-
Vibroacoustic disease (VAD) involves cumulative and systemic pathologies, which have been identified in aircraft technicians, pilots, cabin crewmembers, and disk jockeys, and which are caused by excessive exposure to high intensity [> 110 decibels (dB)] and low frequency [< 200 Hertz (Hz)] noise (HI-LFN)[1,2]. Furthermore, the non-auditory symptomatic effects of HI-LFN exposure on public health are increasing. Studies have shown that HI-LFN exposure leads to annoyance, sleep disturbances, and impairs cognitive performance in humans [3-5]. Furthermore, excessive exposure to noise can impair learning and memory abilities in rats, which is characterized by the activation of glial cells, impaired neurogenesis, and neural apoptosis in the hippocampus [6-8]. However, different types of noise may involve different mechanisms, but the mechanism of how HI-LFN exposure affects cognitive function is still largely unknown, especially after acute exposure.
Memory formation and loss are believed to involve modifications in neural transmission caused by physiochemical and/or structural modifications of synaptic communication within the neuronal network[9], which are mediated by cytoskeleton dynamics in nerve fibers [10,11]. Cytoskeletal components, such as actin, neurofilaments, and microtubules, are important in neurons to maintain the soma and fiber morphology, support axonal transport, and accommodate structural changes of neural circuits that are related to cognitive plasticity[12,13]. Abnormalities in the cytoskeleton contribute to memory deficit, and have been found in the hippocampus in rodent models of Alzheimer's disease[14,15]. Studies have also reported that tissues abundant in cytoskeletons, such as the cochlear stereocilia and respiratory tract brush cell microvilli, which are the main targets for HI-LFN[16,17], suggesting that cytoskeletal injury in nerve fibers is responsible for learning and memory impairment after HI-LFN exposure.
Transient receptor potential vanilloid subtype 4 (TRPV4) is a member of the TRP superfamily of Ca2+-permeable nonselective cation channels[18]. Studies have reported that TRPV4 physically interacted with tubulin, actin, and neurofilament proteins to affect cellular cytoskeletal dynamics and mechanosensitive processes, mechanosensation, osmosensation, and thermosensation[19-21], which can integrate signaling of various intracellular second messengers and signaling cascades in response to external mechanical stimuli. Studies have also suggested that the TRPV4 channel protein might be involved in transmitting intracellular biological signaling of acoustic stimuli[7,22,23]. We therefore hypothesized that as a type of acoustic stimulus, HI-LFN exposure impaired cellular cytoskeleton and nerve fibers via the TRPV4 channel.
This study therefore aimed to characterize changes in morphology and cognitive function of the hippocampus after acute HI-LFN exposure in a mouse model. Taken together, the results showed that TRPV4 up-regulation induced neurofilament injury, and mediated nerve fiber and memory impairment of the hippocampus after HI-LFN exposure.
-
This study used 130 adult male mice (8−10 weeks of age, 20−25 g). C57/BL6 wild-type (WT) mice were obtained from the Experimental Animal Center of the Third Military Medical University (Chongqing, China). The TRPV4-/- mice were established by Cyagen Biosciences (Santa Clara, CA, USA). Female mice were excluded from the study for the following reasons: 1) estrogen levels of female mice fluctuate with the menstrual cycle and behavior; 2) estrogen has a neuroprotective effect[24]; and 3, to guarantee the homogeneity of mice and avoid behavioral bias caused by different gonadal hormones. The mice were housed in a specific temperature-controlled room under a standard 12-h light/12-h dark cycle, and were provided with free access to food and water. All experiments were in compliance with the Animal Research: Reporting in vivo Experiments guidelines. Our experimental protocols were approved by the Laboratory Animal Welfare and Ethics Committee of the Third Military Medical University (AMUWEC20201153) and conducted according to the Guide for the Care and Use of Laboratory Animals. Randomization of animals was conducted using odd/even numbers.
-
The device used for noise exposure has been previously described in detail[25], using the same instruments. Exposure to HI-LFN vibration is a prerequisite for VAD. Therefore, noise with frequencies of 100, 150, or 200 Hz and a pressure of 140 dB were used as previously described[25], which has been shown to induce biological function in animals. To identify the mechanism of memory impairment after acute exposure to HI-LFN, the exposure period was 2 h for 3 consecutive days, as previously described[26]. To minimize sound pollution during exposure to HI-LFN, the instrument was insulated with sound-proof glass. The mice in the control group were also insulated with sound-proof glass, next to the HI-LFN instrument. The animals were used for behavioral tests, or the brains were collected for morphological and biochemical experiments after the animals were deeply anesthetized and euthanized.
-
Western blotting analysis was performed as described by Yang et al.[27]. The brains were obtained after noise exposure, then proteins of hippocampus tissues were analyzed. The primary antibodies included rabbit anti-TRPV4 polyclonal antibody (1:1,000, #ab39260, RRID: AB_1143677; Abcam, Cambridge, UK), rabbit anti-neurofilament 200 polyclonal antibody (NF200, 1:1,000; #N4142, RRID: AB_477272; Sigma-Aldrich, St. Louis, MO, USA), goat anti-myelin basic protein polyclonal antibody (MBP, 1:1,000, #sc-13914-R, RRID: AB_784681; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-β-actin (1:1,000, #BM0627, RRID: AB_2814866; Boster Biological Technology, Pleasanton, CA, USA), and mouse anti-glyceraldehyde3-phosphate dehydrogenase (GAPDH, 1:2,000, #BM1623, RRID: AB_2885058; Boster Biological Technology) for 12 h at 4 °C. The secondary antibodies were anti-mouse, goat, or rabbit IgG horseradish peroxidase-conjugated antibodies (1:10,000; Abcam). Finally, an enhanced chemiluminescence reagent kit (Millipore, Temecula, CA, USA) for western blotting was used to visualize the immunoreactive bands. The relative densities of the bands were then analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
-
Immunofluorescence was conducted according to Gao et al.[28]. Thirty micron cryostat sections were incubated with the following primary antibodies in 1% bovine serum albumin (overnight, 4 °C): rabbit anti-NF200 polyclonal antibody (1:1,000; Sigma-Aldrich) and goat anti-MBP polyclonal antibody (1:1,000; Santa Cruz Biotechnology). After the sections were washed with 0.01 mol/L phosphate buffer, they were probed with the appropriate Cy3-, 488-conjugated, or Cy3-SABC secondary antibodies (1:500, 4 h, at room temperature; Jackson ImmunoResearch, West Grove, PA, USA). Finally, the sections were mounted in Vectashield medium (Vector Laboratories, Newark, CA, USA). For HE staining, after being deparaffinized in xylene and rehydrated using graded alcohol, the 5 µm paraffin sections were stained using HE reagent. The stained cells were viewed and photographed using an Axivert microscope equipped with a Zeiss AxioCam digital color camera connected to the AxioVision 3.0 system (Zeiss, Oberkochen, Germany).
-
The New Object Recognition (NOR) Task Mice were acclimatized to the experimental room for one night before each test. The NOR task was performed as previously described with minor modifications [29]. Mice were tested in an open field box located in a sound-attenuated room to measure short-term memory. Briefly, after a 5 min habituation period, the mice were tested in the first trial; two identical cylinders were used in diagonal corners, and each mouse was placed in the box and allowed to freely explore the objects for 10 min. In the second trial, one of the cylinders was replaced with a new cuboid object, and the time for exploring freely was 10 min. The two trials were separated by 10 min. Only the second trial (retention session) was analyzed, and the discrimination index was defined as the ratio of the time spent exploring the cuboid object to the total time spent exploring both objects. Individual movement tracks were analyzed by Ethovision 10.0 (Noldus, Hof, Germany).
The Morris Water Maze (MWM) Test The
MWM test was performed as previously described[30]. Briefly, the water maze included a 1.3 m diameter white pool and a 10 cm diameter hidden platform, which was submerged approximately 1 cm below water level. The mice were trained with four trials (one trial means that the mouse was released into the water at water level from one quadrant to explore for 1 min) per day for 5 days to find and remember the hidden platform. On the 7th day, the animals were subjected to a probe trial. The platform was removed, and the mice were allowed to explore the platform location for 60 s. During the probe trial, the platform was moved, and the number of times the animal crossed the location of the hidden platform and the duration of time in each quadrant was calculated. All trials were recorded with a ceiling-mounted video camera and analyzed using Ethovision 10.0. -
All data were collected in a double-blind manner, and statistical analyses were performed using SPSS statistical software for Windows, version 25.0 (SPSS, Chicago, IL, USA). Data are presented as the mean ± SEM and analyzed using one-way or two-way analysis of variance (ANOVA). The Bonferroni test was used as a post-hoc test following ANOVA. For two-way ANOVA, the effects of HI-LFN exposure (main effect) and different strains (WT or TRPV4-/- mice) were analyzed. P < 0.05 was considered to indicate a statistically significant difference.
-
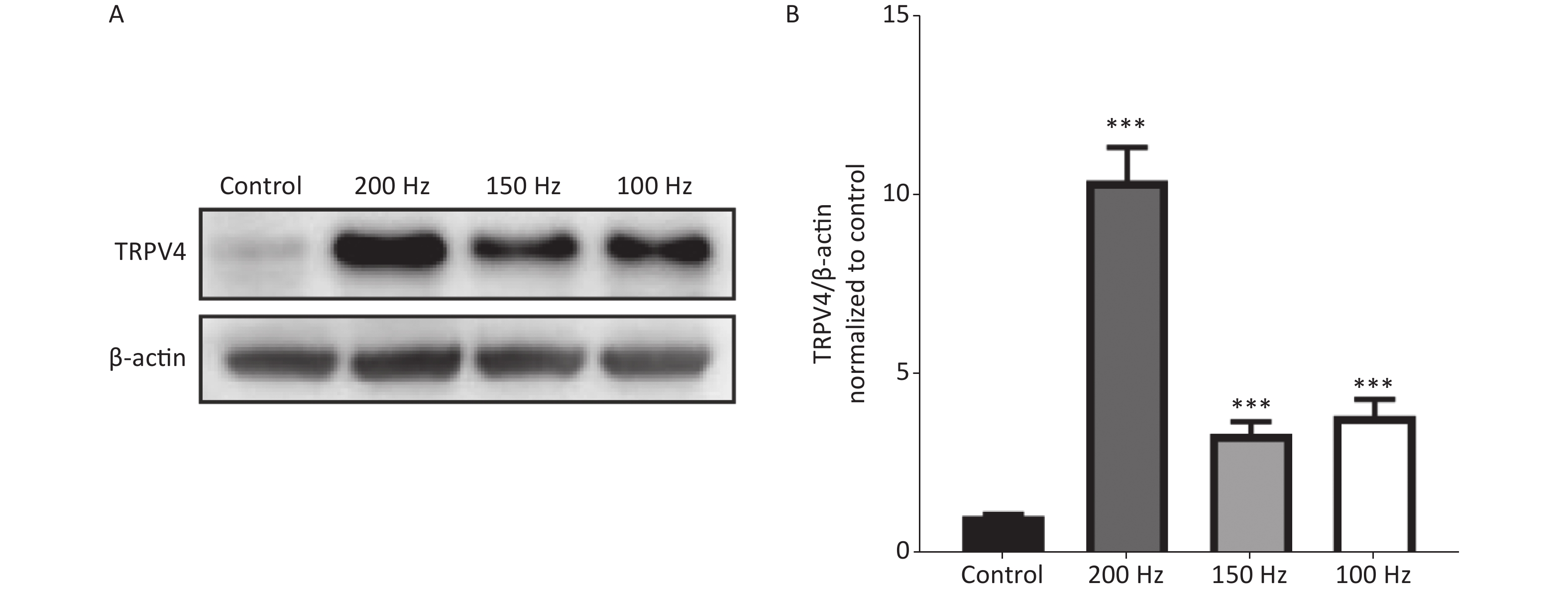
To determine changes in expression of TRPV4 after acute exposure to HI-LFN, WT mice were exposed to 140 dB noise at 100, 150, or 200 Hz for 2 h for 3 consecutive days. We found that the expression of TRPV4 in hippocampus tissue was significantly increased after HI-LFN exposure [Figure 1A–B, P < 0.001, F (3, 16) = 53.69] when compared with that in the control group. TPRV4 expression was the highest when exposed to 200 Hz noise (Figure 1A–B, P < 0.001). We next used the mice in the 140 dB and 200 Hz groups to analyze morphological and functional changes of the hippocampus.
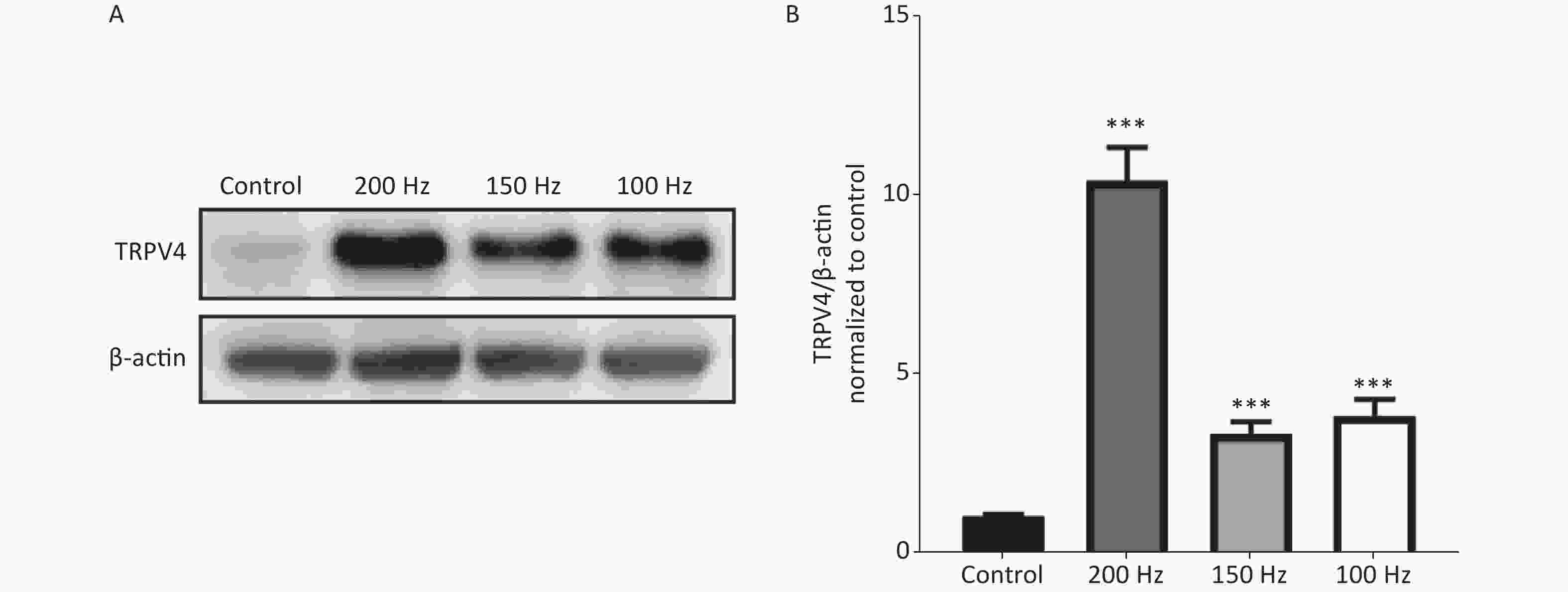
Figure 1. The expression of transient receptor potential vanilloid subtype 4 (TRPV4) after high intensity, low frequency noise exposure. Western blot photographs (A) and quantitative analyses (B) of TRPV4 and β-actin in the Control, and 200 Hz, 150 Hz, and 100 Hz groups. Data are shown as the mean ± SEM, one-way analysis of variance, followed by the Bonferroni post hoc test (n = 5 for each group; ***P < 0.001 vs. the Control group).
-
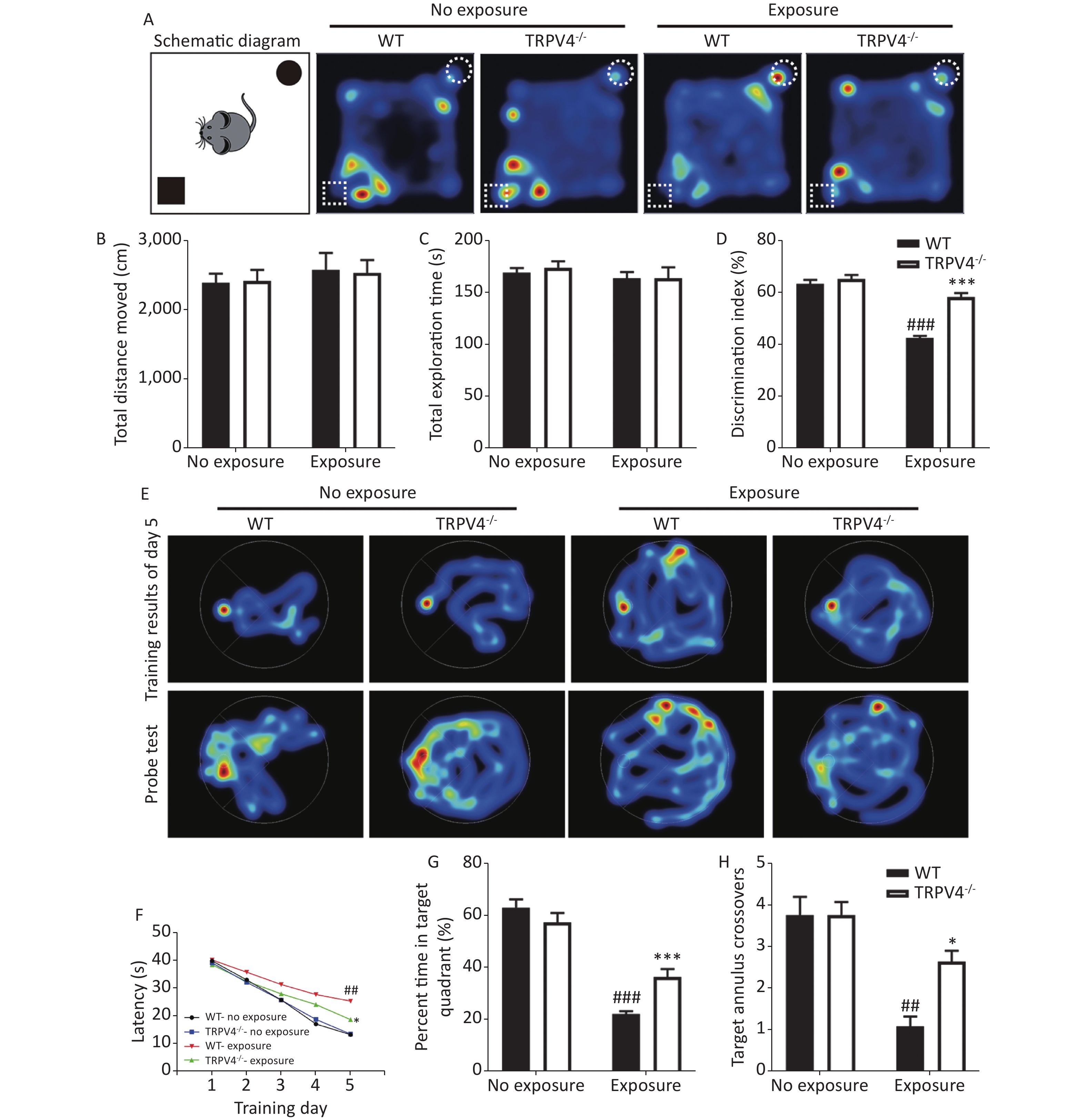
To determine the results of TRPV4 overexpression in the hippocampus after noise exposure, we developed TRPV4-/- mice. Behaviorally, we used the NOR task and MWM test to measure the memory impairments of the WT and TRPV4-/- mice after exposures to 140 dB and 200 Hz noise. The NOR task was used to evaluate recognition memory and short-term memory, and the MWM test was designed to test spatial and long-term memories, and both the NOR task and the MWM were used to assess the cognitive function of the hippocampus. In the NOR task, there was no difference in total distance moved [Figure 2B, two-way ANOVA, F (3, 28) = 0.251, P = 0.86. Between factors: HI-LFN, F (1, 28) = 0.712, P = 0.406; strain, F (1, 28) = 0.002, P = 0.963] and total exploration time [Figure 2C, two-way ANOVA, F (3, 28) = 0.465, P = 0.709. Between factors: HI-LFN, F (1, 28) = 1.171, P = 0.288; strain, F (1, 28) = 0.11, P = 0.742] of two objects between WT and TRPV4-/- mice, with or without HI-LFN exposure, indicating that there was no overall activity or lack of motivation after noise exposure. Furthermore, there was a significant difference in the time of exploration of the novel object [Figure 2D, two-way ANOVA, F (3, 28) = 54.647, P < 0.001. Between factors: HI-LFN, F (1, 28) = 98.516, P < 0.001; strain, F (1, 28) = 40.534, P < 0.001]. In contrast to the WT mice without HI-LFN exposure, HI-LFN exposure of WT mice caused a significant deficit in recognition memory, with a decreased time of exploration (Figure 2D, P < 0.001). However, TRPV4-/- mice exhibited a significant preference for the novel object after HI-LFN exposure (Figure 2D, P < 0.001), when compared with that in the WT group. Similarly, using the MWM test, there were significant differences for HI-LFN exposure and strain in exploration of the platform time on day 5 during training [Figure 2F, two-way ANOVA, F (3, 28) = 21.13, P < 0.001. Between factors: HI-LFN F (1, 28) = 48.808, P < 0.001; strain F (1, 28) = 6.825, P = 0.014], the time of exploration in the target quadrant [(Figure 2G, two-way ANOVA, F (3, 28) = 61.48, P < 0.001). Between factors: HI-LFN, F (1, 28) = 165.275, P < 0.001; strain F (1, 28) = 6.168, P = 0.019] and the number of crossing annulus [Figure 2H, two-way ANOVA, F (3, 28) = 15.445, P < 0.001. Between factors: HI-LFN, F (1, 28) = 34.683, P < 0.001; strain F (1, 28) = 5.826, P = 0.023]. In contrast to the WT mice without HI-LFN exposure, HI-LFN exposure induced a significant delay in WT mice when exploring the platform on day 5 during the training trial (Figure 2F, P = 0.001), with less exploring time in the target quadrant (Figure 2G, P = 0.001) and less number of crossing annulus (Figure 2H, P = 0.002) during the probe trial. Nevertheless, with exposure to HI-LFN, the TRPV4-/- mice showed a significantly shorter time for exploring the platform on day 5 during training (Figure 2F, P = 0.047), with more exploring time in the target quadrant (Figure 2G, P < 0.001) and increased number of crossing the annulus (Figure 2H, P = 0.04) during the probe trial, when compared with that in the WT group. Together, the results indicated that TRPV4 overexpression mediated memory deficits after noise exposure, and that TRPV4 knockout alleviated the memory impairments.
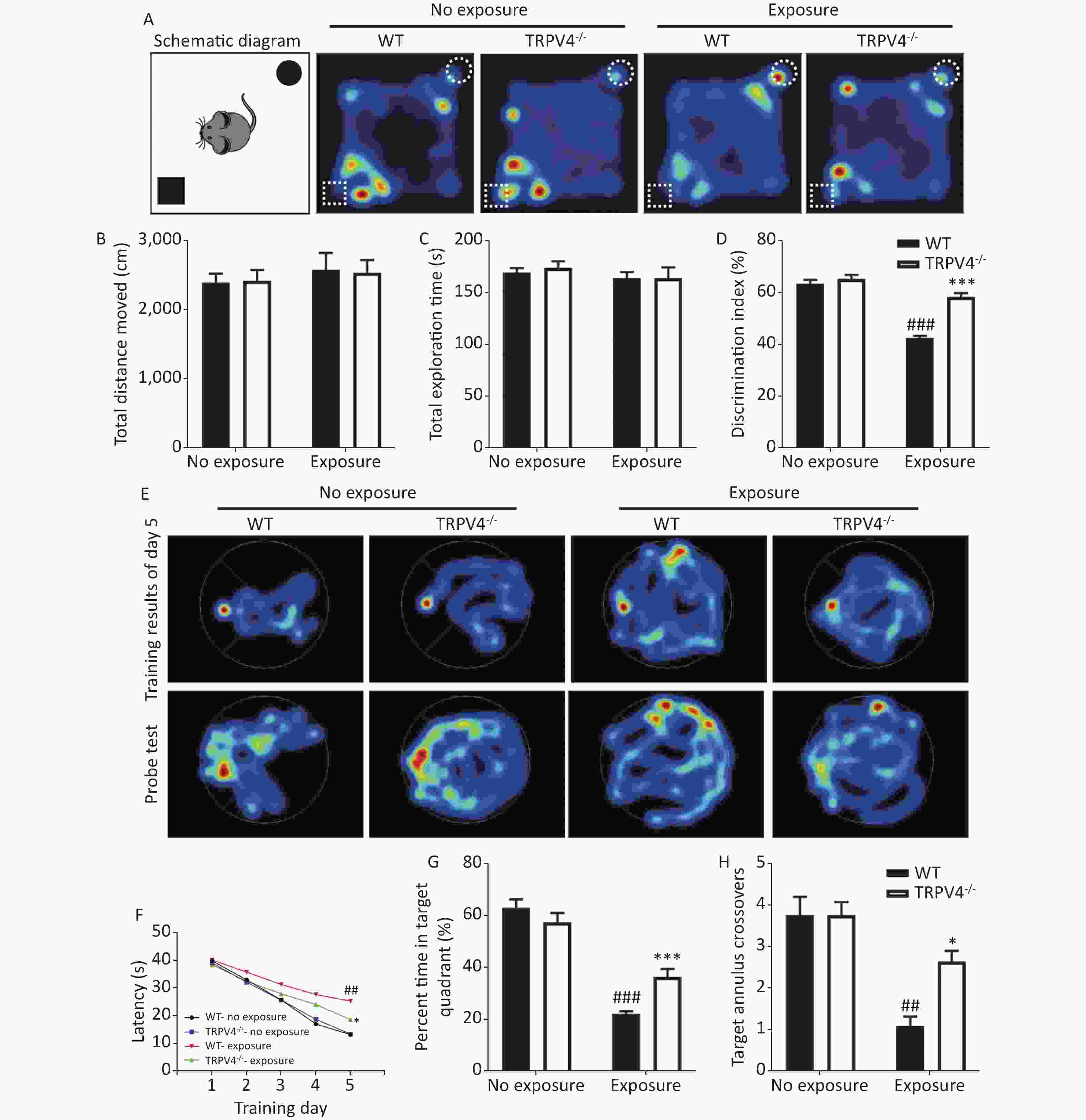
Figure 2. Learning and memory tests of wild-type (WT) and TRPV4-/- mice after high intensity, low frequency noise (HI-LFN) exposure. (A) Schematic diagram and representative hot maps of the novel object recognition (NOR) task in WT and TRPV4-/- groups with or without HI-LFN exposure. (B)–(D) Quantitative data of total distance moved (B), total exploration time of two objects (C), and discrimination index of the two objects (D) during the NOR task in WT and TRPV4-/- groups with or without HI-LFN exposure. ###P < 0.001 vs. the WT-no exposure group, ***P < 0.001 vs. the WT-exposure group; n = 8, two-way analysis of variance followed by post-hoc Bonferroni correction. (E) Representative Morris water maze (MWM) test spatial learning at day 5 (up) and memory (down) images. (F) Quantitative date of latency for 5 days of training, ##P < 0.01 vs. the WT-no exposure group, *P < 0.05 vs. the WT-exposure group; n = 8, two-way analysis of variance (ANOVA) followed by post hoc Bonferroni correction. (G)–(H) The percentage time in the target quadrant (G) and the number of target annulus crossovers (H) during the MWM test in the WT and TRPV4-/- groups with or without HI-LFN exposure. ##P < 0.01, ###P < 0.01 vs. the WT-no exposure group, *P < 0.05, *P < 0.001 vs. the WT-exposure group; n = 8, two-way ANOVA followed by post hoc Bonferroni correction. Data are shown as the mean ± SEM.
-
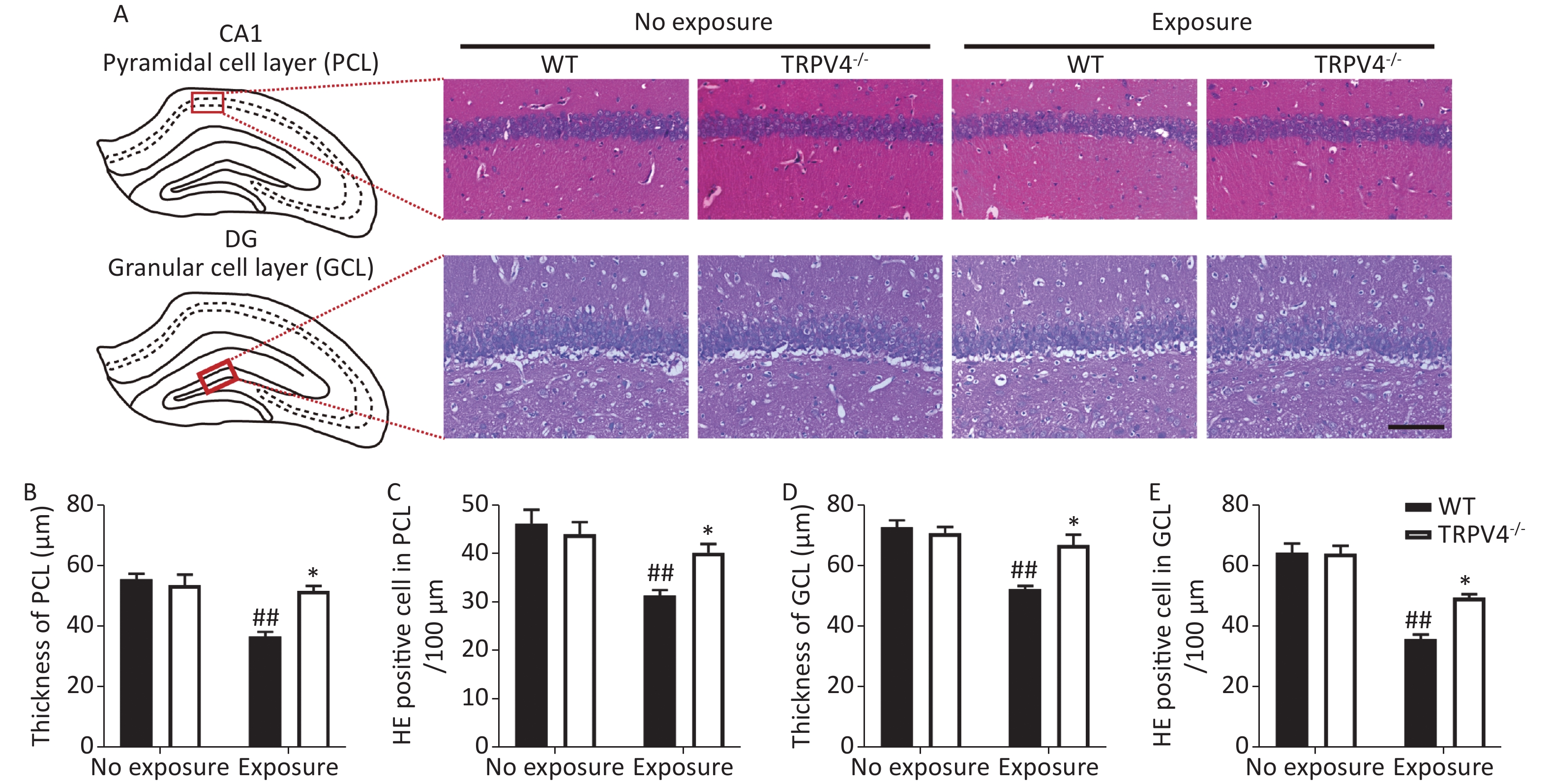
Next, HE staining was used to analyze morphological changes in the pyramidal cell layer (PCL) of the CA1 and granular cell layer (GCL) of the DG in the hippocampus, which play important roles in learning and memory[31]. Generally, there were significant differences for HI-LFN exposure and strain in the thickness of cellular layers in the PCL [Figure 3B, two-way ANOVA, F (3, 16) = 15.056, P < 0.001]. Between factors: HI-LFN, F (1, 16) = 1.823, P < 0.001; strain, F (1, 16) = 8.755, P = 0.009] and GCL [Figure 3D, two-way ANOVA, F (3, 16) = 15.348, P < 0.001. Between factors: HI-LFN, [F (1, 16) = 26.577, P < 0.001; strain, F (1, 16) = 7.214, P = 0.016]. Additionally, there were significant differences for HI-LFN exposure and strain in the number of pyramidal neurons in the PCL [Figure 3C, two-way ANOVA, F (3, 16) = 0.369, P < 0.001. Between factors: HI-LFN, F (1, 16) = 9.91, P < 0.001; strain, F (1, 16) = 5.344, P = 0.034] and the number of granular neurons in the GCL [Figure 3E, two-way ANOVA, F (3, 16) = 36.942, P < 0.001. Between factors: HI-LFN, F (1, 16) = 92.48, P < 0.001; strain, F (1, 16) = 8.634, P = 0.01]. In contrast to WT mice without HI-LFN exposure, HI-LFN exposure induced a significant decrease in the thickness of cellular layers in the PCL (Figure 3A–B, P = 0.009) and GCL (Figure 3A and D, P = 0.008). Furthermore, the numbers of pyramidal neurons in the PCL (Figure 3A and C, P = 0.003) and granular neurons in the GCL (Figure 3A and E, P = 0.002) per 100 μm in the HI-LFN group were also decreased, when compared with that in the WT group. However, with HI-LFN exposure, TRPV4 knockout significantly alleviated the PCL and GCL changes in thicknesses (PCL: Figure 3B, P = 0.021; GCL: Figure 3D, P = 0.026) and neural density (PCL: Figure 3C, P = 0.022; GCL: Figure 3E, P = 0.036) compared with that in the WT group.
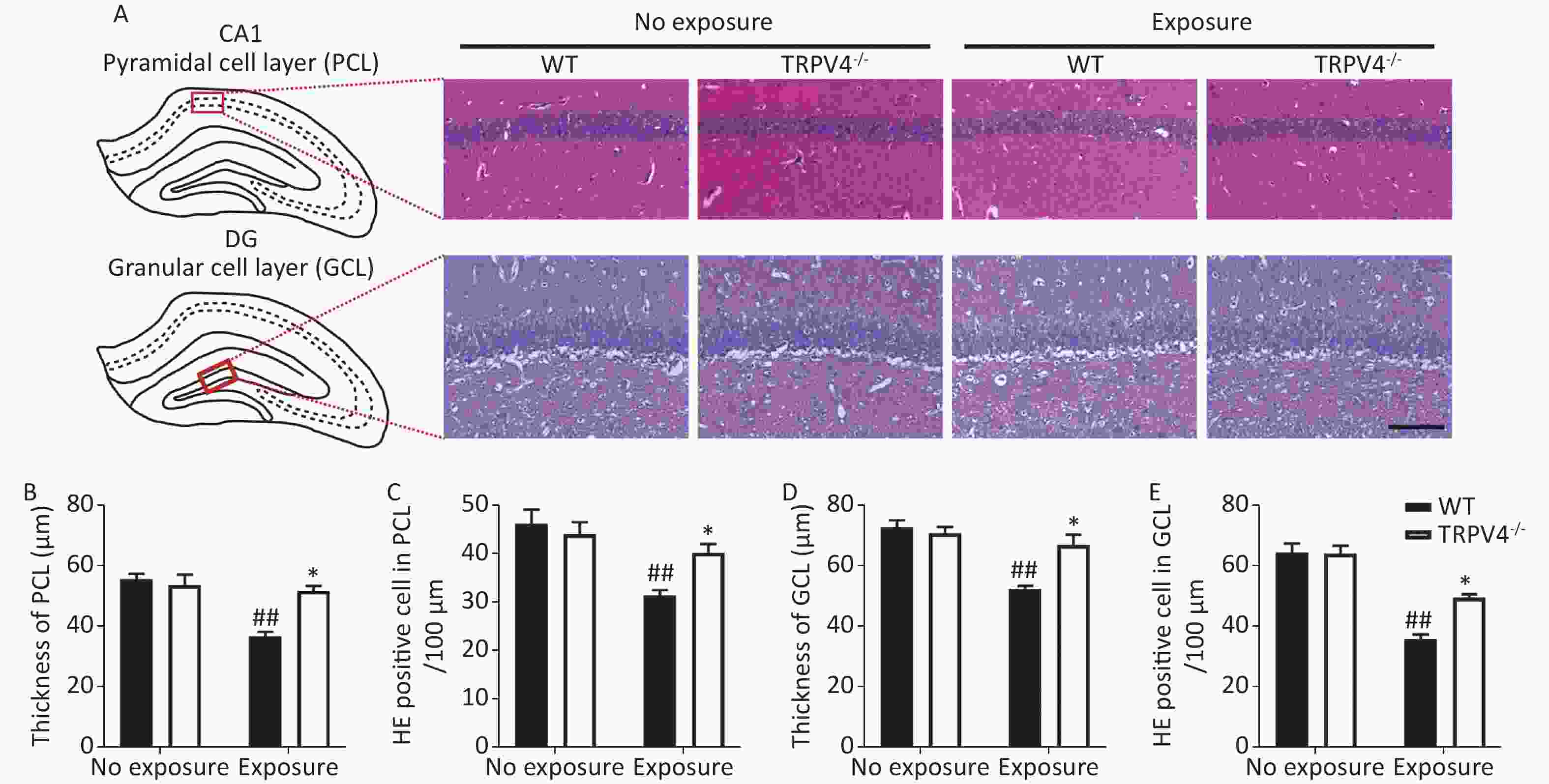
Figure 3. Transient receptor potential vanilloid subtype 4 knockout alleviated the impairment of the pyramidal cell layer (PCL) and granular cell layer (GCL) induced by high intensity, low frequency noise (HI-LFN) exposure. (A) Representative hemoxylin & eosin (HE) staining images of the PCL of CA1 and GCL of the dentate gyrus (DG) in the wild-type and TRPV4-/- groups with or without HI-LFN exposure (scale bar: 100 µm). (B) Quantitative data of the thickness of the PCL for the four groups. (C) Quantitative data of the HE positive neurons in the PCL per 100 μm for the four groups. (D) Quantitative data of the thickness of the GCL for the four groups. (E) Quantitative data of the HE positive neurons in the GCL per 100 μm for the four groups. Data are shown as the mean ± SEM, n = 5 for each group, two-way analysis of variance followed by post hoc Bonferroni correction. ##P < 0.01 vs. the WT-no exposure group, *P < 0.05 vs. the WT-exposure group.
-
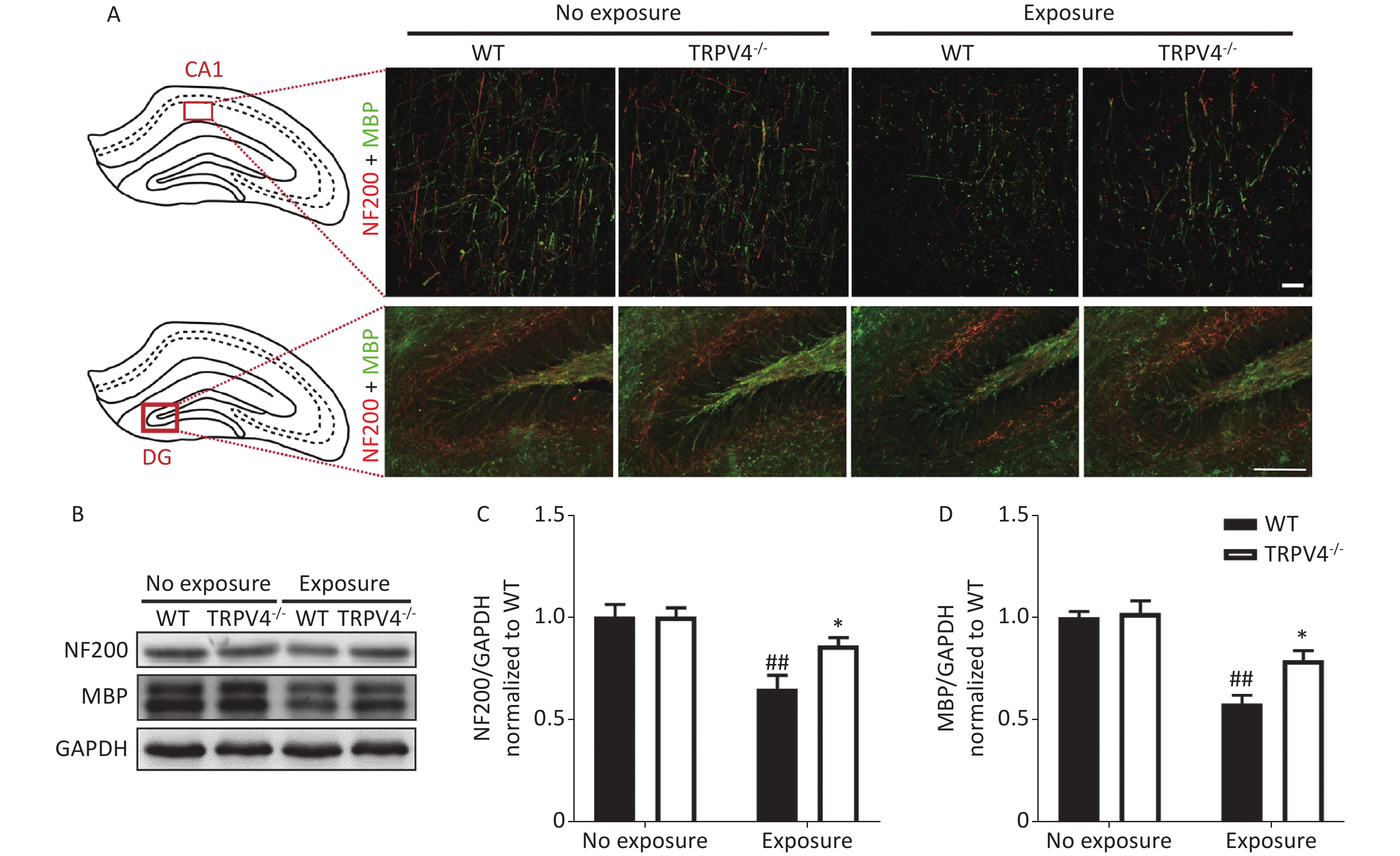
The memory function of the hippocampus is mediated by neural circuits that are composed of massive nerve fibers. NF200 is a marker for neurofilaments; the neurofilaments are believed to function primarily to provide structural support for axons. MBP is a marker for the myelin membrane and is thought to be important in the process of nerve myelination. We labeled the nerve fibers with NF200 and MBP antibodies to characterize morphological changes of neural circuits in the CA1 and DG after HI-LFN exposure. HI-LFN exposure induced disorder in the arrangement of neurofilaments of positive nerve fibers (Figure 4A) of pyramidal cells in the CA1 and granular cells in the DG. Generally, there were significant differences for HI-LFN exposure and strain in protein expression levels of NF200 [Figure 4C, two-way ANOVA, F (3, 16) = 9.812, P = 0.001. Between factors: HI-LFN, [F (1, 16) = 21.308, P < 0.001; strain, F (1, 16) = 4.064, P = 0.041] and MBP [Figure 4D, two-way ANOVA, F (3, 16) = 19.937, P < 0.001. Between factors: HI-LFN, F (1, 16) = 49.185, P < 0.001; strain, F (1, 16) = 6.297, P = 0.023]. In detail, there was significantly decreased protein expression of NF200 (Figure 4A–C, P = 0.003) and MBP (Figure 4A–B and D, P = 0.003) in the hippocampus of WT mice. Importantly, the TRPV4 knockout mice suffered less damage to neurofilament-positive nerve fibers, had lower underexpression of NF200 (Figure 4A–C, P = 0.022) and MBP (Figure 4A–B and D, P = 0.036) in the CA1 and DG areas, when compared with those in the WT group with HI-LFN exposure, indicating that TRPV4 overexpression induced neurofilament and nerve fiber injury in the hippocampus, which could mediate memory impairment after HI-LFN exposure.
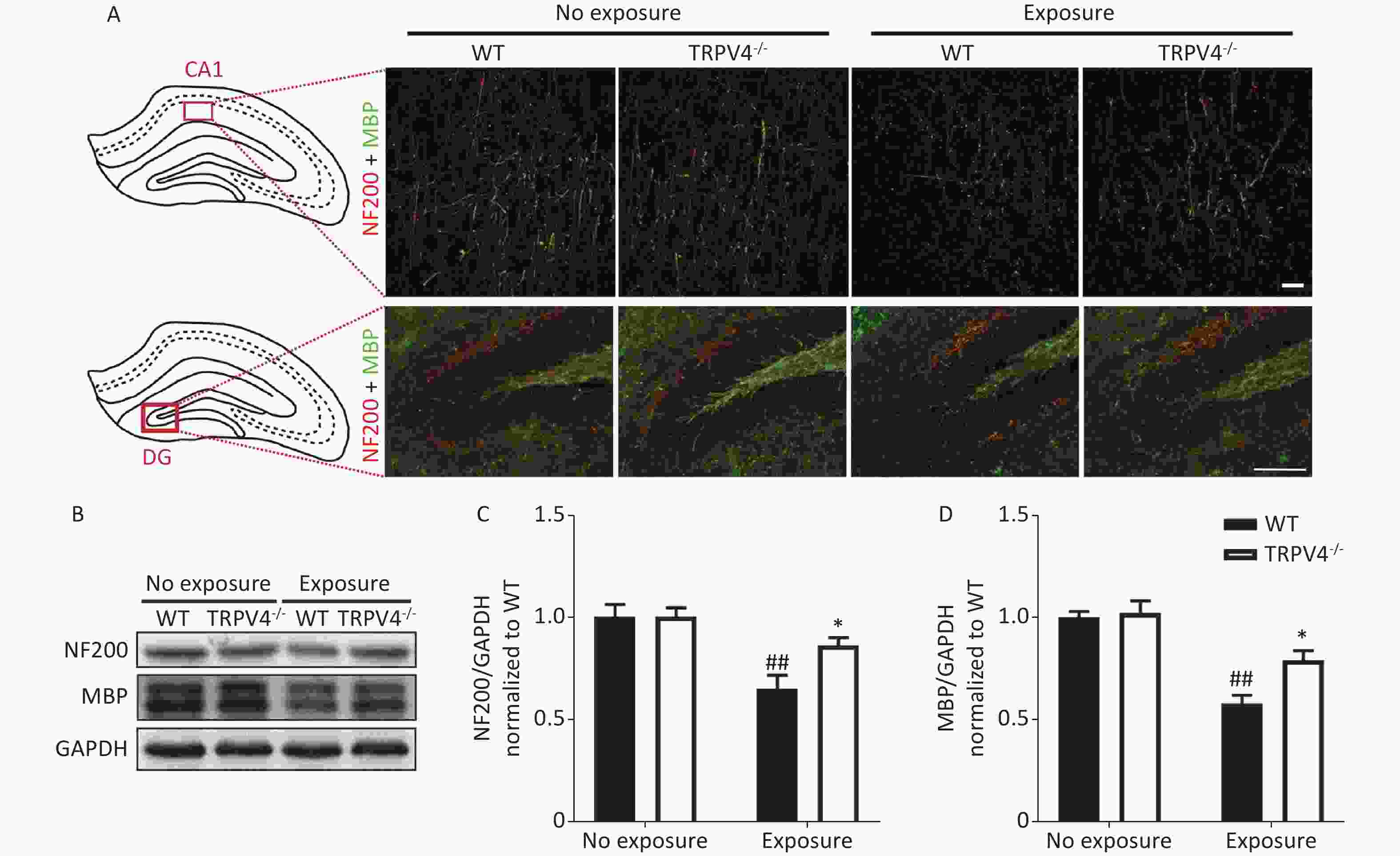
Figure 4. Transient receptor potential vanilloid subtype 4 (TRPV4) knockout alleviated the neurofilaments and nerve fiber injury in the hippocampus induced by high intensity, low frequency noise (HI-LFN) exposure. (A) Representative double immunofluorescence staining of the NF200 and myelin basic protein (MBP) of the CA1 and DG areas in wild-type (WT) and TRPV4-/- groups with or without HI-LFN exposure [scale bar: 20 µm (up) and 100 µm (down)]. (B)–(D) Western blot photographs (B) and quantitative analysis of NF200 (C), MBP (D), and GAPDH in the hippocampus of the WT and TRPV4-/- groups with or without HI-LFN exposure. Data are shown as the mean ± SEM, n = 5 for each group, two-way ANOVA followed by post-hoc Bonferroni correction. ##P < 0.01 vs. the WT-no exposure group, *P < 0.05 vs. the WT-exposure group.
-
In the present study, we investigated how TRPV4-induced neurons and nerve fiber injury in the hippocampus mediated memory impairment after HI-LFN exposure in mice. The results showed that the expression of TRPV4 was upregulated in the hippocampus after HI-LFN exposure. Furthermore, significant memory deficits and lower neurofilament-positive nerve fiber densities in the hippocampus were found in WT mice after HI-LFN exposure. However, TRPV4-/- mice showed better performance in memory tests and more integrated neurofilament-positive nerve fibers in the hippocampus after HI-LFN exposure.
Some studies have reported that noise exposure affected the physiological structure and subsequently the function of the hippocampus in non-auditory systems[32,33]. Excessive exposure to noise can impair learning and memory by impairing hippocampal neurogenesis[34], promoting neuronal death, activating glial cells[35], disrupting hippocampal neurotransmission[36], and altering the plasticity of synapses[37]. However, the hippocampus is composed of several regions and is connected to many brain regions, with thousands of nerve fibers and synaptic connections associated with these complex circuits for memory formation and storage[38]. Therefore, it seems more rational that noise exposure leads to cognitive dysfunction by impairing hippocampal neural circuits. In the present study, we found that HI-LFN exposure led to lowered densities of nerve fibers in the CA1 and DG. The densities of pyramidal neurons and granular neurons were also decreased in the CA1 and DG of the hippocampus after HI-LFN exposure. Several mnemonic functions have been shown to be closely related to the CA1 in the hippocampus, such as novelty detection [39], binding of information to spatial context[40], and working memory[41]. We found that mice developed significant mnemonic impairments in the new object recognition task and the Morris water maze test after HI-LFN exposure. Thus, we hypothesized that injury of neural circuits and neurons in the hippocampus might be the primary trigger of memory deficits after HI-LFN exposure.
Mechanotransduction is a fundamental biological process in sensing and responding to the physical environment of cells. Cells express many channel proteins in the membrane for sensing mechanical inputs, such as the extensively documented mechanically activated PIEZOs and TRPV4 ion channels[42]. These channels can be activated by a diverse range of physical forces such as vibration, stretching, or sound waves, and transduce the mechanical inputs into biochemical signals. Importantly, a previous study reported that TRPV4 was activated in glial cells in the hippocampus, which then induced learning and memory deficits after infrasound exposure, but both pharmacological and genetic inhibition of the expression of TPRV4 could rescue memory[7]. Similarly, our results showed that TRPV4 expression was significantly increased after HI-LFN exposure. Additionally, suggestive evidence showed that TRPV4 physically interacted with cytoskeleton proteins to affect mechanosensitive processes, and tissues that were abundant in the cytoskeleton were specific targets for HI-LFN[19]. The hippocampus contains extensive cytoskeletal networks, which form complex neural circuits, and neural cytoskeletons are extensively involved in the molecular-level processing of information required for learning and memory storage[31,43,44]. We therefore hypothesized that TRPV4 activation directly induced cytoskeleton injury, and then caused nerve fiber impairment and memory deficits after HI-LFN exposure. Our hypothesis was supported by the finding that neurofilaments in the hippocampus were significantly decreased after HI-LFN exposure. In addition, the more integrated structure of neurofilaments and nerve fibers in the hippocampus resulted in better performance in memory tests in TRPV4-/- mice, when compared with WT mice after noise exposure. These results suggested a crucial role of TRPV4 activation in neurofilament injury and memory impairment after HI-LFN exposure.
There were some concerns of our study. First, the hippocampus receives direct or indirect neural input from the auditory cortex[33]. Further studies therefore need to investigate the morphological changes of these circuits, to explain hippocampal injury after auditory system injury. Second, we mainly investigated whether acute exposure to HI-LFN exposure also induced memory impairment and other potential mechanisms. However, VAD needs long-term exposure in humans (≥ 10 years)[45]. Therefore, criteria for confirmation of the VAD model in mice are needed. Third, we only investigated TRPV4 up-regulation-mediated injury of neurofilaments after HI-LFN exposure using TRPV4 knockout mice. Neuroinflammation, neuronal apoptosis, and degeneration should also be studied using specific inhibitors and conditional TRPV4 knockout mice.
-
In summary, the results suggested that up-regulation of TRPV4 played an important role in nerve fiber injury and memory impairment after HI-LFN exposure, which could provide a promising therapeutic candidate for treating cognitive dysfunction of VAD patients after HI-LFN exposure.
TRPV4-induced Neurofilament Injury Contributes to Memory Impairment after High Intensity and Low Frequency Noise Exposures
doi: 10.3967/bes2023.005
- Received Date: 2022-02-12
- Accepted Date: 2022-07-11
-
Key words:
- Low frequency noise /
- Memory impairment /
- TRPV4 /
- Neurofilament /
- Nerve fibers /
- Hippocampus
Abstract:
The authors declare that they have no competing interest.
The experimental protocols were approved by the Ethics Committee of the Third Military Medical University.
AD: Alzheimer's disease; dB: decibels; CA1: cornu ammonis 1; PCL: pyramidal cell layer; Hz: Hertz; HI-LFN: high-intensity, low-frequency noise; MBP: myelin basic protein; MWM: Morris water maze; NF200: neurofilament 200; NOR: new object recognition; TRPV4: transient receptor potential vanilloid 4; VAD: Vibroacoustic disease; WT: wild type.
&These authors contributed equally to this work.
| Citation: | YANG Yang, WANG Ju, QUAN Yu Lian, YANG Chuan Yan, CHEN Xue Zhu, LEI Xue Jiao, TAN Liang, FENG Hua, LI Fei, CHEN Tu Nan. TRPV4-induced Neurofilament Injury Contributes to Memory Impairment after High Intensity and Low Frequency Noise Exposures[J]. Biomedical and Environmental Sciences, 2023, 36(1): 50-59. doi: 10.3967/bes2023.005 |







 Quick Links
Quick Links
 DownLoad:
DownLoad: