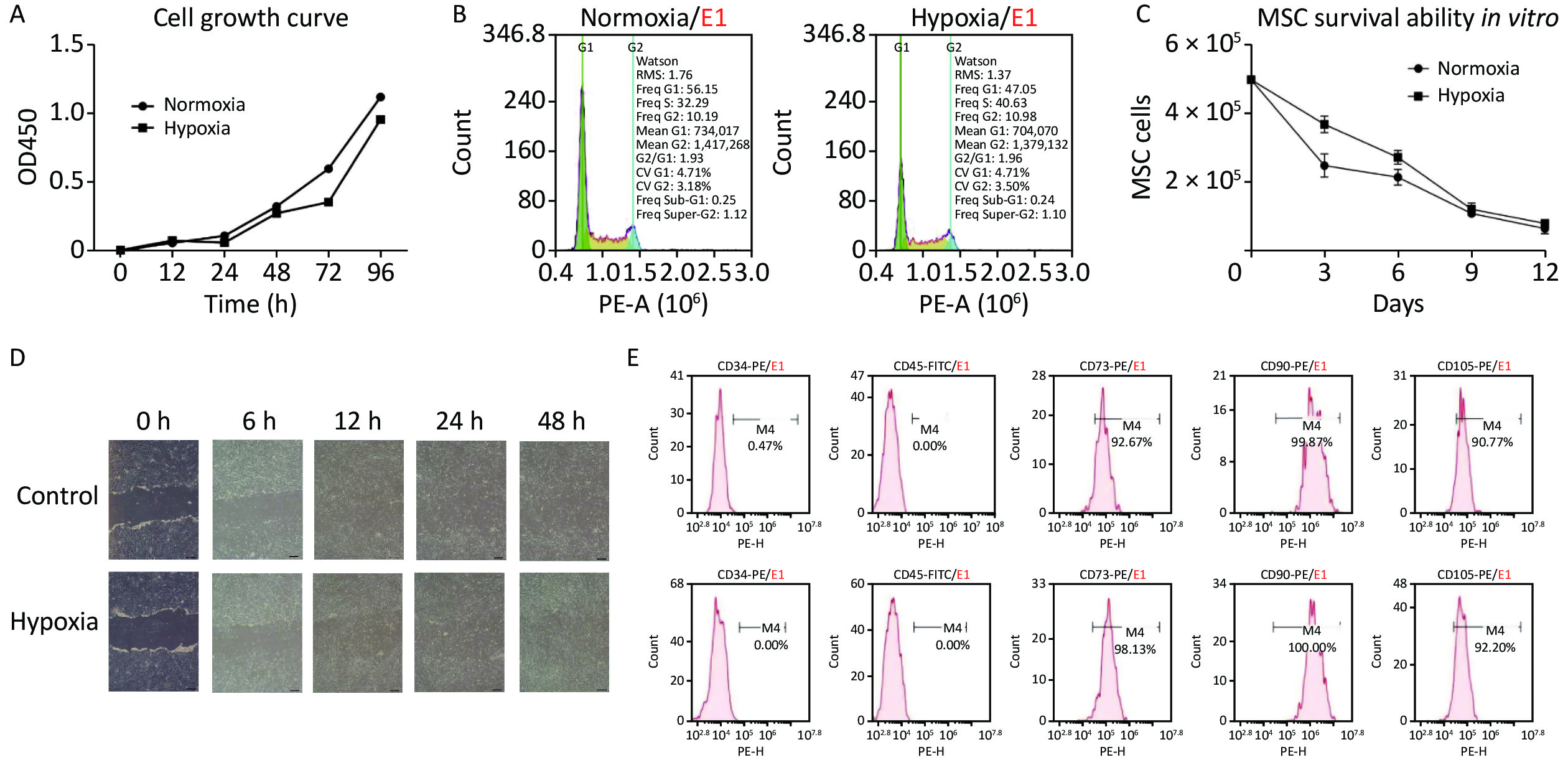
The industrially processed hypoxia-MSCs used in this study were prepared from human umbilical cord, and written informed consent was obtained from the donors. First, the normoxia-MSCs were prepared with essential medium and 5% serum substitute at 37 °C, 5% CO2, and 95% humidity. The medium was replaced every second day and when confluence reached 80%, the cells were collected, cryopreserved in dimethyl sulfoxide (DMSO), and underwent quality evaluation. When hypoxia-MSCs were needed, cryopreserved normoxia-MSCs were resuscitated and cultured in an incubator with 1% oxygen for 96 h. The proliferation, survival ability, and migration of hypoxia-MSCs were evaluated to assess their biological functions. The proliferation of hypoxic and normoxic MSCs was tested by determining the growth curve and cell cycle using the CCK-8 method and flow cytometry. The growth curve (Figure 1A) demonstrated that the proliferation trends of the two groups within 96 h were similar, and the cell number under hypoxia was slightly lower than that under normoxia; however, this was not statistically significant. The cell cycle was measured using flow cytometry, and the results (Figure 1B) revealed that the number of hypoxic cells in the G1 phase accounted for 47.05% and G2/G1 was 1.96, indicating that cell proliferation was similar to normoxia and that most cells were in a state of proliferation. The in vitro survival ability was tested by counting the cell numbers of hypoxic and normoxic MSCs within 12 days. The results (Figure 1C) showed that more cells survived in the hypoxia group than in the normoxia group, indicating that hypoxia improves the survival ability of MSC, but the difference was not statistically significant. Cell migration was assessed using a scratch assay, and hypoxic MSCs (Figure 1D) exhibited good migration ability as normoxic MSCs after 24 h. Studies have reported that hypoxia enhances the function of MSCs by increasing proliferation, survival, homing, differentiation, and paracrine activities[7]. The above results also support these conclusions, indicating that hypoxia does not influence the biological function of MSCs in proliferation and migration, and it improves their survival ability in vitro, which is a key factor in cell therapy.

Figure 1. Cell proliferation, survival ability, and migration under normoxic and hypoxic conditions. (A) Cell growth curve; (B) Cell cycle detection; (C) Survival of MSC in vitro; (D) The scratch assay of MSC (Scale bar, 200 μm); (E) The surface markers of normoxia (up) and hypoxia MSC (down).
To ensure that the hypoxic MSC products met quality standards and were suitable for preclinical testing, we examined the morphology, number, viability, and surface markers of cells as well as bacterial/fungal contamination to identify the quality attributes. The hypoxic MSCs presented typical MSC morphology, with cell viability above 90% and positive expression of CD73, CD90, and CD105 but negative expression of CD34 and CD45 (Figure 1E), which was in line with the unified international standards for MSCs and could be used in subsequent animal experiments.
The effectiveness of hypoxic MSCs in wound healing was assessed in diabetic rat models, established using Sprague–Dawley (SD) rats (male, 253.42 ± 9.57 g, aged approximately 7 weeks), with streptomycin (STZ, 30 mg/kg) injected intraperitoneally on the day after the rats were fed a high-fat diet for 8 weeks and fasted for 12 h. We injected 800 μL (1 × 107/mL) cells subcutaneously into the four corners of the back wound of the diabetic rats on days (D) 1 and 9 after the wound surgery. The wounds were inspected at D0, D3, D6, D9, D12, and D15, and the results of wound healing are shown in Figure 2A. We analyzed the wound area and healing rate in the control (solvent), normoxic, and hypoxic groups. The area of the wound was gradually shrinking, and on D9 after surgery, the mean wound area was 1.97 cm2, 2.09 cm2, and 1.31 cm2 in the control, normoxic, and hypoxic groups, respectively, and the hypoxic MSCs showed a statistically significant difference compared with the control group (Figure 2B, P < 0.01). The mean rate of wound healing increased gradually, and on D9, the rates were 54.06% ± 4.20%, 52.71% ± 12.96%, and 69.93% ± 5.93% for the control, normoxic, and hypoxic groups, respectively, with a statistically significant difference between hypoxic MSC and the control group (Figure 2C, P < 0.01). The healing rate of hypoxic MSCs reached 92.29% on D15; however, the difference between hypoxic and normoxic MSC groups was not statistically significant.

Figure 2. Hypoxia-MSC enhances wound healing in diabetic rats. (A) The results of wound healing on D3, D6, D9, and D12 days after injection; (B) Changes in the area of wound; (C) Changes in wound healing rate; (D) The histological structure of the wounds in skin treated with Normoxia-MSC and Hypoxia-MSC under high and low power; (E) The histopathological score of the wound. Data were presented as the mean; N = 5. (*P <0.05 , **P < 0.01)
Further, we tested blood glucose levels at D5, D10, and D15 after surgery, and there was no statistically significant difference among the three groups (Supplementary Figure S1, available in www.besjournal.com). This not only indicates the success of modeling but also indicates that cell therapy has no effect on blood glucose levels.
Histopathological examination was performed on the D15 after surgery with hematoxylin and eosin (HE) staining, and the histological structure of the wound is shown in Figure 2D. The histopathological scoring standard of the wound was referred to Guo HF, et al. (PMID: 33224609). In brief, the score was based on the epidermal structure, derma-epidermal adjacency and microblisters, collagen bundle and skin structure, epidermal regeneration, and number of granulocyte infiltrations, which were scored as 0, 1, or 2 according to the situation of the wound. As shown in Figure 2E, for the wound skin score, the average score of the three aspects of epidermal structure, epidermal adjacency and microblister, collagen bundle, and skin structure of rats in each group was approximately 1.0, and there was no statistically significant difference between the two MSC groups and control. The epidermal regeneration score in the control and normoxic MSC groups was 0.0, but the average score in the hypoxic MSC group was 0.6, showing a statistically significant difference compared with the control group (P < 0.05). The average score of granulocyte infiltration in each group was 0.0. The significant difference in epidermal regeneration between the hypoxic and control groups supported the improved wound-healing function of hypoxic MSC.
It is generally accepted that MSCs promote tissue repair primarily through paracrine. It has been reported that wound healing is related to the secretion of cytokines and that hypoxic preconditioning could enhance vascular endothelial growth factor (VEGF) secretion and promote vascular regeneration and tissue healing[5,8,9]. Therefore, we detected the expression of VEGF, epidermal growth factor (EGF), hepatocyte growth factor (HGF), and fibroblast growth factor (FGF) in hypoxic MSC products to explore the possible mechanism. The results (Figure 3A) revealed that compared with normoxic MSCs, the VEGF of hypoxic MSCs at 48 h was significantly increased, approximately 20 times that of normoxic MSC. Therefore, we further tested VEGF expression in hypoxic MSCs at different time points in 1% oxygen and that at different oxygen concentrations for 96 h. The results showed that VEGF expression in hypoxic MSCs was significantly increased when cultured in 1% oxygen for 96 h, reaching 53 times that of normoxic MSCs (Figure 3E). The VEGF in 1% group was the highest among the four different oxygen concentration groups (Figure 3F). High levels of VEGF at different time points indicated that hypoxic-MSCs improved wound angiogenesis, which was confirmed through the histological analysis of epidermal regeneration (Figure 2). In addition, we also tested the immunomodulatory ability of hypoxic MSCs by co-culturing them with peripheral blood mononuclear cell (PBMC). The results (Supplementary Figure S1) showed that Th1 and Th17 were downregulated, whereas Th2 and Treg were upregulated; however, the difference was not significant. Based on the results of the current and previous studies, we hypothesized that the paracrine effect of VEGF is the most likely reason for the promotion of diabetic wound healing in rats.

Figure 3. The secretion of cytokines in MSC under different oxygen concentrations and times. (A) VEGF in 1% O2, 48 h; (B) EGF in 1% O2, 48 h; (C) HGF in 1% O2, 48 h; (D) FGF in 1% O2, 48 h; (E) VEGF in different times, 1% O2; (F) VEGF in different oxygen concentrations, 96 h. (*P < 0.05 , **P < 0.01)
Using industrially processed MSCs to treat diabetic wounds was an innovative aspect of our research. There are several quality control measures for clinical-grade cell therapy products, including minimizing the risk of starting cells and the key materials used in cell preparation[10]. In our process, all donors of the starting cells were tested for infectious diseases, and family and genetic histories were evaluated before the umbilical cord was sampled, which ensured the safety of the cells at the source. Key materials such as clinical-grade basic medium and serum substitute were used in the process of cell preparation, and hypoxia alone was used for preconditioning, with no other risk factors introduced, which ensured that the hypoxic MSCs were xeno-free products. Quality tests before hypoxic MSC release and their effect on wound healing in rats verified the applicability of this industrial process. The hypoxia pretreatment period is hardly 3–4 days, which allows the manufacturing facility to quickly provide large quantities of cellular products to meet clinical and scientific needs.
In conclusion, our results indicated that industrially processed hypoxic MSCs enhance VEGF secretion and promote diabetic wound healing in rats. Using hypoxic MSCs may be a promising cell therapy strategy for chronic wounds caused by diabetes mellitus.
doi: 10.3967/bes2023.100
Hypoxic Mesenchymal Stem Cells Promote Diabetic Wound Healing in Rats by Increasing VEGF Secretion
-
-
Figure 2. Hypoxia-MSC enhances wound healing in diabetic rats. (A) The results of wound healing on D3, D6, D9, and D12 days after injection; (B) Changes in the area of wound; (C) Changes in wound healing rate; (D) The histological structure of the wounds in skin treated with Normoxia-MSC and Hypoxia-MSC under high and low power; (E) The histopathological score of the wound. Data were presented as the mean; N = 5. (*P <0.05 , **P < 0.01)
Figure 3. The secretion of cytokines in MSC under different oxygen concentrations and times. (A) VEGF in 1% O2, 48 h; (B) EGF in 1% O2, 48 h; (C) HGF in 1% O2, 48 h; (D) FGF in 1% O2, 48 h; (E) VEGF in different times, 1% O2; (F) VEGF in different oxygen concentrations, 96 h. (*P < 0.05 , **P < 0.01)
-
[1] Azevedo MM, Lisboa C, Cobrado L, et al. Hard-to-heal wounds, biofilm and wound healing: an intricate interrelationship. Br J Nurs, 2020; 29, S6−13. [2] Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes. Lancet, 2022; 400, 1803−20. doi: 10.1016/S0140-6736(22)01655-5 [3] Li SX, Mohamedi AH, Senkowsky J, et al. Imaging in chronic wound diagnostics. Adv Wound Care (New Rochelle), 2020; 9, 245−63. doi: 10.1089/wound.2019.0967 [4] Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells, 2015; 33, 1818−28. doi: 10.1002/stem.1976 [5] Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord, 2019; 20, 207−17. doi: 10.1007/s11154-019-09492-1 [6] Fernández-Santos ME, Garcia-Arranz M, Andreu EJ, et al. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front Immunol, 2022; 13, 918565. doi: 10.3389/fimmu.2022.918565 [7] Yang YM, Lee EH, Yang Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng Part B: Rev, 2022; 28, 966−77. doi: 10.1089/ten.teb.2021.0145 [8] Ishiuchi N, Nakashima A, Doi S, et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther, 2020; 11, 130. [9] Yusoff FM, Nakashima A, Kawano KI, et al. Implantation of hypoxia-induced mesenchymal stem cell advances therapeutic angiogenesis. Stem Cells Int, 2022; 2022, 6795274. [10] Tan LS, Chen JT, Lim LY, et al. Manufacturing clinical-grade human induced pluripotent stem cell-derived beta cells for diabetes treatment. Cell Prolif, 2022; 55, e13232. -
 23048+Supplementary Materials.pdf
23048+Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links