-
A number of epidemiological studies have investigated an association of self-reported sleep duration and risk of diabetes[1-2], hypertension[3-4], myocardial infarction[5-6], stroke[7-8], and mortality[9-10]. With increasing life expectancy in many societies, cognitive impairment or neurodegenerative disease has become a growing problem. Esposito and Carotenuto found that sleep was associated with cognition processes, particularly in cognitive borderline dysfunction[11-12]. Moreover, more and more researchers have begun to explore the association between night sleep duration and cognitive impairment, and a commonly reported finding is that both short and long sleep duration is associated with cognitive impairment[13-19]. However, the evidence is mixed and inconsistent regarding the association of sleep duration with the risk of cognitive impairment. Different cross-sectional studies have suggested that only long night sleep duration[15-16, 20], only short night sleep duration[17, 19, 21], or both short and long sleep duration (U-shaped relationship or V-shaped relationship)[13, 18, 22] was associated with an increased risk of cognitive impairment. In addition, a small number of longitudinal studies have begun to examine whether habitual short or long night sleep duration increases the risk for cognitive decline[23-25] in the elderly, but the results were also mixed. Recently, a cross-sectional study from southern China showed that short or long night sleep duration was an important sleep-related factor independently associated with memory impairment in older people (50-85 years of age)[13]. It is evident that lifestyle, living conditions, and food intake differs between southern and northern Chinese populations. And in their study[13], carotid plaque, a very important factor with respect to cognitive impairment[26], was not considered a confounding factor. It is still unknown whether there is an association between night sleep duration and cognitive impairment in people in northern China. Therefore, we used data from the Kailuan Study to explore whether night sleep duration is associated with cognitive impairment.
-
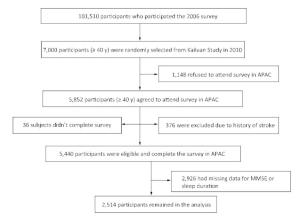
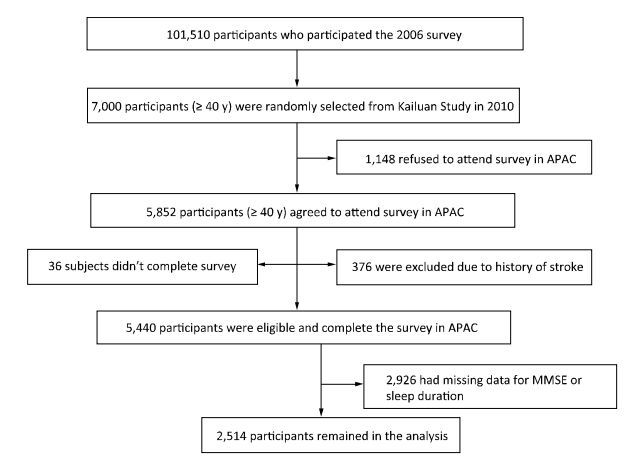
We conducted a cross-sectional analysis of baseline data in the Asymptomatic Polyvascular Abnormalities Community (APAC) Study. The APAC Study is a community-based, ongoing observational study that aims to investigate asymptomatic polyvascular abnormalities in Chinese adults[27]. From June 2010 to June 2011, 7, 000 eligible adults over 40 years of age (stratified by age and sex) were randomly sampled from a reference population of 101, 510 participants (20, 400 women and 81, 110 men, aged 18-98 years) who had been participating in a long-term follow-up study (Kailuan Study[28]) since 2006 in the Kailuan Coal Group in Tangshan, a modern coastal city in northern China. The sample size was calculated based on detection of a 7% event rate with 0.7% precision and an α value of 0.05. The response rate was assumed to be > 80%. A total of 5, 852 individuals agreed to participate in the APAC Study and 5, 816 people eventually completed the baseline data collection. Among these 5, 816 individuals, 376 subjects did not meet the following inclusion criteria: (1) no history of stroke, transient ischemic attack, or coronary disease at baseline as assessed by a validated questionnaire; and (2) absence of neurologic deficits for stroke as estimated by experienced doctors. Finally, 5, 440 participants were eligible and included in the APAC Study[29]. After excluding individuals with missing data for the Mini-Mental State Examination (MMSE) or sleep duration (n = 2, 926), 2, 514 participants remained in the analysis (1, 172 women and 1, 342 men) (Figure 1). The study was approved by the Ethics Committee of Kailuan General Hospital, following the guidelines outlined by the Helsinki Declaration. All participants agreed to participate in this study and provided written informed consent.
-
Night sleep duration was obtained through a self-reported answer to the question, 'How many hours of sleep did you have on an average night in the preceding 3 months?' Based on participants' responses, sleep duration was categorized into five groups: ≤ 5, 6, 7, 8, and ≥ 9 h[30].
-
Cognitive function was measured using the MMSE in 2012. The MMSE is a measure of general cognitive function and includes orientation to time and place, attention and calculation, language, and memory[31]. Higher scores indicate greater cognitive function. Cognitive impairment was defined as a score of < 24.
-
All participants underwent a clinical examination and a standardized interview. The information on physical activity, smoking, and drinking status was evaluated as detailed previously[30]. Education level was categorized as illiteracy or primary, middle school, and high school or above. The family per-member monthly income was categorized as < ¥1, 000, ¥1, 000-3, 000, or ≥ ¥3, 000. Anthropomorphic parameters such as body height and weight and waist circumference were measured. Body weight (kg) was divided by the square of height (m2) to determine the body mass index (BMI). Fasting blood glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride, and total cholesterol levels were evaluated. Diabetes mellitus was defined as a self-reported history or current treatment with insulin or oral hypoglycemic agents. Hyperlipidemia was defined as a history of hyperlipidemia, total blood cholesterol level ≥ 220 mg/dL, triglyceride level ≥ 150 mg/dL, or use of antihyperlipidemic medications. Hypertension was defined as a history of hypertension, a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications[32].
-
High-resolution B-mode ultrasounds (Philips iU-22 ultrasound system, Philips Medical Systems, Bothell, WA) with a 5-to 12 MHz linear array transducer was used to detect plaques bilaterally on three segments: the common carotid artery, the bifurcation, and the internal carotid artery. Atherosclerotic plaque was defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or a thickness > 1.5 mm as measured from the media-adventitia interface to the intima-lumen interface[33].
-
Data are presented as means ± standard deviations for continuous variables and frequencies and percentages for categorical variables. One-way analysis of variance was used for unpaired samples of normally distributed parameters and the Kruskal-Wallis test for non-parametric variables. The χ2 test was applied to compare categorical variables. A multivariate analysis was performed using three models. Model 1 was adjusted for age and sex, and model 2 for age, sex, BMI, family per-member monthly income, education level, smoking status, drinking status, physical activity, and snoring. In addition to the independent parameters analyzed in model 2, model 3 also included history of hypertension, diabetes mellitus, hyperlipidemia, stroke, and carotid plaque. We used a logistic regression model to calculate the odds ratios and 95% confidence intervals (CI) of cognitive impairment (the group reporting 7-h night sleep duration was used as the reference category). All interactions were analyzed by multiplicative logistic regression modeling. The level for statistical significance was set at P ≤ 0.05 (two-tailed). Statistical analysis was performed using SAS version 9.3 statistical software (SAS Institute Inc., Cary, NC, USA).
-
In total, 2, 514 participants (1, 342 men and 1, 172 women) were enrolled in this study (mean age, 52.37 ± 9.40 years). Among the study population, 122 (4.85%) patients were diagnosed with cognitive impairment, defined as an MMSE score < 24. Table 1 summarizes the characteristics of participants and non-participants. Compared with the group of nonparticipants, the group of study participants was significantly younger; had a higher proportion of women, higher BMI, and a higher prevalence of married family status and high-school graduation; and had a lower reported income, SBP, and fasting blood glucose concentration and a lower proportion of participants with a carotid plaque (Table 1).
Table 1. Comparison of Demographic and other Characteristics of Participants and Non-participants
Variable Overall Participants Non-participants P Value No. 5, 440 2, 514 2, 926 Age (y) 57.7 ± 11.2 55.8 ± 9.5 59.6 ± 12.5 < 0.001 Men, n (%) 3, 257 (59.9) 1, 342 (53.4) 1, 915 (65.4) < 0.001 Marital status (married), n (%) 4, 165 (76. 6) 2, 466 (98.1) 1, 699 (58.1) < 0.001 Body mass index (kg/m) 24.9 ± 3.2 25.0 ± 3.3 24.8 ± 3.2 0.090 Systolic blood pressure (mmHg) 130.9 ± 19.6 128.6 ± 18.6 133.4 ± 20.4 < 0.001 Diastolic blood pressure (mmHg) 82.8 ± 10.9 82.4 ± 10.5 83.1 ± 11.3 0.180 Fasting blood glucose (mmol/L) 5.8 ± 1.9 5.7 ± 1.7 5.8 ± 2.3 < 0.010 Education, n (%) < 0.001 Illiteracy/primary school 1, 104 (21.9) 149 (5.9) 955 (37.6) Middle school 2, 657 (52.6) 1, 544 (61.4) 1, 113 (43.9) High-school graduate 1, 290 (25.5) 821 (32.7) 469 (18.5) Family per-member monthly income ≥ ¥3, 000, n (%) 1, 074 (21.3) 447 (17.8) 627 (24.7) < 0.001 Physical activity > 4 times/week, n (%) 846 (16.8) 311 (12.4) 535 (21.1) < 0.001 Current smoker, n (%) 1, 600 (31.7) 838 (33.3) 762 (30.0) 0.050 Current alcohol drinker, n (%) 1, 444 (28.6) 802 (31.9) 642 (25.3) < 0.001 History of disease Hypertension, n (%) 2, 529 (50.1) 1, 225 (48.7) 1, 304 (51.4) 0.060 Diabetes mellitus, n (%) 703 (13.9) 353 (14.0) 350 (13.8) 0.800 Hyperlipidemia, n (%) 2, 251 (44.6) 1, 281 (50.9) 970 (38.2) < 0.001 Stroke, n (%) 71 (1.3) 24 (1.0) 47 (1.6) < 0.050 Carotid plaque, n (%) 1, 886 (46.6) 1, 053 (42.3) 833 (53.6) < 0.001 Snoring, n (%) 410 (9.6) 273 (10.9) 137 (7.8) < 0.010 Night sleep duration, n (%) ≤ 5 h 684 (12.6) 282 (11.2) 402 (13.7) 6 h 1, 511 (27.8) 675 (26.8) 836 (28.6) 7 h 2, 300 (42.3) 1, 165 (46.3) 1, 135 (38.8) 8 h 881 (16.2) 367 (14.6) 514 (17.6) ≥ 9 h 64 (1.2) 25 (1.0) 39 (1.3) Table 2 presents the characteristics of patients with good cognition and cognitive impairment. Age, sex, marital status, SBP, education, income level, alcohol use, and history of hypertension and carotid plaque were associated with cognitive impairment (P < 0.05). Compared with patients with good cognition, patients with cognitive impairment had a higher proportion of participants with a night sleep duration of ≥ 9 h (4.10% vs. 0.84%, P < 0.001).
Table 2. Comparisons between Patients with and without Cognitive Impairment in the Kailuan Study
Variable Overall Cognitively Intact
(MMSE ≥ 24)Cognitively Impaired
(MMSE < 24)P Value No. 2, 514 2, 392 122 Age (y) 55.8 ± 9.5 55.3 ± 9.1 65.3 ± 11.9 < 0.001 Men, n (%) 1, 342 (53.4) 1, 260 (52.7) 82 (67.2) < 0.001 Marital status (married), n (%) 2, 466 (98.1) 2, 352 (98.3) 114 (93.4) < 0.001 Body mass index (kg/m) 25.0 ± 3.3 25.0 ± 3.3 24.9 ± 3.5 0.850 Systolic blood pressure (mmHg) 128.6 ± 18.6 128.2 ± 18.4 137.4 ± 20.1 < 0.001 Diastolic blood pressure (mmHg) 82.4 ± 10.5 82.4 ± 10.4 84.2 ± 11.1 0.080 Fasting blood glucose (mmol/L) 5.7 ± 1.7 5.7 ± 1.6 6.1 ± 2.4 0.080 Education, n (%) < 0.001 Illiteracy/primary school 149 (5.9) 121 (5.1) 28 (23.0) Middle school 1, 544 (61.4) 1, 458 (61.0) 86 (70.5) High-school graduate 821 (32.7) 813 (33.9) 8 (6.6) Family per-member monthly income ≥ ¥3, 000, n (%) 447 (17.8) 432 (18.1) 15 (12.3) < 0.050 Physical activity > 4 times/week, n (%) 311 (12.4) 299 (12.5) 12 (9.8) 0.120 Current smoker, n (%) 838 (33.3) 790 (33.0) 48 (39.3) 0.240 Current alcohol drinker, n (%) 802 (31.9) 764 (31.9) 38 (31.2) < 0.001 History of disease Hypertension, n (%) 1, 225 (48.7) 1, 145 (47.9) 80 (65.6) < 0.001 Diabetes mellitus, n (%) 353 (14.0) 330 (13.8) 23 (18.9) 0.140 Hyperlipidemia, n (%) 1, 281 (50.9) 1, 227 (51.3) 54 (44.3) 0.130 Stroke, n (%) 24 (1.0) 20 (0.8) 4 (3.3) < 0.050 Carotid plaque, n (%) 1, 053 (42.3) 982 (41.4) 71 (59.2) < 0.001 Snoring, n (%) 273 (10.9) 255 (10.7) 18 (14.8) 0.370 Night sleep duration, n (%) < 0.001 ≤ 5 h 282 (11.2) 255 (10.7) 27 (22.1) 6 h 675 (26.8) 643 (26.9) 32 (26.2) 7 h 1, 165 (46.3) 1, 125 (47.0) 40 (32.8) 8 h 367 (14.6) 349 (14.6) 18 (14.8) ≥ 9 h 25 (1.0) 20 (0.8) 5 (4.1) Note.MMSE: mini-mental state examination. Table 3 shows the baseline characteristics of participants according to mean night sleep duration. The baseline characteristics of the participants varied significantly between sleep categories except marital status (P = 0.22), BMI (P = 0.30), and history of hyperlipidemia (P = 0.11). In particular, most of the short-duration sleepers or long-duration sleepers were older and male participants, and they had a greater likelihood of having hypertension, diabetes mellitus, snoring, and carotid plaque.
Table 3. Characteristics (2012) according to Night Sleep Duration
Variable Night Sleep Duration ≤ 5 h 6 h 7 h 8 h ≥ 9 h P No. 282 675 1, 165 367 25 Age (y) 58.0 ± 10.9 56.3 ± 9.6 55.4 ± 9.0 54.1 ± 8.8 58.1 ± 11.4 < 0.001 Men, n (%) 175 (62.1) 385 (57.0) 593 (50.9) 173 (47.1) 16 (64.0) < 0.001 Marital status (married), n (%) 274 (97.2) 657 (97.3) 1, 149 (98.6) 361 (98.4) 25 (100) 0.220 Body mass index (kg/m2) 25.2 ± 3.4 25.1 ± 3.3 24.8 ± 3.3 25.1 ± 3.2 25.7 ± 3.9 0.300 Systolic blood pressure (mmHg) 130.4 ± 19.6 129.9 ± 18.2 127.7 ± 18.4 127.6 ± 18.8 133.6 ± 18.8 < 0.050 Diastolic blood pressure (mmHg) 82.8 ± 10.8 83.3 ± 10.1 81.9 ± 10.5 82.0 ± 10.5 85.9 ± 11.3 < 0.050 Fasting blood glucose (mmol/L) 5.8 ± 1.7 5.8 ± 1.7 5.6 ± 1.6 5.7 ± 1.7 6.9 ± 3.8 < 0.050 Education, n (%) < 0.001 Illiteracy/primary school 22 (7.8) 39 (5.8) 58 (5.0) 29 (7.9) 1 (4.0) Middle school 194 (68.8) 438 (64.9) 670 (57.5) 221 (60.2) 21 (84.0) High-school graduate 66 (23.4) 198 (29.3) 437 (37.5) 117 (31.9) 3 (12.0) Family per-member monthly income ≥ ¥3, 000, n (%) 40 (14.2) 112 (16.6) 232 (19.9) 60 (16.4) 3 (12.0) < 0.001 Physical activity > 4 times/week, n (%) 52 (18.4) 97 (14.4) 121 (10.4) 38 (10.4) 3 (12.0) < 0.001 Current smoker, n (%) 110 (39.0) 246 (36.4) 370 (31.8) 105 (28.6) 7 (28.0) < 0.050 Current alcohol drinker, n (%) 111 (39.4) 241 (35.7) 343 (29.4) 99 (26.9) 8 (32.0) < 0.050 History of disease Hypertension, n (%) 151 (53.6) 349 (51.7) 553 (47.5) 158 (43.1) 14 (56.0) < 0.050 Diabetes mellitus, n (%) 37 (13.1) 107 (15.8) 148 (12.7) 53 (14.4) 8 (32.0) < 0.050 Hyperlipidemia, n (%) 148 (52.5) 372 (55.1) 569 (48.8) 180 (49.1) 12 (48.0) 0.110 Stroke, n (%) 2 (0.7) 12 (1.8) 8 (0.7) 1 (0.3) 1 (4.0) < 0.050 Carotid plaque, n (%) 154 (55.0) 292 (43.9) 461 (39.8) 135 (37.1) 11 (44.0) < 0.001 Snoring, n (%) 64 (22.7) 86 (12.7) 88 (7.6) 32 (8.7) 3 (12.0) < 0.001 A multivariate regression analysis was performed to investigate the association between night sleep duration and cognitive function (Table 4). After adjusting for age, sex, family per-member monthly income, education level, marital status, smoking status, drinking status, physical activity, BMI, history of diabetes, hypertension, hyperlipidemia, stroke, and carotid plaque, compared with the participants sleeping 7 h, the multivariable adjusted odds ratios (in model 3) of cognitive impairment among participants were 2.14 (95% CI, 1.20-3.83) for ≤ 5 h, 1.13 (95% CI, 0.67-1.89) for 6 h, 1.51 (95% CI, 0.82-2.79) for 8 h, and 5.37 (95% CI, 1.62-17.80) for ≥ 9 h sleep duration.
Table 4. Odds Ratios (95% CI) for Cognitive Impairment according to Night Sleep Duration
Items 95% CI (according to Night Sleep Duration) ≤ 5 h 6 h 7 h 8 h ≥ 9 h Cases, n (%) 27 (9.57) 32 (4.74) 40 (3.43) 18 (4.90) 5 (20.00) Model 1† 2.27 (1.34-3.86) 1.26 (0.78-2.06) Reference 1.65 (0.92-2.96) 5.54 (1.78-17.28) Model 2‡ 2.12 (1.20-3.77) 1.18 (0.71-1.95) Reference 1.46 (0.79-2.68) 5.86 (1.83-18.72) Model 3§ 2.14 (1.20-3.83) 1.13 (0.67-1.89) Reference 1.51 (0.82-2.79) 5.37 (1.62-17.80) Note.CI, confidence interval; †Model 1 was adjusted for age and sex; ‡Model 2 was adjusted for age, sex, family per-member monthly income, education level, marital status, smoking status, drinking status, physical activity, and body mass index; §Model 3 was adjusted for the variables in model 2 plus history of diabetes, hypertension, hyperlipidemia, stroke, and carotid plaque. We also analyzed the interactions between night sleep duration and sex/age for cognitive impairment and no significant interactions were observed (P= 0.32/0.95).
-
In this large cross-sectional study, we observed a U-shaped association between night sleep duration and cognitive impairment assessed by MMSE score among 2, 514 participants. This association was sustained after taking into account potential confounders, including age, sex, BMI, education level, smoking status, drinking status, physical activity, hypertension, diabetes mellitus, hyperlipidemia, and carotid plaque. Our data suggest that night sleep duration is associated with cognitive impairment and may be used as an additional clinical indicator in assessing increased risk of cognitive impairment.
Previous studies have examined cross-sectional associations between sleep problems and cognitive function[13, 15-22]. Among them, one study conducted in a population sample of individuals aged 50 and over in southern China demonstrated a U-shaped association between sleep duration and cognitive impairment in older people[13]. The prevalence of cognitive impairment was 12.9% in southern China, which was higher than the 4.85% observed in our study. The difference in prevalence may be due to the different methods used to evaluate cognitive impairment. Although the prevalence of cognitive impairment, lifestyle, living conditions, and food intake differ between south and north China, we also found an association between night sleep duration and cognitive impairment in northern China. The overall association between night sleep duration and all cognitive impairment was U-shaped, with the lowest scores consistently associated with short and long sleep duration. On the other hand, Blackwell et al.[34] did not find a cross-sectional association between night sleep duration and cognitive impairment in older women. In a study of 3, 212 Spanish people aged > 60 years, the risk of cognitive impairment increased across night sleep duration groups from 7 to ≥ 11 h, whereas no association was found between short sleep duration and cognitive impairment[18]. Tworoger et al. observed that sleeping < 5 h was associated with poorer results on various cognitive tests administered to 1, 852 women in the United States. However, the association was no longer observed after 2 years of prospective follow-up[21]. These differences may arise from differences in the ethnicities of study participants. Participants in the present study are mainly Han-ethnic Chinese, who probably have different sleep patterns, such as Chinese naptime, and lifestyles compared with other ethnic populations.
The exact pathophysiological explanations underlying the association of night sleep duration with cognitive impairment has not yet been clarified. First, one important biological pathway is through inflammation, as both short sleep duration[35-36] and long sleep duration[37-38] have been associated with an increased level of inflammatory biomarkers. Elevated inflammatory cytokine levels increase the risk of cognitive decline[39-41] and have been associated with increased white matter hyperintensity[42] as well as reduced gray matter volume[42-43]. Second, sleep restriction causes fatigue and daytime sleepiness, which translates to poorer cognitive performance[17]. Third, sleep fragmentation, which can result in poor quality sleep, depression, and underlying disease processes, such as cardiopulmonary disorders, appear to be relevant to the association between sleep duration and cognitive function[44]. Further prospective research is needed to corroborate these mechanisms that underlie these associations.
The strengths of our study include a large sample size, Asian ethnicity of the participants, and a broad spectrum of potential confounding parameters, especially carotid plaque. However, potential limitations of our study should also be mentioned. First, a high number of participants were excluded owing to the lack of MMSE and sleep data, which may generate bias in the results of the statistical analysis. After comparing participants and non-participants, we found that the majority of the non-participants were older, had higher SBP, fasting blood glucose concentrations, and prevalence of carotid plaque, and had lower education level, which are risk factors of cognitive impairment. Second, our study did not exclude participants with obstructive sleep apnea syndrome (OSAS) or parasomnias. OSAS was associated with cognitive decline in a 15-year follow-up study among 966 participants with an average age of 61 years[45]. Third, depression has been shown to be associated with poor sleep, including long sleeping duration[46], and with cognitive function in a previous cohort[47]. However, in our study, we did not collect data on depression, which may generate bias in the results. Fourth, we collected information only on night sleep duration using self-reported questionnaires, and data with respect to midday napping and sleep quality were not collected. Thus, nocturnal sleep duration may be different from the duration of sleep during the entire day, especially in China where napping and poor sleep quality are not unusual. Finally, this was a cross-sectional study, which limits our ability to conclude a cause-and-effect association between night sleep duration and cognitive impairment. Furthermore, all participants were employees of the Kailuan Coal Company, and their health insurance policies were covered by the Kailuan Medical Group; therefore, they cannot be viewed as a representative sample of the Chinese general population. However, studying such a geographically confined and controlled population greatly reduces residual confounding due to diverse socioeconomic factors and lifestyle patterns.
-
In summary, our study indicates that both short sleep duration and long night sleep duration are asso-ciated with an increased risk of cognitive impairment. The underlying neurobiology and mechanistic media-tors linking habitual sleep duration with increased risk of cognitive impairment need to be investigated.
-
Conceived and designed the experiments: WANG Xi Zhu and WU Shou Ling. Performed the experiments and collected data: HU Wan Ning, LIU Xiao Xue, ZHOU Wen Hua, HAN Xiao Chen, and LU Ai Dong. Analyzed the data and wrote the paper: SONG Qiao Feng and LIU Xiao Xue. Reviewed manuscript: SONG Qiao Feng, LIU Xiao Xue, HU Wan Ning, HAN Xiao Chen, LIU Ai Dong, WANG Xi Zhu, and WU Shou Ling. Edited manuscript: LIU Xiao Xue.
-
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group, and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
-
No conflict of interest to declare.
doi: 10.3967/bes2017.100
Night Sleep Duration and Risk of Cognitive Impairment in a Chinese Population:A Cross-sectional Study
-
Abstract:
Objective Although sleep is one of the most important health-related behavioral factors, the association between night sleep duration and cognitive impairment has not been fully understood. A cross-sectional study was conducted with a random sample of 2, 514 participants (≥ 40 years of age; 46.6% women) in China to examine the association between night sleep duration and cognitive impairment. Methods Night sleep duration was categorized as ≤ 5, 6, 7, 8, or ≥ 9 h per night. Cognitive function was measured using the Mini-Mental State Examination. A multivariate regression analysis was used to analyze the association of night sleep duration with cognitive impairment. A total of 122 participants were diagnosed with cognitive impairment. Results A U-shaped association between night sleep duration and cognitive impairment was found. The odds ratios (95% confidence intervals) of cognitive impairment (with 7 h of daily sleep being considered as the reference) for individuals reporting ≤ 5, 6, 8, and ≥ 9 h were 2.14 (1.20-3.83), 1.13 (0.67-1.89), 1.51 (0.82-2.79), and 5.37 (1.62-17.80), respectively (P ≤ 0.01). Conclusion Short or long night sleep duration was an important sleep-related factor independently associated with cognitive impairment and may be a useful marker for increased risk of cognitive impairment. -
Key words:
- Night sleep duration /
- Cognitive /
- Impairment /
- Cross-sectional
-
Table 1. Comparison of Demographic and other Characteristics of Participants and Non-participants
Variable Overall Participants Non-participants P Value No. 5, 440 2, 514 2, 926 Age (y) 57.7 ± 11.2 55.8 ± 9.5 59.6 ± 12.5 < 0.001 Men, n (%) 3, 257 (59.9) 1, 342 (53.4) 1, 915 (65.4) < 0.001 Marital status (married), n (%) 4, 165 (76. 6) 2, 466 (98.1) 1, 699 (58.1) < 0.001 Body mass index (kg/m) 24.9 ± 3.2 25.0 ± 3.3 24.8 ± 3.2 0.090 Systolic blood pressure (mmHg) 130.9 ± 19.6 128.6 ± 18.6 133.4 ± 20.4 < 0.001 Diastolic blood pressure (mmHg) 82.8 ± 10.9 82.4 ± 10.5 83.1 ± 11.3 0.180 Fasting blood glucose (mmol/L) 5.8 ± 1.9 5.7 ± 1.7 5.8 ± 2.3 < 0.010 Education, n (%) < 0.001 Illiteracy/primary school 1, 104 (21.9) 149 (5.9) 955 (37.6) Middle school 2, 657 (52.6) 1, 544 (61.4) 1, 113 (43.9) High-school graduate 1, 290 (25.5) 821 (32.7) 469 (18.5) Family per-member monthly income ≥ ¥3, 000, n (%) 1, 074 (21.3) 447 (17.8) 627 (24.7) < 0.001 Physical activity > 4 times/week, n (%) 846 (16.8) 311 (12.4) 535 (21.1) < 0.001 Current smoker, n (%) 1, 600 (31.7) 838 (33.3) 762 (30.0) 0.050 Current alcohol drinker, n (%) 1, 444 (28.6) 802 (31.9) 642 (25.3) < 0.001 History of disease Hypertension, n (%) 2, 529 (50.1) 1, 225 (48.7) 1, 304 (51.4) 0.060 Diabetes mellitus, n (%) 703 (13.9) 353 (14.0) 350 (13.8) 0.800 Hyperlipidemia, n (%) 2, 251 (44.6) 1, 281 (50.9) 970 (38.2) < 0.001 Stroke, n (%) 71 (1.3) 24 (1.0) 47 (1.6) < 0.050 Carotid plaque, n (%) 1, 886 (46.6) 1, 053 (42.3) 833 (53.6) < 0.001 Snoring, n (%) 410 (9.6) 273 (10.9) 137 (7.8) < 0.010 Night sleep duration, n (%) ≤ 5 h 684 (12.6) 282 (11.2) 402 (13.7) 6 h 1, 511 (27.8) 675 (26.8) 836 (28.6) 7 h 2, 300 (42.3) 1, 165 (46.3) 1, 135 (38.8) 8 h 881 (16.2) 367 (14.6) 514 (17.6) ≥ 9 h 64 (1.2) 25 (1.0) 39 (1.3) Table 2. Comparisons between Patients with and without Cognitive Impairment in the Kailuan Study
Variable Overall Cognitively Intact
(MMSE ≥ 24)Cognitively Impaired
(MMSE < 24)P Value No. 2, 514 2, 392 122 Age (y) 55.8 ± 9.5 55.3 ± 9.1 65.3 ± 11.9 < 0.001 Men, n (%) 1, 342 (53.4) 1, 260 (52.7) 82 (67.2) < 0.001 Marital status (married), n (%) 2, 466 (98.1) 2, 352 (98.3) 114 (93.4) < 0.001 Body mass index (kg/m) 25.0 ± 3.3 25.0 ± 3.3 24.9 ± 3.5 0.850 Systolic blood pressure (mmHg) 128.6 ± 18.6 128.2 ± 18.4 137.4 ± 20.1 < 0.001 Diastolic blood pressure (mmHg) 82.4 ± 10.5 82.4 ± 10.4 84.2 ± 11.1 0.080 Fasting blood glucose (mmol/L) 5.7 ± 1.7 5.7 ± 1.6 6.1 ± 2.4 0.080 Education, n (%) < 0.001 Illiteracy/primary school 149 (5.9) 121 (5.1) 28 (23.0) Middle school 1, 544 (61.4) 1, 458 (61.0) 86 (70.5) High-school graduate 821 (32.7) 813 (33.9) 8 (6.6) Family per-member monthly income ≥ ¥3, 000, n (%) 447 (17.8) 432 (18.1) 15 (12.3) < 0.050 Physical activity > 4 times/week, n (%) 311 (12.4) 299 (12.5) 12 (9.8) 0.120 Current smoker, n (%) 838 (33.3) 790 (33.0) 48 (39.3) 0.240 Current alcohol drinker, n (%) 802 (31.9) 764 (31.9) 38 (31.2) < 0.001 History of disease Hypertension, n (%) 1, 225 (48.7) 1, 145 (47.9) 80 (65.6) < 0.001 Diabetes mellitus, n (%) 353 (14.0) 330 (13.8) 23 (18.9) 0.140 Hyperlipidemia, n (%) 1, 281 (50.9) 1, 227 (51.3) 54 (44.3) 0.130 Stroke, n (%) 24 (1.0) 20 (0.8) 4 (3.3) < 0.050 Carotid plaque, n (%) 1, 053 (42.3) 982 (41.4) 71 (59.2) < 0.001 Snoring, n (%) 273 (10.9) 255 (10.7) 18 (14.8) 0.370 Night sleep duration, n (%) < 0.001 ≤ 5 h 282 (11.2) 255 (10.7) 27 (22.1) 6 h 675 (26.8) 643 (26.9) 32 (26.2) 7 h 1, 165 (46.3) 1, 125 (47.0) 40 (32.8) 8 h 367 (14.6) 349 (14.6) 18 (14.8) ≥ 9 h 25 (1.0) 20 (0.8) 5 (4.1) Note.MMSE: mini-mental state examination. Table 3. Characteristics (2012) according to Night Sleep Duration
Variable Night Sleep Duration ≤ 5 h 6 h 7 h 8 h ≥ 9 h P No. 282 675 1, 165 367 25 Age (y) 58.0 ± 10.9 56.3 ± 9.6 55.4 ± 9.0 54.1 ± 8.8 58.1 ± 11.4 < 0.001 Men, n (%) 175 (62.1) 385 (57.0) 593 (50.9) 173 (47.1) 16 (64.0) < 0.001 Marital status (married), n (%) 274 (97.2) 657 (97.3) 1, 149 (98.6) 361 (98.4) 25 (100) 0.220 Body mass index (kg/m2) 25.2 ± 3.4 25.1 ± 3.3 24.8 ± 3.3 25.1 ± 3.2 25.7 ± 3.9 0.300 Systolic blood pressure (mmHg) 130.4 ± 19.6 129.9 ± 18.2 127.7 ± 18.4 127.6 ± 18.8 133.6 ± 18.8 < 0.050 Diastolic blood pressure (mmHg) 82.8 ± 10.8 83.3 ± 10.1 81.9 ± 10.5 82.0 ± 10.5 85.9 ± 11.3 < 0.050 Fasting blood glucose (mmol/L) 5.8 ± 1.7 5.8 ± 1.7 5.6 ± 1.6 5.7 ± 1.7 6.9 ± 3.8 < 0.050 Education, n (%) < 0.001 Illiteracy/primary school 22 (7.8) 39 (5.8) 58 (5.0) 29 (7.9) 1 (4.0) Middle school 194 (68.8) 438 (64.9) 670 (57.5) 221 (60.2) 21 (84.0) High-school graduate 66 (23.4) 198 (29.3) 437 (37.5) 117 (31.9) 3 (12.0) Family per-member monthly income ≥ ¥3, 000, n (%) 40 (14.2) 112 (16.6) 232 (19.9) 60 (16.4) 3 (12.0) < 0.001 Physical activity > 4 times/week, n (%) 52 (18.4) 97 (14.4) 121 (10.4) 38 (10.4) 3 (12.0) < 0.001 Current smoker, n (%) 110 (39.0) 246 (36.4) 370 (31.8) 105 (28.6) 7 (28.0) < 0.050 Current alcohol drinker, n (%) 111 (39.4) 241 (35.7) 343 (29.4) 99 (26.9) 8 (32.0) < 0.050 History of disease Hypertension, n (%) 151 (53.6) 349 (51.7) 553 (47.5) 158 (43.1) 14 (56.0) < 0.050 Diabetes mellitus, n (%) 37 (13.1) 107 (15.8) 148 (12.7) 53 (14.4) 8 (32.0) < 0.050 Hyperlipidemia, n (%) 148 (52.5) 372 (55.1) 569 (48.8) 180 (49.1) 12 (48.0) 0.110 Stroke, n (%) 2 (0.7) 12 (1.8) 8 (0.7) 1 (0.3) 1 (4.0) < 0.050 Carotid plaque, n (%) 154 (55.0) 292 (43.9) 461 (39.8) 135 (37.1) 11 (44.0) < 0.001 Snoring, n (%) 64 (22.7) 86 (12.7) 88 (7.6) 32 (8.7) 3 (12.0) < 0.001 Table 4. Odds Ratios (95% CI) for Cognitive Impairment according to Night Sleep Duration
Items 95% CI (according to Night Sleep Duration) ≤ 5 h 6 h 7 h 8 h ≥ 9 h Cases, n (%) 27 (9.57) 32 (4.74) 40 (3.43) 18 (4.90) 5 (20.00) Model 1† 2.27 (1.34-3.86) 1.26 (0.78-2.06) Reference 1.65 (0.92-2.96) 5.54 (1.78-17.28) Model 2‡ 2.12 (1.20-3.77) 1.18 (0.71-1.95) Reference 1.46 (0.79-2.68) 5.86 (1.83-18.72) Model 3§ 2.14 (1.20-3.83) 1.13 (0.67-1.89) Reference 1.51 (0.82-2.79) 5.37 (1.62-17.80) Note.CI, confidence interval; †Model 1 was adjusted for age and sex; ‡Model 2 was adjusted for age, sex, family per-member monthly income, education level, marital status, smoking status, drinking status, physical activity, and body mass index; §Model 3 was adjusted for the variables in model 2 plus history of diabetes, hypertension, hyperlipidemia, stroke, and carotid plaque. -
[1] Ferrie JE, Kivimäki M, Akbaraly TN, et al. Change in Sleep Duration and Type 2 Diabetes:The Whitehall Ⅱ Study. Diabetes Care, 2015; 1467-72. http://care.diabetesjournals.org/content/diacare/38/8/1467.full.pdf [2] Lou P, Zhang P, Zhang L, et al. Effects of sleep duration and sleep quality on prevalence of type 2 diabetes mellitus:A 5-year follow-up study in China. Diabetes Res Clin Pract, 2015; 178-84. https://www.ncbi.nlm.nih.gov/pubmed/25934527 [3] Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension:the Sleep Heart Health Study. Sleep, 2006; 1009-14. https://es.scribd.com/document/361157089/Association-of-Usual-Sleep-Duration-With-Hypertension-the-Sleep-Heart-Health [4] Kawada T, Otsuka T. Sleep duration and hypertension with special emphasis on sex and obesity. Am J Hypertens, 2013; 1362. https://www.ncbi.nlm.nih.gov/pubmed/25314720 [5] Xie D, Li W, Wang Y, et al. Sleep duration, snoring habits and risk of acute myocardial infarction in China population:results of the interheart study. BMC Public Health, 2014; 531. http://www.biomedcentral.com/imedia/4543563771301800_manuscript.pdf [6] Meisinger C, Heier M, Lowel H, et al. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population:the MONICA/KORA Augsburg cohort study. Sleep, 2007; 1121-7. http://www.ncbi.nlm.nih.gov/pubmed/17910384 [7] Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke, 2008; 3185-92. http://www.ncbi.nlm.nih.gov/pubmed/18635832 [8] Leng Y, Cappuccio FP, Wainwright NW, et al. Sleep duration and risk of fatal and nonfatal stroke:a prospective study and meta-analysis. Neurology, 2015; 1072-9. https://www.ncbi.nlm.nih.gov/pubmed/?term=Sleep+duration+and+risk+of+fatal+and++nonfatal+stroke%3A+a+prospective+study+and+meta-analysis. [9] Kawada T. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology, 2015; 388. http://www.ncbi.nlm.nih.gov/pubmed/25253755 [10] Cai H, Shu XO, Xiang YB, et al. Sleep duration and mortality:a prospective study of 113 138 middle-aged and elderly Chinese men and women. Sleep, 2015; 529-36. http://www.ncbi.nlm.nih.gov/pubmed/25348122 [11] Esposito M, Carotenuto M. Borderline intellectual functioning and sleep:the role of cyclic alternating pattern. Neurosci Lett, 2010; 89-93. https://www.ncbi.nlm.nih.gov/pubmed/20813159 [12] Esposito M, Carotenuto M. Intellectual disabilities and power spectra analysis during sleep:a new perspective on borderline intellectual functioning. J Intellect Disabil Res, 2014; 421-9. https://eric.ed.gov/?id=EJ1022895 [13] Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese:the Guangzhou Biobank Cohort Study. Sleep, 2011; 575-80. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079936/ [14] Ferrie JE, Shipley MJ, Akbaraly TN, et al. Change in sleep duration and cognitive function:findings from the Whitehall Ⅱ Study. Sleep, 2011; 565-73. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3079935/ [15] Kronholm E, Sallinen M, Suutama T, et al. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res, 2009; 436-46. https://www.ncbi.nlm.nih.gov/pubmed/19732318 [16] Schmutte T, Harris S, Levin R, et al. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults:results from the Bronx aging study. Behav Sleep Med, 2007; 39-56. https://einstein.pure.elsevier.com/en/publications/the-relation-between-cognitive-functioning-and-self-reported-slee-2 [17] Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med, 2002; 201-8. https://www.ncbi.nlm.nih.gov/pubmed/11802754 [18] Faubel R, López-García E, Guallar-Castillón P, et al. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res, 2009; 427-35. http://www.ncbi.nlm.nih.gov/pubmed/19691473/ [19] Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep, 2005; 981-9. https://www.ncbi.nlm.nih.gov/pubmed/16218081 [20] Sternberg DA, Ballard K, Hardy JL, et al. The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Front Hum Neurosci, 2013; 292. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3687527/ [21] Tworoger SS, Lee S, Schernhammer ES, et al. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord, 2006; 41-8. https://www.ncbi.nlm.nih.gov/pubmed/16493235 [22] Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the mini-mental score:the Northern Manhattan study. J Clin Sleep Med, 2013; 669-73. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3671331/pdf/jcsm.9.7.669.pdf [23] Devore EE, Grodstein F, Duffy JF, et al. Sleep duration in midlife and later life in relation to cognition. J Am Geriatr Soc, 2014; 1073-81. http://www.ncbi.nlm.nih.gov/pubmed/24786726 [24] Keage HA, Banks S, Yang KL, et al. What sleep characteristics predict cognitive decline in the elderly? Sleep Med, 2012; 886-92. https://www.ncbi.nlm.nih.gov/pubmed/22560827 [25] Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep, 2012; 491-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3296791/ [26] Wang A, Liu X, Chen G, et al. Association between Carotid Plaque and Cognitive Impairment in Chinese Stroke Population:The SOS-Stroke Study. Sci Rep, 2017; 3066. http://www.nature.com/subjects/alzheimers-disease.atom [27] Zhou Y, Li Y, Xu L, et al. Asymptomatic polyvascular abnormalities in community (APAC) study in China:objectives, design and baseline characteristics. PloS One, 2013; e84685. http://www.oalib.com/paper/3007218 [28] Wu S, Huang Z, Yang X, et al. Cardiovascular events in a prehypertensive Chinese population:four-year follow-up study. Int J Cardiol, 2013; 2196-9. [29] Yan N, Zhou Y, Wang Y, et al. Association of Ideal Cardiovascular Health and Brachial-Ankle Pulse Wave Velocity:A Cross-Sectional Study in Northern China. J Stroke Cerebrovasc Dis, 2016; 41-8. [30] Wang, X, Liu, X, Song Q, et al. Sleep duration and risk of myocardial infarction and all-cause death in a Chinese population:the Kailuan study. Sleep Med, 2016; 13-6. http://www.sleep.theclinics.com/article/S1556-407X(15)00138-1/fulltext [31] Folstein MF, Folstein SE, McHugh PR. 'Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975; 189-98. https://jhu.pure.elsevier.com/en/publications/mini-mental-state-a-practical-method-for-grading-the-cognitive-st-5 [32] Song Q, Liu X, Wang X, et al. Age-and gender-specific associations between sleep duration and incident hypertension in a Chinese population:the Kailuan study. J Hum Hypertens, 2016; 503-7. [33] Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis, 2007; 75-80. [34] Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women:the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sc, 2006; 405-10. http://www.academia.edu/13367377/Poor_Sleep_Is_Associated_With_Impaired_Cognitive_Function_in_Older_Women_The_Study_of_Osteoporotic_Fractures [35] Mullington JM, Simpson NS, Meier-Ewert HK, et al. Sleep loss and inflammation. Best Practice & Research Clinical Endocrinology & Metabolism, 2010; 775-84. [36] Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol, 2004; 678-83. https://www.ncbi.nlm.nih.gov/pubmed/?term=Effect+of+sleep+loss+on+C-reactive+protein%2C+an+inflammatory+marker+of+cardiovascular+risk. [37] Grandner MA, Buxton OM, Jackson N, et al. Extreme sleep durations and increased C-reactive protein:effects of sex and ethnoracial group. Sleep, 2013; 769-79. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624831/ [38] Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep, 2009; 200-4. http://www.ncbi.nlm.nih.gov/pubmed/19238807 [39] Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology, 2003; 76-80. [40] Teunissen CE, van Boxtel MP, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol, 2003; 142-50. http://www.academia.edu/6098328/Inflammation_markers_in_relation_to_cognition_in_a_healthy_aging_population [41] Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev, 2011; 319-29. http://circ.ahajournals.org/content/107/3/499 [42] Satizabal CL, Zhu YC, Mazoyer B, et al. Circulating IL-6 and CRP are associated with MRI findings in the elderly:the 3C-Dijon Study. Neurology, 2012; 720-7. https://www.ncbi.nlm.nih.gov/pubmed/22357713 [43] Taki Y, Thyreau B, Kinomura S, et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum Brain Mapp, 2013; 2418-24. [44] Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev, 2007; 341-60. [45] Lutsey PL, Bengtson LG, Punjabi NM, et al. Obstructive Sleep Apnea and 15-Year Cognitive Decline:The Atherosclerosis Risk in Communities (ARIC) Study. Sleep, 2015; 309-16. https://www2.cscc.unc.edu/aric/node/7089 [46] Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry, 2005; 1254-69. [47] Singh-Manoux A, Akbaraly TN, Marmot M, et al. Persistent depressive symptoms and cognitive function in late midlife:the Whitehall Ⅱ study. J Clin Psychiatry, 2010; 1379-85. -




 下载:
下载:



 Quick Links
Quick Links