-
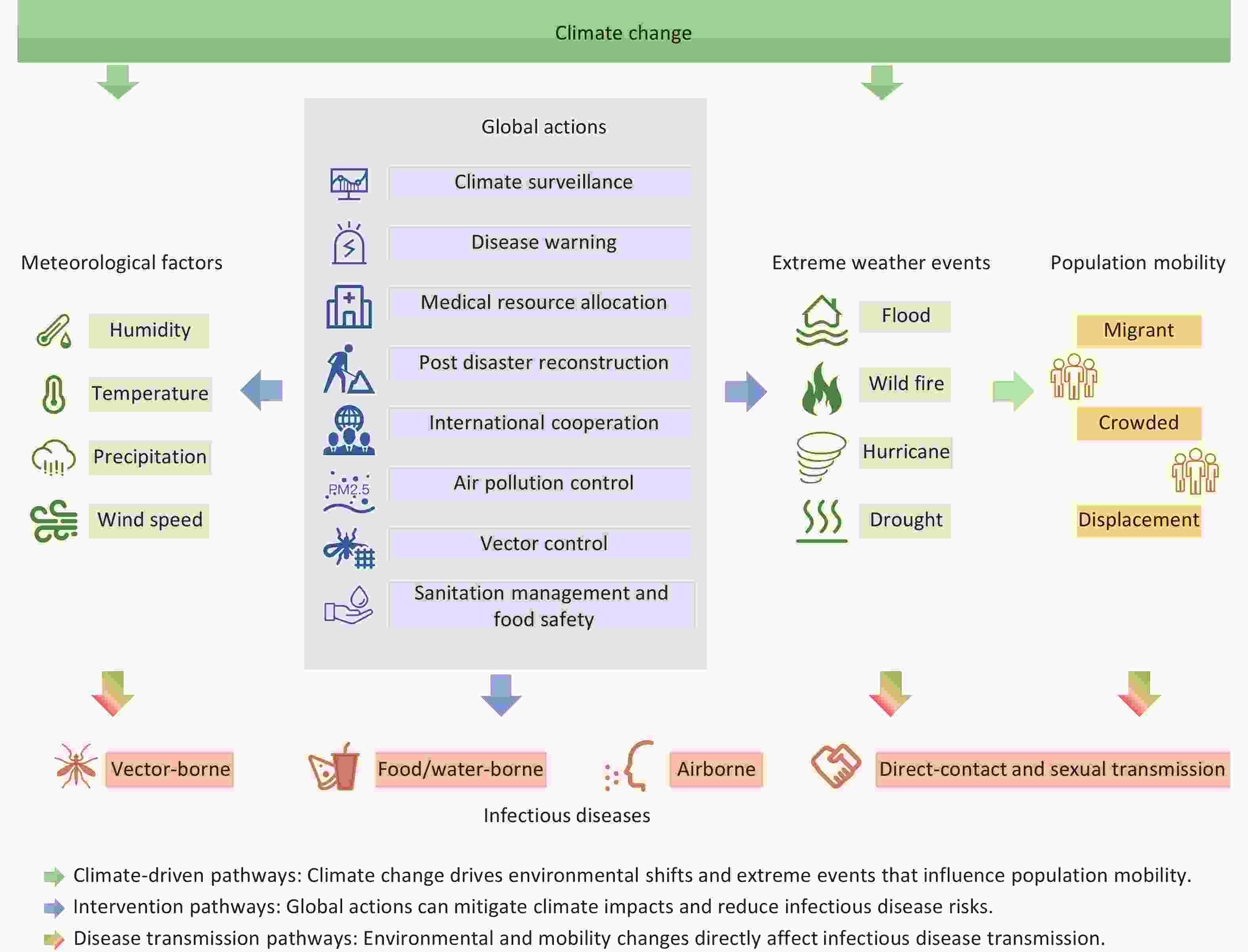
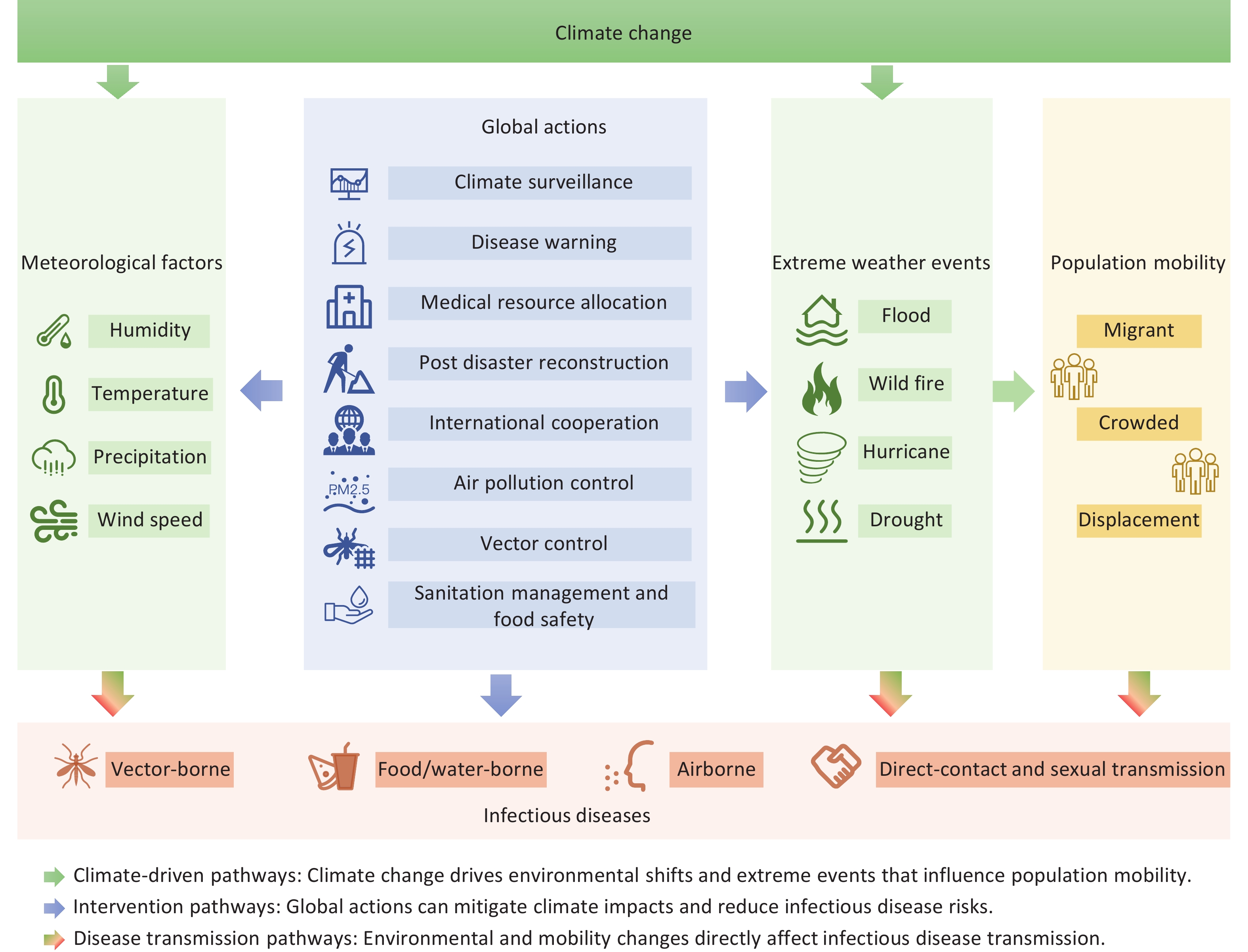
Climate and weather significantly influence the duration, timing, and intensity of disease outbreaks, reshaping the global landscape of infectious diseases[1]. The impact of climate change on the transmission of infectious diseases is complex and multifaceted. Rising temperatures and shifts in precipitation patterns driven by climate change can directly influence the survival and reproduction of pathogens and vector organisms. For instance, increasing temperatures may expand the geographical range of certain vector-borne diseases such as dengue fever and malaria[2,3]. Moreover, climate change is expected to exacerbate extreme weather events, including floods and droughts, which can disrupt infrastructure and elevate the risks of waterborne and foodborne diseases[4-6]. In the Mediterranean region, climate change has already led to significant disruptions in biodiversity and ecosystems, further compounding the risk of infectious disease transmission. The region’s unique geographical characteristics and ongoing socio-political conflicts intensify the adverse effects of climate change[7]. Similarly, in China, climate change has been observed to influence the spread of infectious diseases, particularly in regions with high climatic variability and dense populations, such as Shanxi, Guangdong, Henan, and Anhui Provinces. Rising temperatures and increased precipitation contribute to the resurgence and geographic expansion of vector-borne diseases such as malaria and dengue fever in these areas[8,9]. Health professionals in these regions have recognized potential shifts in the temporal and spatial patterns of infectious disease transmission due to climate change[10,11]. Moreover, climate change exacerbates air pollution and alters environmental conditions, which collectively enhance the transmission risk of infectious diseases by affecting pathogen survival, human immunity, and vector ecology[12]. Furthermore, climate change may alter the epidemiology of vaccine-preventable diseases. Studies suggest that environmental factors such as temperature and humidity, which are subject to climatic fluctuations, can impact the transmission dynamics and burden of these diseases[13]. At a global scale, the impact of climate change on infectious diseases has become a critical public health concern. Researchers have employed bibliometric and co-word analyses to assess global scientific output and emerging trends in this field[14].
The influence of climate change on infectious disease transmission is multifaceted and encompasses changes in temperature, altered precipitation patterns, and extreme weather events. These climatic variations not only affect the ecological characteristics of pathogens and vectors but also indirectly influence human behaviors and socioeconomic conditions, further amplifying disease transmission risks. Addressing this challenge requires an interdisciplinary collaboration and comprehensive public health strategies. This review aims to synthesize the current evidence on the impact of climate change on climate-sensitive infectious diseases and elucidate the underlying mechanisms and transmission pathways. In addition, we explored adaptive policy strategies to mitigate the public health burden of infectious diseases in the context of climate change, offering insights into global health governance and disease control efforts (Figure 1).
-
The influence of climate change on vector-borne disease transmission is complex and multifaceted. Mosquitoes are poikilotherms with life history characteristics that are strongly dependent on ambient temperature, including the length of the gonotrophic cycle, fecundity, biting rate, longevity, and development of immature mosquitoes[15]. As global temperatures rise, the geographic ranges and transmission intensities of many vector-borne diseases are expected to change significantly. For example, mosquito-borne diseases such as dengue fever, Zika virus, and chikungunya are closely linked to temperatures. Studies indicate that the transmission of these diseases is most active within a temperature range of 18–34 °C, peaking at 26–29 °C[16]. A study in China, which used a distributed lag nonlinear model to quantify the effect of different temperature measures on malaria, found that each 5 °C increase in average temperature above 10 °C was associated with a 22% (95% confidence interval [CI], 17% to 28%) increase in malaria cases[17]. A previous worldwide study also showed that National annual average temperatures and precipitation rates were associated with malaria incidences, with an increase in age-standardized incidence rates of 2.01% and 6.04% following a one-unit increase in national annual average temperature and precipitation rate[18]. Additionally, climate change may alter mosquito habitats and extend transmission seasons, further increasing the risk of disease spread[16]. A worrying finding of a previous study was that the impact of the national annual average temperature on malaria incidence increased over time, as indicated by the rising coefficients of annual average temperatures from 0.0067 in 2000 to 0.0348 in 2019[18]. This suggests an escalating effect, with a 1 °C increase in temperature associated with a 0.67% increase in ASIR in 2000 and a 3.54% increase in 2019. This trend aligns with predictions regarding the impact of global warming on vector-borne diseases, suggesting an intensified transmission of malaria associated with temperature increases[19-21].
In Mexico, research has demonstrated the significant impact of climate change on the risk of dengue fever, Zika virus, and chikungunya transmission. Climatic factors such as humidity, temperature, and precipitation directly influence the transmission potential of these diseases[22]. Similarly, in the Mekong Delta region of Vietnam, climate-driven changes in temperature and rainfall patterns are expected to affect the incidence and distribution of dengue and other vector-borne diseases[23]. The relationship between precipitation and vector-borne transmission is intricate. On one hand, precipitation-induced standing water provides a breeding habitat for mosquitoes[24]. On the other hand, high-intensity rainfall may wash away or kill both larvae and adult mosquitoes in some cases[25]. A study reported a positive correlation between rainfall and malaria prevalence in Thailand[26]. Another modeling study found that the probability of the Aedes species’ occurrence increased to nearly 99% when precipitation was less than 100 mm during the warmest quarter of the year; however, this probability quickly dropped as precipitation exceeded 300 mm, reaching 30%[25]. Heavy rainfall may destroy both larvae and adult mosquitoes in some instances. However, under certain conditions, prolonged heavy rainfall can promote the transmission of vector-borne diseases. A study in Sri Lanka reported the ability of A. culicifacies to tolerate changes in salinity during the rainy season[27]. Besides the tolerance of salinity changes, heavy rainfall with a long duration can also provide plenty of breeding sites, which can eventually cause an outbreak. This complex relationship suggests that precipitation plays a dual role in promoting mosquito breeding and vector-borne disease transmission; however, under extreme conditions, it may have adverse effects.
Beyond mosquito-borne diseases, climate change may also influence the spread of other vector-borne infections such as Lyme disease. Studies suggest that rising temperatures could lead to an increased incidence of Lyme disease, as warmer conditions support the survival and activity of ticks[28]. Moreover, the transmission of yellow fever and other arboviral diseases could also be affected, particularly as warming climates facilitate the expansion of mosquito populations and their geographic distribution[29]. Additionally, climate change drives the geographic expansion of Leishmania species beyond their traditionally endemic areas[30]. Shifts in temperature patterns, precipitation levels, and humidity thresholds directly influence vector and reservoir ecology, enabling the spread of these sandfly-borne pathogens into new regions[31]. This environmental-mediated dispersion mechanism underscores the critical role of climate change in reshaping the epidemiology of neglected tropical diseases.
Climate change alters environmental conditions in ways that affect the dynamics and geographic spread of vector-borne diseases. These changes pose significant public health challenges, necessitating integrated disease surveillance and control strategies to mitigate health risks associated with a changing climate[32].
-
Food- and waterborne pathogens are microorganisms, bacteria, viruses, and parasites, that cause illness when ingested through contaminated food or water. These pathogens have caused significant outbreaks and public health concerns worldwide[33]. According to the World Health Organization (WHO), contaminated food leads to illness in 1 in 10 people annually, resulting in 600 million foodborne disease cases and 420,000 deaths each year[34,35]. Common bacterial pathogens associated with foodborne illnesses include Campylobacter jejuni, Clostridium perfringens, Escherichia coli, Salmonella spp., and Staphylococcus aureus[36]. Viruses such as human norovirus and hepatitis A virus (HAV), along with various parasites, also pose significant risks[37].
Climate change influences the survival and distribution of microorganisms and their vectors, thereby increasing the microbial contamination of food and water. These shifts not only threaten human and animal health but also impact food security through direct and indirect pathways[38]. Evidence suggests that climate change affects not only food yields but also food quality and safety[39]. By altering the distribution and prevalence of food-borne pathogens, changing environmental conditions pose challenges to food safety[40,41]. There are concerns that climate change trends worsen the health risks associated with inadequate water, sanitation, and hygiene in many regions of the world[42], while other studies have pointed out the positive correlation between declining water services and increased diarrhea worldwide[43,44].
Rising temperatures and shifting precipitation patterns are key drivers of microbial contamination in food and water[45]. Global warming, alongside an increase in extreme weather events, is projected to impact approximately three-quarters of infectious diseases[46]. Observational studies suggest that while higher temperatures can exacerbate disease risks, arid conditions may mitigate some threats[38]. Additionally, climate change influences optimal conditions for the growth and survival of foodborne pathogens and facilitates the spread of foodborne diseases and plant infections to new regions[47,48]. Rising temperatures and changing precipitation patterns due to climate change create optimal conditions for the growth and survival of foodborne pathogens. Many bacteria, such as Salmonella, E. coli, and Campylobacter jejuni, thrive in warm and humid environments, with their metabolic activity accelerating at higher temperatures[49,50]. Warmer conditions also expand the geographic range of these pathogens, increasing the risk of foodborne disease outbreaks[48]. Additionally, ocean warming and intense precipitation reduce coastal water salinity, fostering the proliferation of Vibrio species, which are linked to vibriosis and cholera outbreaks in previously unaffected regions[51,52]. Humidity plays a crucial role in pathogen survival and transmission, with higher humidity levels amplifying the risk of foodborne diseases such as Salmonella infections, which show a linear association with temperatures[53]. Climate-induced changes in temperatures and moisture also impact fungal pathogens like Fusarium, which produce mycotoxins that contaminate food crops, posing additional health risks[54]. These environmental shifts highlight the need for improved food safety measures and monitoring systems to mitigate the growing burden of foodborne diseases under a changing climate.
Extreme weather events and the rise of sea levels driven by climate change pose significant risks to food safety. Flooding, hurricanes, and wildfires can damage food production infrastructure and contaminate crops, livestock, and aquaculture with pollutants such as sewage, saltwater, and agricultural runoff, creating favorable conditions for the proliferation of foodborne pathogens[55]. Outbreaks of diarrheal diseases have been reported in countries such as India, Brazil, and Bangladesh following flooding episodes, highlighting the public health risks associated with extreme weather events[56]. Droughts can exacerbate food safety risks by reducing water availability, leading to higher pathogen concentrations in water used for irrigation and food processing[57]. Power outages during extreme weather events, such as Hurricane Sandy in 2012, have also been linked to outbreaks of salmonellosis due to compromised refrigeration systems[58]. Additionally, rising sea levels may promote saltwater contamination of freshwater systems, fostering the growth of microbial communities that include opportunistic pathogens[59]. These challenges underscore the need for robust infrastructure, water management systems, and early warning mechanisms to safeguard food safety in a changing climate.
Climate change affects foodborne disease patterns and poses a growing risk to public health. Effective mitigation requires an understanding of climate-pathogen dynamics and the implementation of adaptive measures. The key strategies include strengthening food safety regulations, enhancing disease surveillance, adopting climate-resilient agriculture, and improving water management. Concurrently, the promotion of sustainable aquaculture, public education, and targeted research can address critical knowledge gaps. Multisectoral collaboration among health authorities, agricultural experts, and policymakers is crucial for developing climate-informed interventions. Coordinated efforts can reduce population vulnerability and safeguard global health in warmer climates.
-
Airborne infectious diseases pose a significant challenge to global public health, involving a wide range of pathogens such as influenza viruses, Mycobacterium tuberculosis, SARS-CoV-2, the measles virus, the varicella-zoster virus, and Bacillus anthracis[60-63]. These pathogens are transmitted through respiratory droplets or aerosols, enabling rapid infection of large populations and resulting in severe health consequences. The impact of climate change on their transmission dynamics is complex and multifaceted.
Climate change has profound effects on respiratory infections through temperature variability and the associated environmental stressors. Non-optimal temperatures accounted for 246,000 lower respiratory infection (LRI) deaths globally in 2019, with cold exposure posing a greater burden than heat in most regions[64-68]. In Western Europe, 21% of LRI disability-adjusted life years (DALYs) were attributed to low temperatures, whereas South Asia and Western Sub-Saharan Africa faced higher heat-related burdens (7.8%)[64]. Regional disparities are stark. Cold temperatures ranked as the second-leading risk factor for respiratory infection deaths in the Americas (15.3% of fatalities), surpassed only by smoking[69]. In Oceania, particularly in Australia, it is estimated that 11%–23% of respiratory diseases are attributable to cold temperatures, with climate change expected to shift this burden increasingly toward heat in the future[70]. Temperature extremes disrupt host-pathogen dynamics, with cold conditions increasing LRI hospitalization risks, particularly among children. For instance, a 1 °C rise in maximum temperature correlated with 4.2% and 3.4% increases in pediatric acute LRI admissions during dry and rainy seasons, respectively.71 Conversely, heat waves may paradoxically reduce the tuberculosis (TB) incidence in some settings, although this protective effect remains region-specific[72,73].
The interplay between temperature, humidity, and wind speed creates complex risk profiles for respiratory diseases. Low temperatures are consistently linked to higher TB incidences and hospitalization rates, particularly in males and older adults, with delayed effects peaking 13–15 months post-exposure[72,74-76]. Humidity exhibits nonlinear relationships, where moderate levels at 12–17-month lag times elevate TB risks, while short-term effects are negligible[72,76]. Wind speeds have demonstrated protective properties, with meta-analyses showing a 0.89 relative risk reduction for TB incidence per unit increase in average wind speed.77 Beyond direct meteorological effects, climate change amplifies microbial virulence by promoting biofilm formation in pathogens such as Streptococcus pneumoniae, exacerbating chronic respiratory infections[78,79]. Furthermore, the synergies between rising temperatures and particulate pollution intensify acute LRI risks, as observed in studies linking heat-driven air quality deterioration to infection severity[80].
While cold temperatures dominate respiratory mortality in temperate zones, tropical regions face escalating heat-related burdens that may surpass the effects of cold temperatures under continued warming[81]. Vulnerable populations, including displaced communities, face compounded risks due to climate-impaired immunity and disrupted healthcare access[82]. Critical knowledge gaps persist, particularly regarding the disruption of TB diagnoses and transmission dynamics by extreme weather, underscoring the need for targeted research to inform climate-resilient health policies[82]. These findings highlight the urgency of integrating meteorological surveillance into public health strategies to mitigate the dual threats of temperature extremes and evolving pathogen behaviors in a warming climate.
For infectious diseases such as measles and varicella, climate change may influence disease transmission by altering population immunity and vaccination rates. Studies suggest that climate change could increase the frequency of extreme weather events, which may disrupt the public health infrastructure, thereby affecting vaccine supply and administration[83]. Moreover, climate change can impact infectious disease transmission by modifying human behaviors and socioeconomic conditions. For instance, climate-induced population migration and intensified urbanization may elevate the risk of disease spread[84]. The transmission of diseases such as anthrax may also be affected by climate change, as changes in soil and water conditions in certain regions could influence the survival and dispersal of Bacillus anthracis spores. Climate change, particularly permafrost thawing, has been linked to increasing anthrax outbreaks in the Russian Arctic, as thawed soil releases dormant spores from buried animal carcasses[85]. Additionally, climate-driven shifts in wildlife habitats may alter the transmission patterns of zoonotic diseases[86]. The 2016 Yamal outbreak, triggered by abnormally high temperatures and minimal rainfall, led to mass reindeer mortality and human infections, exacerbated by weakened animal immunity and increased insect vector activity[87]. Additionally, predictive models indicated that rising temperatures, land-use changes, and a lack of vaccination could significantly increase future outbreak risks, highlighting the need for strengthened surveillance and prevention measures[87].
-
Climate change indirectly reshapes the epidemiology of direct contact and sexually transmitted diseases through multifaceted pathways, including exacerbating healthcare disruptions and amplifying the environmental drivers of transmission. For populations living with HIV, a disease intricately tied to both sexual transmission and immunological vulnerability, climate-related disasters pose an existential threat. Natural catastrophes disproportionately affect resource-limited regions with high HIV prevalences, such as sub-Saharan Africa, where cyclones Ida and Kenneth (2019) disrupted antiretroviral therapy (ART) access for over 100,000 patients, abruptly halting critical care and elevating mortality risks[88-90]. ART interruptions not only reverse CD4 count gains but also accelerate disease progression, increasing susceptibility to cardiovascular complications and opportunistic infections such as tuberculosis and herpes simplex[91-93]. Post-disaster displacement compounds these risks, as overcrowded shelters and unsanitary conditions foster bacterial infections (e.g., S. aureus) while malnutrition weakens immune defenses. Critically, rising viral loads in displaced populations heighten transmission risks in confined settings, creating a feedback loop between environmental crises and HIV spread[93]. These dynamics underscore the urgent need for climate-resilient HIV care systems that integrate disaster preparedness into public health strategies.
The intersection of climate-driven migration and dermatological morbidity further highlights systemic vulnerabilities. HIV-positive migrants face a twofold increased risk of skin cancers like Kaposi sarcoma (KS), which remains a leading cause of mortality despite ART’s mitigating effects[94,95]. Disrupted healthcare access impedes early detection of malignancies and adverse cutaneous drug reactions (ACDRs), which affect 8% of patients with HIV and correlate with immunosuppression[96]. Migrants with low CD4 counts are particularly susceptible to morbilliform eruptions (95% of ACDRs) and rare severe reactions like Stevens-Johnson syndrome, often triggered by ART or prophylactic therapies[97,98]. This syndemic of climate displacement and dermatological morbidity illustrates how environmental stressors amplify biologic vulnerabilities, necessitating targeted surveillance programs for migrant populations. Climate-induced migration fuels the spread of direct-contact diseases such as scabies, particularly in tropical regions. Overcrowded living quarters and inadequate sanitation in refugee camps create ideal conditions for Sarcoptes scabiei transmission, as observed among displaced populations in Europe and Africa[99,100]. Such environments, where limited bathing facilities and frequent skin-to-skin contact drive outbreaks, also increase the risk of parasitic infections[101].
Beyond pathogen transmission, climate change indirectly strains the healthcare infrastructure, exacerbating surgical and clinical challenges. Extreme weather events disrupt surgical supply chains and postoperative care, whereas infectious disease outbreaks, such as monkeypox, complicate infection control protocols[102]. In Vietnam, rising temperatures and humidity have been linked to a 7% increase in hand, foot, and mouth disease (HFMD) incidences per 1 °C above 26 °C, though excessive rainfall paradoxically reduces transmission by limiting outdoor activities[103]. These nonlinear climate-disease relationships emphasize the complexity of predicting epidemiologic shifts. The compounding effects of climate change and surgical demand create a vicious cycle: surgical morbidity increases owing to delayed care, whereas infection control measures increase the healthcare sector’s carbon footprint[102]. Addressing this syndemic requires multilevel actions, including climate-adaptive surgical protocols and the advocacy of sustainable practice. Ultimately, the interplay between environmental change and disease transmission demands a paradigm shift in public health planning, one that prioritizes intersectoral collaboration, integrates climate models into disease surveillance, and recognizes migration not merely as a consequence of climate change but as a determinant of global health equity.
-
The intricate links between climate change and infectious diseases underscore the urgency to adopt coordinated global strategies to mitigate these dual threats. While the previous sections have highlighted how rising temperatures, shifting precipitation patterns, and extreme weather events amplify the transmission and geographic spread of climate-sensitive pathogens, addressing these challenges requires systemic interventions spanning climate policies, public health infrastructure, and international cooperation. Mitigation requires dual-track strategies: Climate policies must embed health safeguards, whereas public health systems should adopt climate-resilient designs.
-
Human activities have already warmed the planet by 1 °C above preindustrial levels. The 2015 Paris Agreement, signed by 195 countries, limited global warming below 2 °C; meanwhile, some researchers declared global warming must stay below 1.5 °C[104]. Although the increase in the incidence of infectious diseases did not seem to be too serious compared to extreme weather events, rising sea levels, destruction of coral reefs, loss of biodiversity, ocean acidification and deoxygenation, and extreme heat, efforts are still needed to control these infectious diseases due to global warming[105]. We must reduce CO2 emissions by at least 45% in the next 12 years compared with 2010 levels and achieve net-zero CO2 production by 2050. This is a tall order, requiring action on multiple fronts at all levels of society, both local and global[104]. The 10% of the global population responsible for 50% of global carbon emissions bears a particular responsibility[106]. A synergistic global response must integrate decarbonization technologies (e.g., green hydrogen production, circular water systems) with regenerative agricultural practices such as agroforestry and drought-resistant crop rotations, to stabilize food supply chains amid climatic stressors[107]. Concurrently, healthcare systems should pivot toward climate-adaptive frameworks, deploying AI-driven epidemic forecasting tools and modular field hospitals to address displacement crises triggered by extreme weather[108]. Medical practitioners must champion policies linking emission reduction targets to quantifiable health gains, such as reducing respiratory illnesses through coal phase-outs or curbing malnutrition via climate-smart farming subsidies, both of which can indirectly influence infectious disease risks by weakening host immunity or increasing population vulnerability[109]. Cross-sector partnerships could amplify the impact, for instance, by coupling carbon credit markets with community-led reforestation programs that simultaneously sequester emissions and restore disease-buffering ecosystems.
-
Public health systems frequently break down during and after extreme climate events, and without robust surveillance systems, including active community-based monitoring, it becomes even more challenging to track outbreaks, leading to the underreporting of caseloads and missed opportunities for identification[110]. Therefore, the development of robust climate-disease surveillance networks is critical to preemptively address outbreaks exacerbated by environmental shifts. Integrating meteorological data, such as temperature anomalies, humidity trends, and extreme weather forecasts, with real-time disease surveillance can create dynamic risk maps that highlight regions vulnerable to pathogen emergence or resurgence. For instance, satellite-based tracking of sea surface temperature fluctuations and coastal rainfall patterns has proven effective in predicting cholera outbreaks by correlating Vibrio cholerae proliferation with algal blooms and freshwater contamination[111]. Expanding this approach, health agencies could deploy mobile-enabled community reporting platforms to monitor zoonotic reservoirs (e.g., rodent population surges post-flooding or bat habitat encroachment), enabling early warnings for diseases such as leptospirosis or Nipah virus[112]. To ensure equity, international bodies should fund decentralized data hubs in low-resource regions, providing localized climate-health analysis tools and training for frontline workers. Policy frameworks must mandate data sharing between meteorological offices and public health institutions, whereas innovation funds can incentivize AI-driven models that cross-reference historical outbreak patterns with climate projections. Early warning systems should be linked directly to response protocols, such as pre-positioning oral rehydration salts in cholera-prone coastal zones during El Niño years, or distributing insecticide-treated nets (ITNs) ahead of predicted malaria vector expansions. Moreover, the design of early warning systems needs to consider not only one type of extreme event, but also the compound effects of interacting and successive extreme climate events.110 By embedding climate intelligence into national disease preparedness plans, governments can transform surveillance from passive monitoring to proactive defense, reducing both human suffering and long-term healthcare costs.
-
Vector control remains a cornerstone in mitigating the transmission of climate-sensitive pathogens, particularly as rising temperatures and altered precipitation patterns expand the geographic reach of disease vectors, such as Aedes mosquitoes. Effective strategies must integrate traditional and innovative approaches. Chemical-based tools, such as indoor residual spraying (IRS) and ITNs, continue to play a critical role in reducing adult vector populations and human-vector contact[113]. Concurrently, non-chemical interventions, including habitat modification to eliminate stagnant water and the introduction of biological agents, such as Wolbachia-infected mosquitoes, disrupt breeding cycles while minimizing environmental impact[113,114]. Proactive habitat management is equally vital. Urban planning must prioritize the reduction of heat islands and waterlogged areas to prevent the creation of ideal conditions for vector proliferation. Community-driven efforts to drain standing water and maintain clean environments can significantly diminish larval habitats[114]. Furthermore, the development of novel technologies, such as genetically modified mosquitoes and insecticide-treated eave tubes, offers promising avenues for sustainable vector suppression[113]. To address the interconnected risks posed by climate-driven vector expansion, the One Health Framework is essential. Establishing integrated surveillance networks that harmonize human, veterinary, and entomological data can detect zoonotic spillovers and emerging pathogens early[115]. For instance, insecticide-treated dog collars not only protect animals but also reduce reservoir hosts for diseases such as leishmaniasis. Cross-sector collaboration coupled with public education on preventive measures ensures that interventions are both ecologically sound and culturally adapted. In conclusion, a multipronged strategy, combining targeted vector control, habitat optimization, and interdisciplinary surveillance, is critical for curbing the escalating threat of vector-borne diseases due to global climate change.
-
Climate-driven disruptions to water and food systems require urgent intervention to curb contamination risks and mitigate health crises. Extreme weather events, from floods overwhelming sanitation networks to droughts crippling crop yields, escalate the threat of diarrheal diseases and foodborne pathogens. Building climate-resilient infrastructures, such as elevated water treatment facilities to withstand flooding and drip irrigation systems for drought-prone farmlands, can stabilize access to clean water and sustain agricultural output. Concurrently, strengthening food safety protocols in informal markets through regular inspections and community training on post-flood hygiene practices is vital for minimizing exposure to toxins and pathogens. Studies have highlighted that unsafe water and poor sanitation contribute to over one million annual infectious disease deaths, primarily among children under five years of age[116,117]. Cost-effective solutions, such as point-of-use water filters and handwashing campaigns, have reduced the childhood diarrhea incidence by 30%–50% in low-resource regions[118], demonstrating the efficacy of targeted WASH investments. Beyond immediate health benefits, advancing water security fosters economic resilience, and reliable irrigation and wastewater management systems enhance agricultural productivity, directly supporting poverty reduction and sustainable development[119]. Policymakers must prioritize integrated strategies such as subsidizing rainwater harvesting in arid zones or embedding digital sensors in food supply chains to monitor contamination hotspots. By aligning climate adaptation funding with equitable WASH initiatives, nations can transform their vulnerabilities into opportunities for health equity and ecological stewardship.
-
Comprehensive policy interventions are essential for effectively combating air pollution and reducing the burden of respiratory diseases. In both high- and low-income countries, air pollution is a leading cause of diseases, such as asthma and chronic obstructive pulmonary disease, largely driven by particulate matter (PM2.5) and ozone. Moreover, PM2.5 and ozone contribute significantly to the incidence of infectious respiratory diseases; globally, LRIs account for 8.54% and 17.35% of the disease burden attributable to ambient and household air pollution, respectively[120]. Policymakers must prioritize transitioning to clean energy, phasing out coal-fired power plants, and incentivizing clean transportation systems, such as electric vehicles, to lower emissions and improve public health. For rapidly urbanizing regions, especially in developing countries, stronger regulatory frameworks and better monitoring systems are critical for curbing industrial emissions, which significantly contribute to poor air quality in densely populated urban centers[121]. In low- and middle-income countries (LMICs), addressing household air pollution is particularly urgent, as approximately three billion people rely on solid fuels for cooking and heating, causing significant health risks, especially for women[120,122]. Promoting cleaner cooking technologies, such as liquefied petroleum gas stoves, can reduce PM2.5 levels and health impacts[122]. Additionally, international cooperation and funding are necessary to support LMICs in transitioning to clean energy, ensuring access to affordable and clean fuels, and improving energy efficiency. Enhanced public awareness and adoption of cleaner technologies should be encouraged globally, with a focus on reducing disparities in air quality and health outcomes.
-
After extreme weather events, it is crucial to prioritize healthcare responses to prevent the spread of infectious diseases. Climate extremes, whether caused by the ENSO cycles or human-driven warming, often disrupt essential infrastructures, including healthcare, water supply, and sanitation systems, thereby creating ideal conditions for pathogen transmission. Displaced populations in overcrowded shelters and disrupted preventive measures, such as ITNs, further increase the risk of outbreaks[123]. To minimize this risk, it is essential to quickly deploy medical resources, including vaccines and safe shelters, to prevent overcrowding. Additionally, eliminating breeding grounds for disease vectors such as mosquitoes can help prevent post-disaster outbreaks. Proactively identifying climate outbreak linkages, such as rodent migration following droughts or the spread of Vibrio after flooding, allows health agencies to tailor interventions based on local conditions. Predictive modeling of climatic drivers enables targeted responses such as preemptive vector control or stockpiling of necessary supplies in flood-prone regions. Prepositioning medical supplies and training healthcare workers in disaster-prone areas can enhance preparedness and save lives. By shifting from reactive crisis management to anticipatory strategies, we can reduce both the health burden and systemic collapse following weather events[110].
-
The effects of climate change are not felt equally across the globe, with low-income nations experiencing a disproportionate share of the health impacts, particularly in the context of climate-sensitive diseases[124]. For instance, mortality rates from vector-borne diseases are nearly 300 times higher in developing countries than in industrialized nations, highlighting stark health inequities[125]. Moreover, it was observed that there was a higher risk of people being killed by natural floods in low-income countries, where the risk of over 50 people dying from a single natural flood was 14 times higher than in high-income countries[126]. These disparities exacerbate existing vulnerabilities, overwhelming already fragile healthcare systems and hindering socioeconomic development. Collaboration between wealthier and more vulnerable nations is crucial for addressing this global challenge. Wealthier nations must offer support through technology transfer, funding for climate adaptation initiatives, such as the Green Climate Fund, and platforms for data sharing to help low-income countries bolster their climate resilience. Moreover, international agreements, such as the WHO’s Pandemic Accord, should explicitly incorporate climate resilience strategies to better prepare nations for future health crises, ensuring that climate change is considered a part of pandemic preparedness efforts[127]. It is essential for the global community to work together, ensuring that resources and expertise are shared equally and that no country is left behind in the fight against the impacts of climate change. Only through cooperative efforts can a fair and sustainable response to the intertwined crisis of climate change and health be achieved.
-
This review summarizes the complex interplay between global climate change and climate-sensitive infectious diseases across diverse transmission routes. Rising temperatures, shifting precipitation patterns, and extreme weather events amplify the risks at every level: vector-borne diseases, including malaria and dengue fever, are expanding geographically as arthropod habitats shift; food- and waterborne pathogens exploit disrupted supply chains and contaminated resources; airborne infections face altered transmission dynamics due to air quality fluctuations; and direct-contact diseases confront new exposure risks from climate-driven human displacement. These cascading effects underscore the fact that climate change is not merely an environmental crisis but also a systemic threat to global health security. Therefore, a dual-pronged strategy is required for addressing these challenges. First, climate mitigation must be accelerated through binding international commitments to limit future warming as close to 1.5 °C as possible, acknowledging that this threshold has already been exceeded in several regions, including Australia, by 2024. Every fraction of degree reduction still translates to a measurable decrease in disease burden. Second, adaptive health systems must be prioritized by integrating climate intelligence into core functions: Deploying AI-enhanced surveillance networks that fuse meteorological data with real-time pathogen genomics; implementing climate-resilient infrastructure designs, such as flood-resistant vaccine cold chains and heat-adaptive emergency departments; and establishing equity-focused financing mechanisms, such as climate-health impact bonds, to support vulnerable regions. These efforts require a reimagining of global governance through a One Health lens. By treating climate and health agendas as inseparable, and centering on frontline communities in solution design, we can transform today’s compounding vulnerabilities into tomorrow’s adaptive resilience.
doi: 10.3967/bes2025.077
-
Abstract: Climate and weather significantly influence the duration, timing, and intensity of disease outbreaks, reshaping the global landscape of infectious diseases. Rising temperatures and shifts in precipitation patterns driven by climate change can directly impact the survival and reproduction of pathogens and vector organisms. Moreover, climate change is expected to exacerbate extreme weather events, including floods and droughts, which can disrupt infrastructure and increase the risk of water- and foodborne diseases. There are potential shifts in the temporal and spatial patterns of infectious disease transmission owing to climate change. Furthermore, climate change may alter the epidemiology of vaccine-preventable diseases. These climatic variations not only affect the ecological characteristics of pathogens and vectors but also indirectly influence human behaviors and socioeconomic conditions, further amplifying disease transmission risks. Addressing this challenge requires an interdisciplinary collaboration and comprehensive public health strategies. This review aims to synthesize the current evidence on the impact of climate change on climate-sensitive infectious diseases and elucidate the underlying mechanisms and transmission pathways. Additionally, we explored adaptive policy strategies to mitigate the public health burden of infectious diseases in the context of climate change, offering insights for global health governance and disease control efforts.Qiao Liu searched the literature and drafted the manuscript. Min Liu and Jue Liu conceived and designed the study. Jue Liu supervised the study and interpreted the results. Qiao Liu, Min Liu and Jue Liu revised the manuscript. All authors contributed to the writing of the manuscript.
We declare no competing interests.
Not applicable.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
[1] Bezirtzoglou C, Dekas K, Charvalos E. Climate changes, environment and infection: facts, scenarios and growing awareness from the public health community within Europe. Anaerobe, 2011; 17, 337−40. doi: 10.1016/j.anaerobe.2011.05.016 [2] Cao B, Bai CK, Wu KY, et al. Tracing the future of epidemics: Coincident niche distribution of host animals and disease incidence revealed climate-correlated risk shifts of main zoonotic diseases in China. Glob Chang Biol, 2023; 29, 3723−46. doi: 10.1111/gcb.16708 [3] Samuel GH, Adelman ZN, Myles KM. Temperature-dependent effects on the replication and transmission of arthropod-borne viruses in their insect hosts. Curr Opin Insect Sci, 2016; 16, 108−13. doi: 10.1016/j.cois.2016.06.005 [4] Cissé G. Food-borne and water-borne diseases under climate change in low- and middle-income countries: Further efforts needed for reducing environmental health exposure risks. Acta Trop, 2019; 194, 181−8. doi: 10.1016/j.actatropica.2019.03.012 [5] Hodges M, Belle JH, Carlton EJ, et al. Delays in reducing waterborne and water-related infectious diseases in China under climate change. Nat Clim Chang, 2014; 4, 1109−15. doi: 10.1038/nclimate2428 [6] Liu Q, Yuan J, Yan WX, et al. Association of natural flood disasters with infectious diseases in 168 countries and territories from 1990 to 2019: a worldwide observational study. Global Transi, 2023; 5, 149−59. doi: 10.1016/j.glt.2023.09.001 [7] Dal T, Ramli I, Garaizar J. Effect of climate change on nature and human health with a special focus on infectious diseases in the Mediterranean region. J Infect Dev Ctries, 2023; 17, 1501−10. doi: 10.3855/jidc.17995 [8] Tong MX, Hansen A, Hanson-Easey S, et al. Perceptions of malaria control and prevention in an era of climate change: a cross-sectional survey among CDC staff in China. Malar J, 2017; 16, 136. doi: 10.1186/s12936-017-1790-3 [9] Tong MX, Hansen A, Hanson-Easey S, et al. Perceptions of capacity for infectious disease control and prevention to meet the challenges of dengue fever in the face of climate change: a survey among CDC staff in Guangdong Province, China. Environ Res, 2016; 148, 295−302. doi: 10.1016/j.envres.2016.03.043 [10] Wei JN, Hansen A, Zhang Y, et al. The impact of climate change on infectious disease transmission: perceptions of CDC health professionals in Shanxi Province, China. PLoS One, 2014; 9, e109476. doi: 10.1371/journal.pone.0109476 [11] Yi LP, Xu X, Ge WX, et al. The impact of climate variability on infectious disease transmission in China: current knowledge and further directions. Environ Res, 2019; 173, 255−61. doi: 10.1016/j.envres.2019.03.043 [12] Eguiluz-Gracia I, Mathioudakis AG, Bartel S, et al. The need for clean air: the way air pollution and climate change affect allergic rhinitis and asthma. Allergy, 2020; 75, 2170−84. doi: 10.1111/all.14177 [13] Mahmud AS, Martinez PP, He JX, et al. The impact of climate change on vaccine-preventable diseases: insights from current research and new directions. Curr Environ Health Rep, 2020; 7, 384−91. doi: 10.1007/s40572-020-00293-2 [14] Li F, Zhou H, Huang DS, et al. Global research output and theme trends on climate change and infectious diseases: a Restrospective bibliometric and co-word biclustering investigation of papers indexed in PubMed (1999-2018). Int J Environ Res Public Health, 2020; 17, 5228. doi: 10.3390/ijerph17145228 [15] Ciota AT, Matacchiero AC, Kilpatrick AM, et al. The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol, 2014; 51, 55−62. doi: 10.1603/ME13003 [16] Gizaw Z, Salubi E, Pietroniro A, et al. Impacts of climate change on water-related mosquito-borne diseases in temperate regions: a systematic review of literature and meta-analysis. Acta Trop, 2024; 258, 107324. doi: 10.1016/j.actatropica.2024.107324 [17] Liu ZD, Wang SZ, Zhang Y, et al. Effect of temperature and its interactions with relative humidity and rainfall on malaria in a temperate city Suzhou, China. Environ Sci Pollut Res, 2021; 28, 16830−42. doi: 10.1007/s11356-020-12138-4 [18] Liu Q, Wang YP, Deng J, et al. Association of temperature and precipitation with malaria incidence in 57 countries and territories from 2000 to 2019: A worldwide observational study. J Glob Health, 2024; 14, 04021. doi: 10.7189/jogh.14.04021 [19] Brower V. Vector-borne diseases and global warming: are both on an upward swing?. EMBO Rep, 2001; 2, 755−7. doi: 10.1093/embo-reports/kve193 [20] Aguiar M. The effect of global warming on vector-borne diseases: Comment on “Modeling the impact of global warming on vector-borne” infections by E. Massad et al. Phys Life Rev, 2011; 8, 202−3. [21] Adepoju OA, Afinowi OA, Tauheed AM, et al. Multisectoral perspectives on global warming and vector-borne diseases: a focus on Southern Europe. Curr Trop Med Rep, 2023; 10, 47−70. doi: 10.1007/s40475-023-00283-y [22] Carreto C, Gutiérrez-Romero R, Rodríguez T. Climate-driven mosquito-borne viral suitability index: measuring risk transmission of dengue, chikungunya and Zika in Mexico. Int J Health Geogr, 2022; 21, 15. doi: 10.1186/s12942-022-00317-0 [23] Pham NTT, Nguyen CT, Vu HH. Assessing and modelling vulnerability to dengue in the Mekong Delta of Vietnam by geospatial and time-series approaches. Environ Res, 2020; 186, 109545. doi: 10.1016/j.envres.2020.109545 [24] Kouadio IK, Aljunid S, Kamigaki T, et al. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther, 2012; 10, 95−104. doi: 10.1586/eri.11.155 [25] Velu RM, Kwenda G, Bosomprah S, et al. Ecological niche modeling of Aedes and Culex mosquitoes: a risk map for chikungunya and west Nile viruses in Zambia. Viruses, 2023; 15, 1900. doi: 10.3390/v15091900 [26] Wiwanitkit V. Correlation between rainfall and the prevalence of malaria in Thailand. J Infect, 2006; 52, 227−30. doi: 10.1016/j.jinf.2005.02.023 [27] Jude PJ, Dharshini S, Vinobaba M, et al. Anopheles culicifacies breeding in brackish waters in Sri Lanka and implications for malaria control. Malar J, 2010; 9, 106. doi: 10.1186/1475-2875-9-106 [28] Couper LI, MacDonald AJ, Mordecai EA. Impact of prior and projected climate change on US Lyme disease incidence. Glob Chang Biol, 2021; 27, 738−54. doi: 10.1111/gcb.15435 [29] Valderrama A, Díaz Y, López-Vergès S. Interaction of Flavivirus with their mosquito vectors and their impact on the human health in the Americas. Biochem Biophys Res Commun, 2017; 492, 541−7. doi: 10.1016/j.bbrc.2017.05.050 [30] Steverding D. The history of leishmaniasis. Parasit Vectors, 2017; 10, 82. doi: 10.1186/s13071-017-2028-5 [31] WHO. Leishmaniasis. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. [2025-03-10] [32] Parums DV. Editorial: climate change and the spread of vector-borne diseases, including dengue, malaria, Lyme disease, and West Nile virus infection. Med Sci Monit, 2024; 29, e943546. [33] Awad DA, Masoud HA, Hamad A. Climate changes and food-borne pathogens: the impact on human health and mitigation strategy. Climatic Change, 2024; 177, 92. doi: 10.1007/s10584-024-03748-9 [34] WHO. Food Safetyhttps://www.who.int/news-room/fact-sheets/detail/food-safety. [2025-03-10] [35] Lee H, Yoon Y. Etiological agents implicated in foodborne illness world wide. Food Sci Anim Resour, 2021; 41, 1−7. doi: 10.5851/kosfa.2020.e75 [36] Bintsis T. Foodborne pathogens. AIMS Microbiol, 2017; 3, 529−63. doi: 10.3934/microbiol.2017.3.529 [37] Pexara A, Govaris A. Foodborne viruses and innovative non-thermal food-processing technologies. Foods, 2020; 9, 1520. doi: 10.3390/foods9111520 [38] War JM, Nisa AU, Wani AH, et al. Microbial food-borne diseases due to climate change. In: Parray JA, Bandh SA, Shameem N. Climate Change and Microbes. Apple Academic Press. 2022, 187-234. [39] Vermeulen SJ, Campbell BM, Ingram JSI. Climate change and food systems. Annu Rev Env Resour, 2012; 37, 195−222. doi: 10.1146/annurev-environ-020411-130608 [40] Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci, 2019; 1436, 157−73. doi: 10.1111/nyas.13950 [41] Lake IR, Barker GC. Climate change, foodborne pathogens and illness in higher-income countries. Curr Environ Health Rep, 2018; 5, 187−96. doi: 10.1007/s40572-018-0189-9 [42] Cissé G, Koné B, Bâ H, et al. Ecohealth and climate change: adaptation to flooding events in riverside secondary cities, West Africa. In: Proceedings of the Cities and Adaptation to Climate Change-Proceedings of the Global Forum 2010 on Resilient Cities. Springer. 2011, 55-67. [43] Liu Q, Liu M, Liu J. Association of drinking water services with the disease burden of diarrhea in children under five in 200 countries from 2000 to 2021. Cell Rep Sustain, 2024; 1, 100177. [44] Liu Q, Liu M, Liu J. Global associations between the use of basic drinking water and sanitation services with diarrhoeal disease incidence in 200 countries and territories from 2000 to 2019. Public Health, 2024; 235, 202−10. doi: 10.1016/j.puhe.2024.07.004 [45] Singh BK, Delgado-Baquerizo M, Egidi E, et al. Climate change impacts on plant pathogens, food security and paths forward. Nat Rev Microbiol, 2023; 21, 640−56. doi: 10.1038/s41579-023-00900-7 [46] Nel J, Richards L. Climate change and impact on infectious diseases. Wits J Clin Medicine, 2022; 4, 129−34. doi: 10.18772/26180197.2022.v4n3a1 [47] Peng ZH, Liu Y, Qi JJ, et al. The climate-driven distribution and response to global change of soil-borne pathogens in agroecosystems. Global Ecol Biogeogr, 2023; 32, 766−79. doi: 10.1111/geb.13662 [48] Smith BA, Fazil A. How will climate change impact microbial foodborne disease in Canada?. Can Commun Dis Rep, 2019; 45, 108−13. doi: 10.14745/ccdr.v45i04a05 [49] Dietrich J, Hammerl JA, Johne A, et al. Impact of climate change on foodborne infections and intoxications. J Health Monit, 2023; 8, 78−92. [50] Qiu YJ, Zhou Y, Chang YF, et al. The effects of ventilation, humidity, and temperature on bacterial growth and bacterial genera distribution. Int J Environ Res Public Health, 2022; 19, 15345. doi: 10.3390/ijerph192215345 [51] Vezzulli L, Colwell RR, Pruzzo C. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol, 2013; 65, 817−25. doi: 10.1007/s00248-012-0163-2 [52] Guzman Herrador BR, De Blasio BF, MacDonald E, et al. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: a review. Environ Health, 2015; 27, 29. [53] Ebi K. Climate change and health risks: assessing and responding to them through ‘adaptive management’. Health Aff, 2011; 30, 924−30. doi: 10.1377/hlthaff.2011.0071 [54] Perrone G, Ferrara M, Medina A, et al. Toxigenic fungi and mycotoxins in a climate change scenario: ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms, 2020; 8, 1496. doi: 10.3390/microorganisms8101496 [55] Okaka FO, Odhiambo BDO. Relationship between flooding and out break of infectious diseasesin Kenya: a review of the literature. J Environ Public Health, 2018; 2018, 5452938. [56] Tirado MC, Clarke R, Jaykus LA, et al. Climate change and food safety: a review. Food Res Int, 2010; 43, 1745−65. doi: 10.1016/j.foodres.2010.07.003 [57] Yusa A, Berry P, Cheng JJ, et al. Climate change, drought and human health in Canada. Int J Environ Res Public Health, 2015; 12, 8359−412. doi: 10.3390/ijerph120708359 [58] NYSERDA. Health impacts of power outages and warm weather on food safety. https://www.nyserda.ny.gov/-/media/Project/Nyserda/files/Publications/Research/Environmental/18-25-Health-Power-Outages.pdf. [2025-03-10] [59] Oppenheimer M, Glavovic BC, Hinkel J, et al. Sea Level Rise and Implications for Low-Lying Islands, Coasts and Communities. https://www.ipcc.ch/srocc/chapter/chapter-4-sea-level-rise-and-implications-for-low-lying-islands-coasts-and-communities/. [2025-03-10] [60] Zemouri C, Awad SF, Volgenant CMC, et al. Modeling of the transmission of coronaviruses, measles virus, influenza virus, mycobacterium tuberculosis, and legionella pneumophila in Dental Clinics. J Dent Res, 2020; 99, 1192−8. doi: 10.1177/0022034520940288 [61] Ndeh NT, Tesfaldet YT, Budnard J, et al. The secondary outcome of public health measures amidst the COVID-19 pandemic in the spread of other respiratory infectious diseases in Thailand. Travel Med Infect Dis, 2022; 48, 102348. doi: 10.1016/j.tmaid.2022.102348 [62] Danilenko AV, Kolosova NP, Shvalov AN, et al. Evaluation of HA-D222G/N polymorphism using targeted NGS analysis in A(H1N1)pdm09 influenza virus in Russia in 2018-2019. PLoS One, 2021; 16, e0251019. doi: 10.1371/journal.pone.0251019 [63] Poniedziałek B, Rzymski P, Zarębska-Michaluk D, et al. Viral respiratory infections and air pollution: a review focused on research in Poland. Chemosphere, 2024; 359, 142256. doi: 10.1016/j.chemosphere.2024.142256 [64] Safiri S, Mahmoodpoor A, Kolahi AA, et al. Global burden of lower respiratory infections during the last three decades. Front Public Health, 2022; 10, 1028525. [65] Zhou Z, Gilca R, Deceuninck G, et al. Predictors of hospitalization for lower respiratory tract infection in children aged <2 years in the province of Quebec, Canada. Epidemiol Infect, 2016; 144, 1035−44. doi: 10.1017/S0950268815002204 [66] Kang LY, Jing WZ, Liu Q, et al. The trends of mortality, aetiologies and risk factors of lower respiratory infections in China from 1990 to 2019: findings from the Global Burden of Disease Study 2019. J Infect Public Health, 2022; 15, 870−6. doi: 10.1016/j.jiph.2022.06.016 [67] Shi Y, Zhang LP, Wu D, et al. Systematic analysis and prediction of the burden of lower respiratory tract infections attribute to non-optimal temperature, 1990-2019. Front Public Health, 2024; 12, 1424657. doi: 10.3389/fpubh.2024.1424657 [68] Liu Q, Deng J, Yan WX, et al. Burden and trends of infectious disease mortality attributed to air pollution, unsafe water, sanitation, and hygiene, and non-optimal temperature globally and in different socio-demographic index regions. Glob Health Res Policy, 2024; 9, 23. doi: 10.1186/s41256-024-00366-x [69] Zhong W, Bragazzi NL, Kong JD, et al. Burden of respiratory infection and tuberculosis among US States from 1990 to 2019. Clin Epidemiol, 2021; 13, 503−14. doi: 10.2147/CLEP.S314802 [70] Tong M, Wondmagegn B, Xiang JJ, et al. Hospitalization costs of respiratory diseases attributable to temperature in Australia and Projections for future costs in the 2030s and 2050s under climate change. Int J Environ Res Public Health, 2022; 19, 9706. doi: 10.3390/ijerph19159706 [71] Ngo HKT, Luong LMT, Le HHTC, et al. Impact of temperature on hospital admission for acute lower respiratory infection (ALRI) among pre-school children in Ho Chi Minh City, Vietnam. Int J Biometeorol, 2021; 65, 1205−14. doi: 10.1007/s00484-021-02104-1 [72] Huang K, Hu CY, Yang XY, et al. Contributions of ambient temperature and relative humidity to the risk of tuberculosis admissions: a multicity study in Central China. Sci Total Environ, 2022; 838, 156272. doi: 10.1016/j.scitotenv.2022.156272 [73] Li WX, Wang XD, Bi B, et al. Influence of Temperature and Humidity on the Incidence of Pulmonary Tuberculosis in Hainan, China, 2004-2018. Biomed Environ Sci, 2024; 37, 1080−5. [74] Wagatsuma K. Association of ambient temperature with tuberculosis incidence in Japan: An ecological study. IJID Reg, 2024; 12, 100384. doi: 10.1016/j.ijregi.2024.100384 [75] Ding F, Liu XL, Hu ZY, et al. Association between ambient temperature, PM2.5 and tuberculosis in Northwest China. Int J Environ Health Res, 2024; 34, 3173−87. doi: 10.1080/09603123.2023.2299236 [76] Xu M, Li Y, Liu B, et al. Temperature and humidity associated with increases in tuberculosis notifications: a time-series study in Hong Kong. Epidemiol Infect, 2020; 149, e8. [77] Liyew AM, Clements ACA, Akalu TY, et al. Ecological-level factors associated with tuberculosis incidence and mortality: a systematic review and meta-analysis. PLoS Glob Public Health, 2024; 4, e0003425. doi: 10.1371/journal.pgph.0003425 [78] Kyd JM, Krishnamurthy A, Kidd S. Interactions and mechanisms of respiratory tract biofilms involving Streptococcus pneumoniae and nontypeable Haemophilus influenzae. Microb Biofilms-Importance Appl, 2016. [79] Alotaibi GF, Bukhari MA. Factors influencing bacterial biofilm formation and development. Am J Biomed Sci Res, 2021; 12, 617−26. [80] Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med, 2018; 198, 759−66. doi: 10.1164/rccm.201709-1883OC [81] Burkart KG, Brauer M, Aravkin AY, et al. Estimating the cause-specific relative risks of non-optimal temperature on daily mortality: a two-part modelling approach applied to the Global Burden of Disease Study. Lancet, 2021; 398, 685−97. doi: 10.1016/S0140-6736(21)01700-1 [82] Maharjan B, Gopali RS, Zhang Y. A scoping review on climate change and tuberculosis. Int J Biometeorol, 2021; 65, 1579−95. doi: 10.1007/s00484-021-02117-w [83] Azimi P, Keshavarz Z, Cedeno Laurent JG, et al. Estimating the nationwide transmission risk of measles in US schools and impacts of vaccination and supplemental infection control strategies. BMC Infect Dis, 2020; 20, 497. doi: 10.1186/s12879-020-05200-6 [84] Brattig N, Bergquist R, Vienneau D, et al. Geography and health: role of human translocation and access to care. Infect Dis Poverty, 2024; 13, 37. doi: 10.1186/s40249-024-01205-4 [85] Revich B, Tokarevich N, Parkinson AJ. Climate change and zoonotic infections in the Russian Arctic. Int J Circumpol Heal, 2012; 71, 18792. doi: 10.3402/ijch.v71i0.18792 [86] Wang ZK, Pei SJ, Cui HL, et al. Zoonotic spillover and extreme weather events drive the global outbreaks of airborne viral emerging infectious diseases. J Med Virol, 2024; 96, e29737. doi: 10.1002/jmv.29737 [87] Waits A, Emelyanova A, Oksanen A, et al. Human infectious diseases and the changing climate in the Arctic. Environ Int, 2018; 121, 703−13. doi: 10.1016/j.envint.2018.09.042 [88] UNAIDS. How climate change is affecting people living with HIV. https://www.unaids.org/en/resources/presscentre/featurestories/2019/september/20190920_climate-change-people-living-with-hiv. [2025-03-10] [89] ChildFund. The devastating impact of natural disasters. https://www.childfund.org/stories-and-news/2013/february/the-devastating-impact-of-natural-disasters/. [2025-03-10] [90] AIDS Joint United Nations Programme on HIV and. People living with HIV: HIV prevalence. https://aidsinfo.unaids.org/. [2025-03-10] (查阅网上资料,未找到本条文献信息,请确认) [91] Schooley RT. Our warming planet: is the HIV-1-infected population in the crosshairs. Top Antivir Med, 2016; 26, 67−70. [92] Kaufmann GR, Elzi L, Weber R, et al. Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. Aids, 2011; 25, 441−51. doi: 10.1097/QAD.0b013e3283430013 [93] Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. Aids, 1999; 13, F59−62. doi: 10.1097/00002030-199905280-00001 [94] Crum-Cianflone N, Hullsiek KH, Satter E, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med, 2009; 169, 1130−8. doi: 10.1001/archinternmed.2009.104 [95] Biggar RJ, Chaturvedi AK, Goedert JJ, et al. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst, 2007; 99, 962−72. doi: 10.1093/jnci/djm010 [96] Kwak R, Kamal K, Charrow A, et al. Mass migration and climate change: dermatologic manifestations. Int J Womens Dermatol, 2021; 7, 98−106. doi: 10.1016/j.ijwd.2020.07.014 [97] Yunihastuti E, Widhani A, Karjadi TH. Drug hypersensitivity in human immunodeficiency virus-infected patient: challenging diagnosis and management. Asia Pac Allergy, 2014; 4, 54−67. doi: 10.5415/apallergy.2014.4.1.54 [98] Hoosen K, Mosam A, Dlova NC, et al. An update on adverse cutaneous drug reactions in HIV/AIDS. Dermatopath, 2019; 6, 111−25. doi: 10.1159/000496389 [99] Micali G, Lacarrubba F, Verzì AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis, 2016; 10, e0004691. doi: 10.1371/journal.pntd.0004691 [100] Isenring E, Fehr J, Gültekin N, et al. Infectious disease profiles of Syrian and Eritrean migrants presenting in Europe: a systematic review. Travel Med Infect Dis, 2018; 25, 65−76. doi: 10.1016/j.tmaid.2018.04.014 [101] Arnaud A, Chosidow O, Détrez MA, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol, 2016; 174, 104−12. doi: 10.1111/bjd.14226 [102] Tsagkaris C, Eleftheriades A, Matiashova L. COVID-19, Monkeypox, climate change and surgery: a syndemic undermines the right to be operated in a clean, healthy and sustainable environment. Perioper Care Oper Room Manag, 2023; 30, 100305. doi: 10.1016/j.pcorm.2022.100305 [103] Phung D, Nguyen HX, Nguyen HLT, et al. Spatiotemporal variation of hand-foot-mouth disease in relation to socioecological factors: a multiple-province analysis in Vietnam. Sci Total Environ, 2018; 610-611, 983-91. [104] Law A, Saunders P, Middleton J, et al. Global warming must stay below 1.5°C. BMJ, 2018; 363, k4410. [105] IPCC. Global warming of 1.5°C. https://ipcc.ch/report/sr15/. [2025-03-10] [106] Oxfam. Extreme carbon inequality. https://d1tn3vj7xz9fdh.cloudfront.net/s3fs-public/file_attachments/mb-extreme-carbon-inequality-021215-en.pdf. [2025-03-10] [107] Semenza JC, Lindgren E, Balkanyi L, et al. Determinants and drivers of infectious disease threat events in Europe. Emerg Infect Dis, 2016; 22, 581−9. doi: 10.3201/eid2204.151073 [108] Sarkar A. Climate change: adverse health impacts and roles of health professionals. Int J Occup Environ Med, 2011; 2, 4−7. [109] Roberts I, Stott R. Doctors and climate change. Int J Occup Environ Med, 2011; 2, 8−10. [110] Alcayna T, Fletcher I, Gibb R, et al. Climate-sensitive disease outbreaks in the aftermath of extreme climatic events: a scoping review. One Earth, 2022; 5, 336−50. doi: 10.1016/j.oneear.2022.03.011 [111] Campbell AM, Racault MF, Goult S, et al. Cholera risk: a machine learning approach applied to essential climate variables. Int J Environ Res Public Health, 2020; 17, 9378. doi: 10.3390/ijerph17249378 [112] Gupta S, Kaur R, Sohal JS, et al. Countering zoonotic diseases: current scenario and advances in diagnostics, monitoring, prophylaxis and therapeutic strategies. Arch Med Res, 2024; 55, 103037. doi: 10.1016/j.arcmed.2024.103037 [113] Wilson AL, Courtenay O, Kelly-Hope LA, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis, 2020; 14, e0007831. doi: 10.1371/journal.pntd.0007831 [114] Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: past, present, and future. Insects, 2016; 7, 52. doi: 10.3390/insects7040052 [115] Chevalier V, Pépin M, Plée L, et al. Rift Valley fever--a threat for Europe?. Euro Surveill, 2010; 15, 19506. [116] GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet, 2020; 396, 1223−49. doi: 10.1016/S0140-6736(20)30752-2 [117] Prüss-Ustün A, Wolf J, Bartram J, et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: an updated analysis with a focus on low- and middle-income countries. Int J Hyg Environ Health, 2019; 222, 765−77. doi: 10.1016/j.ijheh.2019.05.004 [118] Wolf J, Hubbard S, Brauer M, et al. Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis. Lancet, 2022; 400, 48−59. doi: 10.1016/S0140-6736(22)00937-0 [119] WHO. Drinking-water. https://www.who.int/news-room/fact-sheets/detail/drinking-water#:~:text=Safe%20and%20readily%20available%20water%20is%20important%20for,growth%20and%20can%20contribute%20greatly%20to%20poverty%20reduction. [2025-03-10] [120] Liu Q, Li D, Xu Z, et al. Disparities in global disease burden attributed to ambient particulate matter pollution and household air pollution from solid fuels. Ecotoxicol Environ Saf, 2025; 291, 117908. doi: 10.1016/j.ecoenv.2025.117908 [121] Mannucci PM, Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health, 2017; 14, 1048. doi: 10.3390/ijerph14091048 [122] WHO. WHO Guidelines for indoor air quality: household fuel combustion. https://iris.who.int/bitstream/handle/10665/141496/9789241548885_eng.pdf?sequence=1. [2025-03-10] [123] McMichael AJ. Extreme weather events and infectious disease outbreaks. Virulence, 2015; 6, 543−7. doi: 10.4161/21505594.2014.975022 [124] Costello A, Abbas M, Allen A, et al. Managing the health effects of climate change. Lancet, 2009; 373, 1693−733. doi: 10.1016/S0140-6736(09)60935-1 [125] Campbell-Lendrum D, Manga L, Bagayoko M, et al. Climate change and vector-borne diseases: what are the implications for public health research and policy?. Philos Trans R Soc B Biol Sci, 2015; 370, 20130552. doi: 10.1098/rstb.2013.0552 [126] Liu Q, Du M, Wang YP, et al. Global, regional and national trends and impacts of natural floods, 1990-2022. Bull World Health Organ, 2024; 102, 410−20. doi: 10.2471/BLT.23.290243 [127] WHO. Pandemic prevention, preparedness and response accord. https://www.who.int/news-room/questions-and-answers/item/pandemic-prevention--preparedness-and-response-accord. [2025-03-10] -




 下载:
下载:



 Quick Links
Quick Links