-
Dyspnea is one of the commonest complaints of patients presenting to the emergency department (ED)[1-3]. Acute heart failure (AHF) accounts for many of these presentations[4] and has relation with high morbidity and mortality[5]. Early discrimination between cardiac and non-cardiac causes of dyspnea is often challenging. A prompt and accurate diagnosis may guide and optimize acute management, avoid delays in care, and improve the outcome. Conventional diagnosis tools, such as history, physical examination, electrocardiogram (ECG), chest X-ray plain film, and serum amino-terminal pro-brain-type natriuretic peptide (NT-proBNP), either lack specificity or sensitivity[6-7]. Due to high requirement of personnel knowledge as well as a special training of cardiac, thoracic and veins ultrasound, it is difficult to popularize the 'gold' standard echocardiography with Doppler examination for a fast-paced and busy ED. However, emergency physicians (EP) often need to make quick and decisive management plans within such limited settings.
In the recent years, a new technique of transthoracic lung ultrasonography (TLS) has emerged and demonstrated promising results in AHF diagnosis at an early stage. Lichtenstein et al. first reported diffuse B-lines as an ultrasound sign of interstitial edema in 1997[8-9]. Increased extravascular lung water could cause thickened subpleural interlobular septa which can be detected by the ultrasound beams. These reflected ultrasound beams create comet-tail reverberation artifact known as B-lines or ultrasound lung comets (ULCs). Subsequently, more studies confirmed that B-lines are ULCs artifacts related to fluid-filled alveoli edema[10-12]. Excessive ULCs have been found to correlate with AHF diagnosis[13]. These artifacts are easily identified with bedside ultrasonography. As mentioned above, TLS through ULCs evaluation is an easy technique for rapid diagnosis of AHF. Moreover, TLS is inexpensive, widely available, and can be repeated without additional radiation to patient. However, the diagnostic value of ULCs for evaluating AHF patients presenting urgently with dyspnea in EDs is uncertain. The objective was to assess the overall performance of the ULCs in the diagnosis of AHF in ED.
-
A literature review and a meta-analysis were conducted. Original articles published in English and Chinese up to the end of July 2017 were searched in PubMed, ProQuest, Cochrane Library, Web of science, OvidSP (EMBASE), EBSCO, Clinicaltrial.gov, CNKI, and WanFang Data. We searched some free text words or medical topic headings separately or in combination: 'ultrasound', 'sonography', 'ultrasonography', 'B-lines', 'comet', 'ultrasound lung comets', 'ULCs', 'dyspnea', 'heart failure', 'heart dysfunction', 'acute cardiogenic pulmonary edema (ACPE)', 'AHF', 'ACPE', 'sensitivity', and 'specificity'. Furthermore, the lists of reference for all eligible articles and reviews were also retrieved for additional studies. No attempt has been made to include unpublished data.
-
We selected articles for analysis that included the following criteria: (1) Population: patients with complaints of acute dyspnea or clinical suspected AHF; (2) Type of studies: prospective diagnostic studies that evaluated the diagnostic efficiency of lung ultrasonography (US) for the detection of AHF in the emergency department or pre-hospital emergency settings (case reports, retrospective investigations, and other types of case-control studies were excluded); (3) Index test: no restriction was made on the protocol of TLS used to detect AHF, as long as the authors addressed that they used the ULCs to make AHF diagnostic decision. In most studies, ULCs was defined as the presence of at least two zones showing the presence of three or more B-lines on both sides of the chest. There is no limit to the type of TLS operating physician or the type of machine and probe which was applied; (4) Comparison: comparison of imaging results with a final diagnosis from clinical follow-up accepted as the reference standard; (5) Outcomes: true positives, false negatives, false positives, and true negatives. If there is no clear report, these data must be calculated to retrieve. Two investigators (GCZ and STY) selected and examined eligible studies independently. Disagreements were resolved through discussion. Repeated citations were deleted and final references were formatted to be compatible with EndNote citation software.
-
We used the Quality Assessment of Studies of Diagnostic Accuracy included in Systematic Reviews-2 (QUADAS-2) tool[14] to assess the methodological quality of all included articles. QUADAS-2 consists of two sections: the risks of bias and concerns regarding applicability. The former part is comprised of four domains: patient selection, index test, reference standard, and flow and timing. The latter is comprised of three domains: patient selection, index test, and reference standard.
-
Two investigators (LCS and SQZ) independently extract the following data items by using a specific data extraction sheet: types of ultrasound machine and probe, characteristics of patients, patient body position, the time between the patient admission to US examination, the TLS criteria for AHF, reference standards, study quality evaluation of included studies, and outcomes. When there are discrepancies, reaching consensus with a third author of this review (STY) was adopted.
-
The forest plots and summary receiver operating characteristic (SROC) curves were analyzed by using Meta-DiSc (version 1.4) software (http://www.hrc.es/investigacion/metadisc_en.htm). The threshold/cutoff effect was presented by calculating the Spearman correlation coefficient between the logit of sensitivity and the logit of specificity. The heterogeneity in each study was evaluated through performing χ2 tests. If there was significant heterogeneity existing among studies, the random-effects model was applied. On the contrary, the fixed effect model was adopted. We also conducted Meta-DiSc to calculate the inconsistency index (I2) to quantify the amount of heterogeneity. P < 0.1 or I2 > 50% suggested a prominent heterogeneity. Publication bias was checked using a Deeks' funnel plot asymmetry test. Fagan plot analysis was also applied. We assumed the pretest probabilities of 20%, 45%, and 70%. The corresponding post-test probabilities were calculated following a 'positive' or 'negative' TLS outcome based on the summary sensitivity and specificity which showed the relationship among the prior probability specified, the likelihood ratio, and posterior test probability[15]. The level of low (20%), moderate (45%), and high pre-test probability (70%) were defined according to the PRIDE AHF scores[16].
Most studies included in our meta-analysis have reported the time between the patients' admission and bedside TLS examination. As it is widely known, this time interval is crucial for the EPs to make prompt evaluation and could immensely influence the volume of extravascular lung water and thus, affect US pathologic findings because of initial treatment effect. Accordingly, the following subgroup analysis was established: comparisons between studies performed within 90 min of patient admission and beyond this time interval.
-
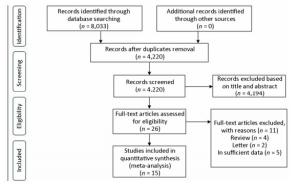
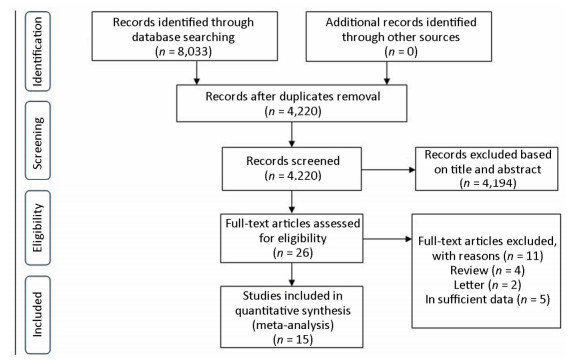
Fifteen studies were finally identified for this meta-analysis[10, 16-30]. The flow diagram of search results is summarized in Figure 1. The number of cases ranged from 42 to 1, 005, involving a total of 3, 309 patients. The detailed characteristics of selected studies are showed in Table 1 and Table 2.
Table 1. Characteristics of Eligible Studies
Authors Year Patients
(n)Study Country US Operator Study Type Consecutive Time Interval Scanning Protocol Reference Standard Probe Type Pivetta et al.[17] 2015 1, 005 Italy EP Cohort study 2 < 90 min 6 zones Final hospital diagnosis Curvilinear transducer
(3-5 MHz)Russell et al.[18] 2014 99 America EP Observational study 2 < 90 min 8 zones Blinded chart review Curvilinear probe
(3-5 MHz)Mumoli et al.[19] 2015 226 Italy Nurse Observational study 1 < 90 min 8 zones Final hospital diagnosis Curved array transducer
(2-5.5 MHz)Arrgarwal et al.[20] 2016 42 India Cardiologist Cross-sectional study 1 IMR 28 scan sites Blinded chart review Portable machine
(not specified)Prosen et al.[21] 2011 218 Slovenia EP Cohort study 1 < 90 min 8 zones Final hospital diagnosis Not specified Liteplo et al.[10] 2009 94 America EP Observational study 2 > 90 min 8 zones Blinded chart review Curved array transducer
(2-5 MHz)Shah et al.[22] 2016 117 America Internal medicine resident Cohort study 2 NR 8 zones Final hospital diagnosis Phased array transducer Pirozzi et al.[23] 2014 167 Italy EP Observational study 2 < 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz)
Cardiac transducer
(2.5-3.5MHz)Kajimoto et al.[24] 2012 90 Japan Cardiologist Observational study 1 < 90 min 8 zones Phased array probe
(l.7-3.5MHz)Sartini et al.[25] 2016 236 Italy EP Observational study 2 > 90 min 12 zones Final hospital diagnosis Convex probe (3.5-5 MHz) Volpicelli et al.[30] 2006 300 Italy EP and radiologist Observational study 1 > 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz) Cibinel et al.[26] 2011 56 Italy EP Observational study 2 > 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz) Chiem et al.[27] 2015 380 America EM resident Cross-sectional study 2 < 90 min 8 zones Blinded chart review Curvilinear transducer
(2-5 MHz)Li et al.[28] 2016 187 China Sonographer Cohort study 2 > 90 min 28 scan sites Echocardiography with Doppler S5-1 probe (1-5 MHz) Unluer et al.[29] 2014 96 Turkey Nurse Cross-sectional study 2 < 90 min 6 zones Provisional diagnosis at the end of the ED stay Microconvex probe
(3.6 MHz)Note. NR: not reported; 1: yes: 2: no; EP: emergency physician; EM: emergency medicine; Scanning protocol: The different zones of thoracic ultrasonography considered in each study. Table 2. Patient Characteristics
Study Setting Age, y (range) Inclusion Pivetta et al.[17] ED 77 (IQR13) acute dyspnea Russell et al.[18] ED 56 ± 13 (22-91) undifferentiated dyspnea Mumoli et al.[19] ED 78.7 ± 12.7 acute dyspnea Arrgarwal et al.[20] ED 64.4 ADHF suspected Prosen et al.[21] Prehospital emergency setting 70.9 ± 11.7 shortness of breath Liteplo et al.[10] ED 74 ± 14 acute dyspnea Shah et al.[22] ED 36 (17-58) undifferentiated dyspnea Pirozzi et al.[23] ED 74.3 ± 4.3 acute dyspnea Kajimoto et al.[24] ED 78.1 ± 8.1 acute dyspnea Sartini et al.[25] ED 79.98 acute dyspnea not related to any trauma Volpicelli et al.[30] ED 68.4 ± 8.4 alveolar-interstitial syndrome suspected Cibinel et al.[26] ED 82.1 (38.7-94.3) acute dyspnea Chiem et al.[27] ED 55 ± 5 dyspnea Li et al.[28] ED 62.4 ± 2.4 acute dyspnea Unluer et al.[29] ED 70.59 acute dyspnea Note. IQR: interquartile range; ADHF: Acute decompensated heart failure; SD: Standard deviation. -
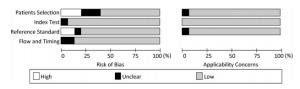
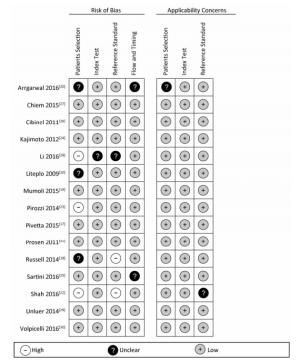
Figure 2 and Figure 3 show the quality assessment of individual studies.
-
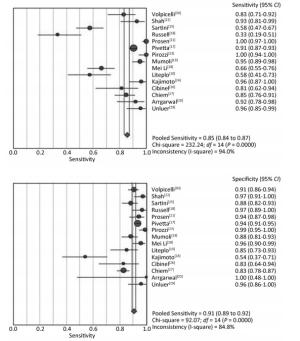
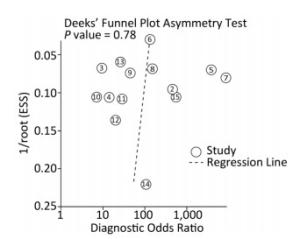
The summary sensitivity and specificity values were 0.85 [95% confidence interval (CI), 0.84-0.87], and 0.91 (95% CI, 0.89-0.92) (Figure 4). The summary PLR, NLR, and diagnostic odds ratio (DOR) were 8.94 (95% CI, 5.64-14.18), 0.14 (95% CI, 0.08-0.26) and 67.24 (95% CI, 31.78-142.28). The HSROC was 0.9587 (SE, 0.0130) (Figure 5). The Deeks' funnel plot asymmetry test showed no evidence of significant publication bias (P = 0.783) (Figure 6).
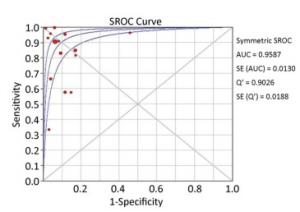
Figure 5. Summary receiver operating characteristic (SROC) curves for the detection of AHF using ULCs.
We detected significant heterogeneity among included studies, and therefore, all these results were analyzed under the random-effect model. Spearman rank correlation was -0.214 (P = 0.443), which indicated no significant threshold effect among individual studies. We also explored possible sources of heterogeneity among the studies by using meta-regression analysis with the following covariates as predictor variables: patient number (e.g., > 100 vs. < 100), study quality, operator (experienced vs. inexperienced), scanning protocol and the time interval between the patient's admission to bedside TLS examination (< 90 min vs. > 90 min). Results suggest that the time interval was closely related to accuracy (relative DOR, 3.56; 95% CI, 1.01-12.51; P = 0.0480).
-
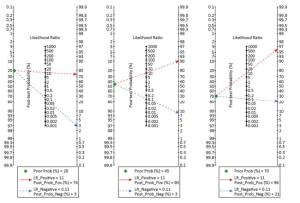
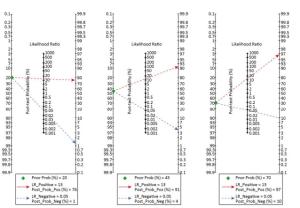
According to the definition of PRIDE AHF scores[16], the pretest probabilities of 20%, 45%, and 70% were evaluated in contrast with post-test probabilities based on a 'positive' or 'negative' ultrasound result. The Fagan plot analysis demonstrated that when the pre-test probabilities were 20%, 45%, or 70%, the positive post-test probabilities of AHF were 74%, 90%, or 96%, respectively (Figure 7). Furthermore, ULCs was helpful to reduce the negative post-probability of AHF to as low as 3% and 8% when the pre-probabilities of 'negative' measurement were 20% and 45%. Although the probability of correctly diagnosing AHF based on a 'positive' ULCs results is as high as 96% when the pretest probability was 70%, the diagnosis would be incorrect in 21% of patients whose ULCs results were 'negative' (Figure 8).
-
Five studies reported a time interval between the patient's admission to bedside TLS examination > 90 min[10, 25-26, 28, 30], and the pooled sensitivity, specificity, PLR, NLR, and DOR were 0.67 (95% CI, 0.61-0.72), 0.90 (95% CI, 0.87-0.92), 6.42 (95% CI, 4.14-9.95), 0.35 (95% CI, 0.25-0.50), and 20.14 (95% CI, 9.17-44.24), respectively. The HSROC was 0.8924 (SE, 0.0631). Conversely, eight studies reported a time interval between the patient's admission to bedside TLS examination < 90 min[17-19, 21, 23-24, 27, 29], and the pooled sensitivity, specificity, PLR, NLR, and DOR were 0.91 (95% CI, 0.89-0.92), 0.90 (95% CI, 0.89-0.92), 10.13 (95% CI, 4.87-21.08), 0.07 (95% CI, 0.02-0.24), and 127.8 (95% CI, 46.10-354.30), respectively. The HSROC was 0.9712 (SE, 0.0258). The studies with the evaluation time < 90 min have an overall better sensitivity than the others. According to the summary likelihood ratio calculated from included studies reported the time interval < 90 min (Figure 7), we evaluated the pretest probabilities of 20%, 45%, and 70% against the corresponding post-test probabilities based on 'positive' or 'negative' ULCs results. When the pretest probability was only 20%, the accurate diagnosis probability of AHF was up to 76% based on the positive ULCs result, while the probability was only 1% based on the negative ULCs result; when pretest probability was 45%, the accurate diagnosis probability of AHF was up to 91% based on the positive ULCs result, while the probability was 4% based on the negative ULCs result; when pretest probability was 70%, the accurate diagnosis probability of AHF was up to 97% based on the positive ULCs result, while the probability was 10% based on the negative ULCs result.
-
The results demonstrated that the ULCs is reliable in the diagnosis of AHF. The overall sensitivity, specificity, and DOR were 85%, 91%, and 67.24, respectively as well as the AUC was 0.91. Positive ULCs results could increase the probability of AHF in low and moderate risk groups by approximately 50%. If TLS was performed without delay, negative ULCs results could decrease the probability of AHF in low and moderate risk groups to approximately 1%-4%; As a result, the diagnostic process may change in some patients. In our analysis, even in low-risk AHF patients, ULCs has been shown to have appropriate accuracy. Further subgroup analyses showed a significantly better diagnostic accuracy for the diagnosis of AHF for the studies that reported a time interval between the patient's admission to bedside TLS examination < 90 min than for those > 90 min.
Early diagnosis of AHF is critical to successfully identify underlying diseases or causes and to prevent further myocardial dysfunction and clinical deterioration in some patients. However, initial diagnosis may be difficult because of the nonspecific, highly variable, and probably observer dependent signs and symptoms[31-32]. NT-proBNP and BNP could not accurately differentiate AHF from other etiologies of dyspnea. Moreover, other factors, such as the presence of a 'gray zone' of uncertainty in its reference range, comorbid illnesses, age, renal dysfunction, and expensive assay kit may affect the use of NT-proBNP[33]. Chest radiography (CR) can be used in most acute dyspneic patients in ED, but it has been proved to be an insensitive and inaccurate test. Other imaging examinations are not always available in most EDs and transferring a critically ill and unstable patient for scanning is impractical[34]. Moreover, large amount radiation also cannot be ignored. It is considered that echocardiography with doppler examination maybe the gold standard for the diagnosis of a patient with AHF. However, an immediate extensive echo testing and a consultant cardiologist is not always available in the emergency department. Additionally, echo may not be indicated for all of the patients presenting to the emergency department with acute undifferentiated dyspnea[35]. In summary, none of these tests considered singularly seems sufficiently accurate to identify AHF because each method exhibits an imbalance between sensitivity and specificity characteristics[36-38]. Therefore, it was estimated that heart failure is correctly diagnosed initially in only 50% of affected patients[39]. Thus, it is imperative to find a simple, secure, and portable diagnostic tool in ED. TLS performed by EPs emerged as the times require.
TLS could identify ULCs easily even by novice sonographers or nurses. In our study, the average quality of all included literature is at a high level. According to the included literature description, AHF could be easily diagnosed by the presence of at least three B-lines in an intercostal space in two or more regions for each side of the thorax regardless of numbers of the chest wall are divided. In our predefined subgroup analyses, study quality and scanning protocol did not significantly alter the point estimates for sensitivity and specificity. The above results indicated that TLS is repeatable and easily interpretable. More importantly, TLS could effectively rule out ACPE when no multiple ULCs are detected. However, early studies were mostly conducted in intense care unit (ICU) or cardiopulmonary ward where patients have already been treated with diuretics and nitrates. Thus, the diagnostic efficiency may be altered. Moreover, most patients transferred to the ward may have not been in the critical stage because of the emergency management and plenty of time was available for intensivist or cardiologist to perform TLS. As a result, the true TLS test utility and practicability for a fast-paced and busy ED might be affected.
Al Deeb et al. also conducted a meta-analysis to evaluate the diagnostic performance of point-of-care US for ACPE in 2014[40]. The majority of their included studies were conducted in ICU or inpatient ward. Only two studies in this article were completed in the ED. They reported that a sensitivity of US using B-lines to diagnosis ACPE of 94.1% and a specificity of 92.4%. There are several strengths in our study compared to the study by Al Deeb et al. First, we included more studies in the present study. Second, Al Deeb et al. admitted that further studies with more undifferentiated dyspneic patients from ED were necessary to gain more valid and reliable estimates of test accuracy in ED patients. Thus, in the present meta-analysis we reported the diagnostic value of ULCs mostly performed by frontline physicians other than radiologists in detecting AHF in EDs or even prehospital emergency settings. The results showed that for the early diagnosis of AHF, the overall accuracy of TLS performed by EPs who was not specialized in cardiac US is also good. The final noteworthy strength of our study was that the time interval between ED admission and bedside TLS performance (< 90 min vs. > 90 min) was found to be strongly associated with accuracy of TLS, because ULCs rapidly resolve with decongestion. It is widely known this time window is vital for EPs quick evaluation and is of great clinical utility. The Fagan plot analysis indicated that when the TLS was performed at the time of a patient's initial presentation to the ED, the presence of AHF among low to moderate risk populations would be accurately excluded due to a negative TLS result. We chose the time interval 90 min prior to TLS as we thought this was more applicable to daily practice, as patients may be treated by emergency medical services or other pre-hospital emergency personnel prior to initial evaluation by EPs. Delayed TLS after an initial diuretic therapy could affect final US pathologic findings, but it is considered that almost two hours are necessary to appreciate B-lines resolution after medical or mechanical ventilation therapy[41-42]. Therefore, in clinical routine TLS may represent a valuable addition to the current diagnostic work-up of patients with suspected AHF. This non-invasive test is likely to help patients triage and draw a preliminary diagnosis. In spite of its simplicity, safety, and mobility, US has limitations in the diagnosis of AHF. TLS results were generated from the quantification of lung B-lines. Other causes of interstitial and alveolar thickening which may accompany or cause AHF, such as pulmonary fibrosis, can provide false positive results. Therefore, ULCs findings may not be representative of general spectrum of disease.
Some limitations of this study should be taken into consideration. First, we did not identify unpublished studies, and no attempt was made to include articles published in other languages. Second, the accuracy of ULCs in the diagnosis of AHF usually depends on the skill of the operators. The differences between the operators (their skill, experience, knowledge of chest US, etc.) were not interpreted in detail in this study and might result in such significant heterogeneity in our study. However, no sufficient details were available in the studies focus to make a stratification of the operators' skills. Therefore, in future studies we encourage the researchers to give a more detailed description. Thirdly, the diagnostic accuracy of ULCs was compared with that of the 'gold standard', namely comprehensive examination results, whereas the standard was not the unique one. This might explain the high heterogeneity found in this meta-analysis. Chart review is fairly common for determining final diagnosis in AHF studies, so while imperfect, it may be the best method that we currently have. Therefore, it is necessary to continue this meta-analysis with more unified criterion articles, and the results of our study should be interpreted with caution.
-
To our knowledge, this is the first meta-analysis of ULCs detected in ED for the diagnostic evaluation of AHF. Despite above-mentioned limitations, this study strongly supports the routine detection of AHF using TLS performed at the bedside and early test performance during the evaluation of patients presenting to the ED with acute dyspnea together with routine examinations. Eps or other medical professionals performed portable ultrasonography is a reliable method in the diagnosis of AHF, but the accuracy of ULCs in the diagnosis of AHF depends on the time interval between patient's admission to bedside TLS examination.
-
We are grateful to Beijing Municipal Science and Technology Commission and National Natural Science Foundation of China which funded this study. We appreciate the assistance of GAO Wen MD in the preparation of this article.
-
RL MD and ZGQ MD contributed to the conception and design of the study. RL MD, ZGQ MD, and YST MD contributed to the retrieval of articles, the extraction of data, the calculation of data, and the design of the figures and tables. RL MD and ZGC MD wrote the original manuscript. SLC PhD and ZSQ MD revised the manuscript. All authors approved the final manuscript.
-
No conflict of interest to declare.
doi: 10.3967/bes2018.081
Role of Ultrasound Lung Comets in the Diagnosis of Acute Heart Failure in Emergency Department: A Systematic Review and Meta-analysis
-
Abstract:
Objective A new technique of transthoracic lung ultrasonography (TLS) has emerged and demonstrated promising results in acute heart failure diagnosis at an early stage. However, the diagnostic value of ultrasound lung comets (ULCs) for acute heart failure (AHF) performed in busy emergency department (ED) is uncertain. The present meta-analysis aimed to assess the diagnostic efficiency of ULCs in AHF. Methods We conducted a search on online journal databases to collect the data on TLS performed for diagnosing AHF published up to the end of July 2017. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and summary receiver operating characteristic (SROC) curve were calculated. The post-test probability of AHF was calculated by using Bayes analysis. Results We enrolled a total of 15 studies involving 3, 309 patients. The value of sensitivity, specificity, PLR, NLR, DOR, area under the SROC curve, and Q* index was 85%, 91%, 8.94, 0.14, 67.24, 0.9587, and 0.9026, respectively. We detected significant heterogeneity among included studies, and therefore, all these results were analyzed under the random-effect model. We also explored possible sources of heterogeneity among the studies by using meta-regression analysis. Results suggest that the time interval between patient's admission to bedside TLS examination was closely related to TLS accuracy. Conclusion This meta-analysis demonstrated that detecting ULCs is a convenient bedside tool and has high accuracy for early AHF diagnosis in ED. TLS could be recommended to be applied for early diagnosis of AHF in ED. -
Key words:
- Transthoracic lung ultrasonography /
- Lung comets sign /
- Dyspnea /
- Acute heart failure /
- Diagnostic test /
- Meta-analysis
-
Table 1. Characteristics of Eligible Studies
Authors Year Patients
(n)Study Country US Operator Study Type Consecutive Time Interval Scanning Protocol Reference Standard Probe Type Pivetta et al.[17] 2015 1, 005 Italy EP Cohort study 2 < 90 min 6 zones Final hospital diagnosis Curvilinear transducer
(3-5 MHz)Russell et al.[18] 2014 99 America EP Observational study 2 < 90 min 8 zones Blinded chart review Curvilinear probe
(3-5 MHz)Mumoli et al.[19] 2015 226 Italy Nurse Observational study 1 < 90 min 8 zones Final hospital diagnosis Curved array transducer
(2-5.5 MHz)Arrgarwal et al.[20] 2016 42 India Cardiologist Cross-sectional study 1 IMR 28 scan sites Blinded chart review Portable machine
(not specified)Prosen et al.[21] 2011 218 Slovenia EP Cohort study 1 < 90 min 8 zones Final hospital diagnosis Not specified Liteplo et al.[10] 2009 94 America EP Observational study 2 > 90 min 8 zones Blinded chart review Curved array transducer
(2-5 MHz)Shah et al.[22] 2016 117 America Internal medicine resident Cohort study 2 NR 8 zones Final hospital diagnosis Phased array transducer Pirozzi et al.[23] 2014 167 Italy EP Observational study 2 < 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz)
Cardiac transducer
(2.5-3.5MHz)Kajimoto et al.[24] 2012 90 Japan Cardiologist Observational study 1 < 90 min 8 zones Phased array probe
(l.7-3.5MHz)Sartini et al.[25] 2016 236 Italy EP Observational study 2 > 90 min 12 zones Final hospital diagnosis Convex probe (3.5-5 MHz) Volpicelli et al.[30] 2006 300 Italy EP and radiologist Observational study 1 > 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz) Cibinel et al.[26] 2011 56 Italy EP Observational study 2 > 90 min 8 zones Final hospital diagnosis Convex probe (3.5 MHz) Chiem et al.[27] 2015 380 America EM resident Cross-sectional study 2 < 90 min 8 zones Blinded chart review Curvilinear transducer
(2-5 MHz)Li et al.[28] 2016 187 China Sonographer Cohort study 2 > 90 min 28 scan sites Echocardiography with Doppler S5-1 probe (1-5 MHz) Unluer et al.[29] 2014 96 Turkey Nurse Cross-sectional study 2 < 90 min 6 zones Provisional diagnosis at the end of the ED stay Microconvex probe
(3.6 MHz)Note. NR: not reported; 1: yes: 2: no; EP: emergency physician; EM: emergency medicine; Scanning protocol: The different zones of thoracic ultrasonography considered in each study. Table 2. Patient Characteristics
Study Setting Age, y (range) Inclusion Pivetta et al.[17] ED 77 (IQR13) acute dyspnea Russell et al.[18] ED 56 ± 13 (22-91) undifferentiated dyspnea Mumoli et al.[19] ED 78.7 ± 12.7 acute dyspnea Arrgarwal et al.[20] ED 64.4 ADHF suspected Prosen et al.[21] Prehospital emergency setting 70.9 ± 11.7 shortness of breath Liteplo et al.[10] ED 74 ± 14 acute dyspnea Shah et al.[22] ED 36 (17-58) undifferentiated dyspnea Pirozzi et al.[23] ED 74.3 ± 4.3 acute dyspnea Kajimoto et al.[24] ED 78.1 ± 8.1 acute dyspnea Sartini et al.[25] ED 79.98 acute dyspnea not related to any trauma Volpicelli et al.[30] ED 68.4 ± 8.4 alveolar-interstitial syndrome suspected Cibinel et al.[26] ED 82.1 (38.7-94.3) acute dyspnea Chiem et al.[27] ED 55 ± 5 dyspnea Li et al.[28] ED 62.4 ± 2.4 acute dyspnea Unluer et al.[29] ED 70.59 acute dyspnea Note. IQR: interquartile range; ADHF: Acute decompensated heart failure; SD: Standard deviation. -
[1] Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA, 2005; 294, 1944-56. doi: 10.1001/jama.294.15.1944 [2] Parshall MB, Schwartzstein RM, Adams L, et al. An Official American Thoracic Society Statement:update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med, 2012; 185, 435-52. doi: 10.1164/rccm.201111-2042ST [3] Currow D, Plummer J, Crockett A, et al. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage, 2009; 38, 533-45. doi: 10.1016/j.jpainsymman.2009.01.006 [4] Gheorghiade M, Zannad F, Sopko G, et al. Acute heart failure syndromes:current state and framework for future research. Circulation, 2005; 112, 3958-68. doi: 10.1161/CIRCULATIONAHA.105.590091 [5] Peacock WF, Braunwald E, Abraham W, et al. National Heart, Lung, and Blood Institute Working Group on emergency department management of acute heart failure. J Am Coll Cardiol, 2010; 56, 343-51. doi: 10.1016/j.jacc.2010.03.051 [6] Collins SP, Lindsell CJ, Storrow AB, et al. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med, 2006; 47, 13-8. doi: 10.1016/j.annemergmed.2005.04.003 [7] Klemen P, Golub M, Grmec S. Combination of quantitative capnometry, N-terminal pro-brain natriuretic peptide, and clinical assessment in differentiating acute heart filure from pulmonary disease as cause of acute dyspnea in prehospital emergency setting:study of diagnostic accuracy. Croat Med J, 2009; 50, 133-42. doi: 10.3325/cmj.2009.50.133 [8] Lichtenstein D, Meziere G, Biderman P, et al. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med, 1997; 156, 1640-6. doi: 10.1164/ajrccm.156.5.96-07096 [9] Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD:the comet-tail artifact. Intensive Care Med, 1998; 24, 1331-4. doi: 10.1007/s001340050771 [10] Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES):sonographic B lines natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med, 2009; 16, 201-10. doi: 10.1111/acem.2009.16.issue-3 [11] Lichtenstein D. Ultrasound in the management of thoracic disease. Crit Care Med, 2007; 35, S250-S261. doi: 10.1097/01.CCM.0000260674.60761.85 [12] Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology, 2004; 100, 9-15. doi: 10.1097/00000542-200401000-00006 [13] Gargani L, Frassi F, Soldai G, et al. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea:a comparison with natriuretic peptides. Eur J Heart Fail, 2008; 10, 70-7. doi: 10.1016/j.ejheart.2007.10.009 [14] Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2:A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med, 2011; 155, 529-36. doi: 10.7326/0003-4819-155-8-201110180-00009 [15] Hellmich M, Lehmacher W. A ruler for interpreting diagnostic test results. Methods Inf Med, 2005; 44, 124-6. doi: 10.1055/s-0038-1633930 [16] Baggish AL, Siebert U, Lainchbury JG, et al. A validated clinical and biochemical score for the diagnosis of acute heart failure:the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Acute Heart Failure Score. Am Heart J, 2006; 151, 48-54. doi: 10.1016/j.ahj.2005.02.031 [17] Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED:a SIMEU multicenter study. Chest, 2015; 148, 202-10. doi: 10.1378/chest.14-2608 [18] Russell FM, Ehrman RR, Cosby K, et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea:a lung and cardiac ultrasound (LuCus) protocol. Acad Emerg Med, 2015; 22, 182-91. doi: 10.1111/acem.12570 [19] Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea:a prospective observational study. Medicine, 2016; 95, e2925. doi: 10.1097/MD.0000000000002925 [20] Aqqarwal M, Gupta M, Vijan V, et al. Use of lung ultrasound for diagnosing acute heart failure in emergency department of southern India. J Clin Diagn Res, 2016; 10, TC05-TC08. http://www.ncbi.nlm.nih.gov/pubmed/28050472 [21] Prosen G, Klemen P, Strnad M, et al. Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit Care, 2011; 15, R114. doi: 10.1186/cc10140 [22] Shah SP, Fils-Aime R, Desir W, et al. Focused cardiopulmonary ultrasound for assessment of dyspnea in a resource-limited setting. Crit Ultrasound J, 2016; 8, 7. doi: 10.1186/s13089-016-0043-y [23] Pirozzi C, Numis FG, Pagano A, et al. Immediate versus delayed integrated point-of-care-ultrasonography to manage acute dyspnea in the emergency department. Crit Ultrasound J, 2014; 6, 5. doi: 10.1186/2036-7902-6-5 [24] Kajimoto K, Madeen K, Nakayama T, et al. Rapid evaluation by lung-cardiac-inferior vena cava (LCI) integrated ultrasound for differentiating heart failure from pulmonary disease as the cause of acute dyspnea in the emergency setting. Cardiovasc Ultrasound, 2012; 10, 49-56. doi: 10.1186/1476-7120-10-49 [25] Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early acute diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance:a prospective study. Intern Emerg Med, 2017; 12, 861-9. doi: 10.1007/s11739-016-1498-3 [26] Cibinel GA, Casoli G, Ella F, et al. Diagnostic accuracy and reproducibility of pleural and lung ultrasound in discriminating cardiogenic causes of acute dyspnea in the emergency department. Intern Emerg Med, 2012; 7, 65-70. http://www.ncbi.nlm.nih.gov/pubmed/22033792 [27] Chiem AT, Chan CH, Ander DS, et al. Comparison of expert and novice sonographers' performance in focused lung ultrasonography in dyspnea (FLUID) to diagnose patients with acute heart failure syndrome. Acad Emerg Med, 2015; 22, 564-73. doi: 10.1111/acem.2015.22.issue-5 [28] Li Mei, Xue Ailing, Zhang Xiaorong. Clinical value of ultrasound lung comets in patients with acute left heart failure. Med J Chin PAP, 2016; 27, 61-4. http://www.en.cnki.com.cn/Article_en/CJFDTotal-WJYX201601021.htm [29] Unluer EE, Karagoz A, Oyar O, et al. Lung ultrasound by emergency nursing as an aid for rapid triage of dyspneic patients:a pilot study. Int Emerg Nurs, 2014; 22, 226-31. doi: 10.1016/j.ienj.2014.03.003 [30] Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med, 2006; 24, 689-96. doi: 10.1016/j.ajem.2006.02.013 [31] Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA, 1989; 261, 884-8. doi: 10.1001/jama.1989.03420060100040 [32] Remes J, Miettinen H, Reunanen A, et al. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J, 1991; 12, 315-21. doi: 10.1093/oxfordjournals.eurheartj.a059896 [33] Raymond I, Groenning BA, Hildebrandt PR, et al. The influence of age, sex and other variables on the plasma level of N-terminal probrain natriuretic peptide in a large sample of the general population. Heart, 2003; 89, 745-51. doi: 10.1136/heart.89.7.745 [34] Jing W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography:a meta-analysis. Chest, 2011; 140, 859-66. doi: 10.1378/chest.10-2946 [35] Ang S-H, Andrus P. Lung ultrasound in the management of acute decompensated heart failure. Curr Cardiol Rev, 2012; 8, 123-36. doi: 10.2174/157340312801784907 [36] Loke I, Squire IB, Davies JE, et al. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender, and heart rate. Eur J Heart Fail, 2003; 5, 599-606. doi: 10.1016/S1388-9842(03)00108-9 [37] Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-Type Natriuretic Peptide testing in patients with acute dyspnea. Arch Intern Med, 2006; 166, 1081-7. doi: 10.1001/archinte.166.10.1081 [38] Siebert U, Januzzi JL Jr, Beinfeld MT, et al. Cost-effectiveness of using N-terminal pro-brain natriuretic peptide to guide the diagnostic assessment and management of dyspneic patients in the emergency department. Am J Cardiol, 2006; 98, 800-5. doi: 10.1016/j.amjcard.2006.06.005 [39] Shamsham F, Mitchell J. Essentials of the diagnosis of heart failure. Am Fam Physician, 2000; 61, 1319-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ026218742 [40] Al Deeb M, Barbic S, Featherstone R, et al. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea:a systematic review and meta-analysis. Acad Emerg Med, 2014; 21, 843-52. doi: 10.1111/acem.12435 [41] Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med, 2008; 26, 585-91. doi: 10.1016/j.ajem.2007.09.014 [42] Liteplo AS, Murray AF, Kimberly HH, et al. Real-time resolution of sonographic B-lines in a patient with pulmonary edema on continuous positive airway pressure. Am J Emerg Med, 2010; 541, e5-e8. http://europepmc.org/abstract/MED/20466266 -




 下载:
下载:



 Quick Links
Quick Links