-
Retinoic acid (RA) is an active metabolite of vitamin A, closely related to the embryonic development of the brain and eye[1-3]. As an important morphogen, RA, at an optimal concentration, regulates central nervous system (CNS) development; however, RA deficiency or excess could lead to brain abnormalities. A previous study reported that cellular effects of retinoids are mediated through binding to their nuclear receptors-RA receptors (RARα, β, and γ) and retinoid receptors (RXRα, β, and γ)-which then bind to retinoic acid responsive elements (RARE) in the promoter regions of the target genes, thereby regulating various biological processes[4]. High expression of RARα in the CNS indicates its important roles in brain development[5].
Nuclear receptor subfamily 2 group E member1 (Nr2e1), also known as Tlx, is a subset of nuclear receptors highly conserved in both the vertebrates and the invertebrates[6], and is prominently expressed in the developing forebrain and eye[7-9]. In mouse, abnormal regulation of Nr2e1 can result in retinal, brain, and behavioral abnormalities[10-12]. Transgenic Nr2e1 expression could lead to an enlarged brain[13]. Moreover, introducing human Nr2e1 gene into null mice could ameliorate brain and eye abnormalities[14]. Besides, several mutations in the Nr2e1 gene are strongly associated with microcephaly[15-16]. However, the possible role of Nr2e1 in RA-induced brain abnormality is largely unknown.
In this study, the mouse model of brain abnormality was established by administering excess RA and regulation of Nr2e1 expression by RA was evaluated. The results of this study indicated that Nr2e1 might play an important role in RA-induced brain abnormality.
-
C57BL/6 mice (44007200007011, 9-10 week-old, weighing 18-23 g), were provided by Guangdong medical laboratory animal center and housed in SPF cage at an approved facility following a 12-h light/dark cycle. Mature male and female C57BL/6 mice were mated overnight, and the vaginal plug was detected the following morning; embryonic day (E) 0.5 was set at noon of the day of vaginal plug detection. On E7.5, mice were randomly divided into two groups: the control group with 9 pregnant mice fed sesame oil and the experimental group with 15 pregnant mice fed 28 mg/kg body weight RA (Sigma, USA) (dissolved in sesame oil) by gavage. Then, pregnant mice were euthanized by cervical dislocation and embryos were dissected from the decidual tissue at E8.5, E9.5, and E10.5, respectively. Embryos from three pregnant miceof the control group and five pregnant mice of the experimental group were collected at each of the time points. For further evaluation, embryos from three different pregnant dams were used for each experiment. All procedures involving animal handling were approved by the Animal Research Ethics Board of Guangdong medical laboratory animal center in China, and were in compliance with the institutional guidelines on the care of experimental animals (GDMLAC/FL01-18-B/0).
-
Primary NSCs were isolated from mouse embryos at E10.5. Briefly, the brain vesicles of mouse embryos were collected under sterile conditions, suspended as single cells, adjusted to a density of about 106 cells/mL, and added to 4 mL DMEM/F12 medium (HyClone, USA) supplemented with 2% B27 (Gibco, USA), EGF (20 ng/mL, Peprotech, USA), and bFGF (20 ng/mL, Peprotech, USA). The cells were incubated at 37 ℃/5% CO2 and passaged every 4 days[17]. NSCs were passaged at least twice before they were used for the subsequent experiments. The cells were treated with 0, 1, 5, 10, 50, and 100 μmol/L RA for 24 h, respectively.
-
Dioxygenin-labeled probes were generated from cDNA derived from the embryo brain using 5'-CGTGG ACACAAGGAAGACAA-3' as the forward primer and 5'-CAGCATTCCGGAAACTTCTC-3' as the reverse primer for Nr2e1. Then the whole mount in situ hybridization was performed as described previously[20].
-
Total RNA was extracted from the brain vesicle tissues or NSCs of mouse embryos using TRIzol method (Ambion, USA). cDNA was synthesized using Oligo (dT) primers and RevertAid First Strand cDNA Synthesis Kit (Thermo, USA). PCR was performed using PCR Master Mix (2×) (Thermo, USA); primers used for the amplification are shown in Table 1. Expression of genes was normalized to that of the Actb gene.
Table 1. Primer sequences used for RT-PCR analysis
Symbol Forward primer (5'-3') Reverse primer (5'-3') Nr2e1 GCACAAAGTCACAGGGTAATGA AGATGGAGACACGGAAATGG RARα CGACGAAGCATCCAGAAGAAC CGCAGAATCAGGATATCCAGG[18] Shh TGCTTTGTAACCGCCACTTT CTACAGGCTCCATCCTCGTG Ptch1 GACTGGGAAACTGGGAGGAT CCAAGCGGTCAGGTAGATGT Gli1 GCCACCAAGCCAACTTTATG ATTACGGTTTGCAGGTCGAG Actb GTCCCTCACCCTCCCAAAAG GCTGCCTCAACACCTCAACCC -
NSCs were resuspended in PM and adjusted to a density of 5 × 105 cells/mL; 2 mL cell suspension was added in each well and transduced with lentivirus carrying shRNA. The duplexes of Nr2e1-specific shRNA 5'-GAATTTGCCTGTCTGAAATGT-3' and a non-specific shRNA 5'-TTCTCCGAACGTGTCACGT-3' were designed, synthesized, and cloned into the pGLV3/H1/GFP+Puro vector (Sangon Biotech, China). The vector was transduced as negative control. Seventy-two hours after transduction, NSCs were harvested for RNA and protein extraction, and Nr2e1 expression at the mRNA and protein levels was detected by RT-PCR and Western blotting, respectively.
-
The proliferation of NSCs was analyzed by colony counting or CCK-8 assays. The neurospheres with diameter > 50 μm were considered to be a colony-forming unit and counted from five or six random fields[19]. For CCK-8 assay, NSCs were adjusted to a density of 2 × 105 cells/mL and 100 μL cell suspension was seeded in each well of 96-well plates. Then, 10 μL CCK-8 solution was added to each well and incubated at 37 ℃ for 4 h. The absorbance was measured at 450 nm immediately by Biotek Eon microwell spectrophotometer (Biotek, USA). Five or seven wells were included for each group.
-
NSCs were resuspended and adjusted to a density of 1 × 105 cells/mL, and 1 mL cell suspension was seeded in each well of the 24-well plate chamber slides pre-coated with polylysine (Solabio, China), and the cells were proliferated for 24 h or differentiated for 7 days by adding 5% FBS. Then NSCs were identified by the standard immunofluorescence staining. The primary antibodies used were the mouse monoclonal anti-Nestin antibody (1:500, Abcam, UK) for neural precursor cells, rabbit monoclonal anti-microtubule-associated protein 2 (Map2; 1:500; Abcam, UK) for neurons, or mouse monoclonal anti-glial fibrillary acidic protein antibody (Gfap, 1:500, Abcam, UK) for astrocytes.
-
Standard Western blotting assay was used to analyze the levels of protein extracted from cells. Thirty micrograms protein from each sample was loaded. The primary antibodies used in the immunoblots were rabbit anti-Nr2e1 monoclonal antibody (1:500, Abcam, UK) or mouse anti-beta actin monoclonal antibody (1:1000, ZSGB-BIO, China).
-
Statistical analysis was performed using GraphPad Prism 5.0. Data were expressed as mean ± SEM. Probabilities (P) were calculated by two-tailed Student's t-test for two-group comparisons or One-way ANOVA with Dunnett's test for multiple comparisons. P < 0.05 was considered statistically significant.
-
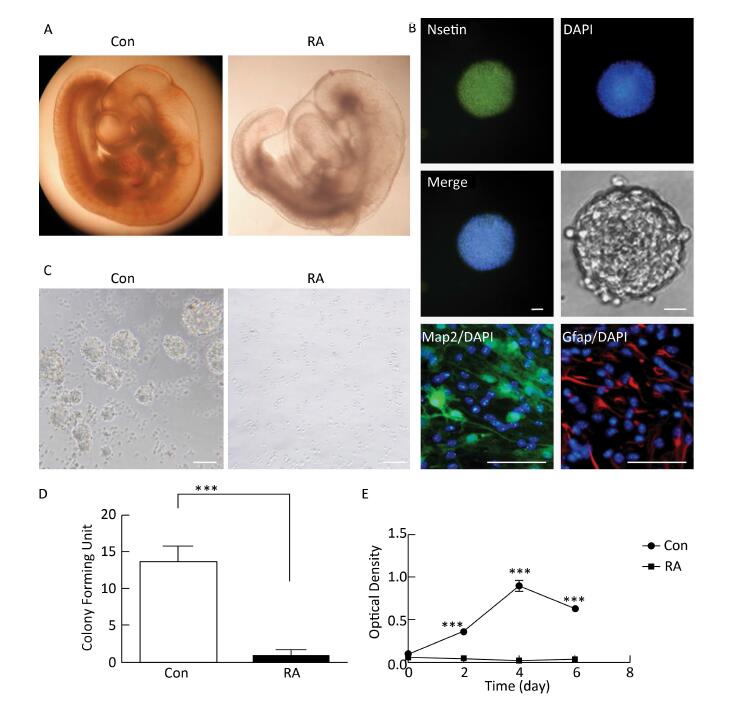
RA is an important morphogen essential for the normal development of the brain. Figure 1A indicated that the brain vesicle of control mouse embryos at E10.5 appeared as distinct bulges. Compared to normal mouse embryos, those treated with 28 mg/kg RA showed obvious overall growth retardation along with a small and hypoplastic brain vesicle. NSCs have the capacity to differentiate into the major cell types during CNS development[20]. Immunofluorescence staining results showed that NSCs isolated from the mouse embryos were positive for the marker Nestin, and were capable of differentiation into Map2-positive neurons and Gfap-positive astrocytes (Figure 1B). In addition, NSCs derived from embryos of RA-treated mice were found to be abnormal (Figure 1C). It was observed that neurospheres derived from embryos of RA-treated mice were significantly fewer with lower proliferation ability than those derived from the embryos of the control mice (Figure 1D and 1E). The results indicated that NSCs derived from embryos of RA-treated mice were impaired.
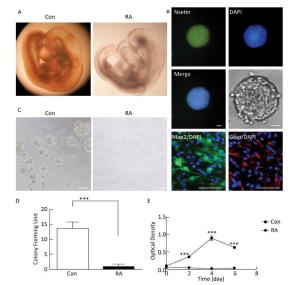
Figure 1. RA induced obvious brain abnormality and NSC impairment in mouse embryos. (A) Obvious abnormality was observed in the brain of embryos of RA-treated mice. Con: 25 ×, RA: 50 ×. (B) Identification of Nestin-, Map2-, and Gfap-positive NSCs by immunofluorescence. Scale bar in the fluorescence field: 50 μm. Scale bar in the bright field: 20 μm. (C) NSCs derived from embryos of RA-treated mice were abnormal. Scale bar: 100 μm. (D) NSCs derived from embryos of RA-treated mice formed few neurospheres compared to that formed by embryos of control mice. (E) NSCs derived from embryos of RA-treated mice had lower proliferation ability than the embryos of control mice, as detected by CCK-8 assay. The data were expressed as mean ± SEM (n = 5), analyzed by Student's t-test, ***P < 0.001 versus control.
-
High expression of Nr2e1 has been reported in the brain of the mouse embryo since E8[8]. To investigate the expression pattern of Nr2e1 in embryos of RA-treated mice, whole mount in situ hybridization and RT-PCR analysis were performed. The results of in situ hybridization showed that Nr2e1 was mainly expressed in the embryonic forebrain, and increased rapidly during the development of the embryos of control mice, but not in embryos of RA-treated mice (Figure 2A). The results of RT-PCR assay also indicated that Nr2e1 expression increased during the normal brain development; in contrast, a significant decrease in expression was observed in embryos of RA-treated mice at E9.5 and E10.5 compared to that in the embryos of the control mice (Figure 2B).
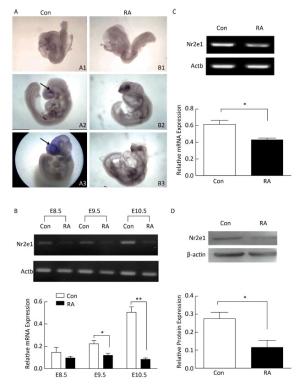
Figure 2. Nr2e1 expression was decreased in embryos and NSCs of RA-treated mice. (A) Whole mount in situ hybridization was performed to detect Nr2e1 in mouse embryos from E8.5 to E10.5. A1, A2, and A3 indicate embryos of control mice at E8.5, E9.5, and E10.5, respectively, and B1, B2, and B3 indicate embryos of RA-treated mice at E8.5, E9.5, and E10.5, respectively. Blue stain (arrowheads) represents Nr2e1 expression. A1, B1: 100 ×, A2, B2, B3: 50 ×, A3: 25 ×. (B) Temporal expression profile of Nr2e1 gene was determined by RT-PCR analysis. The Actb gene was used as a control. (C) Nr2e1 expression in NSCs was analyzed by RT-PCR analysis. The Actb gene was used as a control. (D) Nr2e1 expression in NSCs was analyzed by Western blotting. β-actin was used as a control. The data were expressed as mean ± SEM (n = 3), analyzed by Student's t-test, *P < 0.05, **P < 0.01 versus control.
In addition, Nr2e1 expression at the mRNA and protein levels was detected in NSCs isolated from embryos of control and RA-treated mice by RT-PCR and Western blotting, respectively. Interestingly, Nr2e1 was downregulated in NSCs derived from embryos of RA-treated mice (Figure 2C and 2D), consistent with the change in the protein levels in the brain of embryos of RA-treated mice, suggesting that downregulation of Nr2e1 may play an important role in RA-induced brain abnormality by regulating NSC development.
-
To elucidate the function of Nr2e1, a lentiviral vector with Nr2e1-shRNA was constructed and transduced into NSCs to knockdown endogenous Nr2e1 expression. Additionally, a non-specific shRNA was transduced to serve as a negative control. The results indicated that shRNA plasmid was transduced into NSCs effectively (Figure 3A), and Nr2e1 expression was reduced at the mRNA (Figure 3B) and protein (Figure 3C) levels. Next, theeffect of Nr2e1 on NSC growth was investigated by colony counting and CCK-8 assays. The results indicated that Nr2e1 downregulation inhibited the NSC proliferation (Figure 3D and 3E). In addition, downregulation of sonic hedgehog (Shh), Ptch1, and Gli1, the main components of Shh signaling pathway, was detected in the Nr2e1 shRNA group compared to that in the NC shRNA group (Figure 3F).
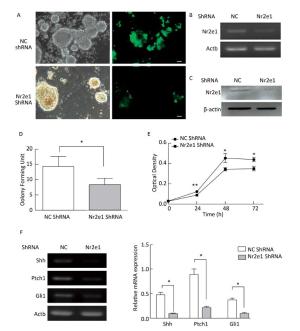
Figure 3. Knockdown of Nr2e1 affected NSC proliferation and Shh signaling pathway. (A) GFP expression images indicate that NSCs were transduced with a lentivirus carrying non-specific or Nr2e1 shRNA effectively. Scale bar: 50 μm. (B) Transduction of Nr2e1 shRNA knocked down Nr2e1 gene expression as analyzed by RT-PCR analysis. The Actb gene was used as a control. (C) Transduction of Nr2e1 shRNA knocked down Nr2e1 expression as analyzed by Western blotting. β-actin was used as a control. (D) NSCs transduced with Nr2e1 shRNA formed fewer neurospheres compared with those transduced with NC shRNA group. (E) Proliferation of NSCs transduced with Nr2e1 shRNA was lower than that of NSCs in NC shRNA group as detected by CCK-8 assay. (F) Relative mRNA levels of Shh, Ptch1, and Gli1 were downregulated after Nr2e1 knockdown as analyzed by RT-PCR analysis. The Actb gene was used as a control. The data were expressed as mean ± SEM (n = 3), analyzed by Student's t-test, *P < 0.05, **P < 0.01 versus NC shRNA.
-
To examine the regulation of Nr2e1 expression by RA, NSCs were exposed to 0, 1, 5, 10, 50, and 100 μmol/L RA for 24 h, respectively. An optimal concentration of RA was found to be essential for NSC proliferation in vitro. The results of the colony counting assay showed that the number of neurospheres increased gradually until 10 μmol/L RA; however, it decreased rapidly at 50 and 100 μmol/L RA (Figure 4A and 4B), and the results of CCK-8 assay also showed a similar tendency (Figure 4C). These results indicated that RA could promote NSC proliferation at lower concentrations, and inhibit NSC proliferation at higher concentrations. Furthermore, the change in Nr2e1 expression induced by different concentrations of RA was noted. The results of RT-PCR analysis showed that Nr2e1 expression increased when induced by low concentration of 0, 1, 5, and 10 μmol/L RA, but decreased when induced by high concentration of 50 and 100 μmol/L RA. To understand whether RARα, an important RA receptor mainly expressed in CNS tissues, is involved in Nr2e1 regulation, the mRNA expression of RARα was further detected. The expression profile of RARα was noted to be similar to that of Nr2e1 (Figure 4D).
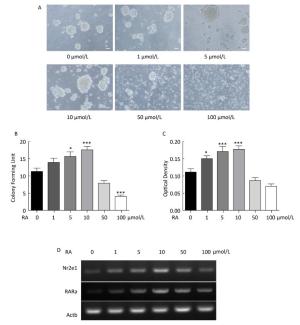
Figure 4. RA regulated Nr2e1 expression in vitro. (A) Morphology of NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA. Scale bar: 50 μm. (B) Formation of neurospheres induced by 0, 1, 5, 10, 50, and 100 μmol/L RA (n = 6). (C) Proliferation of NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA was detected by CCK-8 assay (n = 7). (D) mRNA expression of Nr2e1 and RARα in NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA was analyzed by RT-PCR analysis. The Actb gene was used as a control. The data were showed as mean ± SEM, analyzed by Dunnett's test, *P < 0.05, ***P < 0.001 versus 0 μmol/L RA.
-
Correct organization of the anterior-posterior axis is a crucial step in brain development[21]. RA is an important morphogen involved in patterning the anterior-posterior axis[22]. Maternal insufficiency or excess of RA may cause abnormalities of the CNS, indicating that a precisely regulated supply of RA is required for the normal embryo development[23]. In this study, we observed obvious brain abnormalities in mouse embryos after treatment with excess RA. Because NSCs are important in CNS development[24], we further isolated NSCs from embryos of control and RA-treated mice. After culture, NSCs derived from embryos of RA-treated mice showed lower proliferation than those derived from embryos of control mice, indicating that the former were functionally impaired.
Nr2e1, expressed mainly in the embryonic forebrain, has been reported to play an essential role in body-pattern formation during early embryogenesis[25-26]. Therefore, we hypothesized that Nr2e1 might play an important role in RA-induced brain abnormality. Here, we detected Nr2e1 expression in RA-induced brain abnormality by subjecting the mouse embryos from E8.5 to E10.5, which is the early stage of brain development, to whole mount in situ hybridization and RT-PCR analysis; furthermore, we confirmed that Nr2e1 expression was decreased in embryos of RA-treated mice. In addition, the precise control of NSC behavior is essential to the architecture and function of the CNS[27]. Nr2e1 is required for NSCs development[28]; therefore, the change in Nr2e1 expression in NSCs was next confirmed. Interestingly, we also found that Nr2e1 expression was downregulated in NSCs derived from embryos of RA-treated mice, accompanied by lower cell proliferation ability compared to that in NSCs derived from embryos of control mice.
NSCs are self-renewing multipotent progenitors important for CNS development[24]. Nr2e1 is essential in NSC maintenance and self-renewal in the embryonic brain[29]. The Shh signaling pathway also plays an important role in NSC development[30-31]. A previous study showed that Nr2e1 interacts with Shh during mouse retinal development[32]. In this study, NSCs were transduced with a lentivirus carrying Nr2e1 shRNA, and Nr2e1 reduction considerably inhibited NSC proliferation and Shh signaling pathway. Until now, Nr2e1 has been confirmed to control NSC activation, proliferation, and differentiation by modulating p53, BMP-Smad, and Wnt/β-catenin signaling pathways[33-35]. Our study suggested that Shh signaling pathway might participate in Nr2e1-regulated NSC development.
Interestingly, we observed that RA could regulate Nr2e1 expression in vitro. At a low RA concentration, Nr2e1 expression was upregulated, and at a high RA concentration, Nr2e1 expression was downregulated, which was consistent with the proliferation ability of NSCs. As reported, RA plays roles in various biological functions through different RA receptors[4]. Here, we found that the expression of the RA receptor, RARα, showed a similar trend to that of Nr2e1, indicating that RA might regulate Nr2e1 expression through RARα.
We demonstrated that Nr2e1 was downregulated after treatment with excess RA in vivo and in vitro, which is important in the development of RA-induced brain abnormality. However, the mechanism underlying regulation of Nr2e1 expression by RA is still unclear and should be elucidated in further studies.
-
The induction of brain abnormality by RA is a complex process. Our findings indicated that Nr2e1 could be regulated by RA; this insight may aid in developing a better understanding of the mechanism underlying RA-induced brain abnormality.
doi: 10.3967/bes2017.026
Nr2e1 Downregulation Is Involved in Excess Retinoic Acid-induced Developmental Abnormality in the Mouse Brain
-
Abstract:
Objective This study aimed to investigate the expression pattern and function of Nuclear receptor subfamily 2 group E member 1 (Nr2e1) in retinoic acid (RA)-induced brain abnormality. Methods The mouse model of brain abnormality was established by administering 28 mg/kg RA, and neural stem cells (NSCs) were isolated from the mouse embryo and cultured in vitro. Nr2e1 expression was detected by whole mount in situ hybridization, RT-PCR, and Western blotting. Nr2e1 function was determined by transducing Nr2e1 shRNA into NSCs, and the effect on the sonic hedgehog (Shh) signaling pathway was assessed in the cells. In addition, the regulation of Nr2e1 expression by RA was also determined in vitro. Results Nr2e1 expression was significantly downregulated in the brain and NSCs of RA-treated mouse embryos, and knockdown of Nr2e1 affected the proliferation of NSCs in vitro. In addition, a similar expression pattern of Nr2e1 and RA receptor (RAR) α was observed after treatment of NSCs with different concentrations of RA. Conclusion Our study demonstrated that Nr2e1 could be regulated by RA, which would aid a better understanding of the mechanism underlying RA-induced brain abnormality. -
Key words:
- Retinoic acid /
- Brain abnormality /
- Nr2e1 gene /
- Neural stem cells
注释:1) CONFLICT OF INTEREST: 2) AUTHORS' CONTRIBUTIONS: -
Figure 1. RA induced obvious brain abnormality and NSC impairment in mouse embryos. (A) Obvious abnormality was observed in the brain of embryos of RA-treated mice. Con: 25 ×, RA: 50 ×. (B) Identification of Nestin-, Map2-, and Gfap-positive NSCs by immunofluorescence. Scale bar in the fluorescence field: 50 μm. Scale bar in the bright field: 20 μm. (C) NSCs derived from embryos of RA-treated mice were abnormal. Scale bar: 100 μm. (D) NSCs derived from embryos of RA-treated mice formed few neurospheres compared to that formed by embryos of control mice. (E) NSCs derived from embryos of RA-treated mice had lower proliferation ability than the embryos of control mice, as detected by CCK-8 assay. The data were expressed as mean ± SEM (n = 5), analyzed by Student's t-test, ***P < 0.001 versus control.
Figure 2. Nr2e1 expression was decreased in embryos and NSCs of RA-treated mice. (A) Whole mount in situ hybridization was performed to detect Nr2e1 in mouse embryos from E8.5 to E10.5. A1, A2, and A3 indicate embryos of control mice at E8.5, E9.5, and E10.5, respectively, and B1, B2, and B3 indicate embryos of RA-treated mice at E8.5, E9.5, and E10.5, respectively. Blue stain (arrowheads) represents Nr2e1 expression. A1, B1: 100 ×, A2, B2, B3: 50 ×, A3: 25 ×. (B) Temporal expression profile of Nr2e1 gene was determined by RT-PCR analysis. The Actb gene was used as a control. (C) Nr2e1 expression in NSCs was analyzed by RT-PCR analysis. The Actb gene was used as a control. (D) Nr2e1 expression in NSCs was analyzed by Western blotting. β-actin was used as a control. The data were expressed as mean ± SEM (n = 3), analyzed by Student's t-test, *P < 0.05, **P < 0.01 versus control.
Figure 3. Knockdown of Nr2e1 affected NSC proliferation and Shh signaling pathway. (A) GFP expression images indicate that NSCs were transduced with a lentivirus carrying non-specific or Nr2e1 shRNA effectively. Scale bar: 50 μm. (B) Transduction of Nr2e1 shRNA knocked down Nr2e1 gene expression as analyzed by RT-PCR analysis. The Actb gene was used as a control. (C) Transduction of Nr2e1 shRNA knocked down Nr2e1 expression as analyzed by Western blotting. β-actin was used as a control. (D) NSCs transduced with Nr2e1 shRNA formed fewer neurospheres compared with those transduced with NC shRNA group. (E) Proliferation of NSCs transduced with Nr2e1 shRNA was lower than that of NSCs in NC shRNA group as detected by CCK-8 assay. (F) Relative mRNA levels of Shh, Ptch1, and Gli1 were downregulated after Nr2e1 knockdown as analyzed by RT-PCR analysis. The Actb gene was used as a control. The data were expressed as mean ± SEM (n = 3), analyzed by Student's t-test, *P < 0.05, **P < 0.01 versus NC shRNA.
Figure 4. RA regulated Nr2e1 expression in vitro. (A) Morphology of NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA. Scale bar: 50 μm. (B) Formation of neurospheres induced by 0, 1, 5, 10, 50, and 100 μmol/L RA (n = 6). (C) Proliferation of NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA was detected by CCK-8 assay (n = 7). (D) mRNA expression of Nr2e1 and RARα in NSCs induced by 0, 1, 5, 10, 50, and 100 μmol/L RA was analyzed by RT-PCR analysis. The Actb gene was used as a control. The data were showed as mean ± SEM, analyzed by Dunnett's test, *P < 0.05, ***P < 0.001 versus 0 μmol/L RA.
Table 1. Primer sequences used for RT-PCR analysis
Symbol Forward primer (5'-3') Reverse primer (5'-3') Nr2e1 GCACAAAGTCACAGGGTAATGA AGATGGAGACACGGAAATGG RARα CGACGAAGCATCCAGAAGAAC CGCAGAATCAGGATATCCAGG[18] Shh TGCTTTGTAACCGCCACTTT CTACAGGCTCCATCCTCGTG Ptch1 GACTGGGAAACTGGGAGGAT CCAAGCGGTCAGGTAGATGT Gli1 GCCACCAAGCCAACTTTATG ATTACGGTTTGCAGGTCGAG Actb GTCCCTCACCCTCCCAAAAG GCTGCCTCAACACCTCAACCC -
[1] Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell, 2008, 134: 921-31. doi: 10.1016/j.cell.2008.09.002 [2] Halilagic A, Ribes V, Ghyselinck NB, et al. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol, 2007, 303: 362-75. doi: 10.1016/j.ydbio.2006.11.021 [3] Gupta S, Sen J. Retinoic acid signaling regulates development of the dorsal forebrain midline and the choroid plexus in the chick. Development, 2015, 142: 1293-98. doi: 10.1242/dev.122390 [4] Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J, 1996, 10: 940-54. http://www.fasebj.org/content/10/9/940.abstract?ijkey=2e1f698ba2bad4caa3269203d1bad7bd2ba2e699k10/9/940r10/9/940 [5] Gofflot F, Chartoire N, Vasseur L, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell, 2007, 131: 405-18. doi: 10.1016/j.cell.2007.09.012 [6] Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily:the second decade. Cell, 1995, 83: 835-39. doi: 10.1016/0092-8674(95)90199-X [7] Yu RT, McKeown M, Evans RM, et al. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature, 1994, 370: 375-9. doi: 10.1038/370375a0 [8] Monaghan AP, Grau E, Bock D, et al. The mouse homolog of the orphan nuclear receptor tailless is expressed in the developing forebrain. Development, 1995, 121: 839-53. https://www.ncbi.nlm.nih.gov/pubmed/7720587# [9] Kitambi SS, Hauptmann G. The zebrafish orphan nuclear receptor genes nr2e1 and nr2e3 are expressed in developing eye and forebrain. Gene Expr Patterns:GEP, 2007, 7: 521-8. doi: 10.1016/j.modgep.2006.10.006 [10] Yu RT, Chiang MY, Tanabe T, et al. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Nat Acad Sci US A, 2000, 97: 2621-5. doi: 10.1073/pnas.050566897 [11] Monaghan AP, Bock D, Gass P, et al. Defective limbic system in mice lacking the tailless gene. Nature, 1997, 390: 515-7. doi: 10.1038/37364 [12] Roy K, Thiels E, Monaghan AP. Loss of the tailless gene affects forebrain development and emotional behavior. Physiol Behav, 2002, 77: 595-600. doi: 10.1016/S0031-9384(02)00902-2 [13] Murai K, Qu Q, Sun G, et al. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc Nat Acad Sci USA, 2014, 111: 9115-20. doi: 10.1073/pnas.1406779111 [14] Schmouth JF, Banks KG, Mathelier A, et al. Retina restored and brain abnormalities ameliorated by single-copy knock-in of human NR2E1 in null mice. Mol Cell Biol, 2012, 32: 1296-311. doi: 10.1128/MCB.06016-11 [15] Kumar RA, Leach S, Bonaguro R, et al. Mutation and evolutionary analyses identify NR2E1-candidate-regulatory mutations in humans with severe cortical malformations. Genes Brain Behavior, 2007, 6: 503-16. doi: 10.1111/gbb.2007.6.issue-6 [16] Kumar RA, McGhee KA, Leach S, et al. Initial association of NR2E1 with bipolar disorder and identification of candidate mutations in bipolar disorder, schizophrenia, and aggression through resequencing. Am J Med Genet B Neurops Psychiatr Genet, 2008, 147B, 880-9. doi: 10.1002/ajmg.b.30696 [17] Nakayama T, Momoki-Soga T, Yamaguchi K, et al. Efficient production of neural stem cells and neurons from embryonic stem cells. Neuroreport, 2004, 15: 487-91. doi: 10.1097/00001756-200403010-00021 [18] Kobayashi T, Komori R, Ishida K, et al. Tal2 expression is induced by all-trans retinoic acid in P19 cells prior to acquisition of neural fate. Sci Rep, 2014, 4: 4935. https://www.nature.com/articles/srep04935 [19] Tay Y, Zhang J, Thomson AM, et al. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature, 2008, 455: 1124-8. doi: 10.1038/nature07299 [20] Molyneaux BJ, Arlotta P, Menezes JR, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci, 2007, 8: 427-37. doi: 10.1038/nrn2151 [21] Zaki PA, Quinn JC, Price DJ. Mouse models of telencephalic development. Current opinion in genetics & development, 2003, 13: 423-37. http://www.sciencedirect.com/science/article/pii/S0959437X03000844 [22] Shimozono S, Iimura T, Kitaguchi T, et al. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature, 2013, 496: 363-6. doi: 10.1038/nature12037 [23] Ross SA, McCaffery PJ, Drager UC, et al. Retinoids in embryonal development. Physiological reviews, 2000, 80: 1021-54. [24] Temple S. The development of neural stem cells. Nature, 2001, 414: 112-7. doi: 10.1038/35102174 [25] Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci, 2003, 23: 10568-76. https://www.ncbi.nlm.nih.gov/pubmed/14627641 [26] Islam MM, Zhang CL. TLX:A master regulator for neural stem cell maintenance and neurogenesis. Biochim Biophys Acta, 2015, 1849: 210-6. doi: 10.1016/j.bbagrm.2014.06.001 [27] Klein C, Fishell G. Neural stem cells:progenitors or panacea? Dev Neurosci, 2004, 26: 82-92. [28] Shi Y, Chichung Lie D, Taupin P, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature, 2004, 427: 78-83. doi: 10.1038/nature02211 [29] Li W, Sun G, Yang S, et al. Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol, 2008, 22: 56-64. doi: 10.1210/me.2007-0290 [30] Cheng W, Yu P, Wang L, et al. Sonic hedgehog signaling mediates resveratrol to increase proliferation of neural stem cells after oxygen-glucose deprivation/reoxygenation injury in vitro. Cell Physiol Biochem, 2015, 35: 2019-32. doi: 10.1159/000374009 [31] Wang X, Zhao Y. Umbilical cord blood cells regulate the differentiation of endogenous neural stem cells in hypoxic ischemic neonatal rats via the hedgehog signaling pathway. Brain Res, 2014, 1560: 18-26. doi: 10.1016/j.brainres.2014.02.019 [32] Sehgal R, Sheibani N, Rhodes SJ, et al. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol, 2009, 332: 429-43. doi: 10.1016/j.ydbio.2009.05.579 [33] Niu W, Zou Y, Shen C, et al. Activation of postnatal neural stem cells requires nuclear receptor TLX. J Neurosci, 2011, 31: 13816-28. doi: 10.1523/JNEUROSCI.1038-11.2011 [34] Qu Q, Sun G, Li W, et al. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nature Cell Biol, 2010, 12: 31-40. doi: 10.1038/ncb2001 [35] Qin S, Niu W, Iqbal N, et al. Orphan nuclear receptor TLX regulates astrogenesis by modulating BMP signaling. Front Neurosci, 2014, 8: 74. https://www.researchgate.net/publication/261998609_Orphan_nuclear_receptor_TLX_regulates_astrogenesis_by_modulating_BMP_signaling -




 下载:
下载:



 Quick Links
Quick Links