-
With the progress of science and technology, the ultrasound diagnostic technique has been increasingly used in medical institutions. According to research reports, microbial contamination of ultrasound probes and the couplants is a serious problem, and may be causing nosocomial cross infection[1-3]. Ultrasound probes, couplants, and inspection sheets carry a lot of different kinds of microorganisms, such as Aerobic spore-bearing bacilli, S. aureus, S. epidermidis, E. coli, K. pneumoniae, C. albicans, B. cepacia, P. aeruginosa, etc. Notably, S. aureus appears at a ratio as high as 33.8%, even leading to a risk of infection outbreak[2]. An infection outbreak was confirmed in a German university hospital, three infants suffered infections from S. aureus pyoderma due to cross infection caused by the couplants used in ultrasound detection[3]. Jean-sebastien Casalegno[4] pointed out genital HPV could be spread through the cross infection of ultrasonic diagnostic equipment in hospital, in addition to sexual contact and mother-to-child transmission. However, many are not aware of this route of transmission, and it has not been brought to appropriate attention in the medical community.
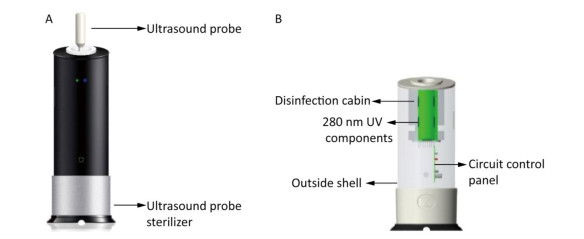
To control nosocomial cross infections caused by ultrasound probes, and to find a quick disinfection method suitable for the ultrasound probe surface, we studied the disinfection effect of a new global pioneering ultraviolet (UV) sterilizer (30 mW power, UV wavelength of 280 nm and refined light-emitting diode (LED) components, referred to as the ultrasound probe sterilizer) (Supplementary Figure S1, available at www.besjournal.com) and its utilization on the ultrasound probe surfaces in our laboratory and the hospitals.

Figure Supplementary Figure S1. Schematic diagrams of the ultrasound probe sterilizer. (A) a scheme of the ultrasound probe sterilizer and a ultrasound probe. (B) a component sketch of the ultrasound probe sterilizer.
The ultrasound probe sterilizer and the probe models were provided by Hunan Health Medical Technology Co., LTD. Experimental bacteria Staphylococcus aureus (CICC21601), Escherichia coli (8099), Pseudomonas aeruginosa (CICC21630), Candida albicans (CMCC98001), and Bacillus subtilis var niger spores (CICC10854, B. subtilis spores for short) were provided by the Disinfection Infection Center, Academy of Military Medical Sciences. Automatic microbial identification and drug susceptibility analysis system (VITEK 2 Compact system) was manufactured by BioMerieux (F-69280 Marcy l'Etoile, France). All procedures were carried out in accordance with the approved technical standards, including any relevant details[4].
To prepare the microbial suspension, an isolated and purified individual typical colony of each experimental bacteria strain was inoculated on agar slant culture medium, while C. albicans was inoculated in Sabouraud liquid medium. All cultures were then placed at 36 ± 1 ℃ for 24 h. Fresh cultures were taken, and the bacterial lawns were washed by tryptone physiology solution (TPS). B. subtilis spores were cultured at 36 ± 1 ℃ for 7 days, then placed at room temperature for 2-3 days, and made into suspension when the spore formation rate was greater than 95%. The bacterial suspension was then diluted to the required concentration with tryptic soy broth (TSB) nutrient broth and ready for used on glass carriers and ultrasound probes before the tests. 0.3% and 3% of bovine serum albumin (BSA) were added to the C. albicans and S. aureus bacteria suspensions, respectively, for the organic substance influence test. Using the drip staining method, 0.02 mL aliquots of bacteria suspension were placed onto distinct areas of the ultrasound probe models (1 cm x 1 cm) and glass carriers (1 cm x 1 cm), making the bacteria count 5 x 105 to 5 x 106 CFU/piece (cm2) until naturally dried. The probe models were then placed in a 37 ℃ incubator for backup.
Carrier quantitative germicidal test was performed on the glass carrier which was put into the ultrasound probe sterilizer, following the procedure for disinfection with the stipulated time. Once disinfection was complete, the carrier was taken out and transferred directly into test tubes with 5 mL sampling liquid (0.5% Tween 80 and 0.1% lecithin in phosphate buffered solution). At the same time, positive control was set at room temperature for the same time as the disinfection. Liquid specimens were used for the viable bacteria count and the Killing Log value (KL) calculation. The disinfection effect of different microbe using the ultrasound probe sterilizer was identified (Table 1). According to the Technical Standard For Disinfection[5], KL above 3.00 was regarded as significant disinfection effects. To meet the standard, the ultrasound probe sterilizer requires varying durations.
Table 1. The Disinfection Effect of the Ultrasound Probe Sterilizer
Microbes Mean and Range of Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control Time 5 10 15 E. coli x±s 3.25 ± 0.04 4.71 ± 0.14 5.92 ± 0.50 6.28 ± 0.47 Range 3.21-3.27 4.61-4.87 5.54-6.48 5.74-6.62 P. aeruginosa x±s 3.07 ± 0.02 4.57 ± 0.18 4.84 ± 0.19 6.29 ± 0.30 Range 3.06-3.10 4.26-4.57 4.69-5.06 5.94-6.49 Time 10 20 30 S. aureus x±s 4.24 ± 0.03 5.53 ± 0.53 6.57 ± 0.04 6.57 ± 0.04 Range 4.20-4.26 5.17-6.14 6.53-6.60 6.53-6.60 C. albicans x±s 3.23 ± 0.15 4.32 ± 0.15 5.70 ± 0.12 5.70 ± 0.12 Range 3.14-3.41 4.22-4.49 5.61-5.84 5.61-5.84 Time 30 60 70 90 B. subtilis spores x±s 1.96 ± 0.01 2.96 ± 0.03 3.54 ± 0.11 5.91 ± 0.16 6.04 ± 0.08 Range 1.95-1.97 2.94-2.99 3.42-3.63 5.73-6.03 5.97-6.12 Note. No microbe grew for negative controls; All numbers are means from three repeated tests. Comparison of the disinfection effect in the testing distance of 4 cm was performed between the ultrasound probe sterilizer and a UV germicidal lamp (30W power, UV wavelength of 253.7 nm). Results showed the sterilizer had better disinfection effects (Supplementary Table S1, available at www.besjournal.com).
Table Supplementary Table S1. The Disinfection Effect of UV Sterilizers with Different Wavelength in the Testing Distance of 4 cm
Name of Sterilizers Microbes Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control 10 20 30 The ultrasound probe sterilizer S. aureus x±s 4.24±0.03 5.53±0.53 6.57±0.04 6.57±0.04 P value 0.0108 0.0987 < 0.001 C. albicans x±s 3.43±0.28 4.16±0.05 5.70±0.12 5.70±0.12 P value 0.5181 < 0.01 0.004 The UV germicidal lamp S. aureus x±s 4.11±0.04 4.87±0.05 5.70±0.12 6.57±0.04 C. albicans x±s 3.25±0.34 3.27±0.05 4.71±0.16 5.70±0.12 Note. No bacteria grew for negative controls; All numbers are mean from 3 repeated tests. Organic substance influence test was performed with the same procedures while the microbial suspensions were prepared with 0.3% or 3% BSA as described above. With an extended 20 s of disinfection time, the ultrasound probe sterilizer achieved disinfection requirements (KL > 3.00) for S. aureus and C. albicans on the carriers in the presence of 0.3% BSA. An extension to 45 seconds was required for 3% BSA (Supplementary Table S2, available at www.besjournal.com).
Table Supplementary Table S2. Organic Substance Influence Tests of the Ultrasound Probe Sterilizer
Experimental Numbers Microbes BSA (%) Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control Time 10 20 30 1 S. aureus 0.3 2.64 3.61 5.09 5.96 C. albicans 0.3 2.24 3.82 4.76 6.11 2 S. aureus 0.3 2.49 3.43 4.53 6.04 C. albicans 0.3 2.16 3.85 4.68 6.22 3 S.aureus 0.3 2.62 3.58 4.66 6.23 C. albicans 0.3 2.11 3.74 4.69 6.36 Time 15 30 45 1 S. aureus 3.0 2.55 2.88 4.39 5.74 C. albicans 3.0 1.84 2.62 4.20 5.82 2 S. aureus 3.0 2.44 2.92 4.63 6.57 C. albicans 3.0 1.98 2.66 4.06 6.67 3 S. aureus 3.0 2.35 2.83 4.54 6.26 C. albicans 3.0 2.16 2.62 4.07 6.69 Note. 1.No bacteria grew for negative controls; 2. All numbers are mean from 3 repeated tests. Simulated on-the-spot trial followed the procedure for 90 seconds of disinfection. Once disinfection was completed, moist sterile cotton swabs were used for sampling and transferred into test tubes with 5 mL sampling liquid. Liquid specimens were processed the same as described above. The sterilizer achieved disinfection requirements (KL > 3.00) for B. subtilis spores artificially infected on the front face of the ultrasound four-dimensional probe, the linear array probe, and the vaginal probe models, but failed to reach requirements for side face (KL values were 1.17-2.95) (Table 2). This is associated with the shadow effect of side UV irradiation, suggesting a full ultrasound probe surface disinfection for better results. The inactivating effect of the ultrasound probe sterilizer on B. subtilis spores was better for the slide (70 s in Table 1) than for the probe models (90 s in Table 2) because the surface of the glass slide is smoother than that of the wooden model probes.
Table 2. Simulated On-the-spot Trials of the Ultrasound Probe Sterilize for B. Subtilis spores
Different Ultrasound Probe Models Experimental Numbers Killing Log Values (KL) to Different Locations for 90 s Sterilization Recovery Log Values of the Positive Control Front Face Long-side Face Short-side Face The four-dimensional probe 1 3.53 1.37 1.33 6.12 2 3.57 1.40 1.37 6.17 3 4.48 1.34 1.35 6.13 The linear array probe 1 5.73 2.18 2.89 6.12 2 5.63 2.28 2.95 6.17 3 5.36 2.25 2.91 6.13 The vaginal probe 1 4.52 1.63 1.17 6.12 2 4.63 1.68 1.55 6.17 3 4.43 1.23 1.22 6.13 Note. 1. No bacteria grew for negative controls; 2. All numbers are means from 3 repeated tests. Survey of hospital ultrasound probe contamination and sterilization application was performed. We investigated 1, 138 people with a total of 2, 616 specimens in 16 hospitals, including 968 subjects for the ordinary ultrasound probe and 170 people for the vaginal ultrasound probe. Samples from the ordinary ultrasound probe were taken before sterilization, after paper towel wiping which was commonly used in most hospitals, and after sterilization. For the vaginal ultrasound probe, specimens were taken from the front and side, before and after sterilization, respectively. The total number of bacteria was counted for the specimens that were taken before disinfection of the ultrasound probe surfaces. Suspected colonies were inoculated and cultured in 36 ± 1 ℃ for 18-24 h. Then representative colonies were selected for identification using the VITEK 2 Compact system. The total number of bacteria was also detected for the specimens taken after paper towel wiping, after sterilization by the ultrasound probe sterilizer for 90 s, and after cleaning by disinfecting wipes wet with (0.2 ± 0.02)% Benzyldimethyl-dodecylammonium chloride. The agar pouring method was used for viable bacteria counting.
Our results indicated that, in 32 kinds of couplants, 46.87% were detected with a total number of bacteria as high as 1.71 x 107 CFU/g including Candida glabrata, Burkholderia cepacia, Aggregatibacter actinomycetemcomitans, Staphylococcus haemolyticus, and Delftia acidovorans. For the 968 specimens obtained from the ordinary ultrasound probe surface before disinfection, the bacteria detection rate was 98.02% with a total number as high as 6.07 x 108 CFU/cm2. In some specimens, 2-3 kinds of bacteria were detected. A total of 23 strains were identified as pathogenic bacteria or conditional pathogenic bacteria, including: Staphylococcus epidermidis, Staphylococcus hominis, Klebsiella pneumoniae, Staphylococcus haemolyticus, Serratia marcescens, Acinetobacter baumannii, Enterobacter cloacae, Staphylococcus capitis, Micrococcus luteus, etc. For the 340 specimens from the vaginal ultrasound probe surface, the bacterial detection rate was 49.41% with a total count up to 7.50 × 108 CFU/pieces, including 4 strains of bacteria: Acinetobacter lwoffii, Bacillus subtilis, Staphylococcus warneri, and klebsiella pneumoniae. In the hospital investigation, the three different disinfection methods had statistically significant differences (P < 0.01). Among these methods, the ultrasound probe sterilizer had the best disinfection effect with negative bacteria detection and a 100% inactivating rate in 676 specimens, while other sterilization methods could not reach the disinfection requirements (Table 3). Comparison of the disinfection effects for different locations of the vaginal ultrasound probe demonstrated disinfection for side face was more serious (Supplementary Table S3, available at www.besjournal.com). In terms of damage observations, the ultrasound probe sterilizer has been used in a hospital for 2 years and showed no notable damage.
Table 3. Comparison of the Disinfection Effects of Three Different Methods for the Ultrasound Probe Surface
Numbers and Disinfection Methods Sample Size Log Values of the Colonies before Disinfection Log Values of the Colonies after Disinfection Inactivating Rate (%) 1. Paper towel wiping 484 4.12 ± 1.42 2.13 ± 1.18 92.59 ± 12.76 2. Wet disinfecting wipes cleaning 148 4.27 ± 1.41 0.91 ± 1.41 98.07 ± 9.86* 3. Disinfection by the ultrasound probe sterilizer 336 4.05 ± 1.42 0 100.00*, # Note. *Compared with 1, P < 0.01; #Compared with 2, P < 0.01. Table Supplementary Table S3. Comparison of the Disinfection Effects for Different Locations of the Vaginal Ultrasound Probe
Types of Ultrasound Locations Sample Size Log Values of the Colonies before Disinfection Log Values of the Colonies after Disinfection Inactivating Rate (%) Vaginal ultrasound Front face 170 1.83 ± 0.59 0 100.00% Side face 170 2.96 ± 1.80 0 100.00% Although there are no reports for nosocomial cross infection outbreaks caused by ultrasonic diagnostic equipment in China, probe contamination is very serious matter. As Julan Yang reported[6], there was a 100% positive rate of bacteria contamination in a total of 104 specimens taken from abdomen ultrasound probes; isolated strains were mainly gram positive cocci, which accounted for 76.93% of bacteria. Li Ming et al.[7] reported on the molecular epidemiology and homology analysis of S. epidermidis and determined that it had caused contamination of the ultrasound probe. From our data showing above, the detection rate, identified numbers and types of bacteria from the surface of ordinary ultrasound probes or vaginal ultrasound probes and from 32 kinds of couplants are different from the results given by Julan Yang et al.[6]. It perhaps due to a more representative choice of hospitals in this study, including the children's hospital, the maternal and child health care hospital, and the general hospital.
To control nosocomial infections caused by microorganism-contaminated surfaces of ultrasonic diagnostic equipment, corresponding operation specifications and standards have been developed both at home and abroad. In 2008, US CDRH/FDA released the industry guideline for manufacturers of ultrasonic instrument and probes[8], which stipulates that if the probe is reused among patients, operation procedures for cleaning and disinfecting should be provided. The US Disease Control and Prevention Center[9] requires medical devices to be classified into non-critical equipment, semi-critical equipment, and critical equipment, according to the Spaulding classification, with corresponding disinfection requirements and methods. The FDA's latest disinfection solution recommends the use of low-temperature plasma sterilization and UV-C light disinfection. Australian[10] ultrasound probe processing standards are based on As/NZs 4187:2014 and As/NZs 4815:2006 which specify that medical equipment must be disinfected. In China, the Technical Standard For disinfection and Measures for the Administration of Disinfection require any equipment or article that contacts skin to be above the disinfection standards, and stipulates that the usage of ultrasound probes should follow the one-person-one-piece mode after sterilization. However, no specific method has been recommended for the disinfection of the ultrasound probes in these standards.
In this study, the ultrasound probe sterilizer with a UV wavelength of 280 nm had a good bactericidal effect, and was fast and easy to use. At present, there are few reports on the surface disinfection of ultrasonic diagnostic equipment with a UV wavelength of 280 nm. The vaginal ultrasound probe surface is easily contaminated due to damage of the protective film, which affects the inspection clarity. To control nosocomial cross infections caused by ultrasound probes, a rapid and effective disinfection method is needed. The advantages of the ultrasound probe sterilizer include fast disinfection, high efficacy, and good compatibility with the ultrasound instrument, while long-term use of disinfectants causes damage to the ultrasound probe. Therefore, the ultrasound probe sterilizer is a powerful tool to control nosocomial cross infections caused by ultrasound probes, and is worthy of increased utilization in medical institutions.
The authors declare no conflict of interest.
The authors would like to sincerely thank the support of LI Xian Bin from Hunan Children's Hospital, individuals from Center for Disease Control and Prevention of Shaoshan City, Changsha city, Xiangtan city, and Loudi city, and the infection control section of the hospital.
CHEN Gui Qiu conceived the study, designed the experiments and the survey of the ultrasound probe surface contamination, and wrote the paper; CHEN Pei Hou and GUO Gui Ping took the responsibility for the guidance of experiment and investigation; CHEN Yu Hao participated in the design of the surface contamination survey and some experimental tests; YI Liang and YIN Jin performed the surface contamination survey in hospitals and experimental tests in the lab; GAO Qiong, SONG Jiang Nan, and LI Shi Kang performed parts of the survey in hospitals and experimental tests in the lab. All authors have read and approved the final paper.
doi: 10.3967/bes2018.021
Study on a New Ultraviolet Sterilizer to the Surface Disinfection of the Ultrasound Probe
-
Abstract: We studied the disinfection effect of a new ultraviolet (UV) sterilizer and its utilization on ultrasound probe surfaces. Carrier quantitative germicidal tests, simulated on-the-spot trials, and organic substance influence tests were used to carry out experimental observation. Artificially infected probes were disinfected using the sterilizer or a germicidal lamp for comparison. The total number and types of bacteria were determined and identified. Our results demonstrated the sterilizer had the best disinfection effect among three different disinfection methods in hospital. The sterilizer has been used in a hospital setting for 2 years with no notable damage to the ultrasound probe instrument. It has the advantages of fast disinfection, high disinfection effect, and good compatibility with the ultrasound instrument, worthy of being a promoted application in medical institutions.
-
Table 1. The Disinfection Effect of the Ultrasound Probe Sterilizer
Microbes Mean and Range of Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control Time 5 10 15 E. coli x±s 3.25 ± 0.04 4.71 ± 0.14 5.92 ± 0.50 6.28 ± 0.47 Range 3.21-3.27 4.61-4.87 5.54-6.48 5.74-6.62 P. aeruginosa x±s 3.07 ± 0.02 4.57 ± 0.18 4.84 ± 0.19 6.29 ± 0.30 Range 3.06-3.10 4.26-4.57 4.69-5.06 5.94-6.49 Time 10 20 30 S. aureus x±s 4.24 ± 0.03 5.53 ± 0.53 6.57 ± 0.04 6.57 ± 0.04 Range 4.20-4.26 5.17-6.14 6.53-6.60 6.53-6.60 C. albicans x±s 3.23 ± 0.15 4.32 ± 0.15 5.70 ± 0.12 5.70 ± 0.12 Range 3.14-3.41 4.22-4.49 5.61-5.84 5.61-5.84 Time 30 60 70 90 B. subtilis spores x±s 1.96 ± 0.01 2.96 ± 0.03 3.54 ± 0.11 5.91 ± 0.16 6.04 ± 0.08 Range 1.95-1.97 2.94-2.99 3.42-3.63 5.73-6.03 5.97-6.12 Note. No microbe grew for negative controls; All numbers are means from three repeated tests. Supplementary Table S1. The Disinfection Effect of UV Sterilizers with Different Wavelength in the Testing Distance of 4 cm
Name of Sterilizers Microbes Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control 10 20 30 The ultrasound probe sterilizer S. aureus x±s 4.24±0.03 5.53±0.53 6.57±0.04 6.57±0.04 P value 0.0108 0.0987 < 0.001 C. albicans x±s 3.43±0.28 4.16±0.05 5.70±0.12 5.70±0.12 P value 0.5181 < 0.01 0.004 The UV germicidal lamp S. aureus x±s 4.11±0.04 4.87±0.05 5.70±0.12 6.57±0.04 C. albicans x±s 3.25±0.34 3.27±0.05 4.71±0.16 5.70±0.12 Note. No bacteria grew for negative controls; All numbers are mean from 3 repeated tests. Supplementary Table S2. Organic Substance Influence Tests of the Ultrasound Probe Sterilizer
Experimental Numbers Microbes BSA (%) Killing Log Values (KL) under Different Time (s) Recovery Log Values of the Positive Control Time 10 20 30 1 S. aureus 0.3 2.64 3.61 5.09 5.96 C. albicans 0.3 2.24 3.82 4.76 6.11 2 S. aureus 0.3 2.49 3.43 4.53 6.04 C. albicans 0.3 2.16 3.85 4.68 6.22 3 S.aureus 0.3 2.62 3.58 4.66 6.23 C. albicans 0.3 2.11 3.74 4.69 6.36 Time 15 30 45 1 S. aureus 3.0 2.55 2.88 4.39 5.74 C. albicans 3.0 1.84 2.62 4.20 5.82 2 S. aureus 3.0 2.44 2.92 4.63 6.57 C. albicans 3.0 1.98 2.66 4.06 6.67 3 S. aureus 3.0 2.35 2.83 4.54 6.26 C. albicans 3.0 2.16 2.62 4.07 6.69 Note. 1.No bacteria grew for negative controls; 2. All numbers are mean from 3 repeated tests. Table 2. Simulated On-the-spot Trials of the Ultrasound Probe Sterilize for B. Subtilis spores
Different Ultrasound Probe Models Experimental Numbers Killing Log Values (KL) to Different Locations for 90 s Sterilization Recovery Log Values of the Positive Control Front Face Long-side Face Short-side Face The four-dimensional probe 1 3.53 1.37 1.33 6.12 2 3.57 1.40 1.37 6.17 3 4.48 1.34 1.35 6.13 The linear array probe 1 5.73 2.18 2.89 6.12 2 5.63 2.28 2.95 6.17 3 5.36 2.25 2.91 6.13 The vaginal probe 1 4.52 1.63 1.17 6.12 2 4.63 1.68 1.55 6.17 3 4.43 1.23 1.22 6.13 Note. 1. No bacteria grew for negative controls; 2. All numbers are means from 3 repeated tests. Table 3. Comparison of the Disinfection Effects of Three Different Methods for the Ultrasound Probe Surface
Numbers and Disinfection Methods Sample Size Log Values of the Colonies before Disinfection Log Values of the Colonies after Disinfection Inactivating Rate (%) 1. Paper towel wiping 484 4.12 ± 1.42 2.13 ± 1.18 92.59 ± 12.76 2. Wet disinfecting wipes cleaning 148 4.27 ± 1.41 0.91 ± 1.41 98.07 ± 9.86* 3. Disinfection by the ultrasound probe sterilizer 336 4.05 ± 1.42 0 100.00*, # Note. *Compared with 1, P < 0.01; #Compared with 2, P < 0.01. Supplementary Table S3. Comparison of the Disinfection Effects for Different Locations of the Vaginal Ultrasound Probe
Types of Ultrasound Locations Sample Size Log Values of the Colonies before Disinfection Log Values of the Colonies after Disinfection Inactivating Rate (%) Vaginal ultrasound Front face 170 1.83 ± 0.59 0 100.00% Side face 170 2.96 ± 1.80 0 100.00% -
[1] Oleszkowicz SC, Chittick P, Russo V, et al. Infections Associated with Use of Ultrasound Transmission Gel:Proposed Guidelines to Minimize Risk. Infect Control Hosp Epidemiol, 2012; 33, 1235-7. doi: 10.1086/668430 [2] L Moshkanbaryans, C Meyers, A Ngu, et al. The importance of infection prevention and control in medical ultrasound. Australas J Ultrasound Med, 2015; 18, 96-9. doi: 10.1002/ajum.2015.18.issue-3 [3] Weist K, Wendt C, Petersen LR, et al. An outbreak of pyodermas among neonates caused by ultrasound gel contaminated with methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol, 2000; 21, 761-4. doi: 10.1086/501729 [4] Jean-sebastien Casalegno, Karine Le Bail Carval, Daniel Eibach, et al. High Risk HPV Contamination of Endocavity Vaginal Ultrasound Probes:An Underestimated Route of Nosocomial Infection? PLoS One, 2012; 7, e48137. doi: 10.1371/journal.pone.0048137 [5] Department of Health Law and Supervision, Ministry of Health, Technical Standard For disinfection, Beijing, the Ministry of Health of the People's Republic of China, 2002. (In Chinese) [6] Julan Yang, Haiyan Qi, Yan Wang. Bacteriological monitoring and measures explore of B-ultrasound probe. Chinese Journal of Disinfection, 2012; 29, 1007-8. (In Chinese) http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZGXD201211022.htm [7] Li M, Liu LH, Gan LP. Homology research of staphyococcus epidermidis molecular epidemiology by ultrasound probes. Journal of Ultrasound in Clinical Medicine, 2010; 12, 222-4. (In Chinese) http://d.wanfangdata.com.cn/Periodical_lccsyxzz201004002.aspx [8] US CDRH/FDA. Guidance for Industry and FDA staff-Information for Manufacturers seeking Marketing Clearance of Diagnostic Ultrasound systems and Transducers, September 9, 2008. https://www.fda.gov/RegulatoryInformation/Guidances/ucm089001.htm [9] Rutala WA, Weber DJ. Guideline for Disinfection and Sterilization in Healthcare Facilities, US Centers for Disease Control and Prevention, 2008. https://www.medscape.com/medline/abstract/20055640 [10] Department of Health & Human Services. Australian/New Zealand Standard: Reprocessing of reusable medical devices in health service organisations 4187, Australia. 2014. http://www.health.gov.au/internet/main/publishing.nsf/Content/About+Us-1 -




 下载:
下载:



 Quick Links
Quick Links