-
Propofol is commonly used in pediatric surgery for the induction and maintenance of anesthesia as an intravenous general anesthetic by enhancing GABAA receptor activation; however, fetal and neonatal exposure to propofol can lead to short- and long-term neurotoxicity, including neuron apoptosis and neurobehavioral deficiencies in adult animals [1-3]. Because an increasing number of newborns and infants are undergoing surgery and medical examinations under general anesthesia with propofol, the safety of neonatal anesthesia with propofol has been a concern among both anesthesiologists and parents. Unfortunately, the mechanism underlying the neurologic effects of propofol has not been established.
The brain growth spurt (BGS) occurs 2 weeks after birth in rodents, with a peak on day 7 after birth[4]. Similarly, the BGS in humans occurs between the last trimester of pregnancy and 2 years of age [5]. During this period the brain develops rapidly, which depends on neural stem cell (NSC) proliferation and differentiation. NSCs persist in the hippocampal dentate gyrus (DG) subgranular zone (SGZ), where NSCs undergo cell proliferation and differentiate into functional neurons that participate in learning and memory after migration into the granule cell layer (GCL) and functional integration with the hippocampal circuit [6].
The serine/threonine protein kinase B ([PKB], also known as Akt) has an important role in cellular processes, such as cell survival, proliferation, and translational regulation [7-9]. Akt is highly expressed in the brain and neuronal survival has been reported to be controlled by the Akt signaling pathway [10]. Akt is also involved in neurogenesis within the central nervous system (CNS)[11]. Akt signaling stimulates cell proliferation via multiple downstream targets involved in cell cycle regulation [12]. p27 (kip1) is a G1 cyclin-dependent kinase (CDK) inhibitor that migrates to the nucleus and controls cyclin-CDK complex activity for proper cell cycle progression [13]. p27 is phosphorylated by Akt at three locations (Ser10, Thr187, and Thr198); phosphorylation of Thr198 promotes cytoplasmic localization of p27[14], which reduces the cell cycle inhibitory effects. Moreover, cyclin D1 inhibition mediates p27 inhibition by phosphorylation of Akt[15]. Thus, we hypothesized that propofol-induced changes in the proliferation and differentiation of NSCs in the hippocampal DG of infant rats is mediated via the Akt/p27 signaling pathway.
-
All animal experiments were approved by the Institutional Animal Care and Use Committee of Xuzhou Medical University. Pregnant Sprague-Dawley rats were housed at 24 °C on a 12 h light: 12 h dark cycle with access to food and water ad libitum. Every time after injection, chambers with computer-controlled heater plates maintained a constant temperature of 36.5 ± 1 ℃ were used to ensure rat comfort and safety.
-
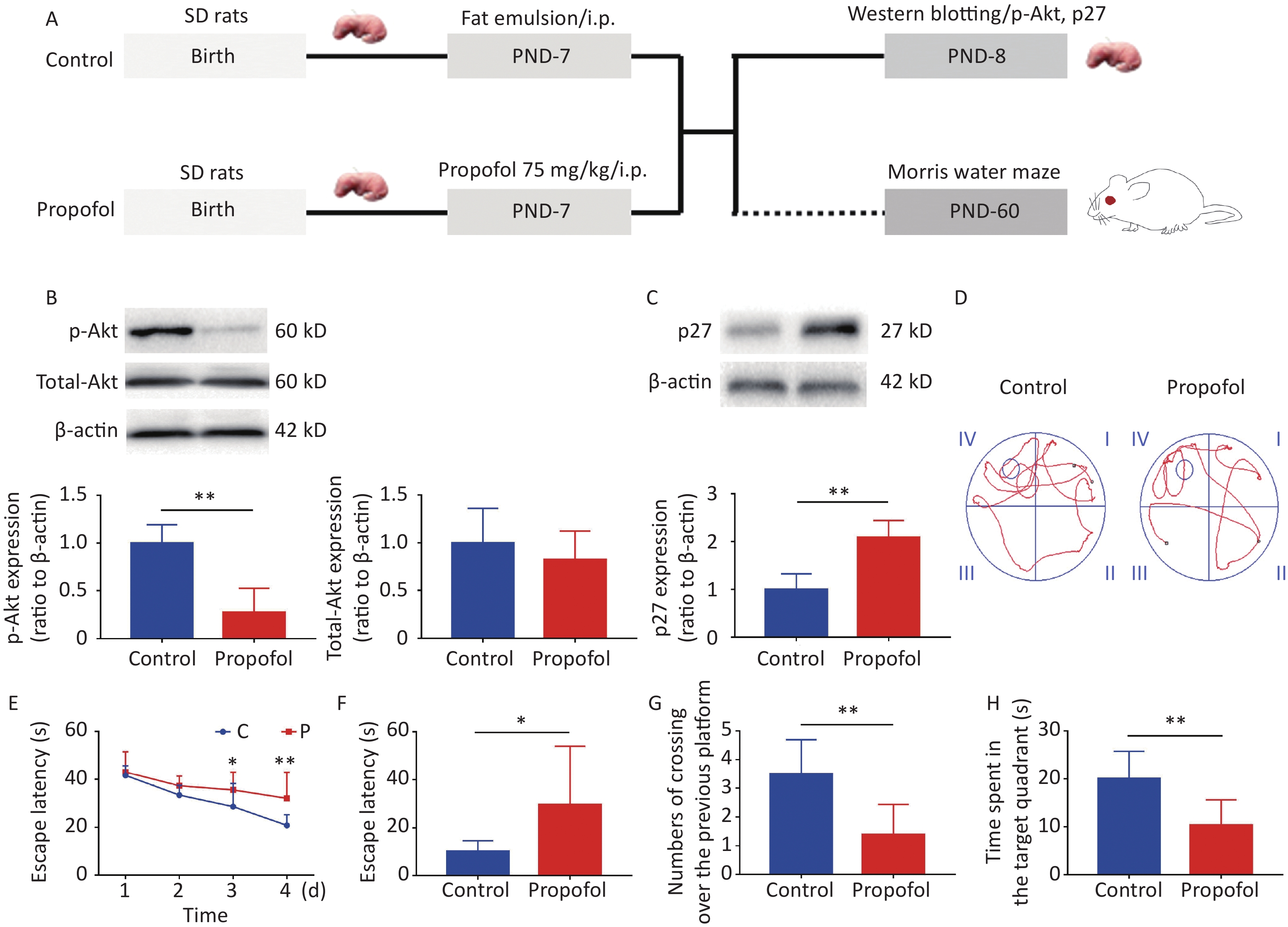
Experiment 1: To evaluate the effect of propofol on the Akt-p27 signaling pathway during the BGS, and the change in learning ability during adulthood, 7-day-old male rats (11–15 g) were divided into two groups (propofol-treated and control groups). In the propofol-treated group, The 7-day-old rats in the propofol-treated group received a single intraperitoneal (ip) injection of propofol (75 mg/kg) diluted in a fat emulsion. The rats in the control group received an equal volume of fat emulsion in a single injection (Figure 1A).
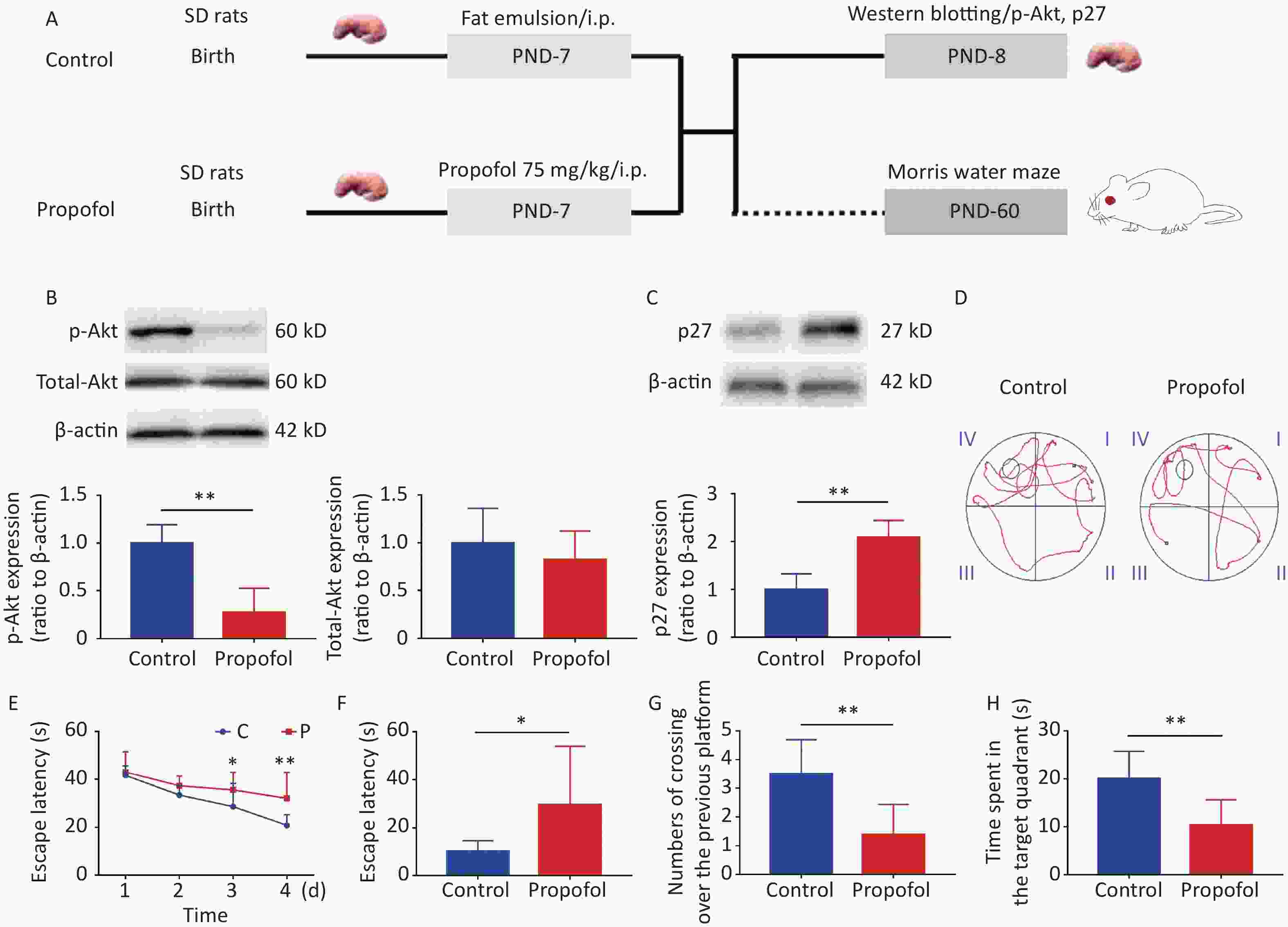
Figure 1. Effect of neonatal anesthesia with propofol on p-Akt/p27 signaling and neurocognitive function in adult rats. (A) Experimental protocol. Western blotting was performed 24 h after each treatment. Sixty-day-old rats underwent Morris water maze (MWM) testing. (B, C) The expression of p-Akt, total-Akt (B), and p27 (C) in the hippocampal DG was determined by western blotting; n = 6. (D–H) Spatial learning and memory function in adult rats was determined by the Morris water maze (MWM) test; n = 8. (D) Representative swimming paths of space exploration. (E) The escape latency of reaching the submerged platform in the training period. (F) The escape latency in the probe period. (G) The crossing over number to the previous platform within 60 s. (H) The time spent in the target quadrant. Data are presented as the mean ± SD. ip, intraperitoneal; PND, postnatal day; *P < 0.05, **P < 0.01
Experiment 2: To determine whether enhancement of Akt phosphorylation influenced the effects of propofol on neurogenesis in the DG and neurocognitive dysfunction in adulthood, male rat pups were randomly assigned to the following four groups: control group; propofol-treated group; SC79 (an Akt activator) group; and SC79 + propofol group. Rats in the SC79 and SC79 + propofol groups were pre-injected with SC79 solution into the hippocampus. Rats in the control and propofol groups received an equal volume of the solvent. Then, the rats in the propofol and SC79 + propofol groups received a single intraperitoneal injection of propofol (75 mg/kg), while the rats in the other groups were given an equal volume of fat emulsion. After injecting propofol and the fat emulsion, all experimental rats were treated with an intraperitoneal injection of 5-bromo-2-deoxyuridine [BrdU], 100 mg/kg; Sigma, St. Louis, MO, USA; (Figure 2A).
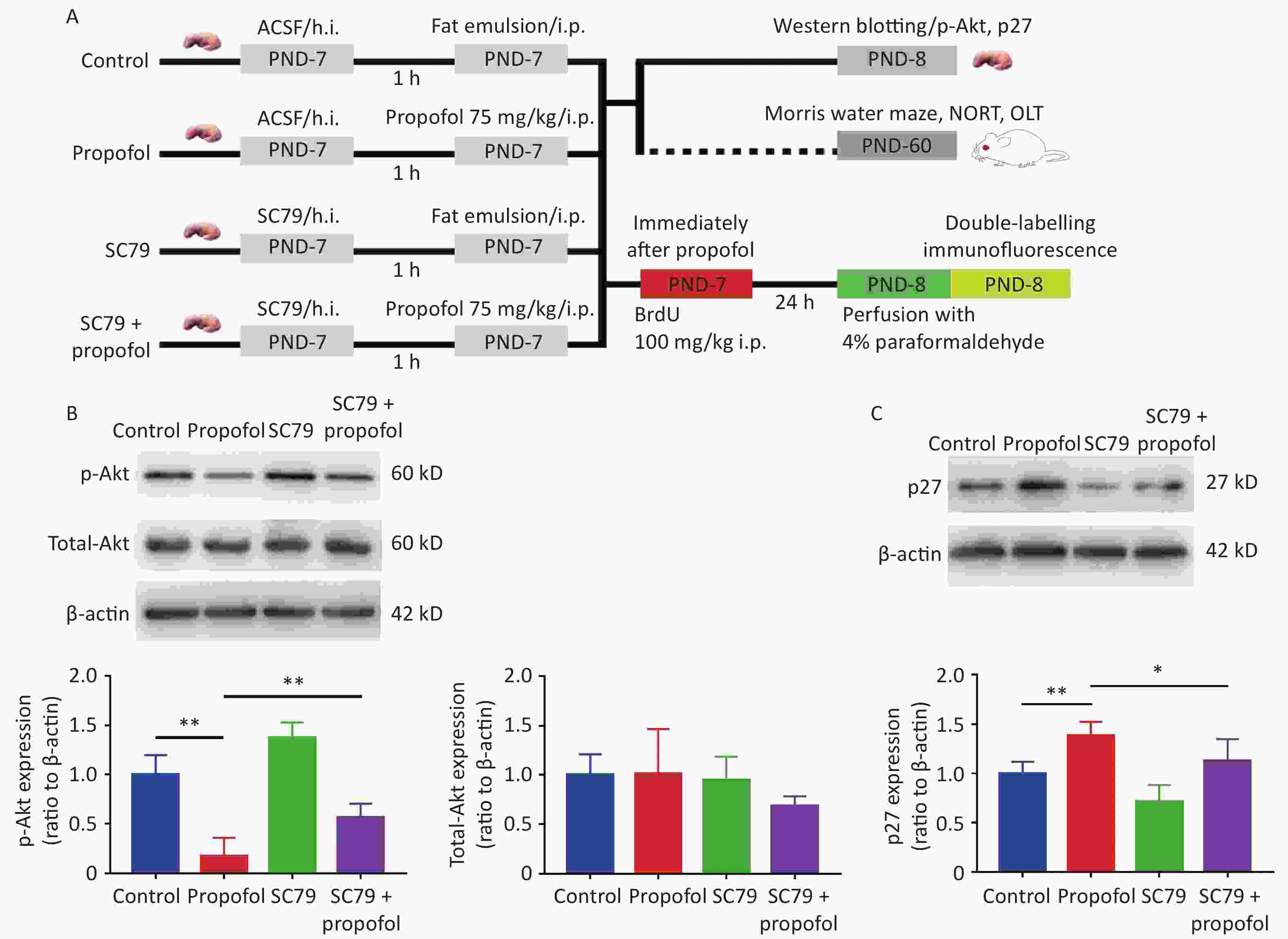
Figure 2. Effect of SC79 pre-treatment on the expression of p-Akt and p27 in the DG of rats exposed to propofol at PND-7. (A) Experimental protocol. (B, C) The expression of p-Akt, total-Akt (B), and p27 (C) in the DG was determined by western blots. Data are presented as the mean ± SD (n = 6). ACSF, artificial cerebrospinal fluid; h.i., hippocampal dentate gyrus injection; ip, intraperitoneal; PND, postnatal day; DG, hippocampal dentate gyrus; *P < 0.05, **P < 0.01.
-
SC79 was injected into the hippocampus of rats using a stereotaxic apparatus. Chloral hydrate (50 mg/kg, ip) was used for anesthesia. The head of the rat was positioned in a stereotaxic frame and a midline sagittal incision on the scalp was made with a scalpel. The layer of connective tissue of newborn rats over the skull was thin and clear, which facilitated easy location of the bregma; coordinates (2.3 mm posterior to the bregma, 2.2 mm lateral to the sagittal suture on the left and right sides, and 2.5 mm beneath the surface of the skull) were adjusted digitally. A puncture needle was used to drill a burr hole on both sides over the hippocampus. A microsyringe was filled with SC79 (20 μL). SC79 (0.045 μg/μL) dissolved in artificial cerebrospinal fluid (ACSF) with 5% DMSO was administered bilaterally (10 µL each side) at a rate of 2 µL/min. The microsyringe was then kept in place for 3−5 min to prevent solution overflow. After removing the microsyringe, we closed the skin incision using a suturing needle and surgical thread. The control animals underwent the same surgical procedure and received the same volume of ACSF with 5% DMSO, but without SC79.
-
Twenty-four hours after injecting BrdU and establishing deep anesthesia with chloral hydrate (50 mg/kg), the animals underwent cardiac perfusion with 4% paraformaldehyde after 0.9% normal saline. The brains were removed carefully, postfixed in 4% paraformaldehyde overnight, and submerged in 30% sucrose. After exposing the hippocampus, the brain was cut in coronal sections on a microtome at a thickness of 30 μm. The sections were incubated in 2N HCl at 37 °C for 30 min to expose the BrdU antigen, then thrice-washed in PBS (5 min/wash). The sections were treated with 10% goat serum in PBS with 0.3% Triton-X for 2 h at room temperature to block non-specific epitopes followed by incubation with the primary antibodies in PBS with 0.3% Triton-X at 4 °C overnight (Table 1). After 3 washes with PBS, the sections were incubated in the appropriate secondary fluorescent antibodies for 2 h at 37 °C. A skilled researcher blinded to the study observed the sections using image stacks on a laser scanning confocal microscope (Fluoview 1000; Olympus, Japan). Image-Pro Plus software was used to calculate the number of double-positive cells in the hippocampal DG.
Table 1. Primary antibodies
Antibody name Specificity Host species Dilution Company Catalog number Nestin Neural stem cells Rabbit 1:100 Santa Cruz sc-23927 Nestin Neural stem cells Mouse 1:200 CST 33475 β-tubulin III Newborn neurons Rabbit 1:200 CST 5568 GFAP Astrocytes Rabbit 1:200 CST 3670 BrdU Newly generated cells Mouse 1:100 Santa Cruz sc-32323 p-Akt Rabbit 1:1,000 CST #4060 p27 Rabbit 1:1,000 CST #3686 -
Twenty-four hours after injection of propofol, the rats were decapitated, and the hippocampal tissues were separated and removed. The tissues were homogenized together with lysis buffer and protease inhibitors, and clarified by centrifugation at 14,000 rpm for 15 min at 4 °C. The liquid supernatant represented the protein sample. Equal amounts of solubilized protein (20 μg) were subjected to sodium dodecyl sulfate 10% polyacrylamide gel, and the target proteins were transferred to nitrocellulose membranes. The blots were blocked with 5% skim milk powder diluted with PBST for 2 h at room temperature, then incubated overnight with the primary antibodies against p-Akt or p27 (Table 1). After thrice-washing with washing buffer (5 min/wash), the membranes were incubated with the appropriate secondary antibodies for 2 h at room temperature. The immunoreactive bands were detected with a chemiluminescence substrate. Image J software was used to calculate the grayscale value of the bands.
-
We used the Morris water maze (MWM) to test hippocampal-dependent spatial memory ability. All the control and propofol-treated animals were tested 2 months after treatment on day 7 after birth. A circular, black pool with a 150-cm diameter and a 50-cm depth was divided into four quadrants. The pool was filled with water at room temperature (23 ± 2) °C until the platform (10 cm diameter), which was placed inside the target quadrant, was submerged 2 cm below the surface. The platform location was kept in the same position throughout all trials. Rats were trained four times/d at 15-min intervals for 4 consecutive days during the memory acquisition trials (60 s/trial). The rats were allotted 60 s to find the platform during each trial from a random starting position; the trials were held in a dark and quiet laboratory. If the platform was located, the rat was allowed to rest on the platform for 15 s. If the rat did not locate the platform, the rat was gently guided to the platform, where the rat remained for 15 s; the escape latency was recorded as 60 s. The mean escape latency from four trials was recorded as the daily outcome. A probing trial was performed on the 5th day with the platform removed; specifically, the starting direction was opposite to the target quadrant. Each rat was allotted 60 s to probe the pool. The time required for the rat to arrive in the labeled quadrant of the previous platform, the number of crossovers to the labeled quadrant, and the time the rat spent in the target quadrant containing the previous platform within 60 s were recorded. A computerized video system was used to track the path of the rats in each trial. After every trial, rats were dried off and normothermia was assured before returning the rat to the chamber.
-
The novel object recognition test (NORT) and the object location test (OLT) are two well-established behavioral tests that are commonly used to evaluate cognitive performance, including memory and spatial cognitive ability[16]. Both of the tests are based on the intrinsic preference of animals to spend more time exploring a novel object or location than a previously encountered object or location. The tests were conducted according to the method of a previous study [17]. The results were recorded as the time spent exploring the new location or object (TN) and the old location or object (TO). The DI, which was used to evaluate the cognitive performance, was calculated as follows: (TN-TO)/(TN+TO).
-
SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) was used to analyze statistics and GraphPad Prism 5 was used to create the graphs. Differences between two groups were analyzed using the Student's t-test and differences in escape latency in the MWM test among more than two groups were analyzed using two-way ANOVA. The other data were analyzed by one-way ANOVA with least significant difference (LSD) post-hoc analysis. The numerical data are presented as means ± SD. Statistical significance was considered at a P-value < 0.05.
-
As shown in Figure 1, propofol exposure downregulated phosphorylation of Akt (Figure 1B, P < 0.01), while increasing the expression of p27 in the hippocampus (Figure 1C, P < 0.01). Moreover, rat memory and learning performance were determined at 2 months of age. During the training stage, the propofol-treated rats had longer escape latency compared with control rats on days 3 and 4 (Figure 1D–E). The escape latency was greatly enhanced during the probing stage (Figure 1F, P < 0.05), but the number of crossovers to the previous platform (Figure 1G, P < 0.01) and the time spent in the target quadrant (Figure 1H, P < 0.01) were significantly decreased in the propofol-treated rats. These results showed that propofol exposure induced marked neurocognitive deficiency, and this effect was possibly associated with inhibition of the Akt/p27 signaling pathway.
-
To further verify that propofol-induced neurocognitive deficiency was mediated by the Akt/p27 signaling pathway in the hippocampus, the Akt activator, SC79, was used to increase phosphorylation of Akt. The results suggested that the propofol-induced decrease in p-Akt (Figure 2B, P < 0.01) and the p27 increase in the hippocampal DG (Figure 2C, P < 0.05) were partially reversed by SC79, suggesting that the propofol effect was mediated via the Akt/p27 signaling pathway.
-
Our previous study showed that exposure to propofol in the infant stage inhibits hippocampal NSC proliferation in vitro [18]. To confirm the function of Akt/p27 signaling in propofol inhibition of hippocampal NSC proliferation in vivo, we enumerated the proliferating NSCs that were co-labeled with BrdU (a proliferation marker) and Nestin (an NSC marker). The representative immunofluorescence images are shown in Figure 3A. As shown in Figure 3B–C, compared to the control group, the percentage (P < 0.01) and the number (P < 0.01) of Nestin+/BrdU+ cells were significantly reduced after treatment with propofol; however, by injecting the Akt activator, SC79, the percentage (P < 0.01) and number (P < 0.05) of Nestin+/BrdU+ cells were significantly increased when compared with the propofol-treated group.

Figure 3. Effect of SC79 pre-treatment on NSC proliferation in the hippocampal dentate gyrus (DG) of neonatal rats exposed to propofol. (A) The primary antibodies against Nestin (green) and BrdU (red) were used to label the proliferation of NSCs. A laser scanning confocal microscope (magnification: × 400; the scale bar is 50 μm) was used to observe the immunoreactive cells. (B) Ratio of Nestin+/BrdU+ cells:Nestin+ cells in the DG. (C) The number of Nestin+/BrdU+ cells in the DG. (D) The number of BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
-
The role of Akt/p27 signaling in propofol inhibition of hippocampal NSC neuronal differentiation using class III beta-tubulin (a marker of early neuronal differentiation phase) [19, 20]. Figure 4A shows representative immunofluorescence images. As shown in Figure 4B–C, compared to the control group, the percentage (P < 0.05) and number (P < 0.01) of β-tubulin III+/BrdU+ cells in the propofol-treated group were reduced; however, the Akt activator, SC79, significantly reversed the propofol-induced decrease in the percentage (P < 0.05) and number (P < 0.05) of β-tubulin III+/BrdU+ cells.
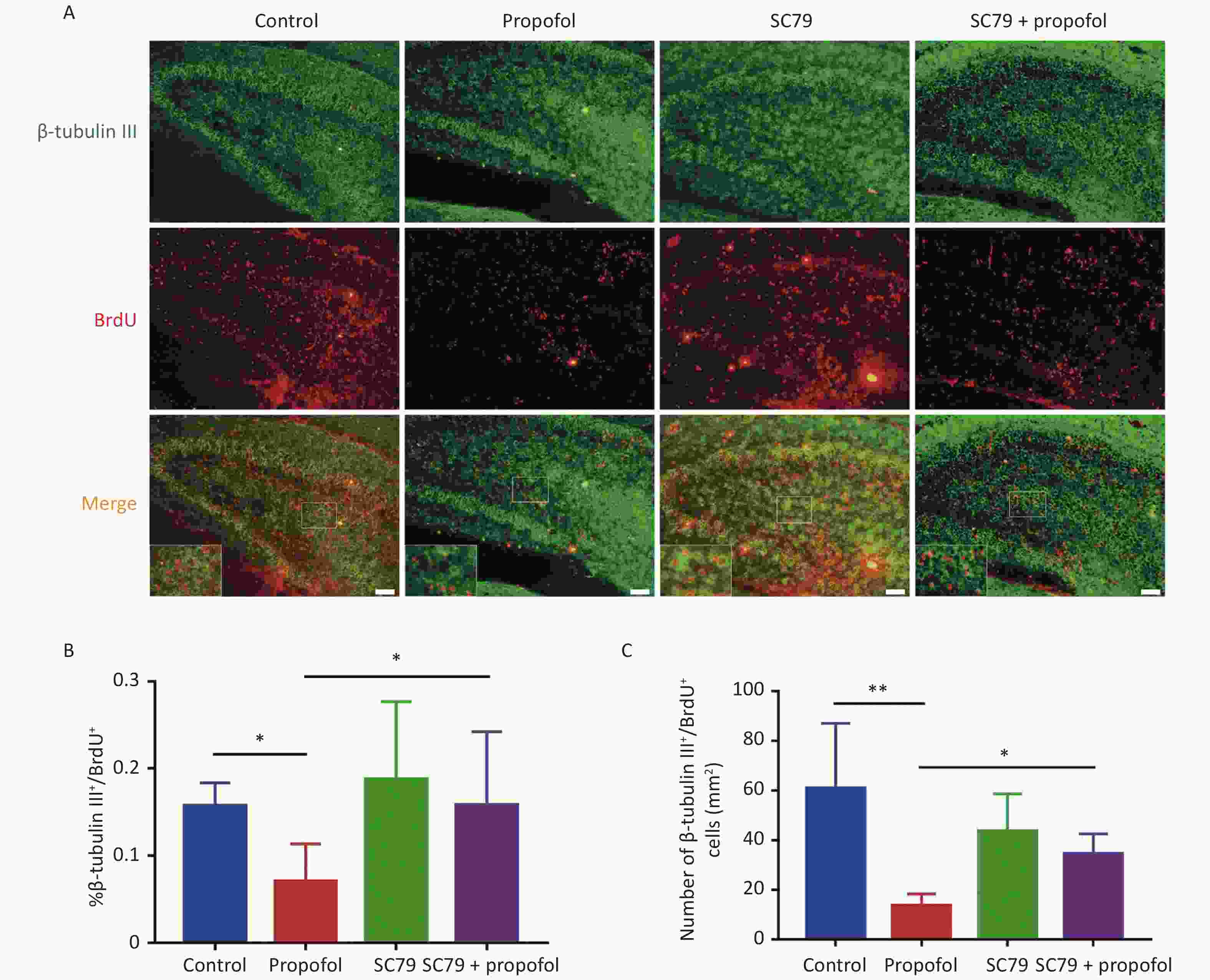
Figure 4. Effect of SC79 pre-treatment of neonatal rats exposed to propofol on neuron differentiation in the hippocampal dentate gyrus (DG). (A) Primary antibodies against β-tubulin III (green) and BrdU (red) were used to label neuronal differentiation of NSCs. A laser scanning confocal microscope (magnification: × 400; scale bar, 50 μm) was used to visualize the immunoreactive cells. (B) The ratio of β-tubulin III+/BrdU+ cells:BrdU+ cells in the DG. (C) The number of β-tubulin III+/BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
-
We further evaluated the role of Akt/p27 signaling on propofol inhibition of astrocytic differentiation among hippocampal NSCs using GFAP (a marker of astrocytes). Similar to the effect of propofol on neuronal differentiation, when compared to the control group, the percentage (P < 0.01, Figure 5B) and number (P < 0.01, Figure 5C) of GFAP+/BrdU+ cells in the propofol-treated group were significantly decreased; however, the percentage (P < 0.05, Figure 5B) and number (P < 0.05, Figure 5C) of GFAP+/BrdU+ cells were increased after injecting SC79 when compared with the propofol-treated group.

Figure 5. Effect of SC79 pre-treatment of neonatal rats exposed to propofol on the astroglia differentiation in the hippocampal dentate gyrus (DG). (A) Astrocytic differentiation of NSCs was determined by labeling with primary antibodies against GFAP (green) and BrdU (red). A laser scanning confocal microscope (magnification: × 400; scale bar, 50 μm) was used to visualize immunoreactive cells. (B) The ratio of GFAP+/BrdU+ cells:BrdU+ cells in the DG. (C) The number of GFAP+/BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
-
The MWM test was used to evaluate the impact of postnatal propofol anesthesia on recognition at 60 days of age. A typical track chart is shown in Figure 6A. During the four training days (Figure 6B), the escape latency in the propofol-treated group was longer than the control group (P < 0.01), while the escape latency in the SC79 + propofol group was shorter than the propofol group (P < 0.05). Compared to the control group, the escape latency in the memory retrieval test was significantly longer in the propofol-treatment group (P < 0.01, Figure 6C), and the number of crossovers from the previous platform site within 60 s was significantly decreased (P < 0.05, Figure 6D). In addition, the time spent in the target quadrant was significantly reduced (P < 0.05, Figure 6E); however, compared with the propofol-treated group, the rats in the SC79 + propofol group took less time to find the previous platform (P < 0.01, Figure 6C), crossed the platform more times (P < 0.05, Figure 6D), and spent longer time in the target quadrant (P < 0.05, Figure 6E).
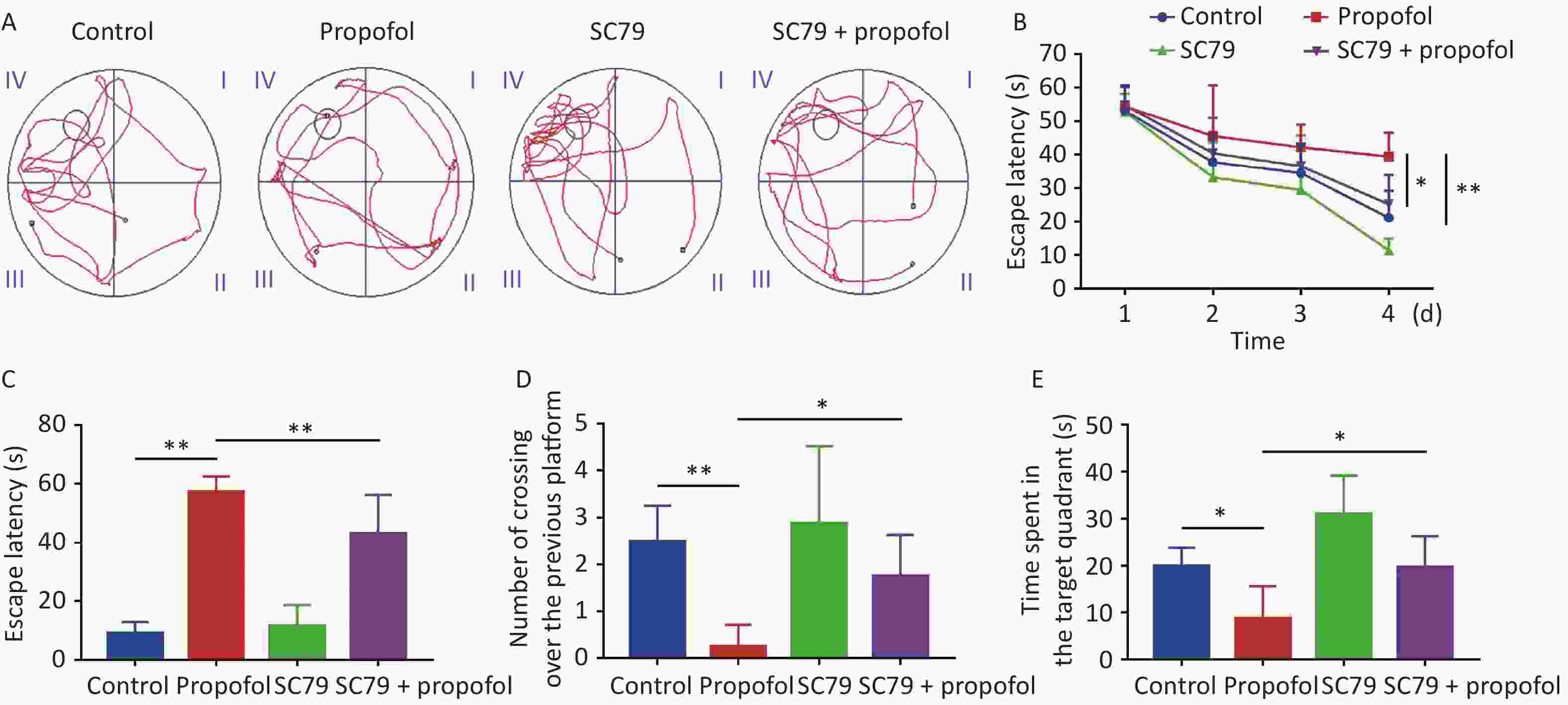
Figure 6. Effect of SC79 pre-treatment on spatial learning and memory of rats exposed to propofol at PND 7. The Morris water maze (MWM) test was performed at 60 days of age. (A) Representative swimming paths of space exploration. (B) The escape latency of reaching the submerged platform in the training period. (C) The escape latency during the probe period. (D) The number of cross-over the previous platform within 60 s. (E) The time spent in the target quadrant. PND, postnatal day. Data are presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01.
The NORT and OLT were used to evaluate the effect of neonatal exposure to propofol on recognition function at 60 days of age. Figure 7B–E show that in both NORT and OLT there were no statistically significant differences in the total object exploration time between the groups; however, the percentage of exploration time was significantly reduced in adult rats after 75 mg/kg of propofol anesthesia on PND-7 compared to control rats. Pre-treatment with the Akt activator, SC79, significantly increased the percentage of investigation time in NORT (P < 0.05, Figure 7C) and OLT (P < 0.05, Figure 7F) in adult rats after neonatal propofol exposure. These data indicated that propofol anesthesia (75 mg/kg) to 7-day-old rats led to neurocognitive dysfunction mediated by a hippocampal-break in the adult stage, which could be significantly attenuated by enhancing the phosphorylation of Akt.
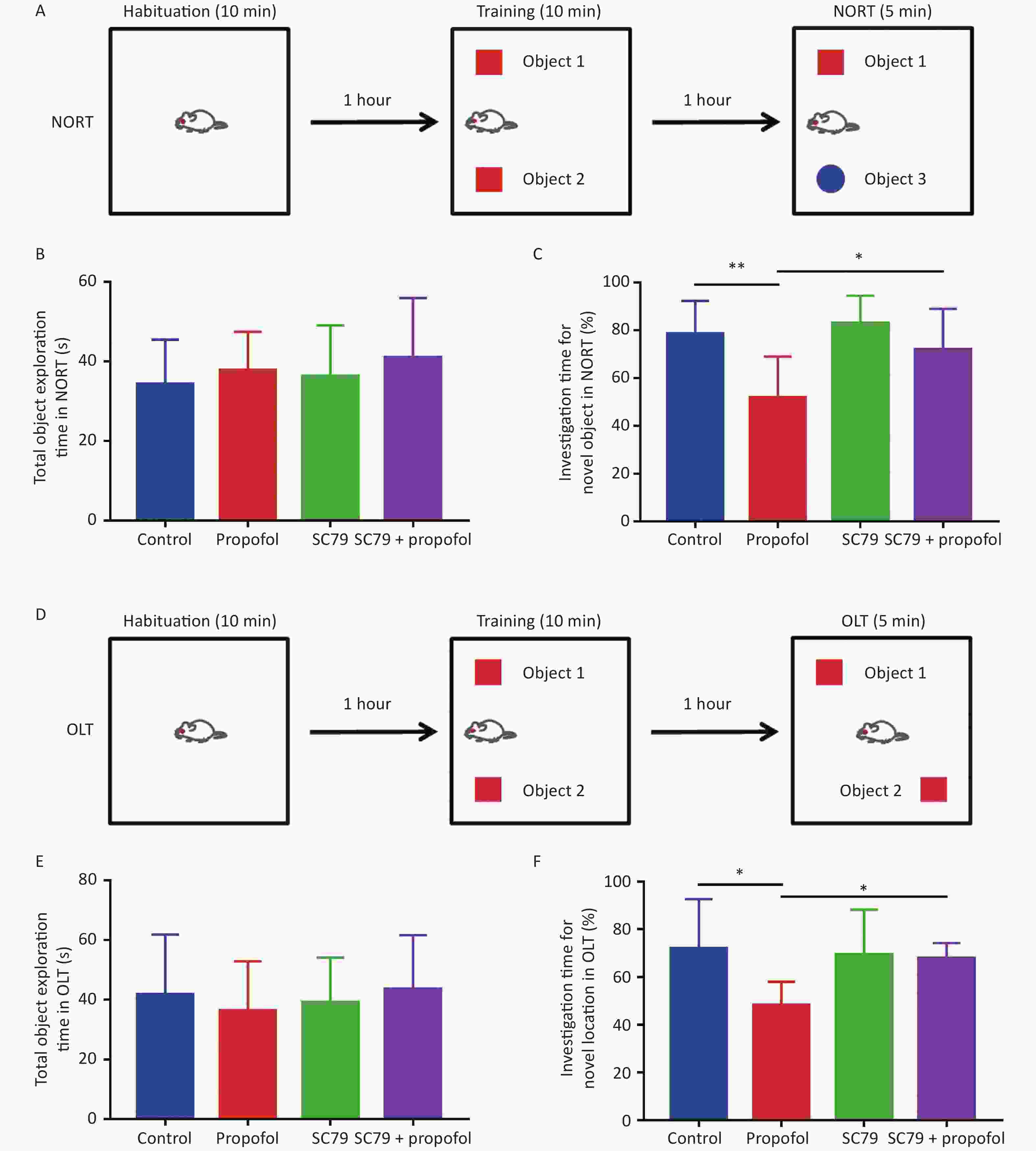
Figure 7. Effect of SC79 pre-treatment on the recognitive function of rats exposed to propofol on PND 7. Novel object recognition test (NORT) and object location test (OLT) were used to evaluate the recognition function for 60 day-old-rats. (A) The diagrammatic picture of NORT. (B) The total time to explore objects in NORT. (C) The percentage of investigation time for exploring novel objects in NORT. (D) The diagrammatic picture of OLT. (E) The total time of exploring objects in OLT. (F) The percentage of investigation time for exploring novel locations in OLT. PND, postnatal day. The data are presented as the mean ± SD (n = 8). *P < 0.05, **P < 0.01.
-
Numerous studies have shown that developing brains are sensitive to propofol exposure and exhibit neurotoxic effects[21, 22], especially the effect on the hippocampus, which is the foundation of learning and memory [23]. In the current study we confirmed that neonatal exposure to propofol induced spatial memory impairment in adult rats; however, the mechanism underlying this effect is not clear. Therefore, we investigated the mechanism underlying propofol-induced spatial memory impairment, which could explain how propofol exposure in infants causes neurocognitive function disorder and is related to the hippocampus.
A number of cellular processes could not be performed without Akt signaling pathway involvement, including neurodevelopment and neuroplasticity [24, 25]. p27 is a G1 cyclin-dependent kinase inhibitor, which is one of the downstream targets of Akt in cell cycle regulation[26]. Akt negatively regulates p27, then opposes p27-mediated G1 arrest [27]. It is not clear, however, if the Akt/p27 signaling pathway contributes to propofol-induced neurocognitive dysfunction. In the current study we showed that neonatal propofol exposure significantly decreased Akt phosphorylation, but augmented the expression of p27 in rat hippocampus, while the Akt activator, SC79, reversed the decrease in p-Akt and increase in p27. Moreover, based on the MWM test, NORT and OLT, we showed that the intra-hippocampal injection of SC79 also attenuated cognitive impairment, including memory and learning in adult rats induced by neonatal exposure to propofol. These results suggested that Akt/p27 signaling pathway had a significant impact in the hippocampal-dependent cognitive impairment induced by infant exposure to propofol.
Neurogenesis in the hippocampus DG is persistent throughout life in humans and animals [28]. Moreover, it has been shown that during childhood, the number of proliferating progenitors and newborn neurons in the DG rapidly decline [29], and a substantial number of granule cells develop after birth and reach a peak in 7 d [30]. Hence, protecting neurogenesis in the DG in neonates is critical for normal hippocampus-dependent function, including learning and memory. Neuronal or astrocytic differentiation from NSCs is a complicated process. NSCs in the hilus/ SGZ of the hippocampal DG partially begin to differentiate into neurons or astrocytes, while others retain the ability to divide. During the process of neurogenesis, the differentiation from NSCs to neurons may change dynamically with age. In the rat, the newly differentiated neurons in the DG are generated from the 14th day of gestation until the adult stage with a peak around 7 d after birth [27]. Propofol has been reported to interfere with hippocampal neurogenesis in both neonatal and adult animals [31, 32]. The present study also showed that Nestin/BrdU double-positive cells was reduced in the propofol-treated group, which indicated that the proliferation of NSCs was markedly inhibited by propofol. We also found that differentiation to astroglia and neurons was weakened because of the decreased number of double-positive cells in GFAP/BrdU and β-tubulin III/BrdU. The results were in agreement with a previous in vitro study [33]. According to our research, the change in neurogenesis in the hippocampal DG caused by postnatal exposure of propofol might be related to neurocognitive dysfunction in adults stage. Notably, only newborn neurons that undergo correct migration into the hippocampal DG GCL play a role in neurogenesis, which indicates that decreased or incorrect migration of neurons has an adverse impact on hippocampal development, then leads to hippocampal-dependent neurocognitive deficits [34]. Glial cells have an important function in guiding the migration of granule cells [28, 35]. Thus, we concluded that suppression of astrocytic differentiation from NSCs influenced neuronal migration and further suppressed neuronal differentiation, leading to the long-term hippocampal-dependent neurocognitive deficits. Therefore, further experiments are needed to prove the propofol’s influence on the neuronal migration.
How is decreased p-Akt activity relevant to cognitive impairment induced by early exposure to propofol? In this study it was shown that neonatal exposure to propofol markedly suppressed neurogenesis in the DG of the hippocampus by inhibiting Akt phosphorylation and increasing the expression of p27. Suppressed neurogenesis impairs the structural development of the hippocampus, which may lead to impairment of synaptic functional plasticity. Actually, our previous study [33] has demonstrated that neonatal exposure to propofol markedly attenuates synaptic functional plasticity evaluated by long-term potentiation (LTP) in the rat hippocampus at 60 days of age, which indicated that synaptic function in the PND-60 rat hippocampus is attenuated by neonatal propofol anesthesia. In the present study propofol significantly inhibited the proliferation of NSCs and decreased the differentiation to neurons and astroglia on PND8. Moreover, the neurocognitive function at 60 days of age was significantly weakened. The suppressive effect of neonatal propofol anesthesia on neurogenesis may be an important reason for the impaired long-term potentiation (LTP) in rat hippocampus at 60 days of age, which may be associated with learning and memory impairment in adults .
Furthermore, SC79 reverses the decrease in p-Akt and the increase in p27 induced by propofol. Downregulated p27 decreased the inhibition to CDK2, then induces proliferation of NSCs as evidenced by an increased number of Nestin/BrdU double-positive cells in the SC79 + propofol group, which was the opposite of the propofol treatment group. In addition, proliferating NSCs might continue to divide, and more NSCs differentiated into neurons and astrocytes as shown by increased number of double-positive cells of β-tubulin III/BrdU and GFAP/BrdU in SC79 + propofol group in contrast to the propofol treatment group. These results indicated that the Akt/p27 signaling pathway might be involved in hippocampal-dependent neurocognitive dysfunction caused by propofol by inhibiting the differentiation and proliferation of NSCs.
-
In summary, the present study showed that peak neurogenesis in the hippocampal DG of infants could be impeded by the exposure of propofol neonatally, contained by the inhibition of NSC proliferation, as well as astrocytic and neuronal differentiation by inhibiting Akt/p27 signaling pathway. These findings could serve as a proper explanation of the neurocognitive damage mediated in the hippocampus after propofol exposure in infants. Thus, a new viewpoint could be given up about neonatal propofol-induced neurotoxicity.
-
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MIAO Hui Hui, LIU Wen Bo, SUN Yin Ying, and JIAO Xin Hao. The first draft of the manuscript was written by SUN Yin Ying and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
-
The authors have no conflicts of interest to declare that are relevant to the content of this article.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
This study was performed in line with the principles of the Declaration of Helsinki. The experimental procedures were approved and performed in accordance with the Institutional Animal Care and Use Committee of Xuzhou Medical University.
doi: 10.3967/bes2022.040
Neonatal Exposure to Propofol Interferes with the Proliferation and Differentiation of Hippocampal Neural Stem Cells and the Neurocognitive Function of Rats in Adulthood via the Akt/p27 Signaling Pathway
-
Abstract:
Objective Neonatal exposure to propofol has been reported to cause neurotoxicity and neurocognitive decline in adulthood; however, the underlying mechanism has not been established. Methods SD rats were exposed to propofol on postnatal day 7 (PND-7). Double-immunofluorescence staining was used to assess neurogenesis in the hippocampal dentate gyrus (DG). The expression of p-Akt and p27 were measured by western blotting. The Morris water maze, novel object recognition test, and object location test were used to evaluate neurocognitive function 2-month-old rats. Results Phosphorylation of Akt was inhibited, while p27 expression was enhanced after neonatal exposure to propofol. Propofol also inhibited proliferation of neural stem cells (NSCs) and decreased differentiation to neurons and astroglia. Moreover, the neurocognitive function in 2-month-old rats was weakened. Of significance, intra-hippocampal injection of the Akt activator, SC79, attenuated the inhibition of p-AKT and increase of p27 expression. SC79 also rescued the propofol-induced inhibition of NSC proliferation and differentiation. The propofol-induced neurocognition deficit was also partially reversed by SC79. Conclusion Taken together, these results suggest that neurogenesis is hindered by neonatal propofol exposure. Specifically, neonatal propofol exposure was shown to suppress the proliferation and differentiation of NSCs by inhibiting Akt/p27 signaling pathway. -
Key words:
- Propofol /
- Neurogenesis /
- Hippocampal dentate gyrus /
- Akt /
- p27
注释: -
Figure 1. Effect of neonatal anesthesia with propofol on p-Akt/p27 signaling and neurocognitive function in adult rats. (A) Experimental protocol. Western blotting was performed 24 h after each treatment. Sixty-day-old rats underwent Morris water maze (MWM) testing. (B, C) The expression of p-Akt, total-Akt (B), and p27 (C) in the hippocampal DG was determined by western blotting; n = 6. (D–H) Spatial learning and memory function in adult rats was determined by the Morris water maze (MWM) test; n = 8. (D) Representative swimming paths of space exploration. (E) The escape latency of reaching the submerged platform in the training period. (F) The escape latency in the probe period. (G) The crossing over number to the previous platform within 60 s. (H) The time spent in the target quadrant. Data are presented as the mean ± SD. ip, intraperitoneal; PND, postnatal day; *P < 0.05, **P < 0.01
Figure 2. Effect of SC79 pre-treatment on the expression of p-Akt and p27 in the DG of rats exposed to propofol at PND-7. (A) Experimental protocol. (B, C) The expression of p-Akt, total-Akt (B), and p27 (C) in the DG was determined by western blots. Data are presented as the mean ± SD (n = 6). ACSF, artificial cerebrospinal fluid; h.i., hippocampal dentate gyrus injection; ip, intraperitoneal; PND, postnatal day; DG, hippocampal dentate gyrus; *P < 0.05, **P < 0.01.
Figure 3. Effect of SC79 pre-treatment on NSC proliferation in the hippocampal dentate gyrus (DG) of neonatal rats exposed to propofol. (A) The primary antibodies against Nestin (green) and BrdU (red) were used to label the proliferation of NSCs. A laser scanning confocal microscope (magnification: × 400; the scale bar is 50 μm) was used to observe the immunoreactive cells. (B) Ratio of Nestin+/BrdU+ cells:Nestin+ cells in the DG. (C) The number of Nestin+/BrdU+ cells in the DG. (D) The number of BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
Figure 4. Effect of SC79 pre-treatment of neonatal rats exposed to propofol on neuron differentiation in the hippocampal dentate gyrus (DG). (A) Primary antibodies against β-tubulin III (green) and BrdU (red) were used to label neuronal differentiation of NSCs. A laser scanning confocal microscope (magnification: × 400; scale bar, 50 μm) was used to visualize the immunoreactive cells. (B) The ratio of β-tubulin III+/BrdU+ cells:BrdU+ cells in the DG. (C) The number of β-tubulin III+/BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
Figure 5. Effect of SC79 pre-treatment of neonatal rats exposed to propofol on the astroglia differentiation in the hippocampal dentate gyrus (DG). (A) Astrocytic differentiation of NSCs was determined by labeling with primary antibodies against GFAP (green) and BrdU (red). A laser scanning confocal microscope (magnification: × 400; scale bar, 50 μm) was used to visualize immunoreactive cells. (B) The ratio of GFAP+/BrdU+ cells:BrdU+ cells in the DG. (C) The number of GFAP+/BrdU+ cells in the DG. Data are presented as the mean ± SD (n = 9). *P < 0.05, **P < 0.01.
Figure 6. Effect of SC79 pre-treatment on spatial learning and memory of rats exposed to propofol at PND 7. The Morris water maze (MWM) test was performed at 60 days of age. (A) Representative swimming paths of space exploration. (B) The escape latency of reaching the submerged platform in the training period. (C) The escape latency during the probe period. (D) The number of cross-over the previous platform within 60 s. (E) The time spent in the target quadrant. PND, postnatal day. Data are presented as mean ± SD (n = 8). *P < 0.05, **P < 0.01.
Figure 7. Effect of SC79 pre-treatment on the recognitive function of rats exposed to propofol on PND 7. Novel object recognition test (NORT) and object location test (OLT) were used to evaluate the recognition function for 60 day-old-rats. (A) The diagrammatic picture of NORT. (B) The total time to explore objects in NORT. (C) The percentage of investigation time for exploring novel objects in NORT. (D) The diagrammatic picture of OLT. (E) The total time of exploring objects in OLT. (F) The percentage of investigation time for exploring novel locations in OLT. PND, postnatal day. The data are presented as the mean ± SD (n = 8). *P < 0.05, **P < 0.01.
Table 1. Primary antibodies
Antibody name Specificity Host species Dilution Company Catalog number Nestin Neural stem cells Rabbit 1:100 Santa Cruz sc-23927 Nestin Neural stem cells Mouse 1:200 CST 33475 β-tubulin III Newborn neurons Rabbit 1:200 CST 5568 GFAP Astrocytes Rabbit 1:200 CST 3670 BrdU Newly generated cells Mouse 1:100 Santa Cruz sc-32323 p-Akt Rabbit 1:1,000 CST #4060 p27 Rabbit 1:1,000 CST #3686 -
[1] Li J, Xiong M, Nadavaluru PR, et al. Dexmedetomidine attenuates neurotoxicity induced by prenatal propofol exposure. J Neurosurg Anesthesiol, 2016; 28, 51−64. doi: 10.1097/ANA.0000000000000181 [2] He L, Wang X, Zheng S. Inhibition of the electron transport chain in propofol induced neurotoxicity in zebrafish embryos. Neurotoxicol Teratol, 2020; 78, 106856. doi: 10.1016/j.ntt.2020.106856 [3] Creeley C, Dikranian K, Dissen G, et al. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth, 2013; 110 Suppl 1, i29-38. [4] Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate, 1977; 32, 166−76. doi: 10.1159/000241012 [5] Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev, 1979; 3, 79−83. doi: 10.1016/0378-3782(79)90022-7 [6] Abbott LC, Nigussie F. Adult neurogenesis in the mammalian dentate gyrus. Anat Histol Embryol, 2020; 49, 3−16. doi: 10.1111/ahe.12496 [7] Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci, 2004; 29, 233−42. doi: 10.1016/j.tibs.2004.03.006 [8] Li HY, Wang R. Blocking SIRT1 inhibits cell proliferation and promotes aging through the PI3K/AKT pathway. Life Sci, 2017; 190, 84−90. doi: 10.1016/j.lfs.2017.09.037 [9] Wei L, Yi Z, Guo KY, et al. Long noncoding RNA BCAR4 promotes glioma cell proliferation via EGFR/PI3K/AKT signaling pathway. J Cell Physiol, 2019; 234, 23608−17. doi: 10.1002/jcp.28929 [10] Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol, 2001; 11, 297−305. doi: 10.1016/S0959-4388(00)00211-7 [11] Xu LX, Ji ZY, Guo LT, et al. Saikosaponin-D-mediated downregulation of neurogenesis results in cognitive dysfunction by inhibiting Akt/Foxg-1 pathway in mice. Toxicol Lett, 2018; 284, 79−85. doi: 10.1016/j.toxlet.2017.11.009 [12] Storm MP, Kumpfmueller B, Thompson B, et al. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: identification of novel regulators of pluripotency. Stem Cells, 2009; 27, 764−75. doi: 10.1002/stem.3 [13] Sekimoto T, Fukumoto M, Yoneda Y. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27Kip1. EMBO J, 2004; 23, 1934−42. doi: 10.1038/sj.emboj.7600198 [14] Fujita N, Sato S, Katayama K, et al. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem, 2002; 277, 28706−13. doi: 10.1074/jbc.M203668200 [15] Medema RH, Kops GJPL, Bos JL, et al. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature, 2000; 404, 782−7. doi: 10.1038/35008115 [16] Denninger JK, Smith BM, Kirby ED. Novel object recognition and object location behavioral testing in mice on a budget. J Vis Exp, 2018; 141. [17] Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1:Behavioral data. Behav Brain Res, 1988; 31, 47−59. doi: 10.1016/0166-4328(88)90157-X [18] Hu Q, Huang L, Zhao C, et al. Ca2+-PKCα-ERK1/2 signaling pathway is involved in the suppressive effect of propofol on proliferation of neural stem cells from the neonatal rat hippocampus. Brain Res Bull, 2019; 149, 148−55. doi: 10.1016/j.brainresbull.2019.04.005 [19] Katsetos CD, Del Valle L, Geddes JF, et al. Localization of the neuronal class III β-tubulin in oligodendrogliomas: comparison with Ki-67 proliferative index and 1p/19q status. J Neuropathol Exp Neurol, 2002; 61, 307−20. doi: 10.1093/jnen/61.4.307 [20] Jirásek T, PísaríkováE, Viklický V, et al. Expression of class III beta-tubulin in malignant epithelial tumours: an immunohistochemical study using TU-20 and TuJ-1 antibodies. Folia Histochem Cytobiol, 2007; 45, 41−5. [21] Bercker S, Bert B, Bittigau P, et al. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res, 2009; 16, 140−7. doi: 10.1007/s12640-009-9063-8 [22] Berndt N, Rösner J, Haq RU, et al. Possible neurotoxicity of the anesthetic propofol: evidence for the inhibition of complex II of the respiratory chain in area CA3 of rat hippocampal slices. Arch Toxicol, 2018; 92, 3191−205. doi: 10.1007/s00204-018-2295-8 [23] Logan S, Jiang CS, Yan YS, et al. Propofol alters long non-coding rna profiles in the neonatal mouse hippocampus: Implication of novel mechanisms in anesthetic-induced developmental neurotoxicity. Cell Physiol Biochem, 2018; 49, 2496−510. doi: 10.1159/000493875 [24] Iaconelli J, Lalonde J, Watmuff B, et al. Lysine Deacetylation by HDAC6 regulates the kinase activity of AKT in human neural progenitor cells. ACS Chem Biol, 2017; 12, 2139−48. doi: 10.1021/acschembio.6b01014 [25] Fares J, Diab ZB, Nabha S, et al. Neurogenesis in the adult hippocampus: history, regulation, and prospective roles. Int J Neurosci, 2019; 129, 598−611. doi: 10.1080/00207454.2018.1545771 [26] Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol Cell Biol, 2000; 20, 9138−48. doi: 10.1128/MCB.20.24.9138-9148.2000 [27] Liang JY, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med, 2002; 8, 1153−60. doi: 10.1038/nm761 [28] Seri B, García-Verdugo JM, McEwen BS, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci, 2001; 21, 7153−60. doi: 10.1523/JNEUROSCI.21-18-07153.2001 [29] Sorrells SF, Paredes MF, Cebrian-Silla A, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature, 2018; 555, 377−81. doi: 10.1038/nature25975 [30] Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol, 1990; 301, 365−81. doi: 10.1002/cne.903010304 [31] Huang J, Jing S, Chen X, et al. Propofol administration during early postnatal life suppresses hippocampal neurogenesis. Mol Neurobiol, 2016; 53, 1031−44. doi: 10.1007/s12035-014-9052-7 [32] Kim JL, Bulthuis NE, Cameron HA. The effects of anesthesia on adult hippocampal neurogenesis. Front Neurosci, 2020; 14, 588356. doi: 10.3389/fnins.2020.588356 [33] Jiang QL, Wang YW, Shi XY. Propofol inhibits neurogenesis of rat neural stem cells by upregulating MicroRNA-141-3p. Stem Cells Dev, 2017; 26, 189−96. doi: 10.1089/scd.2016.0257 [34] Meyer KD, Morris JA. Disc1 regulates granule cell migration in the developing hippocampus. Hum Mol Genet, 2009; 18, 3286−97. doi: 10.1093/hmg/ddp266 [35] Rickmann M, Amaral DG, Cowan WM. Organization of radial glial cells during the development of the rat dentate gyrus. J Comp Neurol, 1987; 264, 449−79. doi: 10.1002/cne.902640403 -




 下载:
下载:



 Quick Links
Quick Links