-
Lymph nodes (LNs), tonsils and Peyer's patches are secondary lymphoid organs that preferentially accommodate naive lymphocytes, allowing these cells to connect with antigen-presenting cells that carry antigens to produce an immune response. LNs play an important role in the immune regulation of the human body, so their development has also drawn the attention of many scholars. Lymphoid tissue and LNs of rodent and human need antigens stimulation to develop and improve[1, 2]. Moreover, the development of lymphoid organs depends on correct expression of several molecules within a given period during ontogeny. This is a very complex process involving adhesion molecules, cytokines, and chemokines. Current studies focus on the interactions of lymphoid-tissue inducer (LTi) cells and lymphoid-tissue organizer (LTo) cells[3].
Most LNs are embedded in adipose tissue, which may indicate that adipose tissue regulates energy balance during adaptive immune response and may perform many key secretion functions[4, 5]. Adipose tissue consists of adipocytes and a variety of cell types, including multiple progenitor cell populations, also known as adipose stromal cells (ASCs)[6]. ASCs are similar to bone marrow mesenchymal stem cells and have the potential to differentiate into mesoderm and exhibit paracrine and immunomodulatory properties[7-10]. Studies have shown that adipose tissue can serve as a source of lymphoid tissue stromal cells or other lymphoid structures associated with fat[11]. The chemokine Cxcl12 and its receptor Cxcr4 may regulate the egress of ASCs from adipose tissue and recruitment into LNs during lymph node activation[12].
The recruitment of hematopoietic LTi cells in the early LN anlage is a key step in the development of LNs. LTi expresses lymphotoxin α1β2 (LTα1β2), interacts with lymphotoxin β-receptors (LTβR), and participates in the maturation of immature stromal cells and other LTo cells[3]. Studies have shown that stromal cells express Cxcl13 to attract LTi cells that express Cxcr5 and so the first LTi cell cluster forms[13]. In addition to Cxcl13-mediated attraction of LTi cells, Ccl21 is also involved in guiding LTi cells to the developing LNs[14, 15]. Through the activation of NF-κB transcription factor via the classical pathway or the alternative pathway[16, 17], the continuous recruitment of LTi cells and the expression of cell adhesion molecules and cytokines necessary for further development of LN anlage increased[1, 18]. Chemokine Cxcl13 and receptor Cxcr5 have been found to be important participants in the development of peripheral LNs.
The development of rodent LN starts during embryogenesis, mesenteric lymph nodes (MLNs) develop first, and then the rest of LNs[3]. At present, most of the researches related to the development of lymph nodes mainly focus on mice or human, and there are few studies in rats. Pref-1 (also known as Dlk-1) is a trans-membrane protein which expresses in mesenchyme and adipocyte progenitors, while its expression is down-regulated in adipocytes. For this reason, it is used for identification of adipocyte progenitors[19]. In this study, we observed the dynamic changes of the Pref-1+ adipocyte progenitor cells in rat MLNs at embryonic day (E) 18.5 (embryonic period), 2 weeks (w) (suckling period) and 6 w (weaning period) after birth. Evidence regarding the differentiation of ASCs into lymphoid tissue stroma cells mainly results from the acquisition of defining features (cytokines, chemokines, etc.) of lymphoid tissue stroma cells[20], morphological evidence is scarce. We try to clarify the differentiation of ASCs into lymphoid tissue stroma cells with ultrastructure evidence. Meanwhile, we detected the changes in the expression of Cxcl12 and its receptor Cxcr4, which may regulate the process of adipocyte progenitor cells mobilizing into MLNs. In addition, we also examined the changes in the expression of chemokines Cxcl13 and Ccl21/Ccr7 axis that draws LTi cells to MLNs and discussed the possible regulatory mechanisms during the development of MLNs. Our study could contribute to a better understanding on the formation of lymph nodes in rat.
-
Antibodies to Pref-1 (bs-2423R), Ccr7 (bs-1305R), Ccl21 (bs-1666R), Cxcl13 (bs-4509R), Cxcr4 (bs-1011R), B220 (bs-0522R) were provided by Biosynthesis Biotechnology (Beijing, China). Rabbit anti-Cxcl12 (BA 1389) was provided by Boster Biotechnology (Wuhan, China). Rat monoclonal antibody MECA-79 (sc-19602) was obtained from Santa Cruz Biotechnology (California, USA). All other chemicals and reagents were supplied by OriGene Technologies, Inc. (Beijing, China) unless otherwise indicated.
-
Twenty adult Wistar Rats (female: age, 11 w, n = 10; male: age, 12 w, n = 10) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd and kept under controlled light (12-hour light/dark cycles) and temperature (22 ℃ - 24 ℃) conditions. All rats were housed in a standard animal laboratory allowing free activity and were provided with standard food and water ad libitum. Adult females were placed with males and checked for vaginal plug every morning. Day of vaginal plug was designated as embryonic day 0.5. All animal protocols were approved by the Institutional Animal Care and Use Committee of Harbin Medical University.
-
The MLN tissues of rats in each group were routinely formalin-fixed, dehydrated, transparentized, immersed in and embedded with paraffin. Ten serial sections of 2.5 μm thickness were obtained by using a paraffin sectioning machine (LEICA RM2255, Germany) and baked for 1 h at 50 ℃. Then, sections were stained for 3 min in Hematoxylin and for 1 min in Eosin, sealed with neutral resin, and observed for morphological changes of MLN tissues under a light microscope (OLYMPUS BX51, Japan).
-
Formalin-fixed, paraffin-embedded MLNs were sectioned (2.5 μm) and placed in series on silanized glass slides. Tissue sections were deparaffinized in xylene and rehydrated through graded alcohol. Antigens were retrieved following the treatment in 10 mmol/L citrate buffer (pH 6.0) for 15 min in a pressure cooker. After rinsing with phosphate-buffered saline (PBS), the sections were immersed in 3% hydrogen peroxide solution for 10 min to block endogenous peroxidases. Non-specific binding was prevented by incubation in 5% normal goat serum for 20 min in a humidified chamber. The sections were then incubated with antibodies for Pref-1 (1:200), Ccl21 (1:150), Ccr7 (1:150), Cxcl12 (1:150), Cxcl13 (1:150), Cxcr4 (1:100), MECA-79 (1:100), B220 (1:150) overnight at 4 ℃, washed, and incubated with secondary antibodies (immediately- used goat anti-rabbit IgG horseradish-peroxidase polymers; PV-6001) at 37 ℃ for 30 min. Diaminobenzidine was used as a chromogen. Tissue sections were counterstained with hematoxylin, dehydrated and mounted. The sections were observed under a light microscope (OLYMPUS BX51, Japan). Immunopositive expression in cells was quantified with Integral optical density (IOD) values by using Image Pro-Plus 6.0 (Media Cybernetics Inc. Rockville, MD, USA).
-
For TEM study, MLNs were dissected and fixed with 2.5% glutaraldehyde for 2 h, postfixed in 1% OsO4 at 4 ℃ for 2 h, and embedded with Epon812 (EM Sciences, Washington, PA, USA) for 72 h at 60 ℃. Ultrathin sections were cut and stained with uranium acetate, followed by lead citrate, and then observed under a transmission electron microscope (JEOL 200, Hitachi, Japan).
-
Statistical differences were evaluated using one-way ANOVA, and P < 0.05 was considered significant. Pearson's correlation analysis was applied to determine the relationship between Pref-1 and Cxcl12 expression.
-
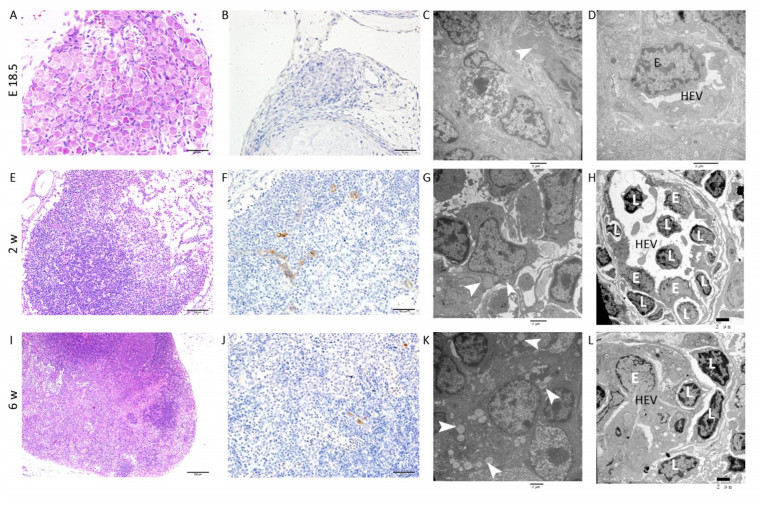
E18.5 HE and IHC: The MLN anlage was very small with a thin capsule. The subcapsular sinus was not obvious and the cells clustered together without significant compartments (Figure 1A). MECA-79 expresses primarily on high endothelial venules (HEVs) in lymphoid tissues. The MECA-79 epitope binds to CD62L and, via this binding, induces leukocyte homing and lymphocyte rolling and tethering[21]. IHC results showed that there was no expression of MECA-79 in the MLN anlage (Figure 1B).
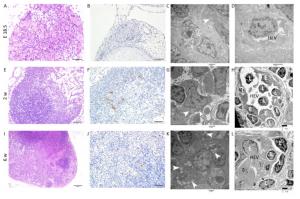
Figure 1. HE, IHC and TEM images of MLNs at E 18.5, 2 w, and 6 w after birth. (A, E, I) HE images of MLNs. (B, F, J) The expression of MECA-79 in MLNs were analyzed by IHC. (C, D, G, H, K, L) TEM images of MLNs. Bars: (A, B, F, J): 50 μm, (E): 100 μm, (I): 200 μm, (C, D, G, H, K, L): 2 μm. White arrowhead: Fat drop, L: Lymphocyte, E: Endothelial cell, HEV: high endothelial venule.
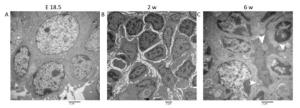
TEM: The nuclei of the lymphocytes were round with little heterochromatin and visible nucleolus. Their mitochondria were abundant, the rough endoplasmic reticulum and the ribosomes were developed. The cells connected with each other, and approximately matured lymphocytes were sometimes observed among them (Figure S1A, available in www besjournal.com). Some cells were long spindle shaped with a few lipid droplets and abundant rough endoplasmic reticulum and mitochondria, whereas their protrusions were not obvious and no obvious collagen fibers were observed (Figure 1C). The wall of the blood vessel was thin, and the basement membrane was complete. No lymphocytes were seen passing through the blood vessel wall (Figure 1D).
Two w after Birth HE and IHC: Compared with E 18.5, the size of MLN increased with thicker capsule and enlarged subcapsular sinus, and the cortex and medulla regions could be distinguished but there was no follicular and paracortical regions (Figure 1E). MECA-79 significantly expressed on the surface of HEV endothelial cells (Figure 1F).
TEM: Lymphocytes were relatively matured with a little cytoplasm and a few mitochondria and were surrounded by some atypical fibroblasts (Figure S1B). Some cells had many small fat drops, clearly visible mitochondria and more developed endoplasmic reticulum in the cytoplasm, the protrusions of which were more obvious, and there were many collagen fiber bundles in and around these protrusions (Figure 1G). The number of blood vessels increased, and there were some atypical HEVs that were in the developmental stage, with thicker endothelial cells and some lymphocytes passing through the vessel wall (Figure 1H).
Six w after Birth HE and IHC: There was a dramatic increase in overall MLN size compared with the MLN at 2w after birth. The capsule was thicker, and subcapsular sinus was enlarged. The peripheral cortex had lymphoid follicles with germinal center, and paracortical area was obvious (Figure 1I). The lumen of HEV was small and the surface of endothelial cells expressed MECA-79 (Figure 1J).
TEM: Lymphocytes were matured with round nuclei, abundant mitochondria, rough endoplasmic reticulum and Golgi complex. Some cells in lymphoid follicle contained small fat drops, the nucleus of which was round and lightly stained and was similar to follicular dendritic cells (FDCs) (Figure 1K). Some nuclei of these cells were long spindle shaped with some protrusions and organelles (Figure S1C). There were many matured HEVs with tall endothelial cells, and a number of lymphocytes were observed migrating through the vessel wall (Figure 1L).
-
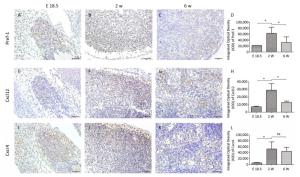
At E18.5, Pref-1+ adipocyte progenitor cells were observed to be predominantly distributed in and around the developing MLN anlages (Figure 2A). The number of Pref-1+ cells in MLNs was significantly increased (P < 0.05, Figure 2B, D) at 2 w after birth, but with the development of MLNs, the number of Pref-1+ cells in MLNs at 6 w after birth was significantly less than that at 2 w after birth (P < 0.05, Figure 2C, D). The expression level of Pref-1 was first increased and then decreased during the development of MLNs.
-
Our results showed that the expression of Cxcl12 and Cxcr4 were significantly increased (P < 0.05, Figure 2E, F, H, I, J, L) at 2 w after birth compared with that at E 18.5, and the expression of Cxcl12 was significantly decreased at 6 w after birth (P < 0.05). The tendency of Cxcl12 expression was consistent with that of Pref-1 and there was a positive correlation between the expression of Cxcl12 and Pref-1 (P < 0.05; r = 0.897). The expression of Cxcr4 did not change significantly at 6 w compared with that at 2 w after birth (P > 0.05, Figure 2L).
-
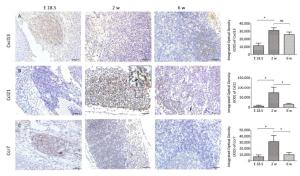
At E 18.5, it was observed that Cxcl13 and Ccr7 were significantly expressed in the MLN anlage (Figure 3A, C), but the expression level of Ccl21 was low (Figure 3B). With the development of MLNs, the expression of Cxcl13, Ccr7 and its ligand Ccl21 in MLNs were significantly increased at 2 w after birth (P < 0.05), but the expression of Ccr7 and Ccl21 were significantly decreased at 6 w after birth (P < 0.05), and the expression of Cxcl13 was not significantly decreased (P > 0.05) (Figure 3).
-
Studies have shown that adipose tissue is closely related to the occurrence of LN anlage[11, 12]. Benezech et al. found that Pref-1+ adipocyte progenitors can be mobilized into LNs from adipose depot surrounding LNs during the development of LNs and express stromal cell marker GP38. Under the action of LTβR signaling pathway, adipocyte progenitor cells are reprogrammed into LN stromal cells by NF-kB2-RelB pathway, thereby inhibiting their differentiation into mature adipocytes[11]. We found Pref-1+ cells were present in MLNs in different periods, which is consistent with the above reports. The highest number of Pref-1+ cells was found in the MLNs at 2 w after birth (Figure 2A-D). In addition, we observed the presence of small lipid droplets in cytoplasm of some cells in MLNs by TEM at E 18.5 and 2 w after birth. Their morphology was similar to FRCs. We also observed some cells containing lipid droplets in LN follicular area at 6 w after birth, which are similar to FDCs (Figure 1). Studies have shown that, after adipocyte progenitor cells being reprogrammed into LN stromal organizer cells in the LNs, stromal organizer cells can give rise to the various stromal cell lineages found in mature lymph nodes, including FRCs, FDCs, lymphatic endothelium and vascular endothelium[22]. We provided ultrastructure evidence to confirm that the adipocyte progenitor cells in the LNs may be differentiated into FRCs and FDCs.
Gil-Ortega et al[12]. found that the ASCs of adipose tissue surrounding LNs can enter the LNs and become LN stromal cells during the LNs activation process through Cxcl12/Cxcr4 axis, and are involved in LN stromal cell proliferation and matrix compartment expansion, and this kind of dynamic can be impaired by Cxcr4 neutralizing antibody. Little is known about the correlation of Cxcl12 and Pref-1+ adipocyte progenitor cells during rat MLN development. So we examined the dynamic expression of Cxcl12, and we found that the expression of Cxcl12 at different time points tended to be consistent with that of Pref-1+ adipocyte progenitor cells, and there was a positive correlation between them. Our results suggest that Cxcl12 may play an important role in the process of adipocyte progenitor cells entering LNs during rat MLN development. To define the specific effect of Cxcl12 on Pref-1+ adipocyte progenitor cells, some intervention experiments will be considered in further in-depth study.
Studies have shown that Cxcl13 play a major role in the initial development of mouse LN anlage, most peripheral LNs fail to develop in the absence of Cxcl13 or its receptor Cxcr5[14, 15, 23]. However, Luther et al[14]. found that other chemokines (including Ccl21 and Ccl19) could recruit enough IL-7Rαhi cells to the MLN anlage in the absence of Cxcl13, in addition to Cxcl13-mediated attracting LTi cells. In addition, the final step in the development of fully mature secondary lymphoid organs involves the recruitment of lymphocytes, the separation of B and T cell regions, and the formation of mature B cell follicles. In mouse lymph nodes this process is delayed until 3 days after birth due to the sequential expression of MAdCAM and MECA-79 on HEVs[18]. Cxcl13 and other chemokines including Ccl19, Ccl21, and Cxcl12 control the stable recruitment of naive B cells and T cells[14] and can induce B and T cell migration in lymphoid organs[24-28]. Our results also showed that Cxcl13 was significantly expressed in MLNs, while the expression level of Ccl21 is very low at E 18.5. At 2 w after birth, Cxcl13 and Ccl21 levels significantly increased, well-developed HEVs with lymphocytes passing through were visible, and HEV significantly expressed MECA-79 (Figure 1H). The high endothelial cells are cubic or columnar, which allows the intercellular space of HEVs to increase within a unit area, contributing to the passage of lymphocytes[29]. Changes in the structure of HEV and the expression levels of MECA-79 and chemokine at 2 w after birth contribute to the recruitment of a large number of naive lymphocytes from blood to LNs, which promotes further development of LNs. However, there was no obvious T/B cell division at 2 w after birth (Figure S2, available in www besjournal.com). Immunohistochemistry showed Cxcl13-was mainly expressed in the cortex near the capsule (the future B cell region, the follicular area) and endothelium of the medullary sinus (Figure 3A). T/B cell division was visible and MLNs were basically mature at 6 w after birth (Figure S2), and at this time point expression of Ccl21 and Cxcl12 were significantly reduced (Figure 3A, B). Here, we speculate that changes in the expression level of chemokines may prevent further expansion of LNs and maintain a stable state.
It has been reported that adipokines were involved in the control of endocrine and metabolic systems and they seem to play a role in the regulation of the immune system, in both the innate and adaptive immune responses[30, 31]. Grases-Pintó B et al.[32] found that dietary supplementation with leptin and adiponectin during the suckling period is able to promote the maturation of the intestinal immune system. Adipokines-related research deserves to be investigated in the future. Moreover, the development of LNs is usually subtle and sometimes there are significant differences among LNs at different sites. These differences are reflected in the diversity of cytokines and chemokines required for the development of various types of LTi cells and LTo cells in LNs at different sites. In this study, we only observed the process of MLNs development. In the next study we plan to analyze LNs at other sites. Understanding these differences and determining the mechanisms for their effects on final structure and function of each secondary lymphoid organ will help us fully understand the body's immune function and provide assistance in the treatment of diseases from an immunological perspective.
-
In summary, cells containing lipid droplets were found in all rat MLNs at E 18.5, 2 w and 6 w after birth, the morphology of which was similar to FRC or FDC, which showed adipocyte progenitor cells are involved in the development and maturation of rat MLNs. There was a positive correlation between the expression of Cxcl12 and Pref-1, and Cxcl12/Cxcr4 axis may guide Pref-1+ adipocyte progenitor cells into rat MLNs. The expression of relevant chemokines during the development of rat MLNs is dynamic and may be related to the maintenance of LNs self- steady state.
-
The authors have declared that no competing interest exists.
-
PENG Yan and JIA Li Min performed experiments, analyzed data, wrote and edited the manuscript. LI Bao Xin participated in the design of methodology and edited the manuscript. XIE Li Ping completed some of the experiments and data analysis. XIE Zun Jiang and ZHENG Jin Hua designed the research goals and aims, provided thoughtful discussion and edited the manuscript.
doi: 10.3967/bes2018.068
Expression of Pref-1 and Related Chemokines during the Development of Rat Mesenteric Lymph Nodes
-
Abstract:
Objective The aim of this study was to investigate the ability of Pref-1+ adipocyte progenitor cells to mobilize into mesenteric lymph nodes (MLNs) and the dynamic expression of related chemokines during the development of rat MLNs. Methods Immunohistochemical analyses were used to detect the expression of Pref-1 and related chemokines. Transmission electron microscopy (TEM) was used to observe the changes in ultrastructure of MLNs. Results Cells containing lipid droplets were found in all rat MLNs at embryonic day (E) 18.5, 2 and 6 weeks (w) after birth, and they were similar to fibroblastic reticular cells (FRCs) or follicular dendritic cells (FDCs) under TEM. Pref-1+ adipocyte progenitor cells were found in all MLNs. The expression level of Pref-1 was significantly increased at 2 w after birth and decreased at 6 w after birth. The tendency of Cxcl12 expression was consistent with that of Pref-1 and was positively correlated with the expression of Pref-1(P < 0.01; r=0.897). At E18.5, Cxcl13, and Ccr7 were significantly expressed in the MLN anlage, but the expression level of Ccl21 was low. The expression level of Cxcl13, Ccr7, and Ccl21 in MLN were significantly increased at 2 w after birth (P < 0.05), while the expression of Ccr7 and Ccl21 were significantly decreased at 6 w after birth (P < 0.05). Conclusion Adipocyte progenitor cells are involved in the rat MLNs development through differentiation into FRC and FDC. The expression of the relevant chemokines during the development of MLNs is dynamic and may be related to the maintenance of lymph nodes self-balance state. -
Key words:
- Mesenteric lymph nodes /
- Development /
- Rat /
- Ultrastructure /
- Adipocyte progenitor cells /
- Chemokines
-
Figure 1. HE, IHC and TEM images of MLNs at E 18.5, 2 w, and 6 w after birth. (A, E, I) HE images of MLNs. (B, F, J) The expression of MECA-79 in MLNs were analyzed by IHC. (C, D, G, H, K, L) TEM images of MLNs. Bars: (A, B, F, J): 50 μm, (E): 100 μm, (I): 200 μm, (C, D, G, H, K, L): 2 μm. White arrowhead: Fat drop, L: Lymphocyte, E: Endothelial cell, HEV: high endothelial venule.
-
[1] van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol, 2010; 10, 664-74. doi: 10.1038/nri2832 [2] McGovern N, Shin A, Low G, et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature, 2017; 546, 662-6. doi: 10.1038/nature22795 [3] Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol, 2003; 3, 292-303. doi: 10.1038/nri1054 [4] Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine, 2002; 17, 131-9. doi: 10.1006/cyto.2001.0999 [5] Pond CM. Paracrine relationships between adipose and lymphoid tissues:implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol, 2003; 24, 13-8. doi: 10.1016/S1471-4906(02)00004-2 [6] Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue:implications for cell-based therapies. Tissue Eng, 2001; 7, 211-28. doi: 10.1089/107632701300062859 [7] Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell, 2002; 13, 4279-95. doi: 10.1091/mbc.e02-02-0105 [8] Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells:physiological and therapeutic perspectives. Circulation, 2004; 109, 656-63. doi: 10.1161/01.CIR.0000114522.38265.61 [9] Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation, 2004; 109, 1292-8. doi: 10.1161/01.CIR.0000121425.42966.F1 [10] Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells:comparison with bone marrow mesenchymal stem cells. Br J Haematol, 2005; 129, 118-29. doi: 10.1111/bjh.2005.129.issue-1 [11] Benezech C, Mader E, Desanti G, et al. Lymphotoxin-beta receptor signaling through NF-kappaB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity, 2012; 37, 721-34. doi: 10.1016/j.immuni.2012.06.010 [12] Gil-Ortega M, Garidou L, Barreau C, et al. Native adipose stromal cells egress from adipose tissue in vivo:evidence during lymph node activation. Stem Cells, 2013; 31, 1309-20. doi: 10.1002/stem.v31.7 [13] van de Pavert SA, Olivier BJ, Goverse G, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol, 2009; 10, 1193-9. doi: 10.1038/ni.1789 [14] Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med, 2003; 197, 1191-8. doi: 10.1084/jem.20021294 [15] Ohl L, Henning G, Krautwald S, et al. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med, 2003; 197, 1199-204. doi: 10.1084/jem.20030169 [16] Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity, 2002; 17, 525-35. doi: 10.1016/S1074-7613(02)00423-5 [17] Yilmaz ZB, Weih DS, Sivakumar V, et al. RelB is required for Peyer's patch development:differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J, 2003; 22, 121-30. doi: 10.1093/emboj/cdg004 [18] Randall TD, Carragher DM, and Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol, 2008; 26, 627-50. doi: 10.1146/annurev.immunol.26.021607.090257 [19] Sul HS. Minireview:Pref-1:role in adipogenesis and mesenchymal cell fate. Mol Endocrinol, 2009; 23, 1717-25. doi: 10.1210/me.2009-0160 [20] Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol, 2015; 15, 350-61. doi: 10.1038/nri3846 [21] Bistrup A, Tsay D, Shenoy P, et al. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates:relationship to the MECA-79 epitope. Am J Pathol, 2004; 164, 1635-44. doi: 10.1016/S0002-9440(10)63722-4 [22] Cupedo T, Jansen W, Kraal G, et al. Induction of secondary and tertiary lymphoid structures in the skin. Immunity, 2004; 21, 655-67. doi: 10.1016/j.immuni.2004.09.006 [23] Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature, 2000; 406, 309-14. doi: 10.1038/35018581 [24] Bajenoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity, 2006; 25, 989-1001. doi: 10.1016/j.immuni.2006.10.011 [25] Pereira JP, Kelly LM, Cyster JG. Finding the right niche:B-cell migration in the early phases of T-dependent antibody responses. Int Immunol, 2010; 22, 413-9. doi: 10.1093/intimm/dxq047 [26] Link A, Vogt TK, Favre S, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol, 2007; 8, 1255-65. doi: 10.1038/ni1513 [27] Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs:partners in immunity. Immunol Rev, 2013; 251, 160-76. doi: 10.1111/imr.2012.251.issue-1 [28] Schulz O, Hammerschmidt SI, Moschovakis GL, et al. Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annu Rev Immunol, 2016; 34, 203-42. doi: 10.1146/annurev-immunol-041015-055649 [29] Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol, 2012; 12, 762-73. doi: 10.1038/nri3298 [30] Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease:facts and expectations at the beginning of the 21st century. Metabolism, 2015; 64, 131-45. doi: 10.1016/j.metabol.2014.10.016 [31] Batra A, Okur B, Glauben R, et al. Leptin:a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology, 2010; 151, 56-62. doi: 10.1210/en.2009-0565 [32] Grases-Pinto B, Abril-Gil M, Rodriguez-Lagunas MJ, et al. Leptin and adiponectin supplementation modifies mesenteric lymph node lymphocyte composition and functionality in suckling rats. Br J Nutr, 2018; 119, 486-95. doi: 10.1017/S0007114517003786 -




 下载:
下载:




 Quick Links
Quick Links