-
At the beginning of the 20th century, the cerebellum was thought to solely be a modulator of motor function, including coordination, diadochokinesia, tonus, and motor speech production[1]. Several neuronal systems are involved in body motion, but the cerebellum is specifically implicated in the voluntary movement. The cerebellum controls the activation, timing, and coordination of distinct muscle groups during body motion. The traditional view of the cerebellum solely as a coordinator of motor function has been substantially redefined in the past decades. Neuroanatomical, neuroimaging, and clinical studies have found that the cerebellum is further involved in the modulation of cognitive and affective processing. Neuroanatomical studies have demonstrated cerebellar connectivity with supratentorial areas involved in higher cognitive and affective functioning, while functional neuroimaging and clinical studies have provided evidence of cerebellar involvement in a variety of cognitive and affective tasks. Neuroanatomical studies have also revealed that the cerebellum is linked in a reciprocal way to the autonomic, limbic, and associative regions of the supratentorial cortex[2].
The cerebellum was chosen as a region of interest for this study because it has higher susceptibility to oxidative stress than other brain regions[3, 4]. In fact, because of its sensitivity to oxidative stress, the cerebellum is known to be vulnerable to damage induced by several chemicals, including xenobiotics. Xenobiotics are important for the production of free hydroxyl radicals in neurodegenerative disorders[5, 6]. Exposure to xenobiotics during development has become an important public health concern because of their possible impact on the development and programming of organ functions[7]. In the cerebellum, reactive oxygen species (ROS) are well known for their role in the pathogenesis of primary neurodegenerative diseases, such as amyotrophic lateral sclerosis[8] and Alzheimer's disease[9]. It has been postulated that neuronal death in these diseases may be mediated by oxidative stress caused by the aberrant metabolism of superoxides. Converging evidence suggests that the cerebellum may also be implicated in anxiety disorders. Potassium bromate (KBrO3) is an oxidizing halogen that has long been used as a food additive, mainly for bread making. In addition, KBrO3 is used worldwide as a neutralizer in home permanent cold wave hair kits[10]. Potassium bromate is known to cause severe and irreversible sensorineural hearing loss as well as renal failure. Moreover, it is thought to be a carcinogen, causing chromosome aberrations and 8-hydroxydeoxyguanosine generation, and is capable of both initiating and promoting rat renal tumorigenesis[11]. Although the adverse effect of KBrO3 on auditory function in animals has been well described[10, 12], to our knowledge, there are no reports on the effects of KBrO3 on the cerebellum.
Vanillin (4-hydroxy-3-methoxybenzaldehyde) is an antioxidant compound isolated from the bean and pod of the tropical vanilla orchid and is widely used in the food and beverage industry[13]. This compound is also used for the synthesis of agrochemicals, antifoaming agents, and pharmaceutical products[14]. Vanillin has been shown to have choleretic, antifungal, antimutagenic, and anticancer effects[15-17] as well as protective effects on the liver and kidney[18, 19]. Most of these effects are attributed to the antioxidant activity of vanillin. Recently, it has been shown that vanillin has neuroprotective effects[20]. Therefore, the role of vanillin in the prevention of neurodegenerative diseases is of great interest. As such, the main goal of this study is to assess oxidative imbalance in the mouse cerebellum after two weeks of KBrO3 exposure. Further, we also explore if vanillin can attenuate any of these effects. To do this, we evaluate the protective role of vanillin on oxidative stress regulation, genes expression of select antioxidant enzymes, and we measure the pro-inflammatory cytokines.
-
This study was performed in accordance with the Institute Ethical Committee for the Care and Use of Laboratory Animals guidelines[21]. Adult mice (40 ± 5 g, 4 weeks old, n = 12) were kept in an air-conditioned room (temperature 22 ± 3 ℃, relative humidity 40%). They were housed in polycarbonate cages and had a daily standard pellet diet (SNA, Sfax, Tunisia) and water ad libitum. The mice were divided into four groups; each group received either no treatment (control group), 2 g/L KBrO3 (Sigma Chemical Co., St. Louis, MO, USA) in drinking water (KBrO3 group), 100 mg/kg body weight vanillin by intraperitoneal injection (vanillin group), or both KBrO3 and vanillin (KBrO3 + vanillin group). KBrO3 and vanillin doses were selected based on previous studies[22, 23]. After 15 days of treatment, the animals were sacrificed by cervical decapitation to avoid stressing the animal. All animals were anesthetized with an intraperitoneal injection of chloral hydrate solution (3.6%) before being sacrificed. The cerebellum was quickly excised, rinsed in Tris-HCl buffer, weighed, and then divided into three parts. One part was homogenized in Tris-HCl buffer, as indicated in the procedures of oxidative stress markers. The other two parts were used in molecular assays and some samples were immediately removed, cleaned, fixed in 10% buffered formalin solution for 48 h, and then embedded in paraffin for histological studies.
-
Beginning 10 days following exposure, open field and rotarod tests were performed. These tests were performed for five consecutive days at the same time (between 9:00 and 11:00 am) and with the same experimenter to minimize the possible effects of stress on mouse performance. By day six (day 15 of treatment) the mice were habituated to the experimental conditions, so we took measurements for the two tests.
Rotarod Activity Motor coordination and grip strength were assessed using a rotarod apparatus. Animals were exposed to the rotarod for 300 s prior to the training session to acclimate them to the apparatus. Animals were then placed on the rotating rod (diameter of 3 cm, speed 20 rpm) for 120 s. The average time to fall off the rotarod was recorded and expressed while per 2 min[24].
Open Field Behavior The open field apparatus was a square-shaped box made of wood, 80 cm in length and 40 cm in height. The floor was divided into 16 squares, each of which measured 20 cm × 20 cm in area. We recorded the number of squares in which each mouse crossed with its paws (crossing); stood on its hind legs (rearing); and wiped, licked, or combed any part of its body (grooming). Each mouse was placed in the center of the apparatus and the number of head dips and head dipping duration (in seconds) within a 3-min period were recorded[25]. A head dip was included only if both eyes are steered down. Between each test, the floor was cleaned with 70% ethyl alcohol and permitted to dry[26, 27].
-
Protein content in the cerebellum was assayed following the methods previously described by Lowry et al.[28].
-
We determined the amount of malondialdehyde (MDA) in the cerebellum using 1, 1, 3, 3-tetraethoxypropane and following the methods described by Draper and Hadley[29]. The values are expressed as nmol MDA/mg protein.
A hydrogen peroxide (H2O2) assay was performed using the ferrous ion oxidation xylenol orange (FOX-1) method[30]. The amount of H2O2 in the cerebellum extract was measured at 560 nm in a spectroph-otometer and expressed as μmol/mg protein.
Lipid hydroperoxides (LOOHs) were quantified using a FOX assay as described by Jiang et al.[31]. The amount of LOOHs produced was calculated using a molar extinction coefficient of 4.59 × 104 mol·L-1·cm-1. Values are expressed as nmol/mg protein.
Advanced oxidation protein products (AOPPs) were assayed using the method described by Witko[32]. The level of AOPP in cerebellar tissue was calculated using an extinction coefficient of 261 mmol·L-1·cm-1 and expressed as μmol/mg protein.
Superoxide dismutase (SOD) enzyme activity was measured using the method described by Beauchamp and Fridovich[33]. One unit of SOD activity was defined as the amount of enzyme required to cause a 50% inhibition of nitro blue tetrazolium photoreduction. SOD activity in cerebellar tissue is expressed as units/mg protein.
Glutathione peroxidase (GPx) enzyme activity was measured using the method described by Flohe and Gunzler[34]. We calculated the modification in absorbance at 340 nm. GPx enzyme activity is expressed as nmol glutathione oxidized/min/mg protein.
-
To determine ATPase activity, cerebellar tissue was homogenized in Tris-HCl buffer of pH 7.4 using the method described by Kawamoto et al.[35] and the resulting supernatants were immediately used. Total ATPase activity was determined by an inorganic phosphate (Pi) assay, in which Pi is released from the enzymatic hydroysis of ATP. Enzyme activity is expressed as μmol Pi liberated/h/mg protein.
-
Total mRNA was isolated from 100 mg of cerebellum using an Invitrogen kit (Pure Link RNA, ref. 12183018A). The integrity of the RNA was examined by electrophoresis and the purity was confirmed by measuring the absorbance at 260 and 280 nm. All samples were required to have an absorbance ratio between 1.7 and 1.9.
-
Total RNA was extracted from the primary astrocytes using a Trizol reagent (Invitrogen, USA). As described above, the purity and concentration of the total RNA were determined by measuring absorbance at 260 and 280 nm. Complementary DNA was synthesized using a total RNA reverse transcription kit following the manufacturer's protocol (Tiangen, China). Approximately 1 μg of total RNA was reverse-transcribed in a reaction volume of 20 μL. PCR reaction cycling conditions were performed first at 94 ℃ for 2 min followed by 35 cycles of denaturation at 94 ℃ for 30 s. The primer sequences were as follows:
(1) SOD, forward 5'-ATGGCGATGAAGGCCGTG TGC-3'; reverse 5'-TTATTGGGCAATCCCAATCAC-3';
(2) GPx, forward 5'-ATGTCTGCTGCTCGGCTCT CC-3'; reverse 5'-TTAGGGGTTGCTAGGCTGCTT-3';
(3) IL-6, forward 5'-TCTTCGAGGCACAAGGCA-3'; reverse 5'-CAGAGGTCCAGGTCCTGGAAT-3';
(4) TNF-α, forward 5'-GGTGATCGGTCCCAACAA GGA-3'; Reverse 5'-CACGCTGGCTCAGCCACTC-3'.
(5) β-Actin, forward 5'-TCCTCCTGAGCGCAAGTA CTC-3'; reverse 5'-GCTCAGTAACAGTCCGCCTAG-3'.
The PCR products were analyzed by electrophoresis (1% agarose gel containingethidium bromide), examined under UV light, photographed, and scanned.
-
Some cerebellar tissue collected from each group was randomly selected for light microscopy. Samples were fixed in formalin solution and embedded in paraffin. The paravermis was then sectioned in the sagittal plane (5 µm thick) and stained with hematoxylin-eosin[36].
-
All experiences and statistical analyses were made in triplicate. All results are expressed as the mean ± standard deviation. Statistical analysis was performed with SPSS 17.0 statistical package for Windows (SPSS, Inc., Chicago, IL). A two-way ANOVA followed by Tukey's post-hoc test was performed to compare treatment and control groups. Statistical significance was set at α = 0.05.
-
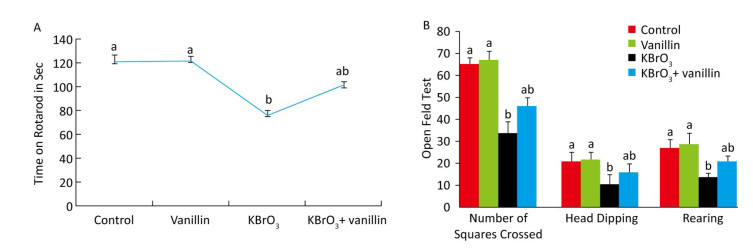
The effect of vanillin on KBrO3-induced motor performance was investigated using a rotarod task on day 15, as shown in Figure 1A. We found that muscle grip strength was significantly reduced (P < 0.01) in the KBrO3 as compared to the control group. Mice that were co-administered vanillin showed significantly increased muscle grip strength as compared to the KBrO3 group (P < 0.01). There was no significant difference in motor performance between vanillin and control groups (Figure 1A).

Figure 1. Effect of vanillin and KBrO3 on mice muscle grip strength (rotarod test, A) and mobility (open field test, B). Values from the rotarod test are expressed as time in seconds and compared to the performance of the control group (mean ± SD). In the open field test, values are expressed as number of squares crossed, head dips, and rearing frequency. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
-
The open field test showed that significantly fewer squares were crossed by mice in the KBrO3 group compared to those in the control group (P < 0.01) (Figure 1B). In mice treated with vanillin, we saw an improvement in mobility, measured by an increase in the number of squares crossed compared to the KBrO3 group (P < 0.01). Similarly, we found that rearing activity decreased in KBrO3-treated animals, whereas vanillin treatment increased the rearing activity and mobility on day 15, as compared to the KBrO3 group (P < 0.01) (Figure 1B). We did not see any significant difference in rearing between the KBrO3+vanillin and the KBrO3 group. In the vanillin group, there was an increase in rearing, however, this difference was not significant when compared to the control group.
-
Lipid Peroxidation The lipid peroxidation product was assessed by measuring MDA levels. As shown in Table 1, mice exposed to KBrO3 had a significant increase in lipid peroxidation in the cerebellum (F = 1.47; P = 0.094) compared to controls. Co-administration of vanillin significantly decreased (P < 0.01) the level of lipid peroxidation compared to the KBrO3 group. Further, there was no significant difference in lipid peroxidation between the vanillin and control groups.
Table 1. Effect of Potassium Bromate (KBrO3), Vanillin and their Combination (KBrO3+vanillin) on Oxidative Stress Markers
Parameters & Treatments Control KBrO3 KBrO3+Vanillin Vanillin F P MDA (nmol MDA/g tissue) 92.920 ± 12.830a 133.540 ± 11.300b 102.330 ± 10.950a 94.510 ± 17.670a 1.470 0.094 LOOH (nmol/mg protein) 1.220 ± 0.130a 3.150 ± 0.180b 2.070 ± 0.080a, b 1.300 ± 0.100a 3.470 0.030 H2O2 (μmol/mg of protein) 0.020 ± 0.007c 0.080 ± 0.009a 0.060 ± 0.008b 0.030 ± 0.007c 37.450 < 0.001 AOPP (µmol/mg of protein) 0.400 ± 0.090a 0.810 ± 0.040b 0.540 ± 0.070a, b 0.380 ± 0.050a 5.670 0.120 Mg2+ ATPase (μmol Pi/h/mg protein) 0.110 ± 0.008 a 0.010 ± 0.003a 0.050 ± 0.004b 0.082 ±0.090a 130.541 < 0.001 Na+-K+ ATPase (μmol Pi/h/mg protein) 0.073 ± 0.004a 0.079 ± 0.008a 0.029 ± 0.009c 0.046 ± 0.005b 35.371 < 0.001 Note. Values are means ± SD for twelve mice in each group. Means not sharing the same letters (a-c) within a column are significantly different (P < 0.05). MDA, Malondialdehyde; LOOH, Lipid Hydroperoxide; H2O2, Hydrogen Peroxide; AOPP, Advanced Oxidation Protein Product. H2O2 Generation As shown in Table 1, we detected higher levels of H2O2 in the cerebellum (F = 3.47; F = 37.45) of KBrO3-treated mice than in controls. Co-administration of vanillin significantly decreased the levels of H2O2 (Table 1).
Protein Oxidative Damage The degree of protein oxidation was estimated by measuring AOPP and LOOH levels. We found that there was a significant increase in AOPP and LOOH levels in the cerebellum (F = 5.67; P < 0.001; P = 0.03; P < 0.001 respectively) of KBrO3-treated mice when compared to controls (Table 1). Co-administration of vanillin significantly decreased these levels (Table 1). Further, there was no significant difference in the AOPP and LOOH levels between the vanillin and control groups.
-
Table 1 shows the activity of the membrane-bound Na+-K+ and Mg2+ ATPase in the cerebellum tissue of mice. KBrO3 administration caused a marked decrease (F = 130.541 and F = 35.371, respectively; P < 0.001) in the Na+-K+ and Mg2+ ATPase activity compared to the control. Vanillin co-administration significantly (P < 0.001) protected against the KBrO3-induced decrease in enzyme activity in the KBrO3+vanillin group. Vanillin administration alone did not produce any significant changes, as there was no significant difference in enzyme activity between the vanillin and control groups.
-
Intracellular activity and gene expression of antioxidant enzymes, such as SOD and GPx, were measured in the cerebellum following 15 days of KBrO3 and vanillin exposure (Figure 2). We found a significant increase (P < 0.01) in SOD and GPx activity in KBrO3-treated mice as compared to controls. Semi-quantitative RT-PCR analysis confirmed our biochemical results and revealed a marked decrease in SOD and GPx mRNA levels after normalization to the β-actin gene in the KBrO3 group (Figure 2). Co-administration of vanillin improved the antioxidant status and gene expression in cerebellar tissue compared to the KBrO3 group. Conversely, the vanillin group showed no significant difference in enzyme activity and gene expression compared to the control group.
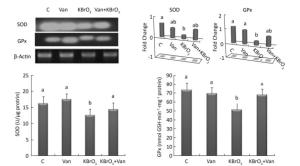
Figure 2. Effect of potassium bromate (KBrO3), vanillin (Van), and their combination (KBrO3+vanillin) on the activities and mRNA expression of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in the cerebellum of adult mice. Values are expressed as mean ± SD (n = 3). C: Controls. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
-
The effect of vanillin, either co-administered with KBrO3 or administered alone, on proinflammatory gene expression was examined by measuring TNF-α and IL-6 mRNA accumulation using RT-PCR (Figure 3). We found a concomitant significant increase in TNF-α and IL-6 mRNA in the cerebellum of KBrO3-treated mice. Importantly, co-administration of vanillin significantly inhibited this increase in proinflammatory cytokines (TNF-α and IL-6 gene expression).
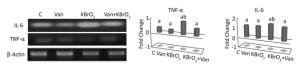
Figure 3. Effect of potassium bromate (KBrO3), vanillin (Van), and their combination (KBrO3+vanillin) on TNF-α and IL-6 mRNA expression in the cerebellum of adult mice. Values are expressed as mean ± SD (n = 3). C: Controls. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
-
Administration of KBrO3 caused cerebellar histological changes in mice (Figure 4C). The most conspicuous damage in KBrO3-treated animals was found in the Purkinje cell layer, where there was a marked reduction in the number of cells. Moreover, edema caused a widening of the Purkinje cell layer (Figure 4C). Co-administration of vanillin protected the cerebellum from severe damage induced by KBrO3 (Figure 4D). The histological pattern in cerebellar tissue was normal in mice treated only with vanillin, similar to the control mice (Figure 4B). Semi-quantitative histological analysis by hematoxylin and eosin staining indicated significant apoptosis and reduction in the number of Purkinje cells in the KBrO3 group.
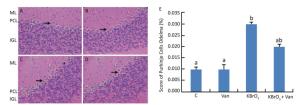
Figure 4. Cerebellum histological sections of controls (A), vanillin (B), KBrO3 (C), and KBrO3+vanillin (D) groups. The score of Purkinje cell edema (E) in adult mice. ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granular layer. Arrows indicate →pyknotic Purkinje cells and

-
In recent years, studies have implicated oxidative stress as a primary mechanism by which xenobiotics exert their deleterious effect on the central nervous system[37, 38]. Antioxidant supplementation is one strategy for maintaining redox homeostasis by directly quenching excessive ROS and protecting or reinforcing the endogenous defense system against oxidative stress. Vanillin, a natural antioxidant, is reported to have broad bioactivity, including antioxidant, anticancer, and anti-inflammatory properties[39]. Chemotherapy-related cognitive deficits are a major neurological problem, but the underlying mechanisms are unclear. Few studies have investigated how to prevent stress-induced cognitive deficit. Thus, we investigated the effects of KBrO3 exposure in addition to the possible protective effects of vanillin on behavior as well as ATPase activity, oxidative stress biomarkers, and gene expression in the cerebellum of adult mice. Our findings provide evidence for, and encourage further exploration of, the benefits of natural antioxidants on behavioral changes and oxidative stress.
Neurobehavioral profiling is an established method for characterizing the influence of various compounds on the motion and locomotion of animals[40]. The cerebellum is active in some forms of motor learning and coordination and is thought to process higher cognitive and emotional functions. Our study shows that exposure to KBrO3 induces behavioral deficits evinced by marked decreases in the maximum speed, total distance travelled, and body rotation of the mice. In an open field test, the KBrO3 group exhibited a smaller number of squares crossed, rearings, and head dips compared to the control group. Co-administration of vanillin (100 mg/kg BW), however, rescued the open field test findings. KBrO3 exposure also causes changes in muscular coordination, as demonstrated by a reduction in rotarod activity. These observations suggest that KBrO3-induced toxicity disrupts the coordination of neuromuscular junctions. However, the present study shows that administration of vanillin reverses the KBrO3-induced behavioral deficits, demonstrated by a significant recovery of the locomotor and motor activity of co-treated mice.
The generation of free radicals following xenobiotic exposure is a well-known process. The brain is susceptible to free radical damage[41] due to its low glutathione content, high polyunsaturated fatty acid proportion in the membrane, high iron content, and ability to consume about 20% of total body oxygen[42]. Excessive ROS formation subsequently attacks almost all cellular components, including the membrane lipids, causing lipid peroxidation[43]. The latter is a consequence of ROS production and is an indirect measure of metal-induced oxidation[44], which has been found to play an important role in the toxicity of many xenobiotics[45]. The capacity to produce ROS can generally be assessed by determining the rate at which respiring mitochondria release H2O2. Our results show that, consistent with the increase in MDA levels, there was also a significant increase in H2O2 production in the cerebellum of KBrO3-treated mice. This finding suggests that KBrO3 induces lipid peroxidation through ROS generation. Indeed, H2O2 is an oxidizing agent that can be easily converted into a hydroxyl radical (OH-), the most reactive free radical molecule that can initiate lipid peroxidation by hydrogen abstraction. Moreover, the enhanced production of ROS likely reflects a mitochondrial dysfunction induced by the metal.
Protein oxidant status is another index that can be used to assess oxidative stress. In fact, due to their fast reaction rates with many radicals and oxidants, proteins are the major targets of oxidative damage which leads to a loss of their specific function. AOPPs and LOOHs are novel biomarkers that can be used to estimate the degree of protein damage. Our results show that AOPP and LOOH levels increased markedly in cerebellar tissue of KBrO3-treated mice when compared to controls. Hence, our findings suggest that KBrO3 promotes ROS generation in the cerebellar tissue of adult mice, subsequently leading to protein oxidation. Our results are consistent with those of previous studies that have reported an increase in AOPP and LOOH levels in kidney tissue after KBrO3 intoxication[46]. Additionally, we found that vanillin reduces the level of reactive oxygen/nitrogen species. These findings lead us to conclude that vanillin is neuroprotective against KBrO3-induced cerebellum toxicity. Such an effect could be attributed to an inhibition of lipid peroxidation.
The brain is an organ that is especially susceptible to peroxide damage due to its richness in lipids, high oxygen turnover, low mitotic rate, and low antioxidant concentration[47]. Brain cells use various mechanisms to combat oxidative stress and repair damaged macromolecules. The primary defense mechanism uses enzymatic antioxidants which have been shown to scavenge reactive oxygen species. One such enzymatic antioxidant is SOD, which is considered the first line of defense against oxidative stress, as it converts toxic superoxide anions to less toxic H2O2 and O2. The H2O2 is then converted to H2O by catalase and GPx. SOD plays a role in preventing free radical damage and generating oxidative stress-like conditions[48]. In the present study, we observe a significant decrease in SOD activity and mRNA gene expression in the cerebellum of adult mice following KBrO3 exposure. The decline of cerebral antioxidant enzyme activity might be due to an enhanced generation of superoxide radicals or the downregulation of antioxidant enzyme synthesis induced by KBrO3 exposure[49].
Along with SOD, GPx is also involved in the scavenging of H2O2. GPx is the most important antioxidant enzyme in the brain[50] because it metabolizes peroxides, such as H2O2, and protects cell membranes from lipid peroxidation. In the present study, we observed a decline in the activity and mRNA gene expression of GPx in KBrO3-exposed animals, which may be attributed to the reduction in the level of substrate and glutathione as well as an increase in the level of peroxides[50].
Following KBrO3 toxicity, ROS are generated by high energy consumption coupled with the inhibition of oxidative phosphorylation and subsequent release of ATP required to meet the body's energy requirement[51]. In the brain, the enzyme Na+-K+ ATPase is responsible for the active transport of Na+ and K+ ions, which maintains the ionic gradient necessary for neuronal excitability and regulates neuronal cell volume[52]. Mg2+ ATPase plays an important role in maintaining high levels of intracellular Mg2+ in the brain. Changes in these Mg2+ levels can modulate the activity of Mg2+-dependent enzymes, thus controlling the rate of protein synthesis as well as cell growth[53].
Our study demonstrates that KBrO3 increases free radical production and consequently alters the structural integrity of membrane lipids. Thus, KBrO3 exposure may affect membrane-bound enzymes, in particular ATPases. In the present study, Na+-K+ and Mg2+ ATPase activity were significantly decreased in KBrO3-treated mice. Therefore, bromate exposure inhibits the activity of ATPases, likely via thiol-and lipid-dependent mechanisms, causing an overproduction of ROS. Similar results were obtained in the erythrocytes and liver of mice exposed to KBrO3 in a study by Ben Saad et al.[18, 54]. Based on our results, we suggest the disruption of the membrane-bound enzymes, Na+-K+ and Mg2+ ATPase, as a possible mechanism mediating KBrO3-induced neurotoxicity. Interestingly, the decrease in ATPase activity induced by KBrO3 in the cerebellar tissue was improved by the co-administration of vanillin. The biological properties of vanillin are commonly attributed to the presence of a phenolic and ether groups as well as aldehyde moieties. In the present study, decreased ROS levels observed following vanillin co-administration suggest that neurotoxicity is attenuated, likely due to the free radical scavenging activity of vanillin. Moreover, vanillin could inhibit singlet oxygen-induced protein and lipid oxidation, as shown by the decrease in cerebellar AOPP levels.
It has been well established that enhanced oxidative stress in the brain may trigger a cascade of events eventually leading to cell death. Our histopathological findings revealed that the morphological features of KBrO3-induced neuronal cell death are dark, apoptotic Purkinje cells in the cerebellum. Apoptosis is a physiological cell death process that plays a critical role in normal development as well as in the pathophysiology of several diseases. Orrenius et al.[55] suggested that KBrO3, a lipophilic molecule, could easily pass through neuronal membranes to reach the mitochondria and induce ROS production, causing apoptosis.
Conventional neuropathological investigations have shown that the cerebellum is invariably involved in these oxidative stress states and leads to the degeneration of the Purkinje cell, the only efferent neuron in the cerebellar cortex[56]. Chaudhary et al.[57] found that cerebellar Purkinje cells were the most affected cell population after the treatment by the aluminum, a metal toxicant.
Cell surface death receptors transmit apoptotic signals initiated by specific ligands, such as Fas, TNF-α, and other related ligands which activate a caspase cascade. In the present study, TNF-α mRNA increased after KBrO3 treatment. Bromate also increased the mRNA expression of IL-6 in brain tissue, which is known to occur in numerous neurological disorders, including Alzheimer's and Parkinson's disease, meningitis, trauma, and stroke[58]. Conversely, co-administration of vanillin led to a significant downregulation of inflammatory cytokines. The present study suggests that KBrO3 induces oxidative stress in the cerebellum and causes lipid peroxidation, leading to a disruption of the cell membrane, loss of ATPase activity, decrease in antioxidant enzyme activity, and increase in inflammatory cytokine expression. In turn, these changes lead to neuronal loss and neurobehavioral dysfunction. Moreover, vanillin reduces the expression of pro-inflammatory cytokines (interleukin-1β and interleukin-6, interferon-β, and tumor necrosis factor-α) and stimulates anti-inflammatory cytokine (IL-4) expression in brain tissue.
-
The present study demonstrates that KBrO3 induces oxidative stress in the cerebellum, resulting in significant learning and memory impairments. Conversely, vanillin is able to ameliorate these deficits, thus improving the motor and cognitive performance in mice. In addition, vanillin reduces the damage caused by bromate-induced oxidative stress in the cerebellum and downregulates abnormal expression of inflammatory markers.
-
No conflict of interest to declare.
doi: 10.3967/bes2018.014
Potassium Bromate-induced Changes in the Adult Mouse Cerebellum Are Ameliorated by Vanillin
-
Abstract:
Objective The current study aimed to elucidate the effect of vanillin on behavioral changes, oxidative stress, and histopathological changes induced by potassium bromate (KBrO3), an environmental pollutant, in the cerebellum of adult mice. Methods The animals were divided into four groups:group 1 served as a control, group 2 received KBrO3, group 3 received KBrO3 and vanillin, and group 4 received only vanillin. We then measured behavioral changes, oxidative stress, and molecular and histological changes in the cerebellum. Results We observed significant behavioral changes in KBrO3-exposed mice. When investigating redox homeostasis in the cerebellum, we found that mice treated with KBrO3 had increased lipid peroxidation and protein oxidation in the cerebellum. These effects were accompanied by decreased Na+-K+ and Mg2+ ATPase activity and antioxidant enzyme gene expression when compared to the control group. Additionally, there was a significant increase in cytokine gene expression in KBrO3-treated mice. Microscopy revealed that KBrO3 intoxication resulted in numerous degenerative changes in the cerebellum that were substantially ameliorated by vanillin supplementation. Co-administration of vanillin blocked the biochemical and molecular anomalies induced by KBrO3. Conclusion Our results demonstrate that vanillin is a potential therapeutic agent for oxidative stress associated with neurodegenerative diseases. -
Key words:
- Cerebellum /
- Behavior /
- Potassium bromate /
- Vanillin /
- ATPases /
- Genes expression
-
Figure 1. Effect of vanillin and KBrO3 on mice muscle grip strength (rotarod test, A) and mobility (open field test, B). Values from the rotarod test are expressed as time in seconds and compared to the performance of the control group (mean ± SD). In the open field test, values are expressed as number of squares crossed, head dips, and rearing frequency. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
Figure 2. Effect of potassium bromate (KBrO3), vanillin (Van), and their combination (KBrO3+vanillin) on the activities and mRNA expression of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in the cerebellum of adult mice. Values are expressed as mean ± SD (n = 3). C: Controls. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
Figure 3. Effect of potassium bromate (KBrO3), vanillin (Van), and their combination (KBrO3+vanillin) on TNF-α and IL-6 mRNA expression in the cerebellum of adult mice. Values are expressed as mean ± SD (n = 3). C: Controls. Means not sharing the same letters (a, b) are significantly different (P < 0.05).
Figure 4. Cerebellum histological sections of controls (A), vanillin (B), KBrO3 (C), and KBrO3+vanillin (D) groups. The score of Purkinje cell edema (E) in adult mice. ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granular layer. Arrows indicate →pyknotic Purkinje cells and

Table 1. Effect of Potassium Bromate (KBrO3), Vanillin and their Combination (KBrO3+vanillin) on Oxidative Stress Markers
Parameters & Treatments Control KBrO3 KBrO3+Vanillin Vanillin F P MDA (nmol MDA/g tissue) 92.920 ± 12.830a 133.540 ± 11.300b 102.330 ± 10.950a 94.510 ± 17.670a 1.470 0.094 LOOH (nmol/mg protein) 1.220 ± 0.130a 3.150 ± 0.180b 2.070 ± 0.080a, b 1.300 ± 0.100a 3.470 0.030 H2O2 (μmol/mg of protein) 0.020 ± 0.007c 0.080 ± 0.009a 0.060 ± 0.008b 0.030 ± 0.007c 37.450 < 0.001 AOPP (µmol/mg of protein) 0.400 ± 0.090a 0.810 ± 0.040b 0.540 ± 0.070a, b 0.380 ± 0.050a 5.670 0.120 Mg2+ ATPase (μmol Pi/h/mg protein) 0.110 ± 0.008 a 0.010 ± 0.003a 0.050 ± 0.004b 0.082 ±0.090a 130.541 < 0.001 Na+-K+ ATPase (μmol Pi/h/mg protein) 0.073 ± 0.004a 0.079 ± 0.008a 0.029 ± 0.009c 0.046 ± 0.005b 35.371 < 0.001 Note. Values are means ± SD for twelve mice in each group. Means not sharing the same letters (a-c) within a column are significantly different (P < 0.05). MDA, Malondialdehyde; LOOH, Lipid Hydroperoxide; H2O2, Hydrogen Peroxide; AOPP, Advanced Oxidation Protein Product. -
[1] Holmes G. Clinical symptoms of cerebellar disease and their interpretation (Croonian lectureⅢ). The Lancet, 1922; 59565. https://www.researchgate.net/publication/227085607_Clinical_symptoms_of_cerebellar_disease_and_their_interpretation [2] Mariën P. A Role for the Cerebellum in Language and Related Cognitive and Affective Functions. In: Mody M. (eds) Neural Mechanisms of Language. Innovations in Cognitive Neuroscience. Springer, Boston, MA. 2017. [3] Atif F, Yousuf S, Agrawal SK. Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol, 2008; 600, 59-63. doi: 10.1016/j.ejphar.2008.09.029 [4] Ben Amara I, Fetoui H, Guermazi F, et al. Dietary selenium addition improves cerebrum and cerebellum impairments induced by methimazole in suckling rats. Inter J Dev Neurosci, 2009; 27, 719-26. doi: 10.1016/j.ijdevneu.2009.07.002 [5] Deng H, Henatati A, Tainer J, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science, 1993; 261, 1047-51. doi: 10.1126/science.8351519 [6] Smith MA, Rudinicka-Nawrot M, Richey PL, et al. Carbonyl-related posttranslational modification of neurofilament protein in the neurofibrillary pathology of Alzheimer's disease. J Neurochem, 1995; 64, 2660-6. doi: 10.1046/j.1471-4159.1995.64062660.x/abstract [7] Becaria A, Bondy SC, Campbell A. Aluminum and copper interact in the promotion of oxidative but not inflammatory events:implications for Alzheimer's disease. J Alzheimer Dis, 2003; 5, 31-8. doi: 10.3233/JAD-2003-5105 [8] Frackowick J, Sukontasup T, Potempska. Lysosomal deposition of Abeta in cultures of brain vascular smooth muscle cells is enhanced by iron. Brain Res, 2004; 1002, 67-75. doi: 10.1016/j.brainres.2003.12.015 [9] Needham LL, Grandjean P, Heinzow B, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol, 2011; 45, 1121-6. doi: 10.1021/es1019614 [10] Matsumoto I, Morizono T, Paparella MM. Hearing loss following potassium bromate:two case reports. Otolaryngol Head Neck Surg, 1980; 88, 625-9. doi: 10.1177/019459988008800519 [11] Cho DH, Hong JT, Chin K, et al. Organotropic formation and disappearance of 8-hydroxydeoxyguanosine in the kidney of Sprague-Dawley rats exposed to adriamycin and KBrO3. Cancer Lett, 1993; 74, 141-5. doi: 10.1016/0304-3835(93)90235-2 [12] Muratsuka Y, Ueda H, Konishi T. Effects of sodium bromate on ionic concentrations and osmolalities of the cochlear fluids in guinea pigs. Hearing Res, 1989; 39, 241-50. doi: 10.1016/0378-5955(89)90044-0 [13] Bythrow JD. Vanillin as a medical plant. Sem Int Med, 2005; 3, 129-31. doi: 10.1016/j.sigm.2006.03.001 [14] Jiankang L, Akitane M. Antioxidant and pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin:Effects on free radicals, brain peroxidation and degradation of benzoate, deoxyribose, amino acids and DNA. Neuropharmacology, 1993; 32, 659-69. doi: 10.1016/0028-3908(93)90079-I [15] Takeda S, Aburada M. The choleretic mechanism of coumarin compounds and phenolic compounds. J Pharmacobiodyn, 1981; 4, 724-34. doi: 10.1248/bpb1978.4.724 [16] King AA, Shaughnessy T, Mure K, et al. Antimutagenicity of cinnamaldehyde and vanillin in human cells:Global gene expression and possible role of DNA damage and repair. Mut Res, 2007; 616, 60-9. doi: 10.1016/j.mrfmmm.2006.11.022 [17] Pereira Bezerra D, Nascimento Soares AK, Damiao Pergentino D. Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxi Med Cell Long, 2016; 9734, 816-9. https://www.ncbi.nlm.nih.gov/pubmed/28077989 [18] Ben Saad H, Driss D, Ben Amara I, et al. Altered hepatic mRNA expression of immune response-associated DNA damage in mice liver induced by potassium bromate:Protective role of vanillin. Environ Toxicol, 2015; 21, 10-22181. doi: 10.1002/tox.22181/abstract [19] Ben Saad H, Gargouri M, Kallel F, et al. Flavonoid compounds from the red marine alga Alsidium corallinum protect against potassium bromate-induced nephrotoxicity in adult mice. Environ Toxicol, 2016; 10, 22368. doi: 10.1002/tox.22368/full [20] Dhanalakshmi Ch, Manivasagam Th, Nataraj J, et al. Neurosupportive Role of Vanillin, a Natural Phenolic Compound, on Rotenone Induced Neurotoxicity in SH-SY5Y. Neuroblastoma. Cells Evid Bas Complem Alter Med, 2015; 626028, 11. [21] Council of European Communities. Council instructions about the protection of living animals used in scientific investigations. Off J Eur Com, 1986; (JO 86/609/CEE), L358, 1-18. http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm [22] Arai T, Kelly VP, Minowa O, et al. The study using wild-type and Ogg1 knockout mice exposed to potassium bromate shows no tumor induction despite an extensive accumulation of 8-hydroxyguanine in kidney DNA. Toxicology, 2006; 221, 179-86. doi: 10.1016/j.tox.2006.01.004 [23] Maury DK, Adhikari S, Nair CK, et al. DNA protective properties of vanillin against γ-radiation under different conditions:Possible mechanisms. Mut Res, 2007; 634, 69-80. doi: 10.1016/j.mrgentox.2007.06.003 [24] Kulkarni SK. Handbook of Experimental Pharmacology. 3rd Edn, Vallabh Prakashan, New Delhi, 1999. [25] Fan LW, Chen RF, Mitchell HJ, et al. alpha-Phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced brain injury and improves neurological reflexes and early sensorimotor behavioral performance in juvenile rats. J Neurosc Res, 2008; 86, 3536-47. doi: 10.1002/jnr.v86:16 [26] Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6-H-2Kmice. Beh Gen, 1999; 26, 263-71. doi: 10.1023/A:1021694307672 [27] Sumathi T, Asha D, Nagarajan G, et al. L-theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting upregulation of inflammatory cytokines and oxidative stress in rat brain. Envir Toxicol Pharmacol, 2016; 42, 99-117. doi: 10.1016/j.etap.2016.01.008 [28] Lowry OH, Rosenbrough NJ, Farr AL. Protein measurement with the Folin phenol reagent. J Biol Chem, 1951; 193, 265-75. https://www.ncbi.nlm.nih.gov/pubmed/14907713 [29] Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods. Enzymol, 1990; 186, 421-31. doi: 10.1016/0076-6879(90)86135-I [30] Ou P, Wolff SP. A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods, 1996; 31, 59-67. doi: 10.1016/0165-022X(95)00039-T [31] Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal Biochem, 1992; 202, 384-9. doi: 10.1016/0003-2697(92)90122-N [32] Witko V, Nguyen AT, Descamps-Latscha B. Microtiter plate assay for phagocytederived taurine chloramines. J Clin Lab Anal, 1992; 6, 47-53. doi: 10.1002/(ISSN)1098-2825 [33] Beauchamp C, Fridovich I. Superoxydedimutase:improved assays and an assay applicable to acrylamide gel. Anal Biochem, 1971; 44, 276-87. doi: 10.1016/0003-2697(71)90370-8 [34] Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol, 1984; 105, 114-21. doi: 10.1016/S0076-6879(84)05015-1 [35] Kawamoto EM, Munhoz CD, Glezer I, et al. Oxidative state in platelets and erythrocytes in aging and Alzheimer's disease. Neurobiol Aging, 2005; 26, 857-64. doi: 10.1016/j.neurobiolaging.2004.08.011 [36] Gabe M. Techniques histologiques. Masson, Paris, 1968; 838-79. http://www.researchgate.net/publication/50339229_Techniques_histologiques [37] Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg induced neurotoxicity. Toxicol Appl Pharma, 2011a; 256, 405-17. doi: 10.1016/j.taap.2011.05.001 [38] Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity:evidence from experimental studies. Life Sci, 2011b; 89, 555-63. doi: 10.1016/j.lfs.2011.05.019 [39] Cheng WY, Hsiang CY, Bau DT. Microarray analysis of vanillin regulated gene expression profile in human hepatocarcinoma. Cells Pharm Res, 2007; 56, 474-82. doi: 10.1016/j.phrs.2007.09.009 [40] Adedara IA, Rosemberg DB, Souza DO, et al. Biochemical and behavioral deficits in lobster cockroach Nauphoetacinerea model of methylmercury exposure. Toxicol Res, 2015; 4, 442-51. doi: 10.1039/C4TX00231H [41] Gupta VB, Anitha S, Hegdea ML, et al. Aluminium in Alzheimer's disease:are we still at a crossroad? Cell Mol Life Sci, 2005; 62, 143-58. doi: 10.1007/s00018-004-4317-3 [42] El-Sayed el-SM, Abo-Salem OM, Abd-Ellah MF, et al. Hesperidin, an antioxidant flavonoid, prevents acrylonitrile-induced oxidative stress in rat brain. J Biochem Mol Toxicol, 2008; 22, 268-73. doi: 10.1002/jbt.v22:4 [43] Hawkins CL, Davies MJ. Generation and propagation of radical reactions on proteins. Biochim Biophys Acta, 2001; 1504, 196. doi: 10.1016/S0005-2728(00)00252-8 [44] Halliwell B, Chirico S. Lipid peroxidation:its mechanism, measurement, and significance. Am J Clin Nutr, 1993; 57, 715-24. doi: 10.1093/ajcn/57.5.715S [45] Anane R, Creppy EE. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures:prevention by superoxide dismutase +-catalase and vitamins E and C. Hum Exp Toxicol, 2001; 20, 477-81. doi: 10.1191/096032701682693053 [46] Ben Saad H, Driss D, Ellouz-Chaabouni S, et al. Vanillin mitigates potassium bromate-induced molecular, biochemical and histopathological changes in the kidney of adult mice. Chem Biol Inter, 2016; 252, 102-13. doi: 10.1016/j.cbi.2016.04.015 [47] Samson FE, Nelson SR. The aging brain, metals and oxygen free radicals. Cell Mol Biol, 2000; 46, 699-707. http://www.ncbi.nlm.nih.gov/pubmed/10875433 [48] Chen KN, Peng WH, Hou CW, et al. Codonopsis javanica root extracts attenuate hyperinsulinemia and lipid peroxidation in fructose-fed insulin resistant rats. J Food Drug Anal, 2013; 21, 347-55. doi: 10.1016/j.jfda.2013.08.001 [49] Farina M, Franco JL, Ribas CM, et al. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol, 2005; 57, 1503-8. doi: 10.1211/jpp.57.11.0017 [50] Rahimi R, Abdollahi M. A review on the mechanisms involved inhyperglycemia induced by organophosphorus pesticides. Pestic Biochem Physiol, 2007; 88, 115-21. doi: 10.1016/j.pestbp.2006.10.003 [51] Lingrel JB. Na+, K+-ATPase:isoform structure, function, and expression. J Bioenerg Biomembr, 1992; 24, 263-70. http://www.ncbi.nlm.nih.gov/pubmed/1328175 [52] Dobrota D, Matejovicova M, Kurella EG. Na+, K+-ATPase under oxidative stress:molecular mechanisms of injury. Cell Mol Neurobiol, 1999; 119, 141-9. http://scialert.net/fulltext/?doi=ijbc.2013.38.46 [53] Sanui H, Rubin H. The role of magnesium in cell proliferation and transformation: Ions, cell proliferation and cancer Boynton AL, McKochan WL and Whitfield JP (eds) New York: Academic Press, 1982; 517-37. [54] Ben Saad H, Kharrat N, Krayem N, et al. Biological properties of Alsidium corallinum and its potential protective effects against damage caused by potassium bromate in the mouse liver. Environ Sci Poll Res, 2015; 23, 3809-23. http://www.ncbi.nlm.nih.gov/pubmed/26498820 [55] Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress:implications for cell death. Annu Rev Pharmacol Toxicol, 2007; 47, 143-83. doi: 10.1146/annurev.pharmtox.47.120505.105122 [56] Oppenheimer DR, Esiri MM. In: J. H. Adams and L. W. Duchen (Eds.), Greenfield's Neuropathology, 5th edn., Edward 29-33. Arnold, London, 1992; 1009-21. [57] Chaudhary M, Joshi DK, Tripathi S, et al. Docosahexaenoic acid ameliorates aluminum induced biochemical and morphological alteration in rat cerebellum. Ann Neurosci, 2014; 21, 5-9. http://pubmedcentralcanada.ca/pmcc/articles/PMC4117144/ [58] Amor S, Fabiola P, David B, et al. Inflammation in neurodegenerative diseases. Immunology, 2010; 129, 154-69. doi: 10.1111/imm.2010.129.issue-2 -




 下载:
下载:



 Quick Links
Quick Links