-
Metabolic Syndrome (MS) is a combination of multiple complex diseases, whose etiology is complicated and has many influencing factors. Randomized controlled trials have shown that vitamin D supplementation could delay the ameliorate symptoms of multiple chronic disease. Therefore, it is reasonable to postulate that the expression levels of vitamin D and its related gene variants might be correlated with MS. MS has always been a serious health burden worldwide, which increases the risk of cardiovascular disease and type 2 diabetes mellitus, the prevalence varies from 20% to 40% across different regions and ethnicities.
Vitamin D functions through the binding with the vitamin D receptor (VDR)[1] which is located on chromosome 12q12-14 (gene ID: 7421). VDR gene contains 12 exons and spans about 252593kb. Once 1, 25-dihydroxyvitamin D [1, 25(OH)2D], the active form of vitamin D, binds to VDR, it's biological functions were activated by modulating the transcription of downstream target genes. The expressions of more than 2, 000 genes (3% of the human genome) are regulated by vitamin D signaling[2]. Several SNPs of VDR genes have already been associated with T2DM, insulin secretion, as well as metabolic changes related to obesity. However, to date, there is paucity in references addressing the association of VDR gene polymorphisms with the risk of MS in Chinese rural population. In China, rural population stands for a special group with large number, which is suffering from the growth trend of T2DM and cardiovascular disease. Therefore, the study on the risk of MS in rural cohort is especially meaningful and important.
Data were obtained from the case-control study performed between January and May 2013 in the cities of Zhengzhou and Jiaozuo, Henan Province (31°-36°N), China. Exclusion criteria: 1) patients with chronic illnesses of kidney or liver; 2) users of antiepileptic drugs or vitamin supplements; 3) pregnant or breastfeeding women. MS was diagnosed according to the IDF metabolic syndrome definition. Finally, 426 patients with MS were included in study. The controls were matched to MS group by number, sex, age (±3), socioeconomic status and residence. Thus, a total of 852 participants were included in the present analysis. A signed consent form was obtained from all participants and the ethics committee of the School of Public Health from Zhengzhou University approved the study protocol. A group of trained technicians administered a standardized questionnaire consisting of demography, lifestyle, family and diseases history and other medical issues were used to obtain the basic information of subjects. Physicians of the investigation area hospital performed anthropometric measurements, including body height, body weight, and waist and hip circumferences. Body mass index [BMI = weight (kg)/height (m)2] was calculated based on measured data. Blood samples were obtained by venipuncture after an overnight fast (> 10 h) and analyzed fasting glucose (GLU), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum levels of 25-hydroxy-vitamin D [25(OH)D]. Homeostasis model assessment of insulin resistance (HOMA-IR) and homeostatic model assessment of β-cell function (HOMA-β).
Genotyping was performed using Applied Biosystems platform (7500 FAST Real-Time PCR system; Applied Biosystems, Foster City, USA). Finally, four SNPs (rs2228570, rs2189480, rs3847987, and rs2239179) were to be studied according to the results of the pre-test (P < 0.05) and related references. Genotyping of four novel SNPs was performed by TaqMan probe assay on the Applied Biosystems platform (7500 FAST Real-Time PCR system; Applied Biosystems, Inc., Carlsbad, USA). The RT-PCR thermal conditions consisted of an initial denaturing at 95 ℃ for 10 min followed by 40 cycles of 95 ℃ for 15 s (denaturing) and 60 ℃ for 1 min (annealing). The genotyping success rates for the 4 SNPs were all > 99.5% and the concordance rates were > 99% based on 5% duplicate samples.
The EpiData 3.1 software (Inc., DK) was used for data inputting. SPSS 21.0 (Inc., Chicago, IL, USA) was used for database management and statistical analyses. Normality of the distribution of each variable was analyzed by Kolmogorov-Smirnov test. Participant characteristics were presented as mean ± SD. Logarithmic transformation was performed to obtain a normal distribution for skewed variables (GLU, TG, Ins, DBP, 25-hydroxy-vitamin D) and presented as median and quadruple spacing. Paired-sample t test was used for continuous variables comparison. Based on observed and expected frequency, chi-square test were used for Hardy-Weinberg equilibrium (HWE) in both case and control groups. Analyses of haplotype construction were examined using the SHEsis (http://analysis.bio-x.cn/myAnalysis.php). The linkage disequilibrium (LD) structure and haplotype interaction analysis was examined using Haploview 4.2. As the rs2239179 was in strong linkage disequilibrium (LD) with (r2= 0.92). Odds ratio weighted gene risk scores (OR-GRS) was used to assess the additive effects of VDR variants on the risk of MS. All P-values were two-tailed, and significance was defend as P ≤ 0.05.
It indicates that the investigated population has reached the genetic balance, that is, the data of this group survey is credible. The detail information was shown in the Supplementary Tables S1 and S2 (available in www.besjournal.com).
Table Supplementary Table S1. Hardy-weinberg Equilibrium of Control Group
Genotype Predictive Value Observed Value χ2 P-value rs2228570 CC 140 133 (31.2%) 0.815 0.665 CT 209 222 (52.1%) TT 77 71 (16.7%) rs2189480 AA 249 169 (39.6%) 2.037 0.361 AC 154 185 (43.4%) CC 24 72 (17.9%) rs3847987 CC 283 279 (64.5%) 1.665 0.435 CA 122 138 (32.4%) AA 13 9 (2.1%) rs2239179 AA 239 237 (55.6%) 0.262 0.877 AG 160 165 (38.7%) GG 27 24 (5.7%) Table Supplementary Table S2. Hardy-weinberg Equilibrium of Case Group
Genotype Predictive Value Observed Value χ2 P-value rs2228570 CC 107 108 (25.4%) 0.019 0.991 CT 213 211 (49.5%) TT 106 107 (25.1%) rs2189480 AA 226 204 (47.9%) 1.541 0.463 AC 168 168 (39.4%) CC 31 54 (12.7%) rs3847987 CC 255 264 (62.0%) 2.968 0.227 CA 150 131 (30.8%) AA 22 31 (7.3%) rs2239179 AA 271 274 (64.3%) 0.482 0.807 AG 138 131 (30.8%) GG 18 21 (4.9%) The general characteristics and biochemical indexes of all subjects were described in Table 1. In our study, compared with controls, BMI, waist circumference, blood pressure, glucose, triglycerides and insulin resistance were significantly higher in individuals with MS (P < 0.05). While the levels of HDL-C, TC, and β Cell function were lower when compared to controls (P < 0.05). Serum 25(OH)D concentrations in MS patients were significantly lower than that in the controls.
Table 1. Characteristics of the Study Population
Variables MS (n = 426) Control (n = 426) t P Valuea Age (year) 54.42 ± 13.54 53.80 ± 13.48 0.67 0.502 Male/Female (n) 191/235 191/235 - - BMI (kg/m2) 28.09 ± 3.05 24.39 ± 3.34 16.85 < 0.001 WC (cm) 95.68 ± 8.42 83.64 ± 10.07 18.80 < 0.001 SBP (mmHg) 136.04 ± 17.08 125.83 ± 17.15 8.71 < 0.001 DBP (mmHg) 86.32 ± 10.01 80.81 ± 9.32 8.32 < 0.001 GLU (mmol/L)b 5.43 (4.50, 7.46) 4.64 (4.07, 5.21) 8.47 < 0.001 INS (mIU/L)b 13.94 (10.07, 19.39) 9.64 (7.06, 13.61) 8.86 < 0.001 HOMA IRb 1.34 (0.84, 1.72) 0.74 (0.33, 1.14) 11.53 < 0.001 HOMA βb 130.61 (62.51, 247.52) 162.02 (88.26, 325.13) -3.65 < 0.001 TG (mmol/L)b 2.12 (1.46, 3.12) 1.08 (0.71, 1.56) 16.69 < 0.001 TC (mmol/L) 4.73 ± 1.12 4.55 ± 0.98 2.45 0.014 HDL-C (mmol/L) 1.20 ± 0.28 1.44 ± 0.27 -13.41 < 0.001 LDL-C (mmol/L) 2.42 ± 0.84 2.52 ± 0.74 -1.90 0.058 25(OH)D (ng/mL)b 17.20 (14.47, 27.39) 20.07 (15.05, 39.40) -4.26 < 0.001 Note. Data are presented as mean ± SD or quartile range; statistical significance (P < 0.05) is shown. n: number of individuals; BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; GLU: glucose; INS: insulin; HOMA IR: insulin resistance; HOMAβ: β Cell function; TG: triglycerides; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; 25(OH)D: Serum 25-hydroxy-vitamin D. aThe paired-sample T test for continuous variables. bVariables were natural log-transformed before statistical analysis. Our finding is in line with the results of several studies, which have shown that vitamin D level is associated with MS. Altieri et al.[3] reported that vitamin D directly influenced insulin secretion in the pancreatic β-cell through increasing intracellular free calcium concentration. However, the causality between vitamin D expression and MS is uncertain.
Additionally, we observed no significantly difference among rs2228570, rs2189480, rs3847987, and rs2239179 of VDR polymorphic genotypes in serum vitamin D level. In a study of 1, 443 Chinese from Yunnan, Li et al.[4] found that there were significant association between VDR-rs2228570 and serum levels of 25(OH)D, which was consistent with our findings.
None of the genotype frequencies was found to deviate from Hardy-Weinberg equilibrium in both case- and control- groups. Table 2 shows that when compared with the CC genotype, the TT of rs2228570 was associated with an increased risk of MS (P = 0.004). As for rs2189480, CC and CA genotypes were protective factors as comparing to AA (P = 0.018, P = 0.043). We also found that allele C of both rs2189480 and rs2239179 were protective factors of MS (P = 0.011, P = 0.009). In rs3847987, individuals who carried AA genotypes had a higher risk of MS than those of CC genotype (P = 0.002). Supplementary Tables S3 and S4 (available in www.besjournal.com) demonstrate the distribution of all clinical variables according to the genotypes observed in MS group and control group.
Table 2. Allele and Genotype Frequencies of Single Nucleotide Polymorphisms of the Vitamin D Receptor (VDR) Gene in the Study Population
SNPs Genotype MS n, (%) Control n, (%) χ 2 P OR a (95% CI) Pa rs2228570 CC 108 (25.4) 133 (31.2) 9.06 0.011 1.00 CT 211 (49.5) 222 (52.1) 1.12 (0.81-1.55) 0.503 TT 107 (25.1) 71 (16.7) 1.82 (1.21-2.715) 0.004 CT + TT 318 293 1.29 (0.95-1.75) 0.106 C 427 (50.1) 488 (57.3) 8.78 0.030 1.00 T 425 (49.9) 364 (42.7) 0.77 (0.57-1.04) 0.092 rs2189480 AA 204 (47.9) 169 (39.7) 6.67 0.036 1.00 CA 168 (39.4) 185 (43.4) 0.73 (0.54-0.99) 0.043 CC 54 (12.7) 72 (16.9) 0.60 (0.40-0.92) 0.018 CC + AC 222 257 0.70 (0.53-0.93) 0.012 A 576 (67.6) 523 (76.5) 2.99 0.084 1.00 C 276 (27.1) 329 (23.5) 0.70 (0.55-0.92) 0.011 rs3847987 CC 264 (62.0) 279 (65.5) 12.70 0.002 1.00 CA 131 (30.8) 138 (32.4) 0.98 (0.73-1.33) 0.911 AA 31 (7.3) 9 (2.1) 3.33 (1.54-7.22) 0.002 CA + AA 162 147 1.13 (0.85-1.51) 0.391 C 659 (77.3) 696 (81.7) 4.93 0.026 1.00 A 193 (22.7) 156 (18.3) 1.17 (0.88-1.55) 0.281 rs2239179 AA 274 (64.3) 237 (55.6) 6.78 0.034 1.00 AG 131 (30.8) 165 (38.7) 0.66 (0.50-0.89) 0.006 GG 21 (4.9) 24 (5.6) 0.76 (0.41-1.42) 0.393 AG + GG 152 189 0.67 (0.51-0.89) 0.006 A 679 (79.7) 639 (75.0) 5.36 0.021 1.00 G 173 (20.3) 213 (25.0) 0.69 (0.52-0.91) 0.009 Note.aAdjusted for age, sex, smoking, alcohol use, high-fat diet, physical activity, hypertension and the family history of obesity and diabetes. Table Supplementary Table S3. The Relationships of the Vitamin D Receptor Genotypes and the Metabolic Syndrome's Components in MS Group

Table Supplementary Table S4. Relationships of the Vitamin D Receptor Genotypes and the Metabolic Syndrome' S Components in Control Group

The biological actions of 1, 25-dihydroxyvitamin D3[1, 25(OH)2D3] are mediated by VDR. The association of VDR gene polymorphisms and MS is little investigated. Our study was the first to investigate the association of rs2228570, rs2189480, rs3847987, and rs2239179 genetic variants in VDR to MS in Chinese rural area.
Among several genetic variants of VDR, rs2228570, located in the initiator codon, may lead to missense. We found that allele TT of VDR-rs2228570 was associated with a risk of MS, which is in accordance with the results of a few studies. In a study of 243 adults from Brazilian, Schuch NJ et al.[5] found that individuals with MS and TT-rs2228570 had lower concentrations of intact parathyroid hormone (iPTH), as well as β Cell dysfunctions, suggesting that TT genotype was associated with insulin resistance. No such conclusion could be drawn from our study. However, Zhao Y et al.[6] found that genotype TT of the rs2228570 polymorphism was correlated with lower BMI in subjects with MS, while rs2228570 was not significantly associated with MS. These discrepancies may due to the frequency distribution of the genotypes and populations difference[7]. In a study of 958 adenoma cases and 909 healthy controls from the Tennessee colorectal polyp study (TCPS), Barry EL et al.[8] found that the A allele of rs2228570 for men was positively correlated to serum adiponecin concentrations, the mechanism of which may be related to the regulating effect of vitamin D signaling on adipogenesis, and thus inhibits adipocyte differentiation[9].
The VDR rs2189480, rs3847987, and rs2239179 are less investigated compared with other SNPs in current studies. The locus rs2189480 is one of the single nucleotide variations (SNVs) located in intron region, which has been found in association with T2DM and cardiovascular disease. We found that genotype AA of rs3847987 was associated with MS, while genotype CC and allele C of rs2189480, allele G of rs2239179 seemed to play a protective role. The rs3847987 located in 3' untranslated region (UTR), has been found to be related to colorectal cancer[10]. The rs2239179, also located in intron region, has been reported to be significantly associated with 25(OH)D concentration on waist-hip ratio (WHR)[11]. All four SNPs are in non-coding region of the VDR gene. Although introns are noncoding regions, interionic SNPs can potentially affect protein synthesis by influencing RNA splicing. Moreover, introns may have their own transcription units that produce regulatory RNAs or small proteins, and thus affect the expressions of the derivates[12]. Polymorphisms at the 3' UTR of VDR gene are associated with its expression, possibly through modulating messenger RNA stability. Hence, the interionic SNPs may still influence the function of VDR gene once they are in high-linkage disequilibrium with alleles of a functional sequence variation in the 3' UTR region. There are too few studies on rs2189480, rs3847987, and rs2239179 of VDR gene to support our conclusions. Therefore, our finding awaits replication.
According to our study, rs2239179 and rs2189480 were in complete LD (r2 = 0.92) (Supplementary Figure S1, available in www.besjournal.com). The frequencies values of haplotypes with a frequency over 0.03 are listed in Table 3. The haplotype A-A significantly increased the risk of MS (P = 0.009) and individuals carrying the haplotype G-C have a lower risk of MS (P = 0.014). Supplementary Table S5 (available in www.besjournal.com) shows that the risk of MS for the second grades (-0.365 ≤ GRS < -0.104) was 1.87 fold (P = 0.003) compared with the first grades (GRS ≥ -0.104).
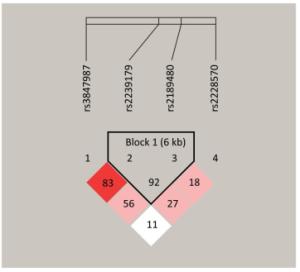
Figure Supplementary Figure S1. Linkage Disequilibrium Plots with r2 Values were generated using Haploview for the VDR Genes.
Table 3. Association Analysis for Haplotypes of the Two Single Nucleotide Polymorphisms of the Vitamin D Receptor (VDR) Gene between Cases and Controls
Haplotype MS 2n (%) Control 2n (%) OR (95% CI) P rs2239179-rs2189480 AA 565.79 (66.4) 514.87 (59.9) 1.31 (1.07-1.60) 0.009 AC 113.21 (13.3) 124.13 (14.6) 0.90 (0.68-1.19) 0.457 GC 162.79 (19.1) 204.87 (24.0) 0.75 (0.59-0.94) 0.014 Table Supplementary Table S5. Association between GRS of Four SNPs and MS
GRS MS 2n (%) Control 2n (%) OR (96% CI) P ≥ -0.104 136 (31.9) 140 (32.9) 1.00 -0.365 to -0.104 101 (23.7) 54 (12.7) 1.87 (1.23, 2.83) 0.003 -0.675 to -0.365 94 (22.1) 127 (19.8) 0.74 (0.51, 1.06) 0.100 < -0.675 95 (22.3) 105 (24.6) 0.88 (0.61, 1.28) 0.515 This was the first research to analyze these four SNPs with MS in one crowd. Our results may help to further understanding of the biological significance of VDR polymorphisms in relation to MS, and contribute to the identification of novel therapeutic strategies for MS. There were some limitations to this study, and therefore, the results should be evaluated with caution. The main limitation lies in the design of case-control studies, which limits the findings to the quality of the pairs between groups. The potential limitations of these data merit consideration. Therefore, our results require further investigation with larger sample sizes. And the present study did not include environmental factors, so we are not able to correlate gene-environment interactions to MS.
In conclusion, we reported that MS was highly prevalent in rural population of Henan province in China. Furthermore, our results showed that the VDR gene (rs2228570, rs3847987, rs2189480, and rs2239179) variants were significantly associated with MS and haplotype of AA rs2239179-rs2189480 were associated with higher MS susceptibility.
doi: 10.3967/bes2019.041
Association of Vitamin D Receptor Gene Polymorphisms with Metabolic Syndrome in Rural Areas of China
-
-
Supplementary Table S1. Hardy-weinberg Equilibrium of Control Group
Genotype Predictive Value Observed Value χ2 P-value rs2228570 CC 140 133 (31.2%) 0.815 0.665 CT 209 222 (52.1%) TT 77 71 (16.7%) rs2189480 AA 249 169 (39.6%) 2.037 0.361 AC 154 185 (43.4%) CC 24 72 (17.9%) rs3847987 CC 283 279 (64.5%) 1.665 0.435 CA 122 138 (32.4%) AA 13 9 (2.1%) rs2239179 AA 239 237 (55.6%) 0.262 0.877 AG 160 165 (38.7%) GG 27 24 (5.7%) Supplementary Table S2. Hardy-weinberg Equilibrium of Case Group
Genotype Predictive Value Observed Value χ2 P-value rs2228570 CC 107 108 (25.4%) 0.019 0.991 CT 213 211 (49.5%) TT 106 107 (25.1%) rs2189480 AA 226 204 (47.9%) 1.541 0.463 AC 168 168 (39.4%) CC 31 54 (12.7%) rs3847987 CC 255 264 (62.0%) 2.968 0.227 CA 150 131 (30.8%) AA 22 31 (7.3%) rs2239179 AA 271 274 (64.3%) 0.482 0.807 AG 138 131 (30.8%) GG 18 21 (4.9%) Table 1. Characteristics of the Study Population
Variables MS (n = 426) Control (n = 426) t P Valuea Age (year) 54.42 ± 13.54 53.80 ± 13.48 0.67 0.502 Male/Female (n) 191/235 191/235 - - BMI (kg/m2) 28.09 ± 3.05 24.39 ± 3.34 16.85 < 0.001 WC (cm) 95.68 ± 8.42 83.64 ± 10.07 18.80 < 0.001 SBP (mmHg) 136.04 ± 17.08 125.83 ± 17.15 8.71 < 0.001 DBP (mmHg) 86.32 ± 10.01 80.81 ± 9.32 8.32 < 0.001 GLU (mmol/L)b 5.43 (4.50, 7.46) 4.64 (4.07, 5.21) 8.47 < 0.001 INS (mIU/L)b 13.94 (10.07, 19.39) 9.64 (7.06, 13.61) 8.86 < 0.001 HOMA IRb 1.34 (0.84, 1.72) 0.74 (0.33, 1.14) 11.53 < 0.001 HOMA βb 130.61 (62.51, 247.52) 162.02 (88.26, 325.13) -3.65 < 0.001 TG (mmol/L)b 2.12 (1.46, 3.12) 1.08 (0.71, 1.56) 16.69 < 0.001 TC (mmol/L) 4.73 ± 1.12 4.55 ± 0.98 2.45 0.014 HDL-C (mmol/L) 1.20 ± 0.28 1.44 ± 0.27 -13.41 < 0.001 LDL-C (mmol/L) 2.42 ± 0.84 2.52 ± 0.74 -1.90 0.058 25(OH)D (ng/mL)b 17.20 (14.47, 27.39) 20.07 (15.05, 39.40) -4.26 < 0.001 Note. Data are presented as mean ± SD or quartile range; statistical significance (P < 0.05) is shown. n: number of individuals; BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; GLU: glucose; INS: insulin; HOMA IR: insulin resistance; HOMAβ: β Cell function; TG: triglycerides; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; 25(OH)D: Serum 25-hydroxy-vitamin D. aThe paired-sample T test for continuous variables. bVariables were natural log-transformed before statistical analysis. Table 2. Allele and Genotype Frequencies of Single Nucleotide Polymorphisms of the Vitamin D Receptor (VDR) Gene in the Study Population
SNPs Genotype MS n, (%) Control n, (%) χ 2 P OR a (95% CI) Pa rs2228570 CC 108 (25.4) 133 (31.2) 9.06 0.011 1.00 CT 211 (49.5) 222 (52.1) 1.12 (0.81-1.55) 0.503 TT 107 (25.1) 71 (16.7) 1.82 (1.21-2.715) 0.004 CT + TT 318 293 1.29 (0.95-1.75) 0.106 C 427 (50.1) 488 (57.3) 8.78 0.030 1.00 T 425 (49.9) 364 (42.7) 0.77 (0.57-1.04) 0.092 rs2189480 AA 204 (47.9) 169 (39.7) 6.67 0.036 1.00 CA 168 (39.4) 185 (43.4) 0.73 (0.54-0.99) 0.043 CC 54 (12.7) 72 (16.9) 0.60 (0.40-0.92) 0.018 CC + AC 222 257 0.70 (0.53-0.93) 0.012 A 576 (67.6) 523 (76.5) 2.99 0.084 1.00 C 276 (27.1) 329 (23.5) 0.70 (0.55-0.92) 0.011 rs3847987 CC 264 (62.0) 279 (65.5) 12.70 0.002 1.00 CA 131 (30.8) 138 (32.4) 0.98 (0.73-1.33) 0.911 AA 31 (7.3) 9 (2.1) 3.33 (1.54-7.22) 0.002 CA + AA 162 147 1.13 (0.85-1.51) 0.391 C 659 (77.3) 696 (81.7) 4.93 0.026 1.00 A 193 (22.7) 156 (18.3) 1.17 (0.88-1.55) 0.281 rs2239179 AA 274 (64.3) 237 (55.6) 6.78 0.034 1.00 AG 131 (30.8) 165 (38.7) 0.66 (0.50-0.89) 0.006 GG 21 (4.9) 24 (5.6) 0.76 (0.41-1.42) 0.393 AG + GG 152 189 0.67 (0.51-0.89) 0.006 A 679 (79.7) 639 (75.0) 5.36 0.021 1.00 G 173 (20.3) 213 (25.0) 0.69 (0.52-0.91) 0.009 Note.aAdjusted for age, sex, smoking, alcohol use, high-fat diet, physical activity, hypertension and the family history of obesity and diabetes. Supplementary Table S3. The Relationships of the Vitamin D Receptor Genotypes and the Metabolic Syndrome's Components in MS Group

Supplementary Table S4. Relationships of the Vitamin D Receptor Genotypes and the Metabolic Syndrome' S Components in Control Group

Table 3. Association Analysis for Haplotypes of the Two Single Nucleotide Polymorphisms of the Vitamin D Receptor (VDR) Gene between Cases and Controls
Haplotype MS 2n (%) Control 2n (%) OR (95% CI) P rs2239179-rs2189480 AA 565.79 (66.4) 514.87 (59.9) 1.31 (1.07-1.60) 0.009 AC 113.21 (13.3) 124.13 (14.6) 0.90 (0.68-1.19) 0.457 GC 162.79 (19.1) 204.87 (24.0) 0.75 (0.59-0.94) 0.014 Supplementary Table S5. Association between GRS of Four SNPs and MS
GRS MS 2n (%) Control 2n (%) OR (96% CI) P ≥ -0.104 136 (31.9) 140 (32.9) 1.00 -0.365 to -0.104 101 (23.7) 54 (12.7) 1.87 (1.23, 2.83) 0.003 -0.675 to -0.365 94 (22.1) 127 (19.8) 0.74 (0.51, 1.06) 0.100 < -0.675 95 (22.3) 105 (24.6) 0.88 (0.61, 1.28) 0.515 -
[1] Norman AW. From vitamin D to hormone D:fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr, 2008; 88, 491S-9S. doi: 10.1093/ajcn/88.2.491S [2] Pike JW, Meyer MB, Lee SM, et al. The vitamin D receptor:contemporary genomic approaches reveal new basic and translational insights. J Clin Invest, 2017; 127, 1146-54. doi: 10.1172/JCI88887 [3] Altieri B, Grant WB, Della Casa S, et al. Vitamin D and pancreas:The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr, 2017; 57, 3472-88. doi: 10.1080/10408398.2015.1136922 [4] Li LH, Yin XY, Wu XH, et al. Serum 25(OH)D and vitamin D status in relation to VDR, GC and CYP2R1 variants in Chinese. Endocr J, 2014; 61, 133-41. http://cn.bing.com/academic/profile?id=97e26d3085e4485a1b9eda59ebd74717&encoded=0&v=paper_preview&mkt=zh-cn [5] Schuch NJ, Garcia VC, Vivolo SR, et al. Relationship between Vitamin D Receptor gene polymorphisms and the components of metabolic syndrome. Nutr J, 2013; 12, 96. doi: 10.1186/1475-2891-12-96 [6] Zhao Y, Liao S, He J, et al. Association of vitamin D receptor gene polymorphisms with metabolic syndrome:a case-control design of population-based cross-sectional study in North China. Lipids Health Dis, 2014; 13, 129. doi: 10.1186/1476-511X-13-129 [7] Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding:Associations with disease and evolution. Genome Res, 2010; 20, 1352-60. doi: 10.1101/gr.107920.110 [8] Barry EL, Peacock JL, Rees JR, et al. Vitamin D Receptor Genotype, Vitamin D3 Supplementation, and Risk of Colorectal Adenomas:A Randomized Clinical Trial. JAMA Oncol, 2017; 3, 628-35. doi: 10.1001/jamaoncol.2016.5917 [9] Belenchia AM, Jones KL, Will M, et al. Maternal vitamin D deficiency during pregnancy affects expression of adipogenic-regulating genes peroxisome proliferator-activated receptor gamma (PPARgamma) and vitamin D receptor (VDR) in lean male mice offspring. Eur J Nutr, 2018; 57, 723-30. doi: 10.1007/s00394-016-1359-x [10] Khan RJ, Riestra P, Gebreab SY, et al. Vitamin D Receptor Gene Polymorphisms Are Associated with Abdominal Visceral Adipose Tissue Volume and Serum Adipokine Concentrations but Not with Body Mass Index or Waist Circumference in African Americans:The Jackson Heart Study. J Nutr, 2016; 146, 1476-82. doi: 10.3945/jn.116.229963 [11] Vimaleswaran KS, Power C, Hyppönen E. Interaction between vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D concentrations on metabolic and cardiovascular disease outcomes. Diabetes Metab, 2014; 40, 386-9. doi: 10.1016/j.diabet.2014.01.003 [12] Wang GS, Cooper TA. Splicing in disease:disruption of the splicing code and the decoding machinery. Nat Rev Genet, 2007; 8, 749-61. doi: 10.1038/nrg2164 -




 下载:
下载:



 Quick Links
Quick Links