-
Tuberculous lymphadenitis (TBLN) is the most common manifestation of extrapulmonary tuberculosis (TB)[1]. Nowadays, several diagnostic methods are available for diagnosis of TBLN, such as fine needle aspiration (FNA) cytology, Ziehl-Neelsen (ZN) staining, mycobacterial culture (culture), and molecular tests like Xpert MTB/RIF (Xpert)[2-4]. However, a limited number of diagnostic methods are available for the basic-level hospitals in the developing countries witnessing high TB burdens. Although FNA cytology and ZN staining are the most commonly used diagnostic methods in the developing countries, low sensitivity and specificity are the two main concerns here[5-6]. Unfortunately, many suspected patients with TB receive the empiric anti-TB treatment based on only clinical findings and without performing the gold standard test[7-8]. As a result, a large proportion of suspected patients with TB visit bigger hospitals where culture or other molecular tests are available after a period of anti-TB treatment. Perhaps, the conversion of sputum culture after 2 months of anti-TB therapy is an important indicator for the effect of treatment undertaken so far. The conversion rate among drug-sensitive patients with TB is reported to range from 30% to over 80% after 2 months of regular anti-TB therapy[9-10]. In fact, wide application of the empiric anti-TB treatment before culture may result in poor sensitivity of the gold standard methods. The Global Tuberculosis Report published by the World Health Organization (WHO) in recent years reveals that only about 30% of reported TB cases are confirmed microbiologically in China, whereas in the developed countries, the positive rate of microbiological methods is above 70%[11-12].
Additionally, paradoxical reactions are often seen in patients with TB[13-14] and their lymph node lesion samples are sent for phenotypic or genotypic drug susceptibility tests (DST) after following a period of anti-TB treatment. However, the influence of anti-TB treatment on these various diagnostic methods is yet unclear.
On the other hand, most commonly used FNA cytology and ZN staining can neither differentiate TB from nontuberculous mycobacterial diseases not identify their drug resistance status. It is reported that molecular tests are able to identify Mycobacterium tuberculosis (M. tuberculosis) from other nontuberculous mycobacteria in formalin-fixed paraffin-embedded (FFPE) tissues[15-17].
Hence, in this study, we enrolled suspected patients with TBLN having different anti-TB treatment background and collected lymph node samples from them by FNA or direct biopsy. All the samples were divided into two halves. While one was processed into FFPE block and tested by histological examination, ZN staining, TB real-time PCR (TB-PCR) for M. tuberculosis identification, and high-resolution melting curve PCR (HRM) test for rifampicin DST, the other was directly processed for culture and Xpert. We compared the diagnostic performance of these methods, and also explored whether initial treatment/retreatment status and the length of anti-TB treatment before sampling affected the performance of these diagnostic tests.
-
All patients clinically suspected to suffer from TBLN were enrolled prospectively in this study between March 2015 and September 2015 at Beijing Chest Hospital, Beijing, China. The patients were classified as TBLN if (1) there was pathological or microbiological evidence; or (2) they were clinically suspected as TBLN and showed good response to anti-TB chemotherapy. The patients were classified into the ‘other diseases' group when histological diagnosis suggested alternative diseases. The patients who did not meet any of the above-mentioned criteria were excluded from the study.
A lymph node sample from each patient was collected by FNA or direct biopsy. All the samples were divided into two parts: one was directly processed for culture and Xpert, whereas the other was processed into an FFPE block for the remaining examinations.
The present study was conducted with the consent of the patients after obtaining an approval from the Ethical and Institutional Review Boards for Human Investigation of the Beijing Chest Hospital.
-
Two 4-μm thick FFPE tissue sections were prepared from each block. The first section was processed with H & E staining. Histological criteria for diagnosis of TB were based on the presence of epithelioid cell granuloma with or without multinucleated giant cells and with or without necrosis (including caseous necrosis and liquefied necrosis)[18]. The other section was stained by a modified acid-fast staining method at room temperature[19]. The presence of acid-fast bacilli (AFB) was confirmed under ×100 power oil immersion lens.
-
The DNA was extracted from FFPE samples utilizing the TIANamp FFPE DNA Kit (TIANGEN Biotech, Beijing, China). Briefly, six 4-μm thick sections were deparaffinized with xylene and washed once with absolute ethanol. Then, the sample was processed according to the manufacturer's instructions.
TB-PCR was performed utilizing the M. tuberculosis fluorescent PCR diagnostic kit (DaAn Gene, Guangzhou, China) according to the manufacturer's instructions. This PCR kit detects IS6110 that is specific for M. tuberculosis complex but is absent in the nontuberculous mycobacteria or in any the other bacteria.
The genotypic rifampin DST was performed utilizing the MeltPro® Mycobacterium tuberculosis Rifampicin-resistance Mutation Test Kit (Zeesan Biotech, Xiamen, China). This kit detects mutations in rpoB gene (codons 507-533), the rifampicin resistance-determining region (RRDR), by high-resolution melting curve analysis. The HRM test was performed according to the manufacturer's instructions.
-
The lymph node samples were homogenized utilizing ceramic sphere (MP Biomedicals, Santa Ana, CA) in 1 mL phosphate buffer saline (pH = 6.8) by the FastPrep-24™ homogenizer (MP Biomedicals, Santa Ana, CA) at 6.5 m/s for 40 s.
Of note, half of the homogenized sample was processed by the standard N-acetyl-L-cysteine (NALC)-NaOH method[20]. The processed samples were resuspended in 0.5 mL phosphate buffer and were cultured in mycobacterial growth indicator tube for 6 weeks (MGIT 960 System, Becton Dickinson, Sparks, MD). All positive cultures were subjected to ZN staining to confirm the presence of AFB.
The other half of the sample was mixed with bactericidal sample reagent at a ratio of 1:2, and subsequently processed according to the Xpert MTB/RIF (Cepheid, Sunnyvale, CA) manufacturer's instructions.
-
All data were analyzed with SPSS software package (V17.0, SPSS Inc., Chicago, IL). The Pearson's chi-squared test and the Fisher's exact test were conducted to determine the significance of categorical variables. The differences were considered statistically significant when P < 0.05.
-
In this study, we enrolled 95 patients with presumptive TBLN. Of these, 2 patients were excluded for not meeting the inclusion criteria and 4 patients were excluded for not having sufficient specimens for the study. Of 89 patients included in this study, females accounted for 57.3% (51 of 89). The age of the included patients ranged from 15 to 68 years, with a mean age of 30.9 years (± 11.7). All the patients tested negative for HIV. The majority [96.6% (86 of 89)] of the cases involved lymph node sites in the cervical regions. The specimens were obtained by FNA and direct biopsy for 50 and 39 cases, respectively. The histological examination demonstrated that 82 patients (92.1%) had inflammation features, 2 (2.2%) were considered as sarcoidosis, and 5 cases (5.6%) were malignancy.
Among patients with TBLN, 52 (63.4%) were selected for initial treatment and the remaining 30 (36.6%) were chosen for retreatment. Among these 82 TBLN lymph node specimens, 22 (26.8%) were obtained before anti-TB treatment, 19 (23.2%) from 1 day to 1 month after treatment, 17 (20.7%) from over 1 month to 3 months treatment, 20 (24.4%) from over 3 months treatment, and 4 (4.9%) cases were unknown.
-
For FFPE samples, granulomas were found in 58.5% (48 of 82) patients with TBLN, whereas AFB were detected in 43.9% (36 of 82) patients. The sensitivity of TB-PCR on FFPE samples (FFPE-PCR) was 69.5% (57 of 82) and was significantly higher than that of ZN staining (χ2 = 10.953, P = 0.001). Not only FFPE-PCR gave positive results in 86.1% (31 of 36) ZN staining positive samples, but also detected additional 56.5% (26 of 46) cases in ZN staining negative samples. When the histological examination was combined with FFPE-PCR, the sensitivity reached up to 82.9% (68 of 82).
The sensitivities of culture and Xpert on fresh samples were 22.0% (18 of 82) and 86.6% (71 of 82), respectively. Apparently, Xpert showed significantly higher sensitivity than culture (χ2 = 69.015, P < 0.0001). Although all the culture positive samples were Xpert positive, Xpert detected almost three times more positive cases compared to culture.
In 2 sarcoidosis and 5 malignant cases, culture, Xpert, ZN staining, and FFPE-PCR tests gave negative results, thereby showing good specificity. In addition, histology also indicated granulomas in 2 sarcoidosis samples, but was ruled out of TB upon combining the clinical information.
These diagnostic methods were also analyzed in patients with TBLN having different anti-TB treatment background. We found that the anti-TB treatment history had a great influence on the sensitivity of culture (Figure 1). In the initially treated group, the sensitivity of culture was 46.2% (24 of 52), whereas in the retreated group, the sensitivity dramatically decreased to 3.3% (1 of 30; χ2 = 16.460, P < 0.0001; Figure 1A). However, histology, ZN staining, Xpert, and FFPE-PCR did not differ significantly in the two groups (P > 0.05; Figure 1A). Furthermore, we compared different diagnostic methods with samples collected before treatment, with anti-TB treatment for 1 day to 1 month, with anti-TB treatment for over 1 month to 3 months, and with anti-TB treatment for over 3 months. The sensitivities of culture were 45.5% (10 of 22), 52.6% (10 of 19), 17.6% (3 of 17), and 0.0% (0 of 20) for the four different time periods, respectively. Yet again, the sensitivity of culture decreased dramatically after 1 month of treatment, and gave no positive results when the treatment lasted over 3 months (Figure 1B). For Xpert, the sensitivity was stable for 3-month treatment with over 86%, but decreased significantly to 70% when the treatment lasted over 3 months (χ2 = 7.798, P = 0.029, Fisher's exact test; Figure 1B). This implies that the length of anti-TB treatment did not affect the sensitivities of histology, ZN staining, and FFPE-PCR (P > 0.05; Figure 1B).
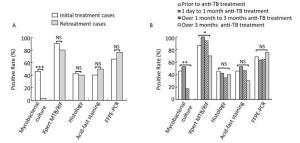
Figure 1. Comparison of sensitivities of different diagnostic methods on lymph node samples with different anti-TB treatment background. (A) Comparison of different diagnostic methods in initial treatment and retreatment groups; (B) Comparison of different diagnostic methods in different anti-TB treatment time groups. *P < 0.05, **P < 0.001, ***P < 0.0001, NSP > 0.05.
In this study, 1 patient with TBLN reported enlargement of the lymph node after 40 days of treatment. To clarify whether it resulted from failure of the treatment or was just a paradoxical reaction, biopsy was performed. After biopsy, both Xpert and FFPE-PCR showed positive results. To elaborate, quantification of the M. tuberculosis amount by FFPE-PCR revealed a huge amount of bacilli, 8.3 × 105 cfu/mL (1660 cfu/per section), in the sample. Detection of Ag85B antigen from M. tuberculosis by immunohistochemistry showed abundant M. tuberculosis bacilli in the tissue (Figure 2B and C) However, culture gave negative results and ZN staining only showed a few AFB in the sample (Figure 2A). Both molecular test and immunohistochemistry only detect nucleic acid or protein but not the intact live bacilli. However, culture only detects live bacilli whereas ZN staining only detects intact bacilli. These results suggested that most bacilli detected in this case were cell wall-deficient and might be in a nonactive state or not alive. Rifampicin susceptibility test by FFPE-HRM also showed no mutation in rpoBRRDR region, suggesting rifampicin sensitive case. From these results, we concluded that anti-TB treatment was effective and the enlargement of lymph node was just a paradoxical reaction.
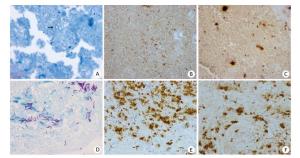
Figure 2. Ziehl-Neelsen staining and M. tuberculosis Ag85B immunohistochemistry staining on lymph node tissues from 2 patients with TBLN having different anti-TB treatment background. (A to C) from a patient with TBLN after 40 days of anti-TB treatment; (D to F) from a patient with TBLN without anti-TB treatment. (A and C) ZN staining of AFB (× 100 oil immersion); (B, C, E, and F) immunohistochemistry staining to detect Ag85B expression (× 40 magnification).
All these results indicated that Xpert was the most sensitive test, and FFPE-PCR, histology, and ZN staining descended from higher sensitivity to lower sensitivity. Of all, culture was the most insensitive method and its sensitivity was dramatically influenced by the anti-TB treatment history.
-
In this study, rifampicin susceptibility was tested by culture, Xpert, and HRM on FFPE samples (FFPE-HRM), all of which gave successful DST results for 22, 69, and 30 samples, respectively (Table 1). Apparently, Xpert showed significantly higher success rate than that of culture (χ2 = 54.535, P < 0.0001) and FFPE-HRM (χ2 = 38.764, P < 0.0001). The success rates of culture and FFPE-HRM were not significantly different (P > 0.05), and these two methods showed rather complementary results. Only eight samples were successful by both culture and FFPE-HRM; however, 14 samples were successful by culture but failed FFPE-HRM, and 22 samples were successful by FFPE-HRM but failed culture (Table 1).
Table 1. Performance of Xpert MTB/RIF, mycobacterial culture, and high-resolution melting curve tests on determining rifampicin susceptibility
Counts Xpert Successful Xpert Failed Culture successful Culture failed Culture successful Culture failed FFPE-HRM successful 8 (1 rifampicin resistant) 22 (0 rifampicin resistant) 0 0 FFPE-HRM failed 14 (1 rifampicin resistant) 25 (1 rifampicin resistant) 0 13 Evidently, the success rate of culture was significantly affected by anti-TB treatment. In the initially treated group, the success rate of culture was 40.4% (21 of 52), whereas in the retreated group, the success rate dramatically decreased to 3.3% (1 of 30; χ2 = 13.304, P < 0.001; Figure 3A). The success rates were 36.4% (8 of 22), 47.4% (9 of 19), 17.6% (3 of 17), and 0.0% (0 of 20) in the four different time periods of receiving anti-TB treatment, respectively. Once again, the success rate of culture decreased dramatically after 1 month of treatment and gave no positive results when the treatment lasted over 3 months (χ2 = 14.948, P = 0.001; Figure 3B). However, the anti-TB treatment history did not affect the success rate of Xpert or FFPE-HRM (P > 0.05; Figure 3).
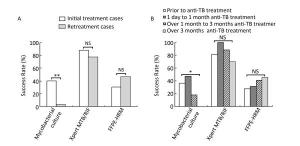
Figure 3. Comparison of performance of different rifampicin susceptibility tests on lymph node samples with different anti-TB treatment background. (A) Comparison of different rifampicin susceptibility tests in initial treatment and retreatment groups; (B) Comparison of different rifampicin susceptibility tests in different anti-TB treatment time groups. *P < 0.01, **P < 0.001, NSP > 0.05.
The Xpert method detected all three rifampicin-resistant samples, two of which were confirmed by culture, and one was confirmed by both culture and FFPE-HRM (Table 1). The rifampicin-resistant rate detected by culture, Xpert, and FFPE-HRM was 9.1% (2 of 22), 4.3% (3 of 69), and 3.3% (1 of 30). All the DST results of culture or FFPE-HRM were in perfect match with Xpert, in addition, the DST result from eight samples that was detected by both culture and FFPE-HRM was in perfect accordance.
These result suggested that the anti-TB treatment background had significant influence on the success rate of culture, but not on molecular DSTs. Moreover, genotypic DSTs were in good accordance with phenotypic DST. The Xpert method was superior to culture and FFPE-HRM in detecting rifampicin resistance.
-
As the most common manifestation of extrapulmonary TB, TBLN is a great health-related problem in the TB endemic area. Perhaps, definite diagnosis of TBLN, according to the WHO guideline, is made available by detecting M. tuberculosis in the lesion[21]. However, in developing countries, culture and molecular tests are not available in basic-level hospitals, and the empiric anti-TB treatment on suspected patients with TBLN is very popular[7-8]. A large amount of lymph node samples are sent to high-level hospitals for further diagnosis after a period of anti-TB treatment. Nevertheless, the influence of the treatment history on histology, microbiology, and molecular tests is not clear. Therefore, in this study, we investigated whether initial treatment/retreatment status and the length of anti-TB treatment before sampling affected the performance of these diagnostic tests.
In this study, we deduced that the treatment history and the length of anti-TB treatment greatly affected the performance of culture but had a little influence on the remaining methods. After 1-month treatment, the sensitivity of culture decreased from over 45% to less than 18%. After 3 months of treatment, no sample gave positive result by culture (Figure 1).
We propose the following reason to explain the results. It is reported that a positive result from culture is associated with live and active M. tuberculosis bacterium, whereas molecular tests can also detect dead or non-active bacteria[22].
In this study, we reported a TBLN case showing a paradoxical reaction after a period of anti-TB treatment. While the culture came out negative, ZN staining only showed a few AFB in the sample (Figure 2A). However, FFPE-PCR showed a huge number of bacilli in the lesion and immunohistochemistry also exhibited abundant expression of M. tuberculosis antigen Ag85B (Figure 2B and C). Usually, the secretory antigen Ag85B can only be detected outside the M. tuberculosis and not inside because the thick cell wall hampers penetration of the antibody (Figure 2E and F)[19, 23]. However, in this case, Ag85B expression was clearly detected in the whole part of bacilli (Figure 2B and C). This expression pattern was dramatically different from active M. tuberculosis bacilli in which Ag85B was highly expressed and widely secreted in the outer space (Figure 2B, C, E, and F). We also noticed that AFB found in this case were shorter than those detected in a patient with no anti-TB treatment (Figure 2A and D). Furthermore, although many M. tuberculosis bacilli were detected by immunohistochemistry, only a few bacilli were shown by ZN staining. It is well known that a thick cell wall is critical for ZN staining. If the cell wall is destroyed, ZN staining cannot stain the bacilli with the dye[24-25]. Hence, we infer that anti-TB treatment was effective in this case and most of the bacilli detected were cell wall-deficient, inactive, or dead organisms. This case supports that culture is not an effective method for diagnosis of TBLN after a period of anti-TB treatment; however, it may reflect treatment outcome more accurately and promptly.
Our study suggests that more attention should be paid to false negative results from culture in diagnosis of suspected patients with TBLN with the anti-TB treatment history. Definite diagnosis of TBLN as per the WHO guideline mainly depends on culture, but it is difficult for the developing countries to follow up in this case. The application of histology and molecular tests will help the developing countries to make more accurate diagnosis of TBLN.
In this study, we also reported the application of molecular tests for the diagnosis of drug-resistant TBLN from tissue samples. A total of three rifampicin-resistant (RR) TBLN cases were detected. While Xpert detected all 3 RR cases, culture detected 2 RR cases with initial treatment and FFPE-HRM detected 1 RR cases with initial treatment. The success rate of culture DST was significantly higher in initial group (40.4%) than in the retreatment group (3.3%); however, there were no significant differences with those of Xpert and FFPE-HRM tests. Although different DST methods gave results in the different samples, the results from the same samples by two or more methods were in perfect accordance (Table 1). These results indicated that genotypic DSTs were reliable with high success rate.
In conclusion, the anti-TB treatment background has great influence on the sensitivity of culture, especially by retreatment status and over 1 month treatment time, but has little influence on pathology and molecular tests. Molecular tests, especially Xpert, show significantly higher sensitivity than other methods. For the drug sensitivity, Xpert also shows highest success rate and is in good accordance with culture and FFPE-HRM. However, culture reflects the treatment effect more correctly and timely than other methods. Our results may contribute to more accurate TBLN diagnosis post-treatment, especially in the developing countries, where the empiric anti-TB treatment is more popular.
doi: 10.3967/bes2017.055
Comparison of Histological, Microbiological, and Molecular Methods in Diagnosis of Patients with TBLN Having Different Anti-TB Treatment Background
-
Abstract:
Objective The influence of anti-tuberculosis (TB) treatment history on tuberculous lymphadenitis (TBLN) diagnosis is unclear. Therefore, this study aims to evaluate the diagnostic methods, including histology, microbiology, and molecular tests, used for TBLN. Methods In this study, suspected patients with TBLN and having different anti-TB treatment background were enrolled. All the samples were tested simultaneously by histology, Ziehl-Neelsen (ZN) staining, mycobacterial culture (culture), Xpert MTB/RIF (xpert), real-time PCR, and high-resolution melting curve PCR (HRM). Thereafter, the performance of these methods on samples with different anti-TB treatment background was assessed. Results In our study, 89 patients were prospectively included 82 patients with TBLN and 7 with other diseases. The overall sensitivities of Xpert, real-time PCR, histology, ZN staining, and culture were 86.6%, 69.5%, 58.5%, 43.9%, and 22.0%, respectively. The anti-TB treatment history revealed dramatic influences on the sensitivity of culture (P < 0.0001). In fact, the treatment that lasted over 3 months also influenced the sensitivity of Xpert (P < 0.05). However, the treatment history did not affect the performance of remaining tests (P > 0.05). For rifampicin drug susceptibility test (DST), the anti-TB treatment showed only significant influence on the success rate of culture DST (P=0.001), but not on those of Xpert and HRM tests (P > 0.05). Conclusion Other tests as well as culture should be considered for patients with TBLN having retreatment history or over 1-month treatment to avoid false negative results. -
Key words:
- Tuberculous lymphadenitis /
- Mycobacterial culture /
- Molecular test /
- Anti-TB treatment /
- Drug resistance
注释:1) COMPETING INTERESTS: -
Figure 1. Comparison of sensitivities of different diagnostic methods on lymph node samples with different anti-TB treatment background. (A) Comparison of different diagnostic methods in initial treatment and retreatment groups; (B) Comparison of different diagnostic methods in different anti-TB treatment time groups. *P < 0.05, **P < 0.001, ***P < 0.0001, NSP > 0.05.
Figure 2. Ziehl-Neelsen staining and M. tuberculosis Ag85B immunohistochemistry staining on lymph node tissues from 2 patients with TBLN having different anti-TB treatment background. (A to C) from a patient with TBLN after 40 days of anti-TB treatment; (D to F) from a patient with TBLN without anti-TB treatment. (A and C) ZN staining of AFB (× 100 oil immersion); (B, C, E, and F) immunohistochemistry staining to detect Ag85B expression (× 40 magnification).
Figure 3. Comparison of performance of different rifampicin susceptibility tests on lymph node samples with different anti-TB treatment background. (A) Comparison of different rifampicin susceptibility tests in initial treatment and retreatment groups; (B) Comparison of different rifampicin susceptibility tests in different anti-TB treatment time groups. *P < 0.01, **P < 0.001, NSP > 0.05.
Table 1. Performance of Xpert MTB/RIF, mycobacterial culture, and high-resolution melting curve tests on determining rifampicin susceptibility
Counts Xpert Successful Xpert Failed Culture successful Culture failed Culture successful Culture failed FFPE-HRM successful 8 (1 rifampicin resistant) 22 (0 rifampicin resistant) 0 0 FFPE-HRM failed 14 (1 rifampicin resistant) 25 (1 rifampicin resistant) 0 13 -
[1] Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician, 2005; 72, 1761-8. http://www.aafp.org/afp/2005/1101/p1761.html [2] Wright CA, Hesseling AC, Bamford C, et al. Fine-needle aspiration biopsy: a first-line diagnostic procedure in paediatric tuberculosis suspects with peripheral lymphadenopathy? Int J Tuberc Lung Dis, 2009; 13, 1373-9. https://www.cabdirect.org/cabdirect/abstract/20093359510 [3] Abdissa K, Tadesse M, Bezabih M, et al. Bacteriological methods as add on tests to fine-needle aspiration cytology in diagnosis of tuberculous lymphadenitis: can they reduce the diagnostic dilemma? BMC Infect Dis, 2014; 14, 720. doi: 10.1186/s12879-014-0720-z [4] Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J, 2014; 44, 435-46. doi: 10.1183/09031936.00007814 [5] Kaur G, Dhamija A, Augustine J, et al. Can cytomorphology of granulomas distinguish sarcoidosis from tuberculosis? Retrospective study of endobronchial ultrasound guided transbronchial needle aspirate of 49 granulomatous lymph nodes. Cytojournal, 2013; 10, 19. doi: 10.4103/1742-6413.119008 [6] Narang S, Solanki A, Kashyap S, et al. Utility of fine needle aspiration cytology to comprehend the pathogenesis of extrapulmonary tuberculosis. Diagn Cytopathol, 2016; 44, 98-102. doi: 10.1002/dc.23403 [7] Peter JG, Theron G, Pooran A, et al. Comparison of two methods for acquisition of sputum samples for diagnosis of suspected tuberculosis in smear-negative or sputum-scarce people: a randomised controlled trial. Lancet Respir Med, 2013; 1, 471-8. doi: 10.1016/S2213-2600(13)70120-6 [8] Onal IK, Cankurtaran M, Cakar M, et al. Fever of unknown origin: what is remarkable in the elderly in a developing country? J Infect, 2006; 52, 399-404. doi: 10.1016/j.jinf.2005.08.021 [9] Micheletti VC, Kritski AL, Braga JU. Clinical Features and Treatment Outcomes of Patients with Drug-Resistant and Drug-Sensitive Tuberculosis: A Historical Cohort Study in Porto Alegre, Brazil. PLoS One, 2016; 11, e0160109. doi: 10.1371/journal.pone.0160109 [10] Velayutham BV, Allaudeen IS, Sivaramakrishnan GN, et al. Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India. Clin Infect Dis, 2014; 59, e142-9. doi: 10.1093/cid/ciu550 [11] World Health Organization, Global tuberculosis report 2014, WHO Press: Geneva, Switzerland, 2014. [12] World Health Organization, Global tuberculosis report 2015, WHO Press: Geneva, Switzerland, 2015. [13] Brown CS, Smith CJ, Breen RA, et al. Determinants of treatment-related paradoxical reactions during anti-tuberculosis therapy: a case control study. BMC Infect Dis, 2016; 16, 479. doi: 10.1186/s12879-016-1816-4 [14] Rajendra A, Sabnis K, Jeyaseelan V, et al. Paradoxical reaction (PR) in tuberculous lymphadenitis among HIV-negative patients: retrospective cohort study. Postgrad Med J, 2016; pii: postgradmedj-2016-134326. http://pmj.bmj.com/content/92/1093/684 [15] Munkhdelger J, Wang HY, Choi Y, et al. Identification of Mycobacterium species in FFPE granulomatous lymphadenitis tissue using REBA Myco-ID®. Int J Tuberc Lung Dis, 2013; 17, 898-902. doi: 10.5588/ijtld.12.0818 [16] Fu YC, Liao IC, Chen HM, et al. Detection of Mycobacterium tuberculosis Complex in Paraffin-Embedded Tissues by the New Automated Abbott RealTime MTB Assay. Ann Clin Lab Sci, 2016; 46, 412-7. https://www.ncbi.nlm.nih.gov/pubmed/27466302 [17] Meghdadi H, Khosravi AD, Ghadiri AA, et al. Detection of Mycobacterium tuberculosis in extrapulmonary biopsy samples using PCR targeting IS6110, rpoB, and nested-rpoB PCR Cloning. Front Microbiol, 2015; 6, 675. https://www.researchgate.net/publication/280220144_Detection_of_Mycobacterium_tuberculosis_in_extrapulmonary_biopsy_samples_using_PCR_targeting_IS6110_rpoB_and_nested-rpoB_PCR_Cloning [18] Gupta AK, Nayar M, Chandra M. Critical appraisal of fine needle aspiration cytology in tuberculous lymphadenitis. Acta Cytol, 1992; 36, 391-4. http://europepmc.org/abstract/MED/1580124 [19] Che N, Qu Y, Zhang C, et al. Double staining of bacilli and antigen Ag85B improves the accuracy of the pathological diagnosis of pulmonary tuberculosis. J Clin Pathol, 2016; 69, 600-6. doi: 10.1136/jclinpath-2015-203244 [20] Tadesse M, Abebe G, Abdissa K, et al. GeneXpert MTB/RIF Assay for the Diagnosis of Tuberculous Lymphadenitis on Concentrated Fine Needle Aspirates in High Tuberculosis Burden Settings. PLoS One, 2015; 10, e0137471. doi: 10.1371/journal.pone.0137471 [21] World Health Organization, Treatment of tuberculosis: guidelines -4th ed, WHO Press: Geneva, Switzerland, 2010. [22] Boyles TH, Hughes J, Cox V, et al. False-positive Xpert® MTB/RIF assays in previously treated patients: need for caution in interpreting results. Int J Tuberc Lung Dis, 2014; 18, 876-8. doi: 10.5588/ijtld.13.0853 [23] Lanéelle MA, Daffé M. Transport assays and permeability in pathogenic mycobacteria. Methods Mol Biol, 2009; 465, 143-51. doi: 10.1007/978-1-59745-207-6 [24] Chandrasekhar S, Ratnam S. Studies on cell-wall deficient non-acid fast variants of Mycobacterium tuberculosis. Tuber Lung Dis, 1992; 73, 273-9. doi: 10.1016/0962-8479(92)90132-4 [25] Seiler P, Ulrichs T, Bandermann S, et al. Cell-wall alterations as an attribute of Mycobacterium tuberculosis in latent infection. J Infect Dis, 2003; 188, 1326-31. doi: 10.1086/jid.2003.188.issue-9 -




 下载:
下载:



 Quick Links
Quick Links