-
With the increasing development of numerous wireless applications, microwave usage has become more prominent in our daily lives[1]. Microwaves are non-ionizing electromagnetic waves and are currently exploited for a variety of applications such as radar, communication, and medical instruments[1]. Despite the benefits of these uses, they have resulted in a significant increase in human exposure to microwave radiation, resulting in questions about the safety of this technology[2]. Evidence has shown that microwaves, especially high power microwaves (HPMs), can induce damaging effects in the central nervous system (CNS)[3-9]. The hippocampus, the area of the brain involved in long-term processing of information about sound, light, and taste, plays a major role in declarative memory. In the mammalian brain, neurogenesis continues in certain regions, namely the hippocampal dentate gyrus (DG) and olfactory bulb. New neurons are generated in the subgranular zone (SGZ) of the DG and integrate into the existing hippocampal circuitry, where they play a major role in learning and spatial memory. The hippocampus is thought to be particularly vulnerable to microwave radiation, which potentially results in spatial learning and memory deficits[3]. Neuronal degeneration and enlarged perivascular spaces have been noted in the hippocampus of microwave-exposed mice. Furthermore, transmission electron microscopy has shown that mitochondria became swollen and cristae become disordered following microwave exposure. In addition, the rough endoplasmic reticulum exhibits sacculated distension, and a decrease in the quantity of synaptic vesicles was noted. Furthermore, the hippocampus can be injured by long-term microwave exposure, which may result in cognitive impairments due to neurotransmitter disruption. Numerous studies have indicated that hippocampus-dependent spatial learning and memory deficits occurring after microwave exposure were associated with the impairment of hippocampal neurotransmission. For example, Wistar rats, rat hippocampal synaptosomes, and differentiated (neuronal) PC12 cells were exposed to microwave radiation for 5 min at a mean power density of 30 mW/cm2, resulting in a decrease in spatial memory performance[10]. In addition, rats exposed at 10 mW/cm2 and 50 mW/cm2 microwave radiation showed significant deficits in spatial learning and memory at 6 h, 1 d, and 3 d after exposure[11]. Chronic low power-density microwave exposure can also induce spatial memory deficits[12]. However, treatments for HPM-induced hippocampal damage have not yet been developed.
Neural stem cells (NSCs)/progenitor cells are self-renewing cells that can differentiate into astrocytes, oligodendrocytes, and neurons[13, 14]. Substantial evidence suggests that adult NSCs persists in two germinal regions in the adult mammalian brain, namely the subventricular zone of the lateral ventricles and the SGZ of the hippocampal DG[15]. The neuronal differentiation of NSCs in the adult hippocampus is associated with learning and memory formation[16]. Therefore, activation of endogenous NSCs may be a potential treatment strategy for hippocampal damage.
Wnt signaling plays an important role in the proliferation of neural progenitor cells during the development of the CNS, and is also involved in dendrite development, synaptogenesis, and the establishment of axons[17]. Recent studies have reported that Wnt signaling plays an important role in the regulation of several aspects of cortical development, such as neural progenitor proliferation and dendritogenesis[18]. Wnt signaling is essential for determining cellular specificity in the hippocampal DG[19]. β-catenin is an important component of the Wnt signaling pathway. In the canonical Wnt signaling pathway, the Wnt peptide interacts with its membrane receptors of low density lipoprotein receptor-related protein and Frizzled[11]. Overexpression of β-catenin can affect the development of the mammalian cortex[20], and a number of studies have demonstrated that Wnt/β-catenin function was crucial for the correct development of the hippocampus[21].
The 20-hydroxyecdysone (20E) is an ecdysteroid hormone produced by insects and some plants, such as Achyranthes, Cyanotis, and Stemmacantha uniflora. It is thought to have a number of beneficial pharmacological properties in mammals, involving stimulation of protein synthesis, inhibition of apoptosis, and even regulation of the fate of some stem cells[22-24]. However, it is unclear whether 20E also affects the proliferation and differentiation of NSCs.
The present study therefore determined whether 20E regulated the fate of adult hippocampal NSCs after HPM radiation exposure, both in vitro and in vivo. Overall, this study investigated if 20E treatment attenuated learning and memory deficits in HPM radiation exposed-rats.
-
All animal procedures were performed in accordance with national regulations regarding the use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of the Third Military Medical University, Chongqing, China (Approval No. AMUWEC20212008), which followed the regulations of the China Laboratory Animal Guidelines (RB/T 019-2019 Edition). All efforts were made to minimize animal suffering and the number of animals used.
A total of 60 male 2-month-old Sprague-Dawley rats from the Experimental Animal Center of the Third Military Medical University, Chongqiong, China, weighing 180−220 g were randomly divided into three groups: normal control (not treated with radiation or 20E), the radiation group, and the radiation + 20E treatment group (n = 20 per group). Four animals per cage were placed in a room with an alternating 12-h light/dark cycle at a temperature of 22−25 °C and humidity of 50%−60%.
-
Rats in the radiation and radiation + 20E treatment groups were exposed to HPM radiation. Briefly, the microwave source, a klystron amplifier model JD 2000 (Wuhu Zhongdian Zhaowei Electronics, Anhui, China), was capable of generating pulsed microwaves at S-band with the frequency of 9.37 GHz ± 40 MHz, a pulse repetition frequency of 1,875 Hz, and a pulse width of 0.6 ± 0.1 μS. Microwave energy was transmitted by a rectangular waveguide and A16-dB standard-gain horn antenna to a standard echoless dark chamber (length = 7 m/width = 6.5 m/height = 4 m) with minimal reflected waves. The interior walls of the chamber were covered with 500 mm and 300 mm pyramidal microwave absorbers to minimize reflections. The emitted power was measured with a semiconductor. The distance from the antenna to the top of the animal cage was 1.4 m, the rats were fixed in an organic glass box, and the radiation table was rotated to assure whole body radiation. The rats were exposed to radiation with an average power density of 30 mW/cm2, an exposure frequency of 9.37 GHz ± 40 MHz, a pulse repetition frequency of 1,875 Hz, and a pulse width of 0.6 ± 0.1 μS for 2 min in a single day. In addition, the specific absorption rate (SAR) calculation was based on the formula: SAR = σE2/ρ (W/kg)[25]. In the formula, E is the electric field strength (V/m), σ (sigma) is the electric conductivity (S/m), and ρ (rho) refers to the sample density (kg/m3). The SAR of the rats was calculated by measuring anal temperatures before and immediately after HPM irradiation using a thermocouple point thermometer (Beijing Xuyang Tiandi Technology Co., LTD, Beijing, China). The average SAR was determined to be 6.08 W/kg. Animals in the control group were also placed in the box but were not exposed to microwave radiation.
Rats in the treatment group received an intraperitoneal injection of 4 mg/kg 20E (CAS No. 5289-74-7, purity: 99.25%; Selleck Chemicals, Houston, TX, USA) twice daily for 7 consecutive d after radiation exposure. Animals in the control and radiation groups were intraperitoneally injected with saline, the solvent in which 20E was dissolved.
Cell proliferation after radiation was assessed by evaluating the cells that incorporated the thymidine analog, 5-bromo-20-deoxyuridine (BrdU). In rats of all three groups, 100 mg/kg BrdU was injected intraperitoneally once daily for 3 consecutive days starting 8 days after radiation exposure.
-
Primary culture of adult rat hippocampal NSCs was performed as previously described with slight modifications[26]. Seven days after radiation exposure, the rats were anesthetized by a 2% isoflurane/air mixture (1−21/min), and perfused with artificial cerebrospinal fluid (Shanghai Maokang Biotechnology, Shanghai, China). The hippocampus was harvested and immediately cut into 1−2 mm3 pieces. After washing three times with D-Hank’s solution, the dissected tissue pieces were suspended in 0.125% trypsin/0.02% EDTA solution. Then, the tissue pieces were incubated for 10 min at 37 °C and triturated using a 5 mL pipette until the cell suspension was free of large tissue pieces. The remaining tissue pieces were removed via filtration using a copper mesh. The filtered cell suspension was centrifuged at 1,000 ×g for 3 min, and the trypsin-EDTA solution was gently removed. The cells were then resuspended in DMEM/F-12 medium containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA) and washed three times. After aspirating the medium, the cells were resuspended in serum-free DMEM/F-12 medium containing 20 ng/mL basic fibroblast growth factor and 20 ng/mL epidermal growth factor (PeproTech, Windsor, NJ, USA). For clonal culture, the suspended cells were seeded at 5 × 104 cells/cm2 on poly-L-ornithine hydrobromide (Sigma-Aldrich)/laminin (Invitrogen, Carlsbad, CA, USA)-coated dishes. Half of the medium in the cultures was replaced with fresh medium every 2−3 days. After 12−14 days, the clones reached the target size (diameter > 50 μm).
To examine the self-renewal and differentiation potential of these cells, primary neurospheres were dissociated and subcultured in serum-free proliferation medium or differentiation medium (containing 5% FBS without growth factors). The more abundant neurospheres were passaged in proliferation medium and stained with rabbit anti-nestin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). In the differentiation medium, neurospheres exhibited adherence and sprouted differentiated neural cells. These were characterized using antibodies against glial fibrillary acidic protein (GFAP), a specific marker of astrocytes, and class III β-tubulin (Tuj1), a specific marker of neurons.
-
The neurospheres were separated using 0.125% trypsin/0.02% EDTA for 1 min and then gently triturated using a glass pipette. Individual NSCs were seeded at 5 × 103
cells/well in 96-well plates and cultured in 100 μL proliferation medium. Four final concentrations (1, 10, 100, and 1,000 μmol/L) of 20E were each applied to six wells of the subcultured cells. For the proliferation assays, the number of clones in five random fields was counted and total cell viability was detected using Cell Counting Kit-8 (CCK-8; Dojindo, Mashiki, Japan), according to the manufacturer’s instructions. Briefly, after culturing for 5 days, 20 μL of CCK-8 buffer was added to each well, followed by incubation for another 2 h. The optical density values at 490 nm were measured using a microplate reader (Thermo MK3; Thermo, Vantaa, Finland). Each OD value reading was obtained in triplicate. For the differentiation assay, the suspended cells of primary neurospheres were seeded with differential media at 5 × 104 cells/well in 6-well plates. The next day, differential medium containing 100 μmol/L 20E or 100 μmol/L 20E + 150 ng/mL Frizzled-related protein (FRZB) was added to subcultured cells and was replaced every 2 days. Ten days after 20E administration, the cells were harvested for immunofluorescence.
-
Total proteins were extracted from cultured cells using CelLytic MT Cell Lysis Reagent (Sigma-Aldrich). Nuclear extracts were obtained as previously reported[23]. Briefly, cells were homogenized in 300 μL of homogenization buffer [10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L DTT, and 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF)] on ice for 1 h. Then, 19 μL of 10% (v/v) NP40 was mixed and centrifuged at 13,000 ×g for 1 min at 4 °C. The supernatant (cytosolic protein fraction) was immediately frozen in liquid nitrogen. The nuclear pellet was washed once with homogenization buffer and then dissolved in 40 μL of buffer [20 mmol/L HEPES, pH 7.9, 400 mmol/L KCL, 1 mmol/L EDTA, 1 mmol/L EGTA, 10% (v/v) glycerin, 1 mmol/L DTT, and 0.5 mmol/L PMSF]. Nuclear proteins were eluted for 1 h on ice and centrifuged at 13,000 ×g for 5 min at 4 °C. Protein concentration was determined using bovine serum albumin as a standard (Sigma-Aldrich). Western blotting was performed as previously described[27]. The protein samples were probed using the following antibodies: rabbit anti-Wnt3a (1:800) (Abcam, Cambridge, MA, USA), mouse anti-β-actin (1:2,000) (Santa Cruz Biotechnology), rabbit anti-β-catenin (1:1,000) (Abcam), and mouse anti-Histone H2A (1:2,000) (EMD Millipore, Burlington, MA, USA). The β-actin was used as an internal control standard. The secondary antibodies were IR Dye 700-conjugated anti-rabbit (1:5,000, LI-COR Biosciences, Lincoln, NB, USA) and IR Dye 800-conjugated anti-mouse (1:5,000, LI-COR Biosciences, Lincoln, NB, USA). The membranes were scanned using an Odyssey imager (LI-COR BiosciencesLI-COR Biosciences, Lincoln, NB, USA), and each band intensity was determined using Quantity One 4.40 software (Bio-Rad, Hercules, CA, USA).
-
For tissue preparation, rats were deeply anesthetized using chloral hydrate (500 mg/kg) and were intracardially perfused with 0.1 mol/L phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were immediately harvested, postfixed in 10% formalin overnight, and cryoprotected in 30% (w/v) sucrose in PBS for 2 days. They were then embedded in paraffin according to standard procedures[28]. Then, 10 μm-thick sections were sliced and stained with hematoxylin and eosin (H&E), toluidine blue, or specific antibodies. H&E staining showed edema, vacuole-like denaturation, and structural disorder in both the cortex and hippocampus after radiation exposure. TUNEL positive cells were detected by a fluorescent technique to assess apoptosis, and Nissl staining was used to show changes in the number of neurons.
-
A standard immunofluorescence protocol was conducted in this study[29]. Briefly, tissue sections or cell culture slides were incubated in 0.1 mol/L PBS containing 0.1% Triton X-100 for 20 min and then treated with primary antibodies in PBS containing 5% horse serum overnight at 4 °C. After removing the unbound primary antibodies with 0.1 mol/L PBS, the sections were incubated with secondary antibodies for 2 h at 37 °C and then mounted using 90% glycerol. Fluorescent staining was examined and photographed using a confocal laser scanning microscope (LSM 710; Carl Zeiss, Oberkochen, Germany). The numbers of positively-stained cells in five random visual fields were counted. The primary antibodies included chicken anti-GFAP (1:250), mouse anti-Tuj1 (1:200) (Abcam), rabbit anti-BrdU (1:350), and rabbit anti-Nestin (1:200) (Santa Cruz Biotechnology). The secondary antibodies included fluorescein isothiocyanate-conjugated goat anti-chicken, cyanine 3 (Cy3)-conjugated goat anti-mouse, and Cy3-conjugated goat anti-rabbit (Santa Cruz Biotechnology). All antibodies were used according to the manufacturer’s instructions.
-
Nine rats from each group were used for behavioral tests. The spatial reference memory of rats was assessed 5 weeks after radiation exposure using a Morris water maze test, performed as previously described[30]. In brief, the maze consisted of a circular water tank (120 cm in diameter and 50 cm in height) with a platform (11 cm in diameter and 25 cm in height) at the center of one quadrant. One d before the test, rats were allowed to swim in the pool for 120 s for training. At the beginning, one rat was placed randomly into the maze facing the wall and allowed to swim until they found the platform. The trial was then terminated, and the rat was allowed to stay on the platform for 15 s. Rats that did not find the platform in 120 s were manually placed on the platform for 15 s. Thirty-two acquisition trials were performed for the place navigation test (PNT, eight trials per day separated by 15 min intervals for 4 d). Their performance was monitored and analyzed with an automated video-tracking system (CleverSys, Reston, VA, USA). The PNT performance of the rats was assessed by measuring the time taken to find the platform (escape latency). One day after the final acquisition trial, the platform was removed, and a 120 s retention trial was conducted for the spatial probe test (SPT). The SPT performance of the rats was assessed by noting the number of times that the rats swam through the target quadrant and the number of crossings over the platform location.
-
The data are presented as the mean ± standard error of the mean (SEM). Differences between two groups were compared using Student's t-test. Multiple comparisons between groups were conducted using one-way analysis of variance. All statistical analyses were performed using SPSS statistical software for Windows, version 17.0 (SPSS, Chicago, IL, USA). The differences were considered statistically significant when P < 0.05 or P < 0.01.
-
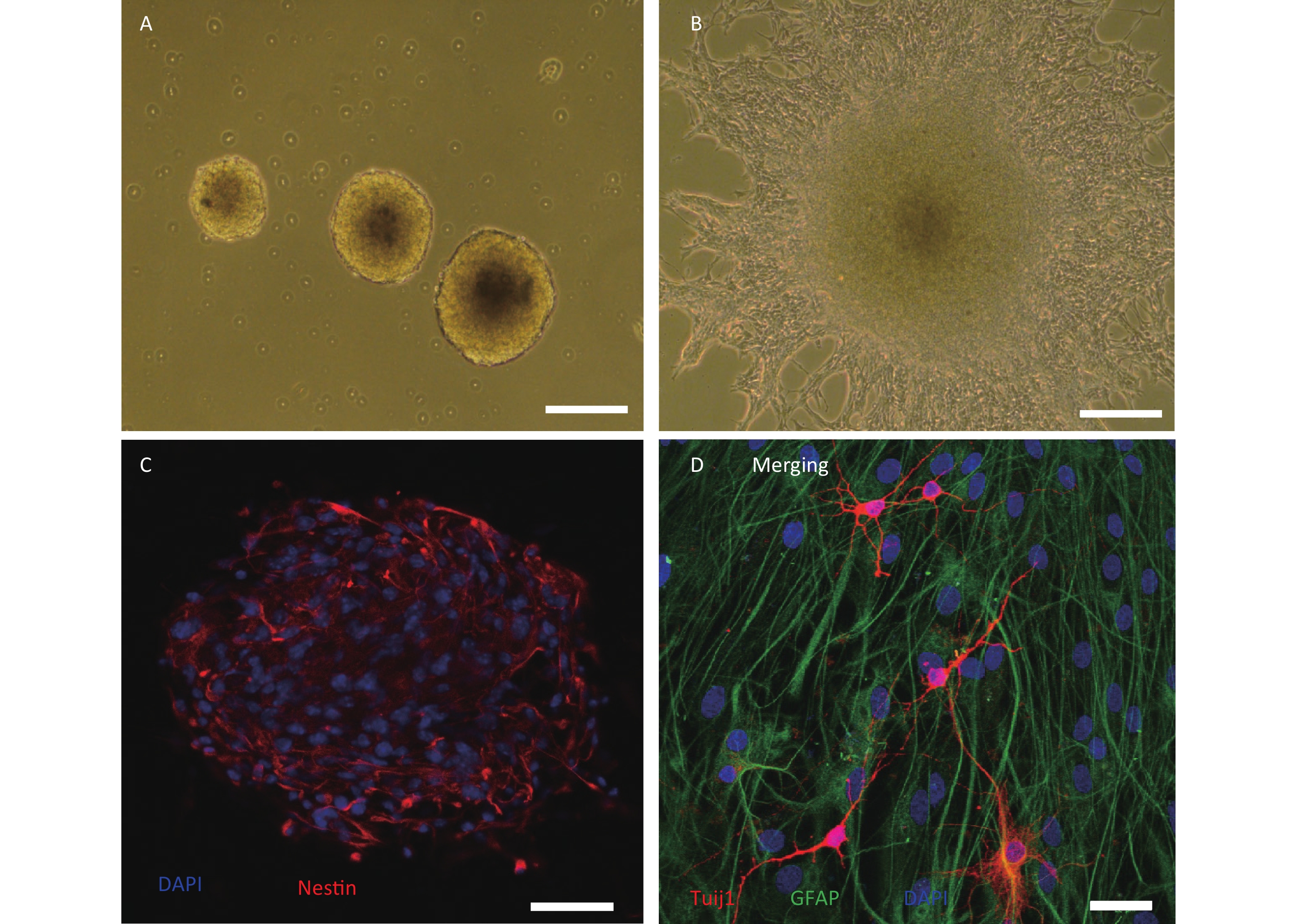
We cultured primary hippocampal NSCs from HMP radiation-exposed rats, and confirmed that these cells could self-renew or differentiate into neural and glial lineages in proliferative and differential media, respectively (Figure 1A–D).
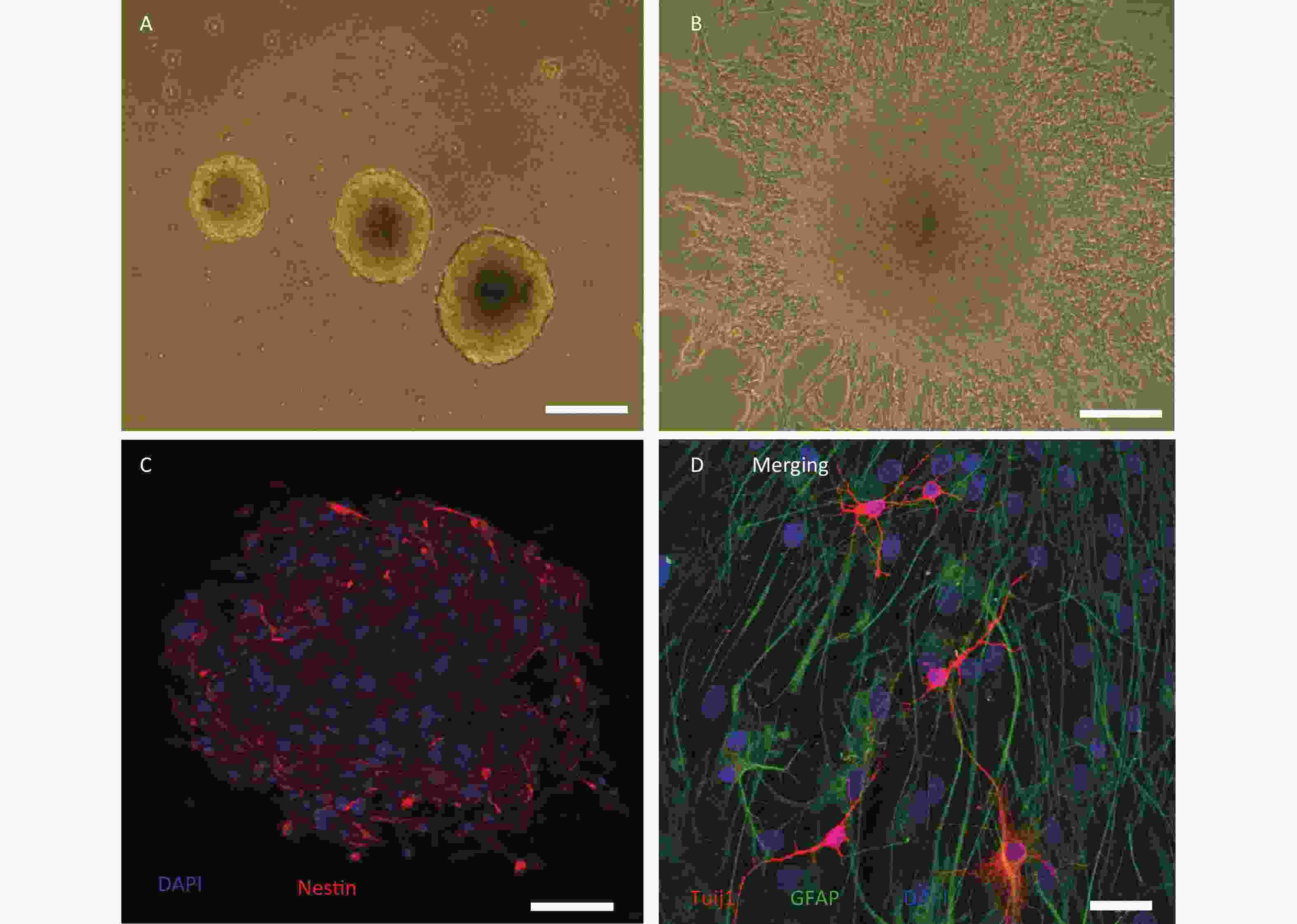
Figure 1. Culture and identification of adult hippocampal neural stem cells from high power microwave radiation-exposed rats. (A) Primary neurospheres in proliferative medium. (B) Adherent neurospheres and migrated cells in differential medium; (C) Nestin labelling (red) of neurospheres. (D) Glial fibrillary acidic protein+ (GFAP, green) and class III β-tubulin+ (Tuj1, red) cells in neurospheres cultured in differential medium. Bar: (A) and (B), 100 μm; (C), 50 μm; (D), 20 μm.
The subcultured cells were treated with four concentrations of 20E (1, 10, 100, or 1,000 μmol/L) in proliferative medium. Seven d later, the viability of the NSCs was assayed using a CCK-8 kit and the number of neurospheres was measured in five random fields using an inverse fluorescent microscope (CKX31-A12PHP; Olympus, Tokyo, Japan). Treatment with 1 μmol/L 20E did not affect the viability of NSCs. Treatment with 10, 100, and 1,000 μmol/L 20E; however, significantly increased the proliferation of NSCs. It was noted that 100 μmol/L 20E exerted stronger stimulatory effects. The 100 μmol/L 20E-treated hippocampal NSCs exhibited a 1.7-fold increase in the number of neurospheres compared to the PBS vehicle-treated control NSCs (Figure 2A–D).
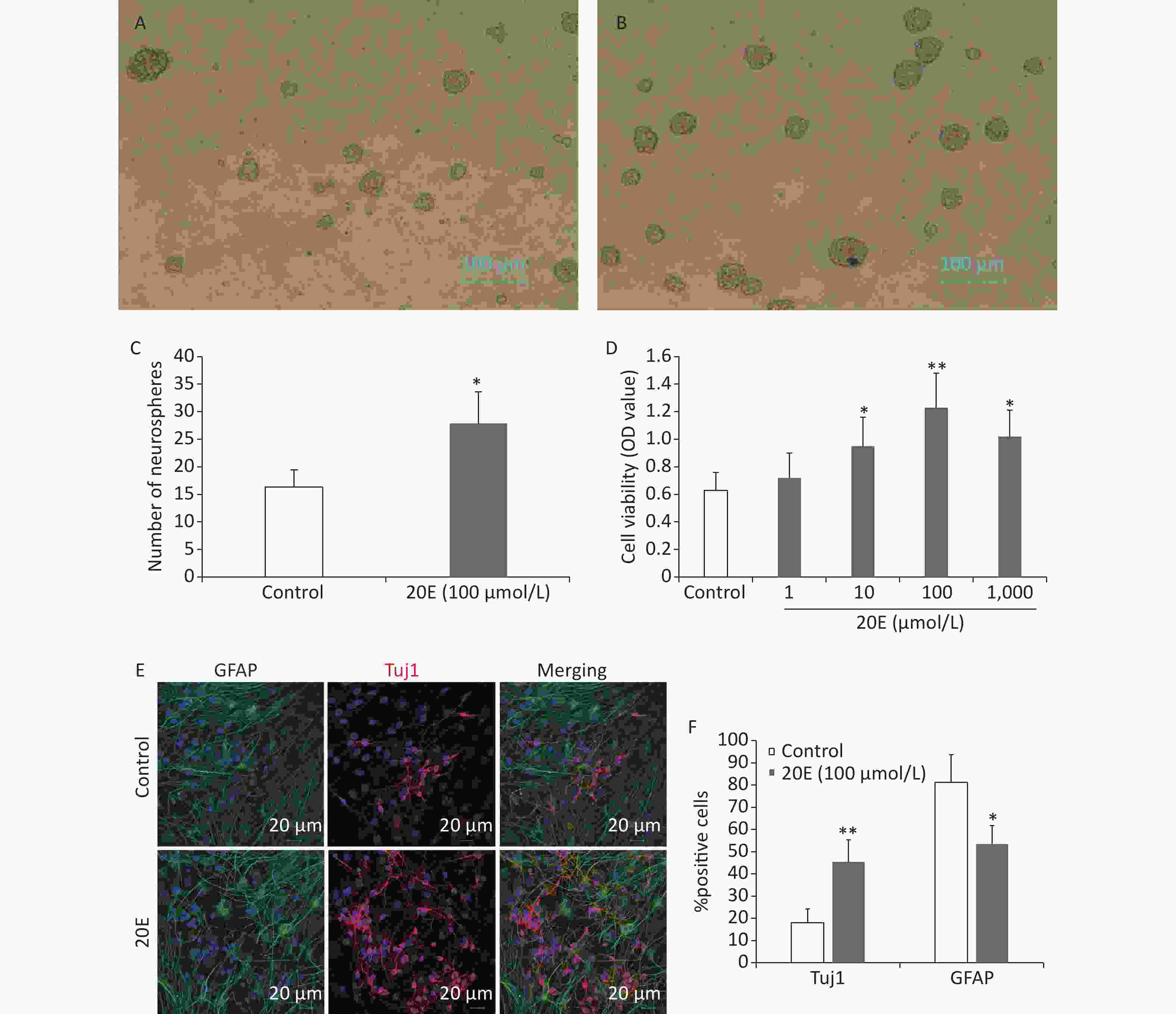
Figure 2. The 20-hydroxyecdysone (20E) promoted the proliferation and neuronal differentiation of neural stem cells (NSCs) in vitro. (A) Neurospheres in normal proliferative medium (control); (B) Neurospheres in proliferative medium containing 100 μmol/L 20E; (C)–(D) The number of neurospheres (C) and total cell viability (D) were significantly increased after 20E treatment. (E) Glial fibrillary acidic protein (GFAP, green) and class III β-tubulin (Tuj1, red) double-labelling of NSCs in differential medium containing 100 μmol/L 20E or control medium. (F) Quantitative analysis of the percentage of Tuj1-positive cells and GFAP-positive cells showing that 100 μmol/L 20E treatment promoted neuronal differentiation of NSCs. *P < 0.05 and **P < 0.01 compared to the control. Bar: (A) and (B), 100 μm; (E), 25 μm.
NSCs were induced to differentiate in medium containing 5% FBS without growth factors for 12 d. Immunofluorescence staining for Tuj1 and GFAP was then conducted to assess differentiated neurons and astrocytes (Figure 2E). The results showed that the 100 μmol/L 20E-treated NSCs exhibited a 2.5-fold increase in the percentage of Tuj1-positive neurons, and a 1.5-fold decrease in the percentage of GFAP-positive astrocytes (Figure 2F).
-
When cultured in differential medium, western blotting showed that the expression of Wnt3a protein in NSCs gradually increased and peaked at day 3. Administration of 100 μmol/L 20E further increased the expression of Wnt3a (Figure 3A and B). Both immunofluorescent staining and western blotting indicated that nuclear β-catenin expression was significantly increased at day 3 when NSCs were treated with 100 μmol/L 20E and cultured in differential medium. Administration of FRZB (150 ng/mL), an antagonist of the Wnt/β-catenin signaling pathway, reduced the 20E-induced nuclear translocation of β-catenin (Figure 3C–E), while FRZB treatment also reversed 20E-induced neuronal differentiation of NSCs in vitro (Figure 3F and G).
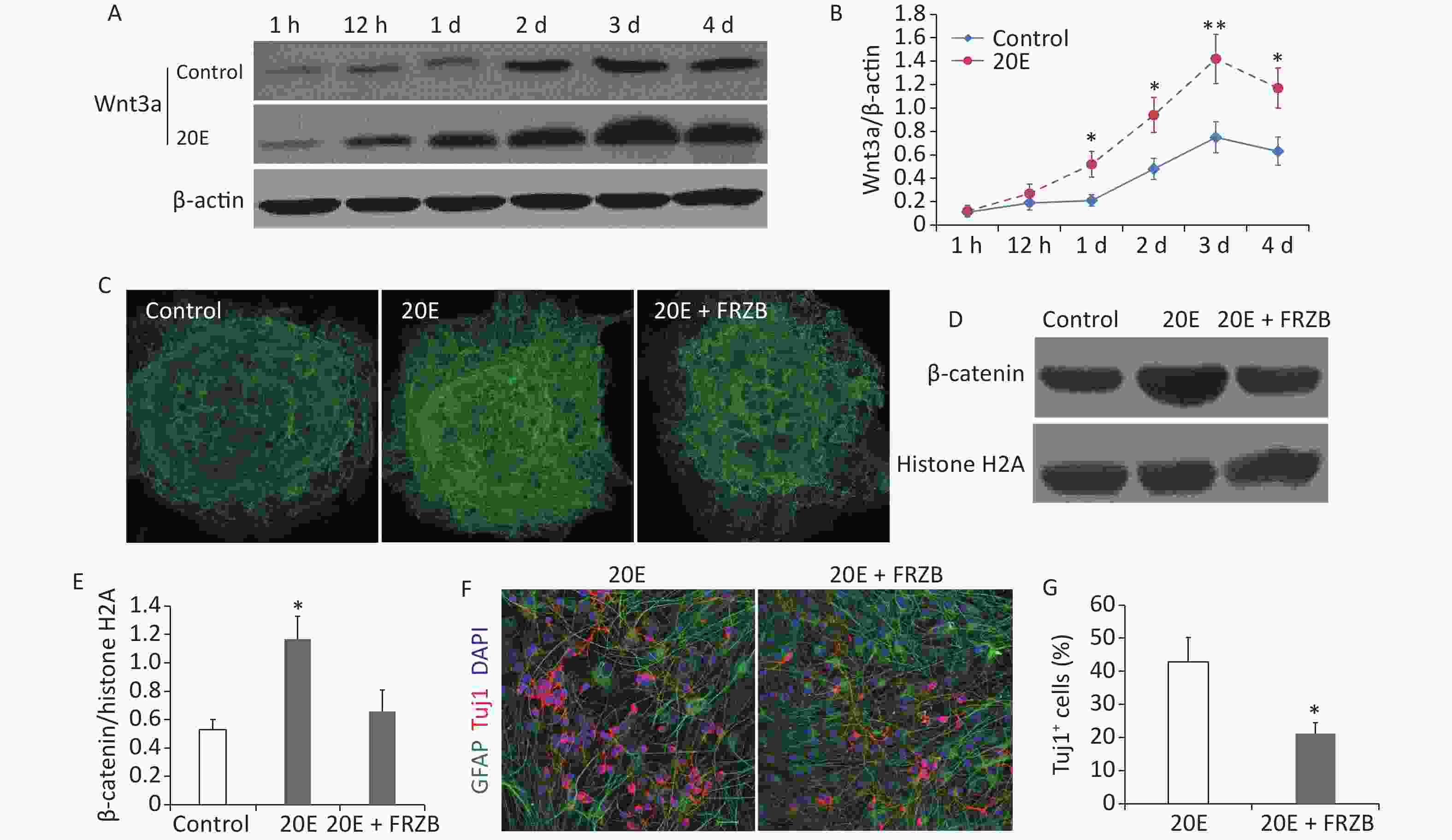
Figure 3. The Wnt3a/β-catenin axis was upregulated in neural stem cells (NSCs) cultured in differential medium after 20-hydroxyecdysone (20E) treatment. (A–B): Western blotting (A) and quantitative analysis (B) of Wnt3a expressions in NSCs treated with 100 μmol/L 20E and control NSCs cultured in differential medium. (C): Immunofluorescent staining of β-catenin in NSCs. (D–E): Western blotting (D) and quantitative analysis (E) of nuclear β-catenin expression in NSCs in control, 20E (100 μmol/L)-treated, and 20E (100 μmol/L) + Frizzled-related protein (FRZB, 150 ng/mL)-treated differential medium. (F): Glial fibrillary acidic protein (GFAP, green) and class III β-tubulin (Tuj1, red) double-labelling of NSCs in differential medium containing 20E (100 μmol/L) or 20E (100 μmol/L) + FRZB (150 ng/mL). (G): Quantitative analysis of the percentage of Tuj1-positive cells showing that administration of FRZB reversed 20E-induced neuronal differentiation of NSCs. *P < 0.05 and **P < 0.01 compared to the control (B), compared to the control and 20E + FRZB (E), and compared to 20E (G). Bar: (C), 40 μm; (F), 25 μm.
-
The brains of the rats that had been exposed to HPM radiation were collected and histological examinations were performed. H&E staining revealed that 24 h after radiation exposure, a number of hippocampal and cortical cells exhibited vacuole-like denaturation (Figure 4A). Nissl staining showed some conspicuous neuronal injuries, including cellular swelling, cell loss, nuclear pyknosis, and karyorrhexis in the hippocampus and cortex 24 h after HPM radiation exposure (Figure 4B). The numbers of TUNEL-positive cells in both the cortex and hippocampus were also significantly increased after HPM radiation exposure (Figure 4C and D).
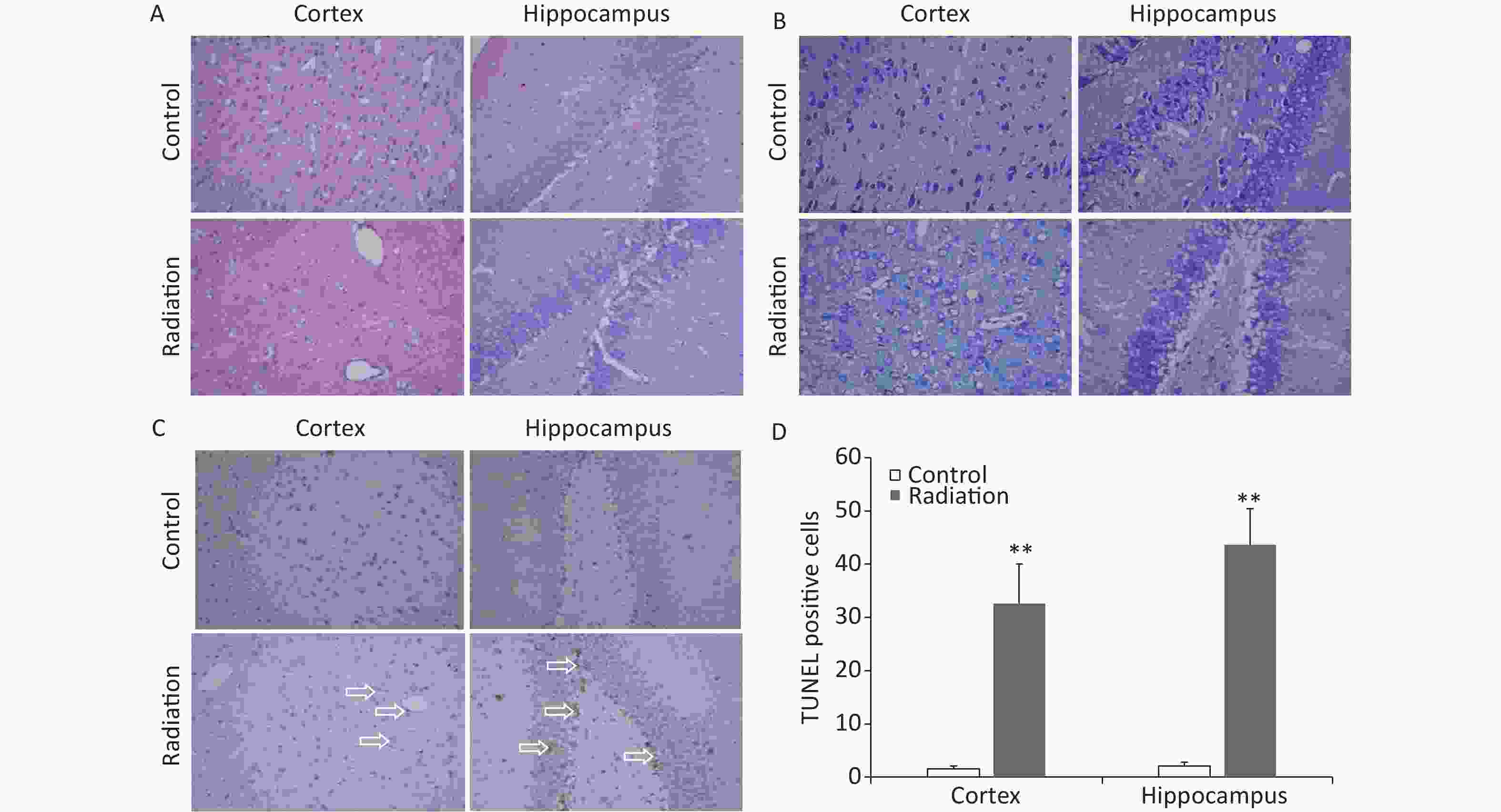
Figure 4. Representative photomicrographs showing changes in the rat brain after high power microwave radiation exposure. (A) Hematoxylin & eosin stained images showing edema, vacuole-like denaturation, and structural disorder in both the cortex (left) and hippocampus (right) after radiation exposure (bottom) compared to the control (top). (B) Nissl staining showing reduced numbers of neurons and thinner neurons in the cortex and hippocampus after radiation exposure. (C–D) The numbers of TUNEL-positive cells were significantly increased in both the cortex and hippocampus after microwave radiation exposure. **P < 0.01 compared to the control. Bar = 50 μm.
-
BrdU was used to measure cell proliferation, and immunofluorescence double staining was used to investigate neurogenesis in the SGZ. After HPM radiation exposure, the number of BrdU+ cells in the SGZ was increased, when compared to the control cells. Furthermore, 20E treatment after radiation significantly increased the number of BrdU+ cells in the SGZ (Figure 5A–D). Twenty-eight d after radiation exposure, Tuj1/BrdU double-labelling was conducted to assess neurogenesis in the SGZ. The results showed that 20E treatment significantly promoted neurogenesis in the SGZ, demonstrating a 2.25-fold increase in the number of Tuj1/BrdU double-positive cells compared to the untreated radiation-exposed group (Figure 5E and F). The ratio of Tuj1/BrdU double-positive cells to BrdU-positive cells in the radiation + 20E group (89.7% ± 9.2%) was greater than in the radiation group (67.9% ± 8.3%) (Figure 5G), implying that 20E treatment promoted neuronal differentiation of NSCs in the SGZ.
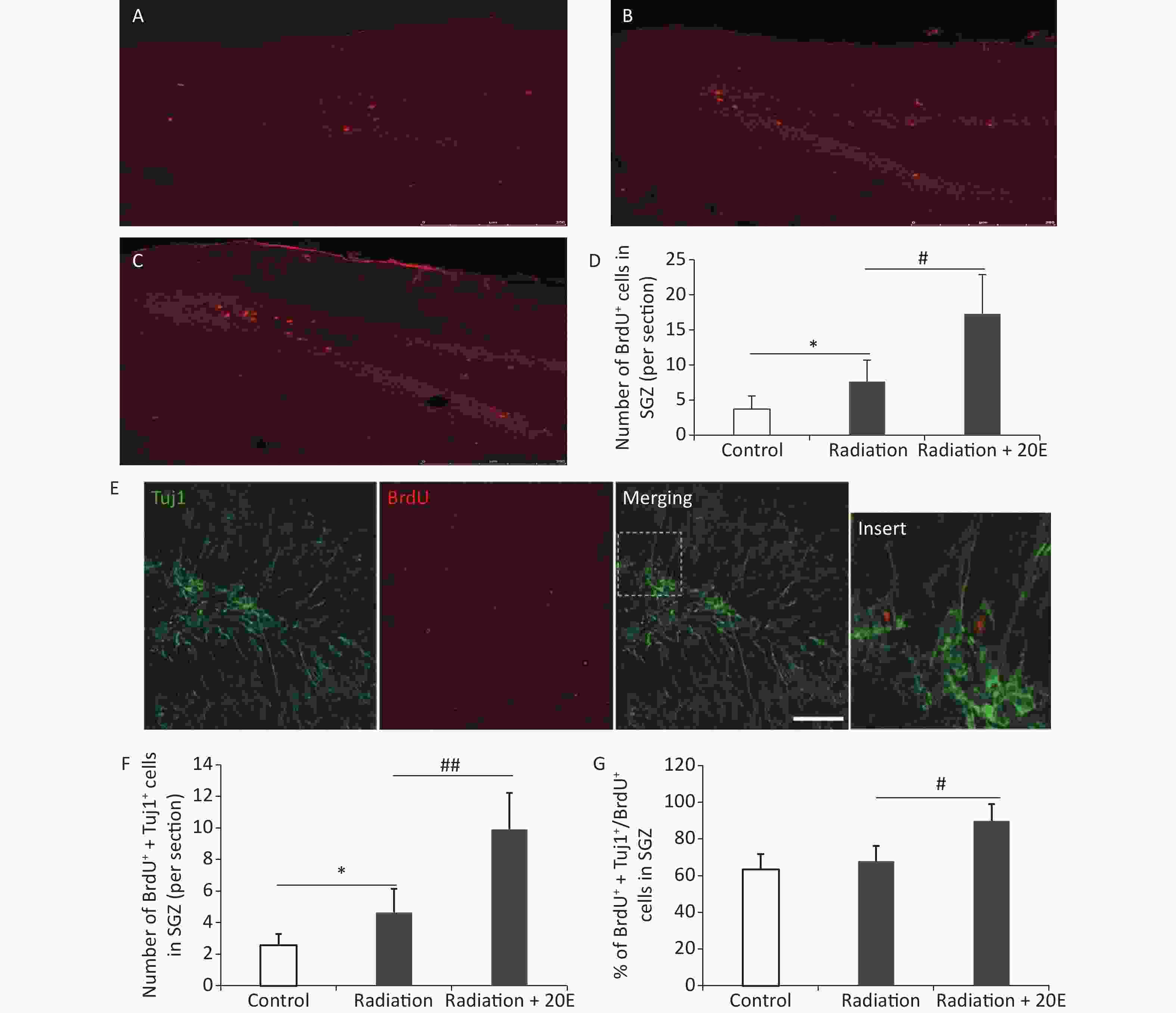
Figure 5. 20-hydroxyecdysone (20E) facilitated neurogenesis in the subgranular zone (SGZ) after radiation exposure. (A–C): Representative images of the SGZ from control (A), radiation (B), and radiation + 20E (C) rats, in red. (D): Quantification of the number of BrdU+ cells in the SGZ in the three groups. 20E treatment significantly increased the number of BrdU+ cells in the SGZ after radiation exposure. (E): Representative Tuj1/BrdU double-labelling images of the SGZ. (F–G): Quantification of the number of Tuj1+/BrdU+ cells (F) and the ratio of Tuj1+/BrdU+ cells to BrdU+ cells (G) in the SGZ in the three groups. 20E treatment significantly promoted neurogenesis (Tuj1+/BrdU+ cells) in the SGZ after radiation exposure. *P < 0.05 compared to the control group. #P < 0.05 and ##P < 0.01 compared to the radiation group. n = 3. Bar: (A–C), 250 μm; (E), 50 μm.
-
The spatial learning and memory of rats were tested using the Morris water maze test. The time taken for the animals to find the platform was recorded in the PNT (Figure 6A–F). In the first 3 days of the PNT, rats in the radiation and radiation+20E groups required a similar length of time to find the hidden platform. On the last day of the PNT (day 4); however, rats in the radiation+20E group found the hidden platform significantly quicker than rats in the radiation group (Figure 6G). The SPT on day 5 of testing showed significant differences between the radiation and radiation+20E groups with respect to the length of time that the rats spent in the quadrant in which the platform was previously placed (Figure 6H) and the number of times that the rats swam across the previous location of the platform (Figure 6I).
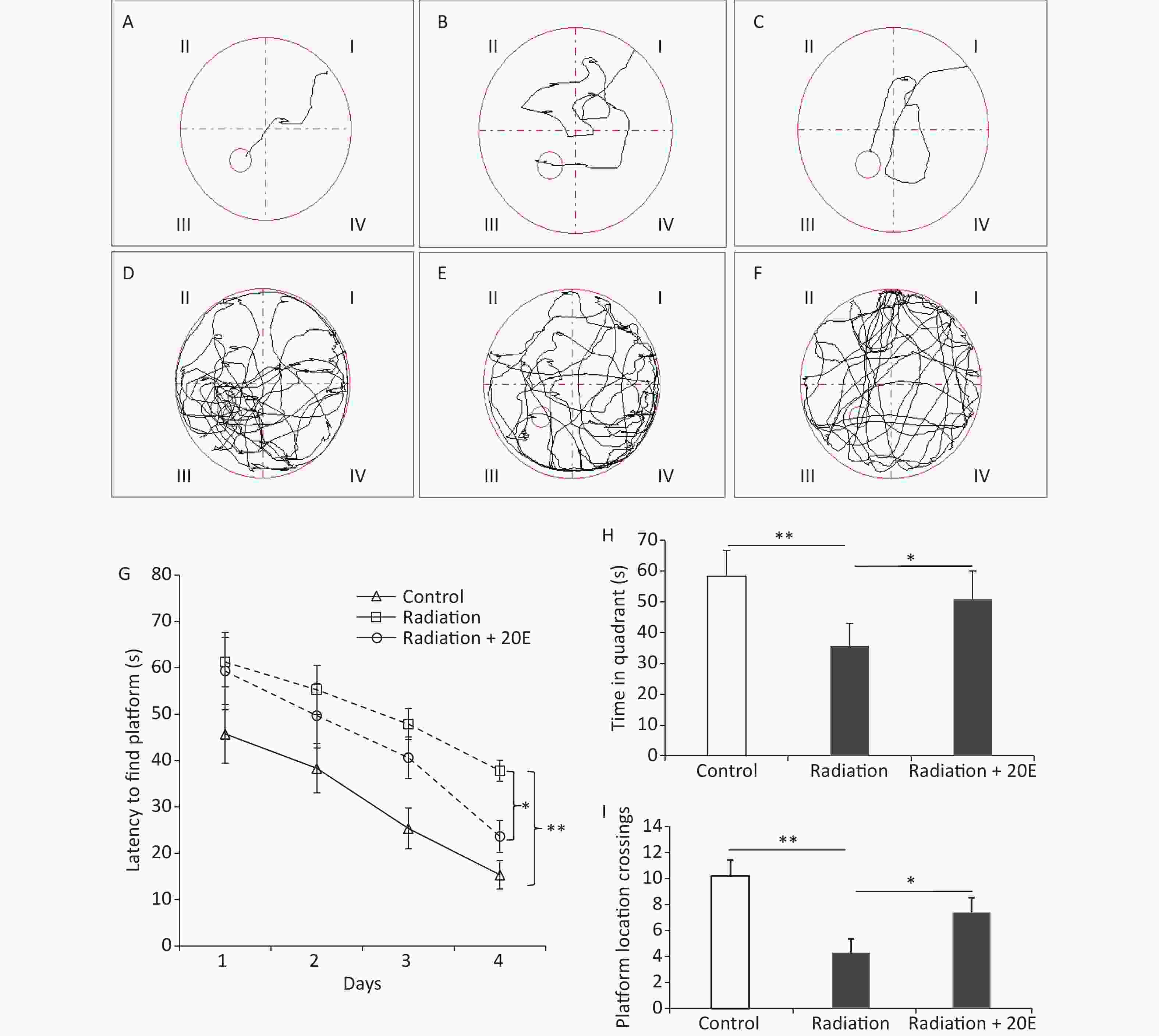
Figure 6. 20-hydroxyecdysone (20E) attenuated high power microwave radiation-induced rat learning and memory deficits as measured using the Morris water maze test. (A–F): Representative swim tracks of the control (A, D), radiation (B, E), and radiation + 20E-treated (C, F) rats in the place navigation trial (A–C) on day 4 and the probe trial (D–F) on day 5 of the Morris water maze test. (G): Length of time required to find the platform during the acquisition phase of the test. (H): Length of time spent in the quadrant of the water tank in which the platform was previously placed during the probe trial of the test. (I): Comparison of the number of crossovers between the three groups in the probe trial test. *P < 0.05 and **P < 0.01 compared to the radiation group. n = 9.
-
The results of this study showed that 20E promoted the neuronal differentiation of adult hippocampal NSCs from HPM radiation-exposed rats via the Wnt3a/β-catenin signaling pathway in vitro. In vivo, we also found that 20E treatment facilitated neurogenesis in the SGZ of the rats after HPM radiation exposure. Additionally, administration of 20E attenuated learning and memory deficits in HPM radiation-exposed rats. Therefore, our research showed that 20E could act as a fate regulator of adult rat hippocampal NSCs and could attenuate HPM radiation-induced learning and memory deficits.
It has been shown that long-term exposure to microwave radiation can exert harmful effects on sensitive organs. These effects depended largely on exposure intensity, frequency of exposure, and exposure duration[31-34]. A number of potentially harmful effects have been reported, namely cognitive impairment, loss of mental concentration, reduced learning ability, and an imbalance in DNA damage and repair, which may ultimately lead to cell death or cancer[35, 36]. However, data on how to control the potential risks of exposure to different sources of microwaves on the brain are limited.
The nervous system, especially the hippocampus, is thought to be particularly vulnerable to HPM radiation exposure. Exposure to HPM radiation disrupts the normal levels of neurotransmitters in the hippocampus, resulting in memory and learning deficits, which may relate to the accumulation of metabolic products or damage to the internal environment[37]. In addition, it has been reported that oxidative stress and the subsequent generation of reactive oxygen species (ROS) can be induced by different kinds of radiation. ROS can cause DNA damage and lead to cognitive impairment[38]. Based on these considerations, the potential role of oxidative stress on the development of the cognitive impairment observed after radiation may include disruption of hippocampal cell proliferation and neurogenesis, hormonal changes, increased oxidative stress and ROS production, chronic increases in inflammation, and alterations in synaptic plasticity and long-term potentiation. While the effects of inflammation and oxidative stress on neurogenesis and their role in cognitive impairment have been widely studied, the HMP-induced cognitive impairment mechanisms that involve mitochondrial dysfunction, estrogen dysregulation, and increased transglutaminase 2 are still unclear. Behavioral and cognitive changes are important outcomes for assessing the effects of microwave exposure on the brain[3,39,40]. In this study, we showed that HPM radiation-exposed rats exhibited hippocampal injury and learning and memory deficits. Further studies are therefore required to identify methods of ameliorating HMP-induced learning and memory impairments.
NSCs are present in two neurogenic regions in the mammalian brain, the subventricular zone and the SGZ of the hippocampal DG, and continue to generate new neurons throughout life[41]. In the SGZ, most NSCs differentiate into dentate granule cells that migrate into the inner granule cell layer and functionally integrate into hippocampal neural circuits[27]. Mice lacking adult-born dentate granule cells exhibit cognitive impairment characterized by an inability to form hippocampus-dependent long-term spatial memory[42]. Adult neurogenesis in the SGZ therefore contributes to at least some types of hippocampus-dependent learning and memory. The hippocampal CA1 area is also closely related to learning and memory. Several animal studies have shown that the CA1 area was selectively vulnerable to the consequences of hypoperfusion[43], indicating that cerebral hypoperfusion played a significant role in cognitive disturbances[44]. Cerebral hypoperfusion has been demonstrated to induce memory impairment; a previous study found that chronic hypoperfusion induced the degeneration of pericytes and the development of capillary deposits in the hippocampal CA1 area but not in the DG[43]. In the present study, HPM radiation-exposed rats exhibited hippocampal damage and consequential learning and memory deficits. Therefore, promoting neurogenesis in the SGZ could improve the learning and memory ability of HPM radiation-exposed rats.
20E, a hydrophobic steroid hormone, is produced in both insects and plants and plays an important role in the regulation of NSCs. It is involved in directing many features of CNS remodeling during insect metamorphosis, and is important in the insect brain for learning and memory[45]. In addition, 20E has been shown to induce osteogenic differentiation in mesenchymal stem cells, to promote the proliferation of human periodontal ligament stem cells in periodontal regenerative therapy[46]. We hypothesized that 20E promoted the neuronal differentiation of adult hippocampal NSCs from HPM radiation-exposed rats. According to our study, administration of 20E promoted the differentiation of NSCs into neurons in the SGZ. Administration of 20E induced a 2.25-fold increase in the number of Tuj1/BrdU double-positive cells, and ameliorated HPM radiation-induced learning and memory impairments. These results indicated that 20E played an important role in the fate regulation of NSCs. In addition, 20E has been shown to have neuroprotective antioxidant potential effects against cerebral ischemia injury by inhibiting ROS production and modulating oxidative stress-induced signal transduction pathways. Therefore, the potential anti-oxidant effect of 20E as a treatment for cognitive impairment is worth investigating.
Wnt/β-catenin signaling plays a pivotal role in adult neurogenesis, the regulation of stem cell pluripotency[47], neural progenitor proliferation in the developing CNS, and is also involved in dendrite development, synaptogenesis, and the establishment of axons. Wnt has also been shown to promote neuronal differentiation of embryonic, somatic, and neural stem cells[48-50]. The Wnt/β-catenin signaling pathway may inhibit cell proliferation and abnormal differentiation by interfering with the cell cycle of NSCs. Indeed, a recent study reported that multiple sevoflurane exposures inhibited proliferation via inhibition of the Wnt/β-catenin signaling pathway in mouse NSCs[51].
The results of our study showed that 20E promoted neurogenesis in the SGZ of HPM-irradiated adult rats, and that Wnt/β-catenin signaling played a key role in this process. The immunofluorescence and western blot results showed a significant increase in nuclear β-catenin on day 3 when NSCs were treated with 100 μmol/L 20E and cultured in differential medium. However, the mechanism by which 20E regulates Wnt expression requires further study.
In conclusion, our study showed that 20E regulated adult NSCs and ameliorated HPM-induced learning and memory impairments, providing a possible therapeutic strategy for HPM-induced deficits. In the present study, the observed learning and memory deficits were, at least in part, due to the marked injury and structural changes observed in the hippocampus following microwave radiation exposure[52]. In addition, disrupted neurotransmitter levels in the hippocampus, which may be closely related to the accumulation of metabolites or damage to the internal environment[53,54], may also have contributed to HMP-induced learning and memory deficits. Overall, future studies in this field should analyze changes in neurotransmitter concentrations to elucidate the relationships between 20E and neurotransmitters[55,39], potentially facilitating the development of new therapeutic strategies for HMP-induced impairment in the hippocampus. Our findings may also aid in the development of alternative treatments for cognitive impairment caused by other types of neurological damage such as trauma and infarction[56]. Our future research will examine the molecular proteomic changes after microwave radiation exposure to determine the associated mechanisms of brain cell dysfunction.
-
HF conceived the paper. WHC wrote the first draft of the paper. JJL analyzed the results and coordinated the construction of the figures. HYZ performed the primary culture of the adult rat hippocampal NSCs. XC and GBZ conducted the experiments. JKL analyzed and processed the data. All authors reviewed the results and approved the final version of the manuscript.
-
The authors declare that they have no conflicts of interest relating to the contents of this article.
doi: 10.3967/bes2022.068
20-Hydroxyecdysone Improves Neuronal Differentiation of Adult Hippocampal Neural Stem Cells in High Power Microwave Radiation-Exposed Rats
-
Abstract:
Objective The hippocampus is thought to be a vulnerable target of microwave exposure. The aim of the present study was to investigate whether 20-hydroxyecdysone (20E) acted as a fate regulator of adult rat hippocampal neural stem cells (NSCs). Furthermore, we investigated if 20E attenuated high power microwave (HMP) radiation-induced learning and memory deficits. Methods Sixty male Sprague-Dawley rats were randomly divided into three groups: normal controls, radiation treated, and radiation+20E treated. Rats in the radiation and radiation+20E treatment groups were exposed to HPM radiation from a microwave emission system. The learning and memory abilities of the rats were assessed using the Morris water maze test. Primary adult rat hippocampal NSCs were isolated in vitro and cultured to evaluate their proliferation and differentiation. In addition, hematoxylin & eosin staining, western blotting, and immunofluorescence were used to detect changes in the rat brain and the proliferation and differentiation of the adult rat hippocampal NSCs after HPM radiation exposure. Results The results showed that 20E induced neuronal differentiation of adult hippocampal NSCs from HPM radiation-exposed rats via the Wnt3a/β-catenin signaling pathway in vitro. Furthermore, 20E facilitated neurogenesis in the subgranular zone of the rat brain following HPM radiation exposure. Administration of 20E attenuated learning and memory deficits in HPM radiation-exposed rats and frizzled-related protein (FRZB) reduced the 20E-induced nuclear translocation of β-catenin, while FRZB treatment also reversed 20E-induced neuronal differentiation of NSCs in vitro. Conclusion These results suggested that 20E was a fate regulator of adult rat hippocampal NSCs, where it played a role in attenuating HPM radiation-induced learning and memory deficits. -
Key words:
- High power microwave /
- Hippocampus /
- 20-hydroxyecdysone /
- Learning and memory
-
Figure 1. Culture and identification of adult hippocampal neural stem cells from high power microwave radiation-exposed rats. (A) Primary neurospheres in proliferative medium. (B) Adherent neurospheres and migrated cells in differential medium; (C) Nestin labelling (red) of neurospheres. (D) Glial fibrillary acidic protein+ (GFAP, green) and class III β-tubulin+ (Tuj1, red) cells in neurospheres cultured in differential medium. Bar: (A) and (B), 100 μm; (C), 50 μm; (D), 20 μm.
Figure 2. The 20-hydroxyecdysone (20E) promoted the proliferation and neuronal differentiation of neural stem cells (NSCs) in vitro. (A) Neurospheres in normal proliferative medium (control); (B) Neurospheres in proliferative medium containing 100 μmol/L 20E; (C)–(D) The number of neurospheres (C) and total cell viability (D) were significantly increased after 20E treatment. (E) Glial fibrillary acidic protein (GFAP, green) and class III β-tubulin (Tuj1, red) double-labelling of NSCs in differential medium containing 100 μmol/L 20E or control medium. (F) Quantitative analysis of the percentage of Tuj1-positive cells and GFAP-positive cells showing that 100 μmol/L 20E treatment promoted neuronal differentiation of NSCs. *P < 0.05 and **P < 0.01 compared to the control. Bar: (A) and (B), 100 μm; (E), 25 μm.
Figure 3. The Wnt3a/β-catenin axis was upregulated in neural stem cells (NSCs) cultured in differential medium after 20-hydroxyecdysone (20E) treatment. (A–B): Western blotting (A) and quantitative analysis (B) of Wnt3a expressions in NSCs treated with 100 μmol/L 20E and control NSCs cultured in differential medium. (C): Immunofluorescent staining of β-catenin in NSCs. (D–E): Western blotting (D) and quantitative analysis (E) of nuclear β-catenin expression in NSCs in control, 20E (100 μmol/L)-treated, and 20E (100 μmol/L) + Frizzled-related protein (FRZB, 150 ng/mL)-treated differential medium. (F): Glial fibrillary acidic protein (GFAP, green) and class III β-tubulin (Tuj1, red) double-labelling of NSCs in differential medium containing 20E (100 μmol/L) or 20E (100 μmol/L) + FRZB (150 ng/mL). (G): Quantitative analysis of the percentage of Tuj1-positive cells showing that administration of FRZB reversed 20E-induced neuronal differentiation of NSCs. *P < 0.05 and **P < 0.01 compared to the control (B), compared to the control and 20E + FRZB (E), and compared to 20E (G). Bar: (C), 40 μm; (F), 25 μm.
Figure 4. Representative photomicrographs showing changes in the rat brain after high power microwave radiation exposure. (A) Hematoxylin & eosin stained images showing edema, vacuole-like denaturation, and structural disorder in both the cortex (left) and hippocampus (right) after radiation exposure (bottom) compared to the control (top). (B) Nissl staining showing reduced numbers of neurons and thinner neurons in the cortex and hippocampus after radiation exposure. (C–D) The numbers of TUNEL-positive cells were significantly increased in both the cortex and hippocampus after microwave radiation exposure. **P < 0.01 compared to the control. Bar = 50 μm.
Figure 5. 20-hydroxyecdysone (20E) facilitated neurogenesis in the subgranular zone (SGZ) after radiation exposure. (A–C): Representative images of the SGZ from control (A), radiation (B), and radiation + 20E (C) rats, in red. (D): Quantification of the number of BrdU+ cells in the SGZ in the three groups. 20E treatment significantly increased the number of BrdU+ cells in the SGZ after radiation exposure. (E): Representative Tuj1/BrdU double-labelling images of the SGZ. (F–G): Quantification of the number of Tuj1+/BrdU+ cells (F) and the ratio of Tuj1+/BrdU+ cells to BrdU+ cells (G) in the SGZ in the three groups. 20E treatment significantly promoted neurogenesis (Tuj1+/BrdU+ cells) in the SGZ after radiation exposure. *P < 0.05 compared to the control group. #P < 0.05 and ##P < 0.01 compared to the radiation group. n = 3. Bar: (A–C), 250 μm; (E), 50 μm.
Figure 6. 20-hydroxyecdysone (20E) attenuated high power microwave radiation-induced rat learning and memory deficits as measured using the Morris water maze test. (A–F): Representative swim tracks of the control (A, D), radiation (B, E), and radiation + 20E-treated (C, F) rats in the place navigation trial (A–C) on day 4 and the probe trial (D–F) on day 5 of the Morris water maze test. (G): Length of time required to find the platform during the acquisition phase of the test. (H): Length of time spent in the quadrant of the water tank in which the platform was previously placed during the probe trial of the test. (I): Comparison of the number of crossovers between the three groups in the probe trial test. *P < 0.05 and **P < 0.01 compared to the radiation group. n = 9.
-
[1] Van Eeghem V, El Arfani A, Arta A, et al. Selective changes in locomotor activity in mice due to low-intensity microwaves amplitude modulated in the EEG spectral domain. Neuroscience, 2017; 359, 40−8. doi: 10.1016/j.neuroscience.2017.06.056 [2] Omer H. Radiobiological effects and medical applications of non-ionizing radiation. Saudi J Biol Sci, 2021; 28, 5585−92. doi: 10.1016/j.sjbs.2021.05.071 [3] Lin YY, Gao P, Guo YC, et al. Effects of long-term exposure to l-band high-power microwave on the brain function of male mice. Biomed Res Int, 2021; 2021, 2237370. [4] Kesari KK, Behari J. Fifty-gigahertz microwave exposure effect of radiations on rat brain. Appl Biochem Biotechnol, 2009; 158, 126−39. doi: 10.1007/s12010-008-8469-8 [5] Lagorio S, Blettner M, Baaken D, et al. The effect of exposure to radiofrequency fields on cancer risk in the general and working population: a protocol for a systematic review of human observational studies. Environ Int, 2021; 157, 106828. doi: 10.1016/j.envint.2021.106828 [6] Dasdag S, Akdag MZ, Ulukaya E, et al. Effect of mobile phone exposure on apoptotic glial cells and status of oxidative stress in rat brain. Electromagn Biol Med, 2009; 28, 342−54. doi: 10.3109/15368370903206556 [7] Ning W, Xu SJ, Chiang H, et al. Effects of GSM 1800 MHz on dendritic development of cultured hippocampal neurons. Acta Pharmacol Sin, 2010; 28, 1873−80. [8] Campisi A, Gulino M, Acquaviva R, et al. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci Lett, 2010; 473, 52−5. doi: 10.1016/j.neulet.2010.02.018 [9] Zhang LH, Pang LL, Zhu SQ, et al. Intranasal tetrandrine temperature-sensitive in situ hydrogels for the treatment of microwave-induced brain injury. Int J Pharm, 2020; 583, 119384. doi: 10.1016/j.ijpharm.2020.119384 [10] Qiao SM, Peng RY, Yan HT, et al. Reduction of phosphorylated synapsin I (Ser-553) leads to spatial memory impairment by attenuating GABA release after microwave exposure in Wistar rats. PLoS One, 2014; 9, e95503. doi: 10.1371/journal.pone.0095503 [11] Verma S, Keshri GK, Karmakar S, et al. Effects of microwave 10 GHz radiation exposure in the skin of rats: an insight on molecular responses. Radiat Res, 2021; 196, 404−16. [12] Lu YH, Xu SC, He MD, et al. Glucose administration attenuates spatial memory deficits induced by chronic low-power-density microwave exposure. Physiol Behav, 2012; 106, 631−7. doi: 10.1016/j.physbeh.2012.04.019 [13] Ganapathi M, Boles NC, Charniga C, et al. Effect of Bmi1 over-expression on gene expression in adult and embryonic murine neural stem cells. Sci Rep, 2018; 8, 7464. doi: 10.1038/s41598-018-25921-8 [14] Park KI, Hack MA, Ourednik J, et al. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal "reporter" neural stem cells. Exp Neurol, 2006; 199, 156−78. doi: 10.1016/j.expneurol.2006.04.002 [15] Mundim MV, Zamproni LN, Pinto AAS, et al. A new function for prokineticin 2: recruitment of SVZ-derived neuroblasts to the injured cortex in a mouse model of traumatic brain injury. Mol Cell Neurosci, 2019; 94, 1−10. doi: 10.1016/j.mcn.2018.10.004 [16] Chen X, Wu H, Chen HS, et al. Astragaloside VI promotes neural stem cell proliferation and enhances neurological function recovery in transient cerebral ischemic injury via activating EGFR/MAPK Signaling cascades. Mol Neurobiol, 2019; 56, 3053−67. doi: 10.1007/s12035-018-1294-3 [17] Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science, 2002; 297, 365−9. doi: 10.1126/science.1074192 [18] Mao YW, Ge XC, Frank CL, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell, 2009; 136, 1017−31. doi: 10.1016/j.cell.2008.12.044 [19] Matsue K, Minakawa S, Kashiwagi T, et al. Dentate granule progenitor cell properties are rapidly altered soon after birth. Brain Struct Funct, 2018; 223, 357−69. doi: 10.1007/s00429-017-1499-7 [20] Durak O, de Anda FC, Singh KK, et al. Ankyrin-G regulates neurogenesis and Wnt signaling by altering the subcellular localization of β-catenin. Mol Psychiatry, 2015; 20, 388−97. doi: 10.1038/mp.2014.42 [21] Ning WJ, Lv RJ, Xu N, et al. lycopene-loaded microemulsion regulates neurogenesis in rats with Aβ-induced Alzheimer's disease rats based on the Wnt/β-catenin pathway. Neural Plast, 2021; 2021, 5519330. [22] Tarkowská D, Strnad M. Plant ecdysteroids: plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta, 2016; 244, 545−55. doi: 10.1007/s00425-016-2561-z [23] Pan J, Di YQ, Li YB, et al. Insulin and 20-hydroxyecdysone oppose each other in the regulation of phosphoinositide-dependent kinase-1 expression during insect pupation. J Biol Chem, 2018; 293, 18613−23. doi: 10.1074/jbc.RA118.004891 [24] Yuan SL, Huang WR, Geng L, et al. Differentiation of lepidoptera scale cells from epidermal stem cells followed by ecdysone-regulated DNA duplication and scale secreting. Cell Cycle, 2017; 16, 2156−67. doi: 10.1080/15384101.2017.1376148 [25] Eşmekaya MA, Seyhan N, Ömeroğlu S. Pulse modulated 900 MHz radiation induces hypothyroidism and apoptosis in thyroid cells: a light, electron microscopy and immunohistochemical study. Int J Radiat Biol, 2010; 86, 1106−16. doi: 10.3109/09553002.2010.502960 [26] Wang P, Chen X. Identification and culture of hippocampal neurons from prenatal rat. Chin J Clin Ration Drug Use, 2018; 11, 29−30,32. (In Chinese [27] Li MQ, Wang YY, Zhang YW, et al. Elevation of plasma corticosterone levels and hippocampal glucocorticoid receptor translocation in rats: a potential mechanism for cognition impairment following chronic low-power-density microwave exposure. J Radiat Res, 2008; 49, 163−70. doi: 10.1269/jrr.07063 [28] Su WP, Foster SC, Xing RB, et al. CD44 transmembrane receptor and hyaluronan regulate adult hippocampal neural stem cell quiescence and differentiation. J Biol Chem, 2017; 292, 4434−45. doi: 10.1074/jbc.M116.774109 [29] L'episcopo F, Serapide MF, Tirolo C, et al. A Wnt1 regulated Frizzled-1/β-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol Neurodegener, 2011; 6, 49. doi: 10.1186/1750-1326-6-49 [30] Taneja R, Gaur D. Robust fuzzy neuro system for big data analytics. In: Aggarwal VB, Bhatnagar V, Mishra DK. Big Data Analytics. Springer. 2018, 543-52. [31] Balietti M, Fattorini G, Pugliese A, et al. Two behavioral tests allow a better correlation between cognitive function and expression of synaptic proteins. Front Aging Neurosci, 2018; 10, 91. doi: 10.3389/fnagi.2018.00091 [32] D'Andrea JA, Chou CK, Johnston SA, et al. Microwave effects on the nervous system. Bioelectromagnetics, 2003; 24, S107−47. doi: 10.1002/bem.10179 [33] Morimoto R, Hirata A, Laakso I, et al. Time constants for temperature elevation in human models exposed to dipole antennas and beams in the frequency range from 1 to 30 GHz. Phys Med Biol, 2017; 62, 1676−99. doi: 10.1088/1361-6560/aa5251 [34] Mortazavi SMJ, Mortazavi SAR. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn Biol Med, 2016; 35, 303−4. doi: 10.3109/15368378.2016.1138125 [35] Behari J. Biological responses of mobile phone frequency exposure. Indian J Exp Bio, 2010; 48, 959−81. [36] Tomay F, Wells K, Duong L, et al. Aged neutrophils accumulate in lymphoid tissues from healthy elderly mice and infiltrate T- and B-cell zones. Immunol Cell Biol, 2018; 96, 831−40. doi: 10.1111/imcb.12046 [37] Bohr H, Bohr J. Microwave enhanced kinetics observed in ORD studies of a protein. Bioelectromagnetics, 2000; 21, 68−72. doi: 10.1002/(SICI)1521-186X(200001)21:1<68::AID-BEM10>3.0.CO;2-9 [38] Zhao L, Peng RY, Wang SM, et al. Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed Environ Sci, 2012; 25, 182−8. [39] Sistani S, Fatemi I, Shafeie SA, et al. The effect of Wi-Fi electromagnetic waves on neuronal response properties in rat barrel cortex. Somatosens Mot Res, 2019; 36, 292−7. doi: 10.1080/08990220.2019.1689116 [40] Wang H, Tan SZ, Dong J, et al. iTRAQ quantitatively proteomic analysis of the hippocampus in a rat model of accumulative microwave-induced cognitive impairment. Environ Sci Pollut Res Int, 2019; 26, 17248−60. doi: 10.1007/s11356-019-04873-0 [41] Tamura Y, Kataoka Y. PET imaging of neurogenic activity in the adult brain: toward in vivo imaging of human neurogenesis. Neurogenesis, 2017; 4, e1281861. doi: 10.1080/23262133.2017.1281861 [42] Cheng JX, Scala F, Blanco FA, et al. The Rac-GEF Tiam1 promotes dendrite and synapse stabilization of dentate granule cells and restricts hippocampal-dependent memory functions. J Neurosci, 2021; 41, 1191−206. doi: 10.1523/JNEUROSCI.3271-17.2020 [43] Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience, 1991; 40, 599−636. doi: 10.1016/0306-4522(91)90001-5 [44] Urbanova BS, Schwabova JP, Magerova H, et al. Reduced cerebrovascular reserve capacity as a biomarker of microangiopathy in alzheimer's disease and mild cognitive impairment. J Alzheimers Dis, 2018; 63, 465−77. doi: 10.3233/JAD-170815 [45] Kraft R, Levine RB, Restifo LL. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurosci, 1998; 18, 8886−99. doi: 10.1523/JNEUROSCI.18-21-08886.1998 [46] Jian CX, Liu XF, Hu J, et al. 20-Hydroxyecdysone-induced bone morphogenetic protein-2-dependent osteogenic differentiation through the ERK pathway in human periodontal ligament stem cells. Eur J Pharmacol, 2013; 698, 48−56. doi: 10.1016/j.ejphar.2012.07.044 [47] Bielen H, Houart C. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev Neurobiol, 2014; 74, 772−80. doi: 10.1002/dneu.22168 [48] García-Cerro S, Rueda N, Vidal V, et al. Normalizing the gene dosage of Dyrk1A in a mouse model of down syndrome rescues several Alzheimer's disease phenotypes. Neurobiol Dis, 2017; 106, 76−88. doi: 10.1016/j.nbd.2017.06.010 [49] Otero JJ, Fu WM, Kan LX, et al. β-catenin signaling is required for neural differentiation of embryonic stem cells. Development, 2004; 131, 3545−57. doi: 10.1242/dev.01218 [50] Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci, 2009; 12, 1097−105. doi: 10.1038/nn.2360 [51] Liu SW, Fang F, Song RX, et al. Sevoflurane affects neurogenesis through cell cycle arrest via inhibiting wnt/β-catenin signaling pathway in mouse neural stem cells. Life Sci, 2018; 209, 34−42. doi: 10.1016/j.lfs.2018.07.054 [52] Wang LF, Wei L, Qiao SM, et al. Microwave-induced structural and functional injury of hippocampal and PC12 cells is accompanied by abnormal changes in the NMDAR-PSD95-CaMKII pathway. Pathobiology, 2015; 82, 181−94. doi: 10.1159/000398803 [53] Ruggiero RN, Rossignoli MT, Marques DB, et al. Neuromodulation of hippocampal-prefrontal cortical synaptic plasticity and functional connectivity: implications for neuropsychiatric disorders. Front Cell Neurosci, 2021; 15, 732360. doi: 10.3389/fncel.2021.732360 [54] Gu JS, Hou ZJ, Zhou XT, et al. Activation of 5-HT1 receptor in lateral habenula impaired contextual fear memory and hippocampal LTP in rat. Neurosci Lett, 2022; 770, 136305. doi: 10.1016/j.neulet.2021.136305 [55] Korkmaz OT, Arkan S, Öncü-Kaya EM, et al. Vasoactive intestinal peptide (VIP) conducts the neuronal activity during absence seizures: GABA seems to be the main mediator of VIP. Neurosci Lett, 2021; 765, 136268. doi: 10.1016/j.neulet.2021.136268 [56] Veenman L. Raloxifene as treatment for various types of brain injuries and neurodegenerative diseases: a good start. Int J Mol Sci, 2020; 21, 7586. doi: 10.3390/ijms21207586 -




 下载:
下载:



 Quick Links
Quick Links