-
The diving operation has a direct bearing on the health of divers due to its particularity, and such effects are mainly related to hyperbaric exposure (HBE)[1], including exposure to high pressure, high oxygen environment, and relevant decompression stress generated from the interaction with gas microemboli.
Many previous studies have reviewed the important role of trace elements in the physiological processes of the human body[2]. As one of the most important trace elements in the human body, iron is involved in a series of biochemical processes, such as ferritin synthesis. These synthetic substances are closely involved in the hematological process in the body. B vitamins in the form of coenzymes are widely involved in various physiological processes. When in a hyperbaric environment, the body is in a state of stress, and its vitamin and mineral levels change accordingly. Previous experiments revealed the changes in the hematological indexes of diving personnel, including the levels of serum iron, serum sodium, serum potassium, vitamin B12, folic acid and hemoglobin, and total calcium of serum[3], after diving under high pressure. This finding may be related to the changes in nutritional intake and pressure hormones. Maintaining the stability of these nutrient indexes in the blood is crucial for body health. Many metabolic and immune diseases are closely related to cecal microbiota. 16S rRNA gene sequencing is an important method to study the composition and distribution of microbiota. The study found that the hyperbaric environment in saturated diving affects cecal microbiota and increases the risk of pathogen infection and thus suggested that supplementing probiotics in diets may prevent these adverse changes[4].
Male SPF SD rats [8-week-old; 250–300 g; laboratory animal production license code SCXK (Shanghai) 2018-0006] were provided by the Navy Special Medical Center Animal Room and fed with standard diets. All animal experiments were approved by the Navy Special Medical Center Medical Ethics Committee [permit license code of using laboratory animals SYXK (Shanghai) 2017-0019]. Twenty rats were randomly divided into four groups of five: Group CK_15 (control check for 15 days), Group CK_30 (control check for 30 days), Group HBE_15 (HBE for 15 days), and Group HBE_30 (HBE for 30 days). The rats in Group HBE_15 and Group HBE_30 were exposed to HBE every day by pressurizing to 60 m depth in 5 min and depressurizing to normal pressure after 1 h of HBE. Meanwhile, the control groups were given blank treatment. The treatment period lasted 15 and 30 days. The rats were weighed before and after the treatment.
At 24 h after the treatment, the rats were anesthetized with 3% pentobarbital sodium. After their eyeballs were clipped, the blood was extracted to a biochemical collecting tube and divided into two parts: one was added with anticoagulation heparin to generate a whole blood sample and then stored at −80 °C, and the other was kept at 37 °C for 30 min and then centrifuged at 10 °C with 3,000 rpm for 20 min by a high-speed low-temperature centrifuge. The supernatant was stored at −20 °C to generate a serum sample. The rats were then killed by decapitation, and the cecum was extracted and stored at −80 °C. The serum sample was used to detect the level of serum ferritin, transferrin, and serum iron, and the whole blood sample was used to detect the level of hemoglobin.
B vitamin standard curve was established by the isotope internal standard method. The serum samples were separated by the Waters ACQUITY UPLC I-Class system (Waters, USA). Mass spectrometry analysis in the positive ion mode was conducted using a 5500 QTRAP mass spectrometer (AB SCIEX).
The cecal contents were collected aseptically and stored at −80 °C. The total DNA of sample microbiota was extracted from feces, and the V3 and V4 regions of the 16S rRNA gene were selected for PCR amplification. The primers were encoded as 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). The obtained data were analyzed on the free online Majorbio Cloud Platform (www. majorbio.com).
During the experiment, the ingestion and activities of the rats were normal. According to the data in Supplementary Table S1 (available in www.besjournal.com), HBE had a certain inhibitory effect on the body growth of adult rats (P > 0.05), and this effect was highly evident after 15 days of exposure. When the rats were exposed to HBE, their mental stress led to high energy consumption and inhibited body growth. With prolonged treatment time, the rats could become familiar with the conditions and HBE. As a result, their body growth could return to normal.
Group Original weight (g) Final weight (g) Weight gain (%) CK_15 276.00 ± 13.42 347.00 ± 35.99 25.72 CK_30 282.00 ± 13.04 369.00 ± 22.80 30.85 HBE_15 268.00 ± 8.37 321.00 ± 21.33 19.77 HBE_30 279.00 ± 13.42 363.00 ± 21.68 30.11 Note. Data is represented by $\bar {\rm{x}} $ ± s (n = 5). Table S1. Effects of HBE on body weight
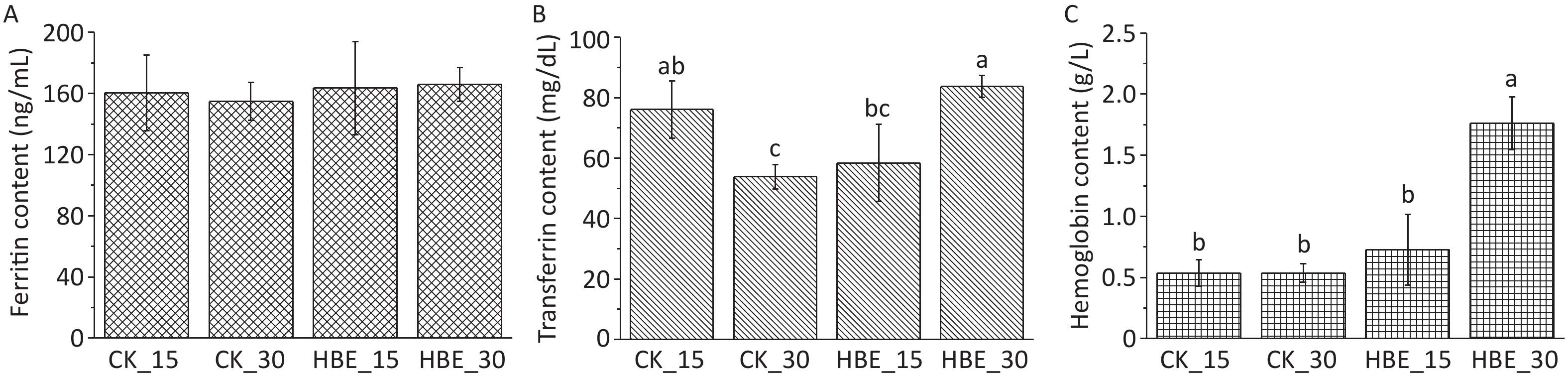
Diving could cause hematological changes due to hyperbaric pressure[3]. S.M. Smith suggested that oxygen exposure in saturated diving may lead to increased iron storage[3]. Ferritin is a widespread iron-containing protein, and its level can reflect the body’s normal iron reserve. As shown in Figure 1A, ferritin levels trended upward but not significantly under HBE (P > 0.05). The increasing rate of ferritin in rats under HBE for 30 days (7.25%) was higher than that in rats under HBE for 15 days (1.94%). Compared with Group CK_15 rats with a serum iron level of 0.187 mg/L, Group HBE_15 rats had a serum iron level of 0.734 mg/L. This finding indicated that the serum iron level was significantly improved by HBE for 15 days (P < 0.05, not shown in the figure), which was consistent with previous research results[5]. Transferrin is responsible for the transport of iron absorbed by the digestive tract and released from the degradation of red blood cells. After HBE for 30 days, the transferrin level in Group HBE_30 rats significantly increased compared with that in Group CK_30 (P < 0.05) (Figure 1B), possibly due to the increase in ferritin release (Figure 1A). Compared with that in the blank groups, the hemoglobin level of the two groups of rats under HBE increased to varying degrees (Figure 1C), possibly due to the increase in the oxygen consumption and body oxygen demand of rats under HBE.
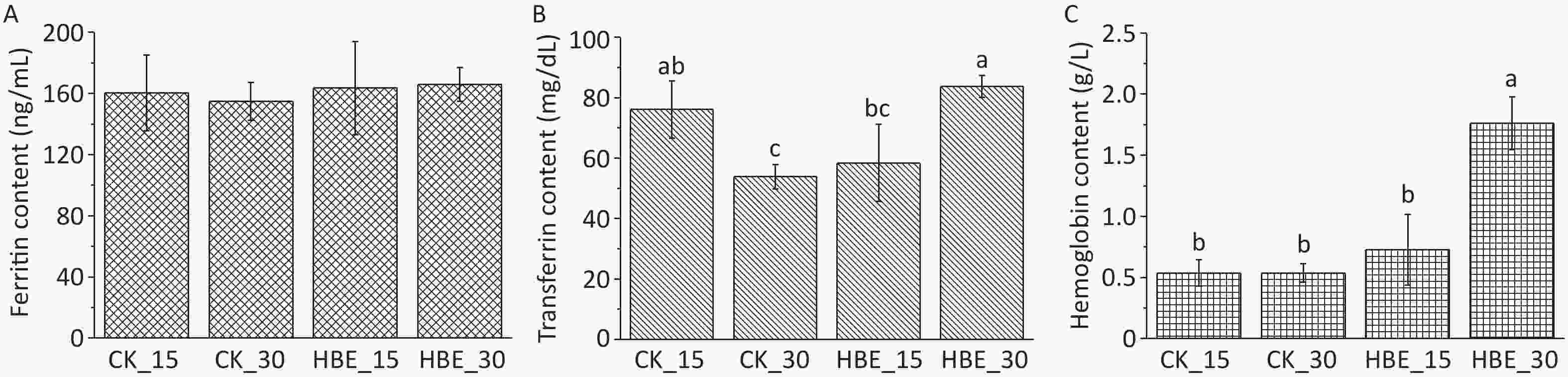
Figure 1. Effects of HBE on iron metabolism (A) Ferritin content; (B) Transferrin content; (C) Hemoglobin content. Data is represented by
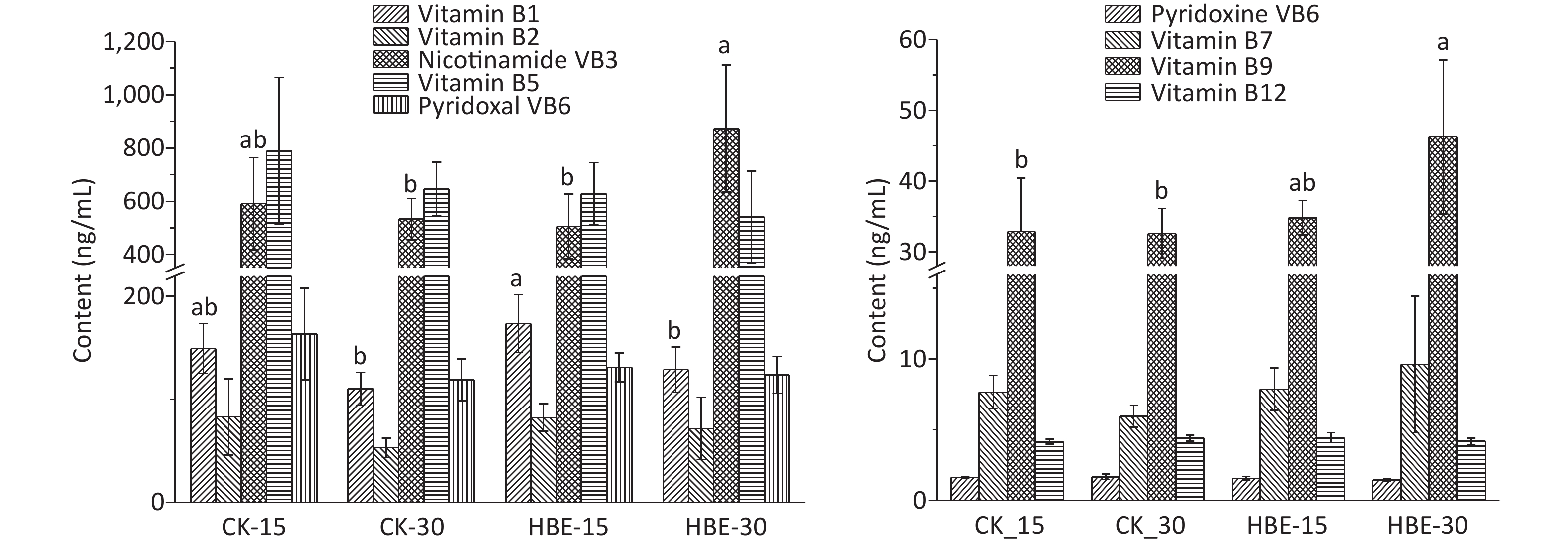
$\bar x $ ± s (n = 5). Different letters (abc) indicate significant difference (P < 0.05), and the same letter indicates no significant difference (P > 0.05).Water-soluble B vitamins are basic substances that promote the conversion of all body substances into energy and maintain the body’s normal operation. The standard curve of each water-soluble B vitamin is shown in Supplementary Table S1. The result (Figure 2) shows that after HBE for 15 days, the serum of rats in Group HBE_15 contained increased levels of thiamine, biotin, folic acid, and cobalamin at varying degrees and decreased levels of other B vitamins compared with that in Group CK_15. After HBE for 30 days, the serum of rats in Group HBE_30 contained increased levels of thiamine, riboflavin, pantothenic acid, pyridoxal VB6, biotin, and folic acid at varying degrees compared with that in Group CK_30. A significant difference was found in the increased amount of pantothenic acid, biotin, and folic acid (P < 0.05). Meanwhile, the level of pyridoxal VB6 in serum decreased by 12.92% (P < 0.05). Compared with that in Group HBE_15, the level of thiamine in the serum of Group HBE_30 rats decreased significantly by 25.772% with prolonged treatment time (P < 0.05). Among the vitamins with increasing levels, the changes in pantothenic acid and folic acid were significant (P < 0.05) at rates of 72.92% and 32.77%, respectively. The decrease in the amount of several substances, such as nicotinic acid and pyridoxal VB6, may result from the rapid metabolism of vitamins and the increasing demand of the body for energy under the HBE stress state. In addition, pyridoxal VB6 and pyridoxine VB6 can transform into each other under certain conditions. Some studies showed that cobalamin has a certain relationship with the level and convertor receptor of ferritin in the body[5].
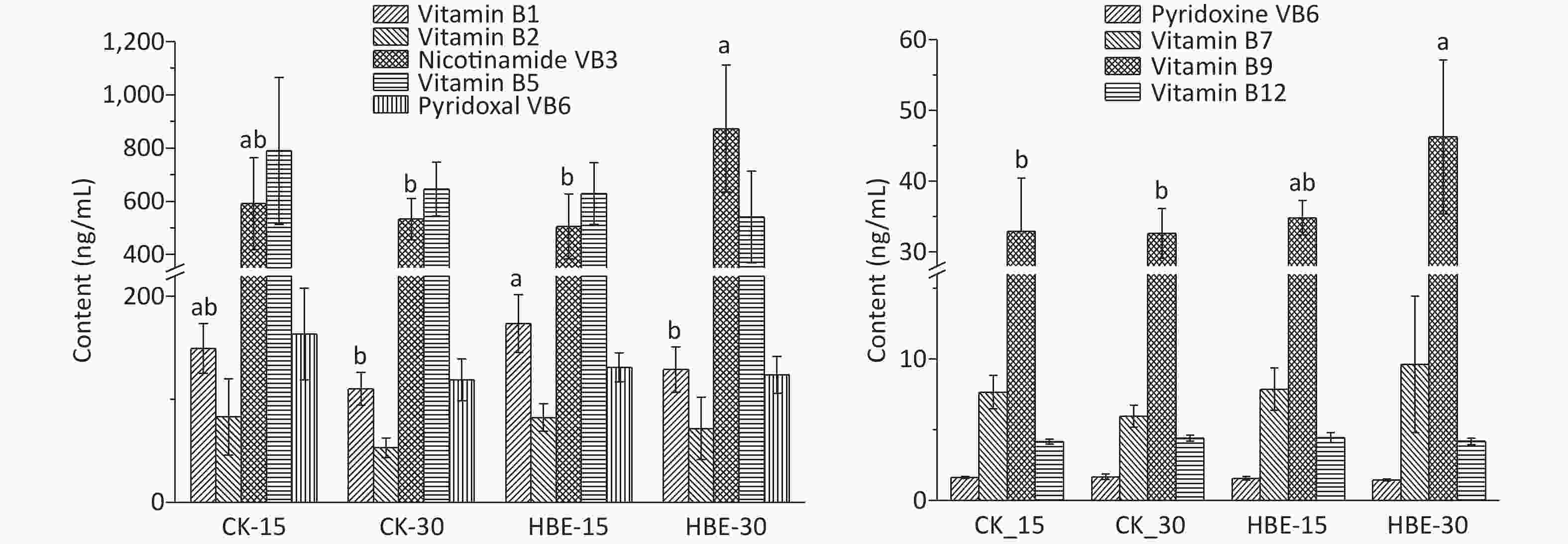
Figure 2. Effects of HBE on content of water-soluble B-vitamin in serum. Data is represented by
$\bar x $ ± s (n = 5). Different letters (ab) indicate significant difference (P < 0.05), and the same letter indicates no significant difference (P > 0.05).16S rRNA gene sequencing was employed to analyze the changes in cecal microbiota and evaluate the effects of HBE on the cecal microbiota of rats.
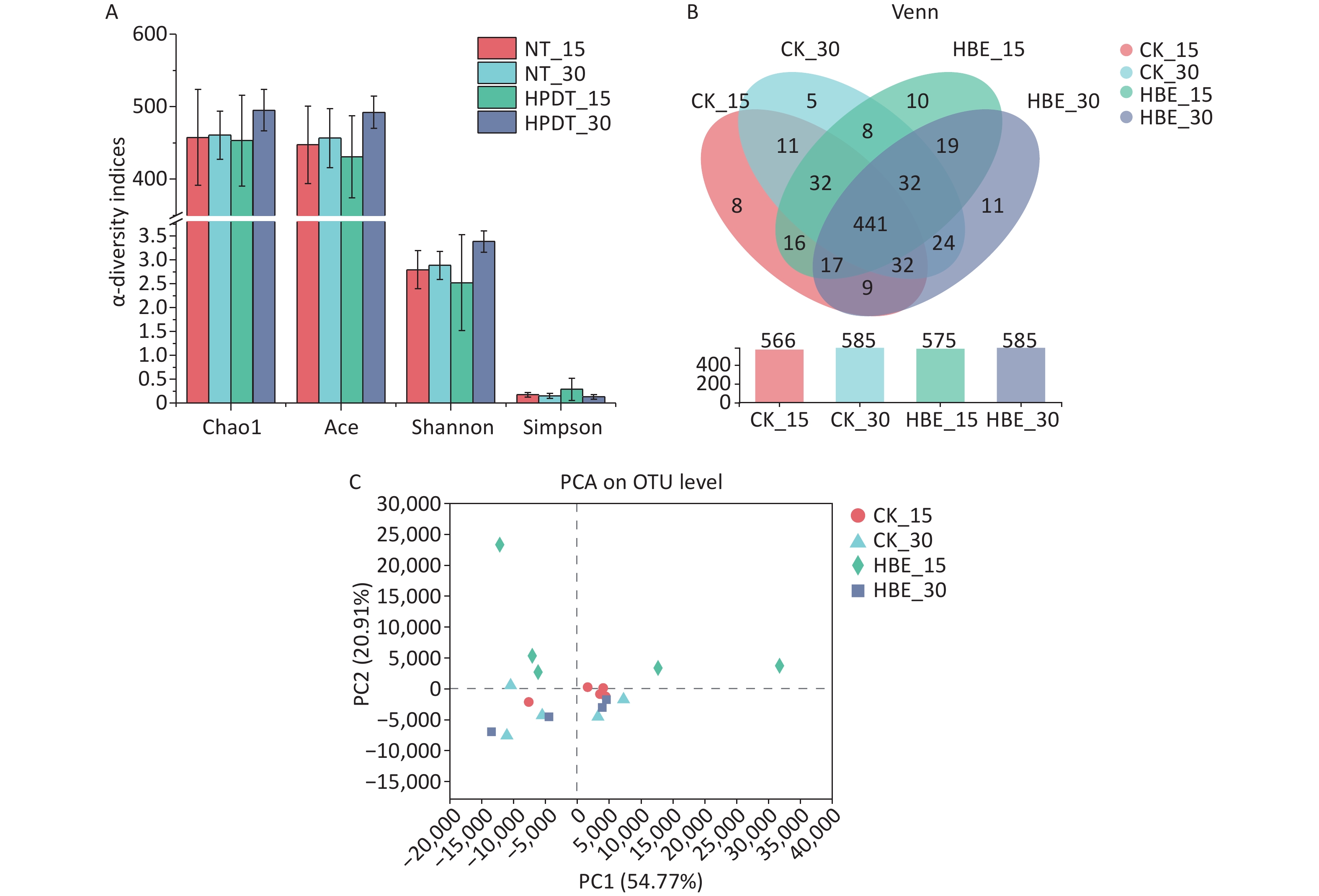
Microbial species richness was mainly determined by Chao1 and ACE indexes, and microbial diversity was examined by Shannon and Simpson indexes. As shown in Figure 3A, the microbial species richness decreased in the cecal contents of rats under HBE for 15 days but increased in the cecal contents of rats under HBE for 30 days. Further comparison between Group HBE_15 and Group HBE_30 showed that the microbial species richness increased with prolonged treatment time. In terms of α-diversity, Group HBE_15 had decreased Shannon index and increased Simpson index compared with the blank group. Meanwhile, Group HBE_30 showed an opposite trend. From a statistical point of view, the Operational Taxonomic Unit (OTU) level of Group HBE_30 was significantly different from the Shannon index of Group CK_30 (P < 0.05).
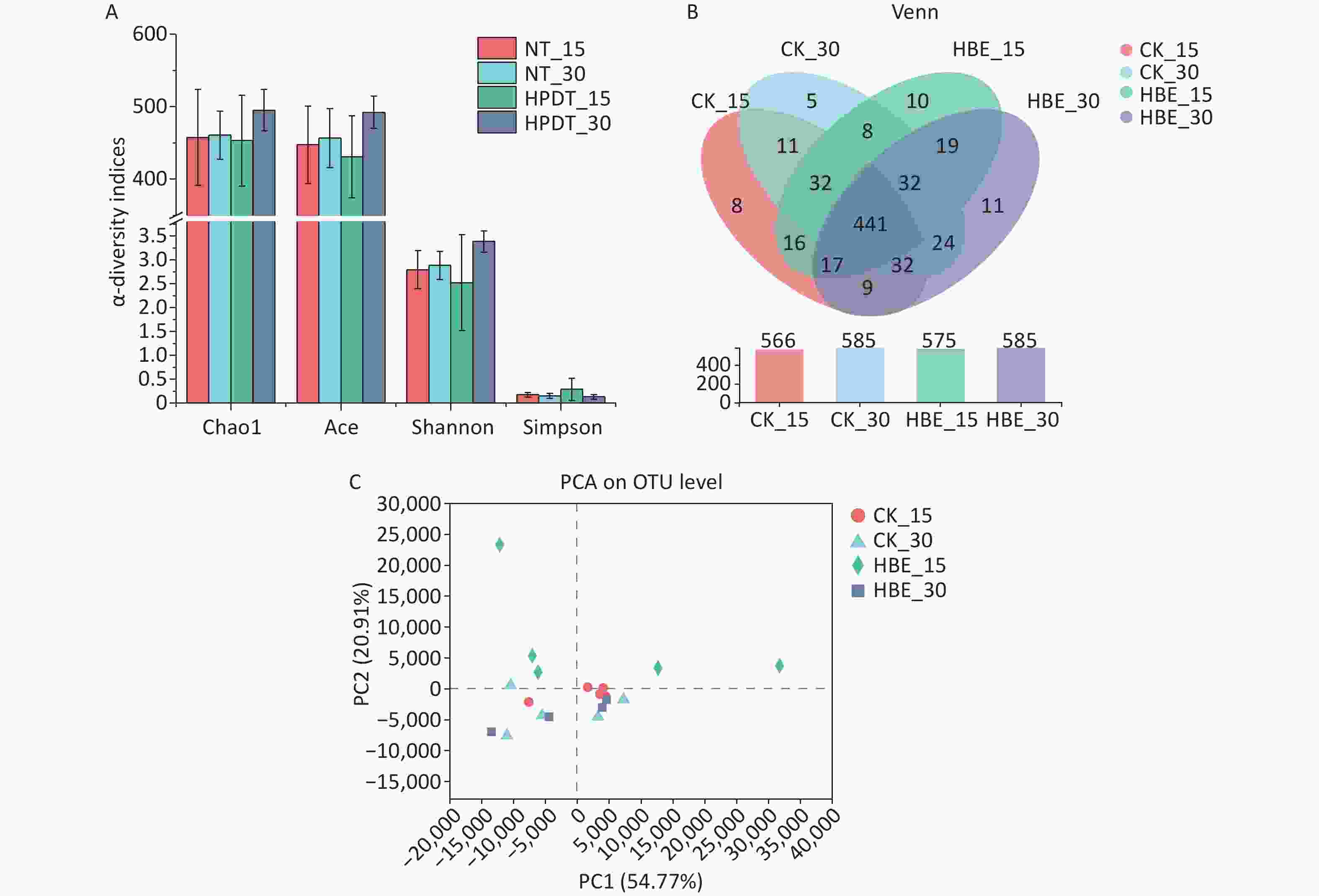
Figure 3. Comparison of α-diversity. (A) α-diversity indices (Chao1, Ace, Shannon and Simpson); (B) Venn map (A) and its comparative analysis of cecal microorganism samples of different groups; (C) PCA on OTU level.
As shown in Figure 3B, 441 of 675 OTUs were common in all groups. Among which, 60 different kinds of microorganisms were found in Group CK_15 and Group HBE_15, and 46 were found in Group CK_30 and Group HBE_30. With prolonged HBE time, the OTUs of Group HBE_15 deviated from those of Group HBE_30 with 76 different kinds of microorganisms. Meanwhile, 10 and 11 unique OTUs were found in Group HBE_15 and Group HBE_30, respectively, which were more than those found in Group CK_15 and Group CK_30. Compared with that in Group CK_15, the total number of OTUs increased in Group HBE_15 (P > 0.05) but remained unchanged in Group HBE_30 and Group CK_30.
Principal Component Analysis (PCA) analysis was used to reflect the differences of multiple sets of data on the 2D coordinate map to show the distribution of dispersion and aggregation and evaluate the difference and distance of microbial structures in rat cecal contents of each group. A close distance between samples reflects good repeatability within the group, and the distance between groups is reflected in the certain differences in microbial structures in the two groups (Figure 3C). The intra-group repeatability of Group HBE_15 was relatively poor but differed from that of Group CK_15, indicating that the structure of microbiota in rats changed after HBE for 15 days. The intra-group repeatability of Group CK_30 and Group HBE_30 was good, indicating that the microbiota in rat cecum contents of the two groups had a minimal difference in OTU level.
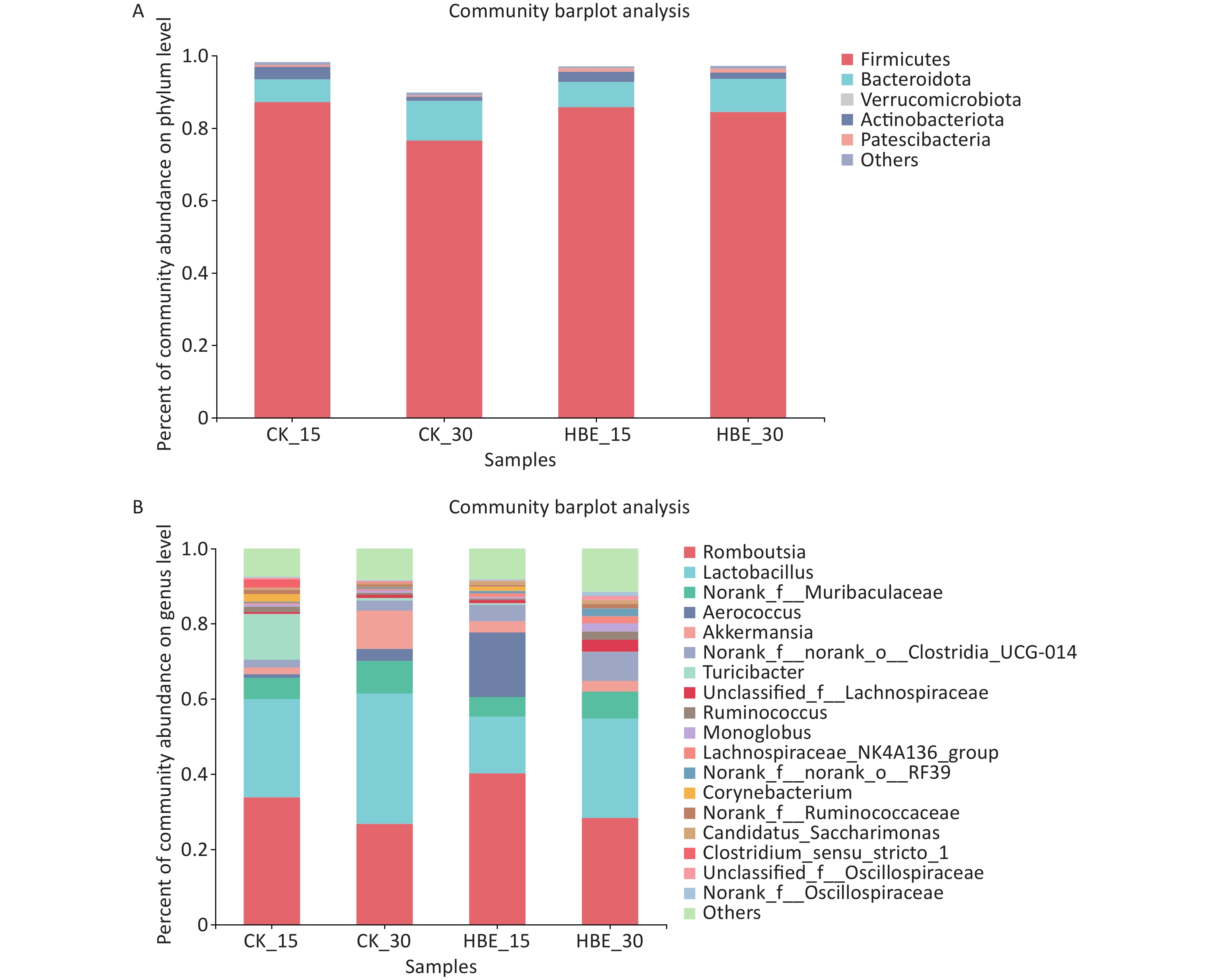
The composition and structure of the microbiota in rat cecal contents were analyzed at different taxonomic classification levels. At the phylum level (Supplementary Figure 1A available in www.besjournal.com), the first four dominant phyla were Firmicutes, Bacteroidota, Verrucomicrobia, and Actinobacteria. Without intervention, the abundance of Firmicutes and Actinobacteria decreased significantly (P < 0.05) with prolonged feeding time. However, HBE reversed this trend to some extent. In the comparison between Group CK_15 and Group HBE_15, the abundance of each phylum presented the minimal change. However, in the comparison between Group CK_30 and Group HBE_30, the abundance of Firmicutes and Actinobacteria increased (P > 0.05), and that of Verrucomicrobia showed an opposite trend. At the genus level (Supplementary Figure 1B), the five dominant genera were Romboutsia, Lactobacillus, Norank_f_Muribaculaceae, Aerococcus, and Akkermansia. Among them, Romboutsia is closely related to gut and kidney diseases[6]. Compared with Group CK_15, Group HBE_15 had increased relative abundance of Romboutsia, Aerococcus, and Akkermansia and decreased relative abundance of Lactobacillus and Norank_f_Muribaculaceae. Compared with Group CK_30, Group HBE_30 had a slightly increased relative abundance of Romboutsia and decreased abundance of Lactobacillus, Norank_f_Muribaculaceae, Aerococcus, and Akkermansia at varying degrees. This finding was partially consistent with the tendency under HBE for 15 days. The decrease in Lactobacillus is related to the conditions of HBE[7], and the results of the present study are consistent with previous findings.
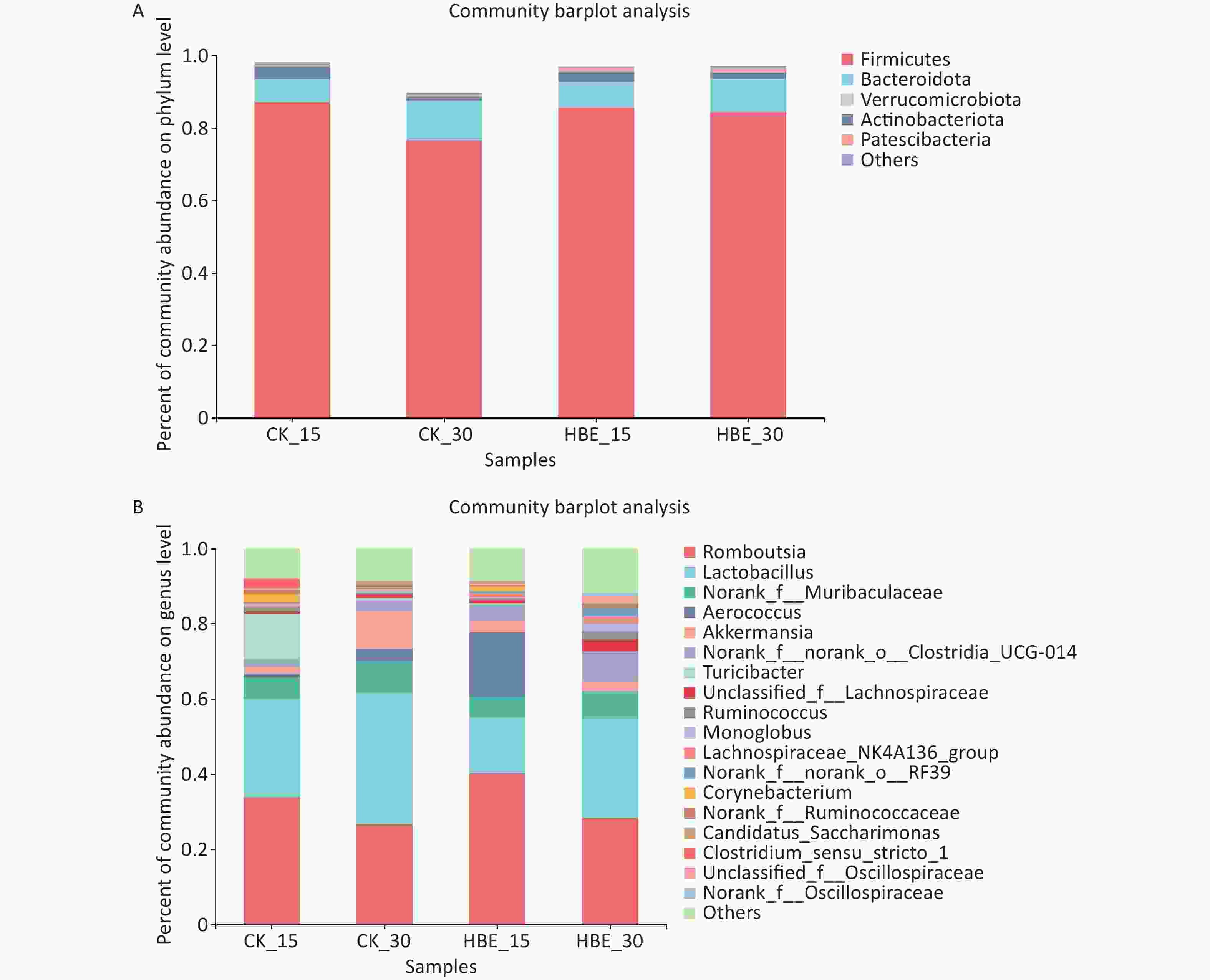
Figure S1. The proportion of dominant bacteria in different groups. (A) On phylum level; (B) On genus level
Analysis at the phylum and genus levels of taxonomic classification revealed that HBE has a certain effect on the microbiota of cecal contents in rats, possibly due to the change in B vitamin levels[8]. The change of cecal microbiota may further affect the related indexes of iron metabolism[9], which is worthy of further study.
The results showed that HBE inhibited the body growth and greatly influenced the hematological indexes of rats such as hemoglobin, transferrin, iron metabolism, vitamin levels, especially water-soluble B vitamins, namely, pantothenic acid, biotin, folic acid, and pyridoxal VB6. These changes varied with the length of treatment time. HBE also changed the microbiota distribution in the cecal contents of rats. This study evaluated the effects of HBE on the physiology of rats, provided experimental ideas for further exploring the influence of hyperbaric diving on the body, and laid a theoretical foundation for the special nutritional guarantee measures of divers.
HTML
Reference
 21289Supplementary Materials.pdf
21289Supplementary Materials.pdf
|

|









 Quick Links
Quick Links
 DownLoad:
DownLoad: