-
Diabetes mellitus (DM), the 10th leading cause of death worldwide, is a major and growing public health problem estimated to affect more than 578 million people in 2030 and 700 million people in 2045[1]. In diabetic animals, protein glycation and glucose autoxidation generate free radicals, which cause lipid peroxidation. Therefore, reactive oxygen species (ROS) can quickly accumulate, decrease antioxidant activity, and cause deterioration of kidney and liver function. A disrupted redox balance in cells also damages crucial biomolecules such as deoxyribonucleic acid (DNA), proteins and lipids. Oxidative stress (OS) is elevated in diabetes mellitus and may be a major factor in the development and evolution of the typical long-term pathophysiology of diabetes and its associated complications[2].
Many compounds isolated from marine algae have shown a variety of biological activities, such as antioxidant, anti-hypertension, anti-coagulant, anti-bacterial, anti-tumor and anti-diabetic functions. For instance, algal polysaccharides exhibit in vitro antioxidant activity that traps ROS in vivo and decreases oxidative damage in living organisms[3,4].
Belhadj et al.[5] have demonstrated that ULPS regulate lipid levels and restore glucose homeostasis in the alloxan diabetic rat model. The same authors have demonstrated that ULPS were a potentially beneficial food supplement in the treatment of obesity and diabetes and may be considered a new active oral anti-diabetic agent.
Over the past 50 years, diabetes has indisputably been shown to also affect bone. Indeed, bone quality in people with diabetes (type 1 and type 2) is affected by poor bone turnover, changes in hormonal responses, incorporation of advanced glycation end products and microvascular complications, which may increase the risk of bone fracture. Recent reviews have documented the etiology of this complication[2]. A novel Ulva lactuca oligosaccharide has been shown to enhance levels of glutathione, total superoxide dismutase, catalase, total antioxidant capacity and telomerase, and to decrease the levels of malondialdehyde and advanced glycation end products, thus ameliorating intracellular ROS accumulation[3].
Given the highly perturbed glycemic control, OS imbalance and risk of bone fracture in diabetes, the objective of this work was to investigate the beneficial effects of ULPS on hepatic, kidney, pancreatic and heart oxidative parameters, and bone mineral density (BMD) in an alloxan diabetic Wistar rat model.
ULPS were isolated from Ulva lactuca collected as previously described by Belhadj et al.[4].
Rats were treated as previously described by Belhadj et al.[5] and divided into four groups (n = 6/group):
Group 1: non-diabetic normal rats (Ctr)
Group 2: diabetic rats (Diab)
Group 3: diabetic rats treated with ULPS by gastric gavage (180 mg/kg of body weight/day for 30 days) (Diab + ULPS)
Group 4: diabetic rats treated with glimepiride by gastric gavage (3 mg/kg of body weight/day for 30 days) (Diab + Gly)
SOD activity was evaluated by assessment of the oxidation of nitro blue tetrazolium and is expressed as U/mg protein. CAT activity was assayed by the decomposition of hydrogen peroxide and is expressed as moles of hydrogen peroxide (H2O2) consumed/minute/mg protein. GPx activity was measured according to Flohé and Günzler[5] and is expressed as nmoles of glutathione oxidized/min/mg protein.
Tissue TBARS concentrations were measured spectrophotometrically at 530 nm according to Esterbauer[6] and are expressed as nmoles/mg protein.
After sacrifice, the femurs were removed and dried for 24 h at 650 °C. Ash bones were precisely weighed and stored in glass tubes. Two mL of HNO3 (Art-chimie, France) was added to 1 g bones. After 10 min, 1 mL of H2O2 (30%), Sigma-Aldrich) was deposited in the same tube. De-ionized water was then added to a 500 mL final volume. Standard range and blank solutions were determined and prepared with Zn, Ca, and Fe standard solutions for equipment calibration. The metal concentrations were measured with inductively coupled plasma optical emission spectrometry (ICP-OES; Ciros; Spectro Analytical Instrument, Germany).
The bone mineral content is expressed in µg/g for Zn and in mg/g for Ca and Fe.
-
Statistical analysis was performed with one-way analysis of variance and Fisher’s test in Statistica Software (StatSoft, Tulsa, OK, USA).
Data are presented as mean ± standard error of the mean. Differences were considered statistically significant at P < 0.05 (*,$), P < 0.01 (**,$$) or P < 0.001 (***,$$$).
The hepatic SOD, CAT and GPx activity (Table 1) in Diab rats was significantly (P < 0.01) lower (58.40%, 41.22% and 48.33%, respectively) than that in the Ctr group. However, ULPS and Gly administration resulted in significantly (P < 0.05 and P < 0.01) higher activity of these enzymes (75.39%, 3.76%, and 70.19%, respectively and 61.91%, 39.21%, and 3.77%, respectively) than that in the Diab group. Notably, the effects of ULPS were more pronounced than those of glimepiride.
Table 1. Effect of Ulva lactuca polysaccharides (ULPS) administration on SOD, CAT, and GPx activity and TBARS levels in the liver, kidney, pancreas and heart in Ctr, Diab, Diab + ULPS, and Diab + Gly rats
Parameters Ctr Diab Diab + ULPS Diab + Gly Liver SOD (U/mg protein) 10.84 ± 0.39 4.51 ± 0.12** 18.33 ± 0.74**$$$ 11.84 ± 0.16$$ CAT (moles of H2O2 consumed/min/mg protein) 37.04 ± 0.50 21.77 ± 0.08** 22.62 ± 0.14$$ 20.95 ± 0.33$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 1.20 ± 0.07 0.62 ± 0.08** 2.08 ± 0.12***$$$ 1.02 ± 0.07$ TBARS (nmol/mg protein) 0.45 ± 0.03 0.66 ± 0.03** 0.47 ± 0.02$$ 0.47 ± 0.01$$ Kidney SOD (U/mg protein) 13.33 ± 0.99 4.90 ± 0.28*** 9.91 ± 0.18*$$ 11.67 ± 0.37$$$ CAT (moles of H2O2 consumed/min/mg protein) 20.15 ± 2.16 6.70 ± 1.41** 22.73 ± 1.71$$$ 18.02 ± 0.27$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 0.83 ± 0.05 0.42 ± 0.05*** 0.69 ± 0.02*$$ 0.67 ± 0.01$$ TBARS (nmol/mg protein) 0.71 ± 0.02 1.11 ± 0.02*** 0.70 ± 0.01$$$ 0.74 ± 0.03$$$ Pancreas SOD (U/mg protein) 12.58 ± 0.17 5.50 ± 0.19*** 11.51 ± 0.19**$$$ 11.28 ± 0.26*$$$ CAT (moles of H2O2 consumed/min/mg protein) 19.76 ± 1.75 6.80 ± 0.55** 25.97 ± 2.29$$$ 15.06 ± 1.35$ GPx (nmoles of glutathione (GSH) oxidized/min/mg protein) 0.57 ± 0.02 0.20 ± 0.02*** 0.66 ± 0.01$$$ 0.51 ± 0.03$$$ TBARS (nmol/mg protein) 0.47 ± 0.02 1.31 ± 0.07*** 0.45 ± 0.03$$$ 0.50 ± 0.07$$$ Heart SOD (U/mg protein) 11.81 ± 0.84 7.21 ± 0.06*** 11.57 ± 0.16$$ 10.88 ± 0.47$$ CAT (moles of H2O2 consumed/min/mg protein) 33.38 ± 2.77 15.46 ± 1.46** 32.62 ± 1.36$$$ 30.13 ± 2.55$$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 0.88 ± 0.06 0.72 ± 0.01* 1.07 ± 0.14$ 0.81 ± 0.03 TBARS (nmol/mg protein) 0.34 ± 0.00 0.79 ± 0.02*** 0.23 ± 0.02**$$$ 0.35 ± 0.00$$$ Note. Ctr: normal rats; Diab: alloxan-induced diabetic rats; Diab + Gly: diabetic rats treated with Glimepiride; Diab + ULPS: diabetic rats treated with ULPS. SOD: Superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase; TBARS: Thiobarbituric acid reactive substances. Values are means ± S.E.M of six samples per group. One-way analysis of variance (ANOVA) followed by Fisher’s test for comparison between groups was performed. *Comparison with Ctr group. $Comparison with Diab group. Statistically significant at *,$P-value < 0.05, **,$$P-value < 0.01, ***,$$$P-value < 0.001. In contrast, TBARS concentrations were significantly (P < 0.01) higher in Diab rats (31.82%) than Ctr rats. The ULPS and Gly treatments resulted in TBARS concentrations 28.79% lower than those in the Diab group (P < 0.001).
The kidney SOD, CAT, and GPx activity (Table 1) was significantly (P < 0.01) lower (63.24%, 66.75%, and 49.40%, respectively) in Diab rats than Ctr rats. ULPS and Gly treatments resulted in significantly (P < 0.01) greater (50.55%, 70.52%, 39.13% and 55.01%, 62.82%, 37.13%, respectively) activity of these enzymes than that in the Diab group.
The TBARS (36.04%, P < 0.001) level was significantly higher in the Diab group than the Ctr group. However, the values significantly (P < 0.001) reverted to near normal in the Diab + ULPS and Diab+Gly groups (36.94% and 33.33%, respectively).
The pancreatic SOD, CAT and GPx activity (Table 1) in Diab rats was significantly (P < 0.01) lower than that in the Ctr group (56.28%, 65.59%, and 64.91%, respectively). In contrast, the TBARS levels were significantly (P < 0.001) higher in Diab rats (64.12%) than Ctr rats. In the Diab + ULPS and Diab + Gly groups, the antioxidant activity was significantly (P < 0.001) higher (by 52.21%, 73.81%, 69.70% and 51.24%, 54.85% and 60.78%, respectively) than that in the Diab rats.
ULPS and Gly treatments resulted in significantly lower (P < 0.001) rates of TBARS (65.65% and 61.83%, respectively) than those in the Diab rats.
The cardiac SOD, CAT and GPx activity in Diab rats (Table 1) was significantly lower (P < 0.05, 38.95%, 53.68%, and 18.18%, respectively) than that in the Ctr group.
The activity of these enzymes was significantly restored (P < 0.01) in the presence of ULPS (37.68%, 52.60%, and 32.71%, respectively) and Gly (33.73%, 48.69%, and 11.11%, respectively). Gly treatment did not result in any improvement in GPx activity in Diab animals.
TBARS levels were significantly (P < 0.001) higher in the hearts of Diab rats (56.96%) than Ctr rats. These levels were significantly improved (P < 0.001) after ULPS (70.89%) and Gly (55.70%) treatments.
The bone Zn content (Figure 1A) was 1.4-fold (P < 0.05) lower in the Diab group (17.94 µg/g) than the Ctr group (25.17 µg/g). After ULPS treatment, the Zn content was 1.86-fold (P < 0.01) higher (33.50 µg/g) than that in the Diab group, but glimepiride had no effect.
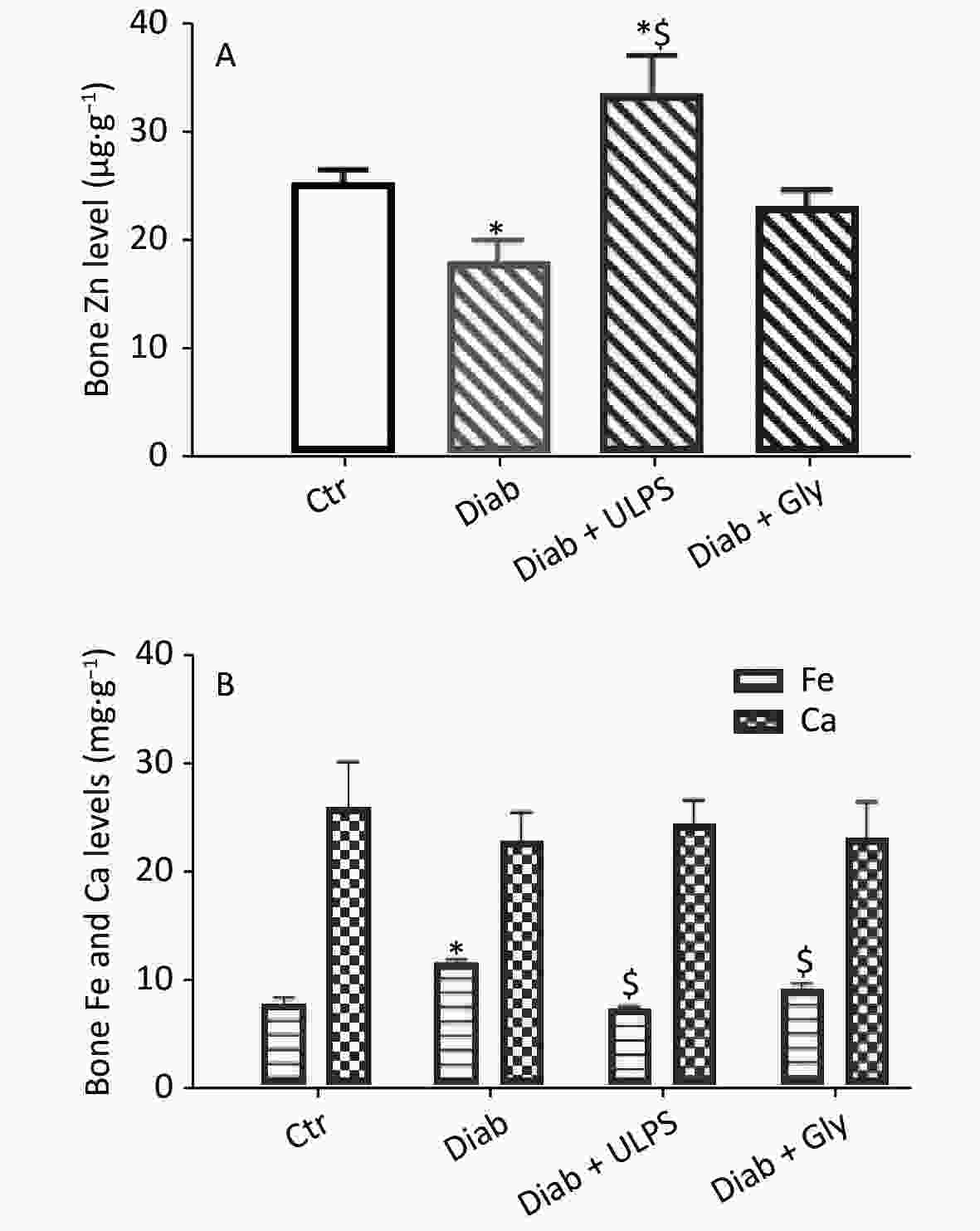
Figure 1. Effects of ULPS on bone zinc, iron and calcium content. Values are means ± S.E.M. *Comparison with Ctr rats. $comparison with Diab rats. Statistically significant at *,$P-value < 0.05.
The Ca content (Figure 1B) in the Diab, Diab + ULPS and Diab + Gly groups did not significantly differ from that in the Ctr group.
In the Diab group, the Fe content (11.52 mg/g) was 1.52-fold (P < 0.001) higher than in the Ctr group (7.6 mg/g). However, the Fe content was significantly (P < 0.01) ameliorated in the Diab + ULPS (7.12 mg/g) and Diab + Gly (9.06 mg/g) groups (Figure 1B).
Administration of ULPS increased the activity of antioxidant enzymes. SOD activity increased in tissues, particularly in the liver. High SOD activity accelerates the dismutation of superoxide radicals into H2O2, which is removed by CAT and finally by GPx. Our results clearly showed that ULPS treatment in diabetic rats not only modified the activity of these enzymes but also decreased OS, thus resulting in a lower TBARS concentration. Our results are in agreement with findings from several studies[3]. The use of these polysaccharides has been found to protect against the hepatic toxicity in rats and to improve the liver antioxidant defense system’s activity against the damage and necrosis induced by d-galactosamine, diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis[7]. These improvements were correlated with increased antioxidant enzyme activity and decreased lipid peroxide, ROS and MDA levels. Our results clearly supported the protective effects of ULPS on the liver and pancreas tissue integrity previously described by Belhadj et al.[4].
One long-term complication of diabetes is bone fracture, although this topic is rarely discussed. Diabetes influences endothelial function, thus contributing to a decrease in BMD. Alterations in calcium metabolism and BMD have been shown to promote the risk of fracture in patients with diabetes[2]. To maintain bone health, a sufficient and persistent supply of certain nutrients, such as vitamin K, vitamin D, calcium, fluoride, magnesium, phosphorus, copper, zinc and potassium, is necessary[3]. In fact, zinc is considered an important mineral for the preservation and growth of bone. Calcium, like zinc, is one of the main minerals composing bones, and 99% of the body's calcium is found in the skeleton. In the bone collagen matrix, iron is a co-factor of a 25-hydroxycholcalciferol hydroxylase, which is involved in the transformation of vitamin D into its active form. In the present study, the results showed that alloxan causes a highly significant decrease in zinc levels in the bone, thus promoting bone fracture. However, ULPS in diabetic rats significantly increased the zinc levels, and decreased the iron but not the calcium levels in bones, thus clearly indicating the beneficial effects of these polysaccharides. Our results associated with calcium levels in bone are in agreement with those of Ferreira et al.,[8] who have shown that zinc supplementation is an effective treatment against bone loss in type 1 diabetes. Leão et al.[9] have reported a BMD (calcium, phosphorus and magnesium) in people with type 1 diabetes was almost lower than that of the controls. The authors have also shown that the decrease in calcium levels in the bones of patients with diabetes is influenced by the duration of the disease. Because we did not record the decrease in Ca bone content in our rats, we concluded that a duration of 1 month of diabetes was not sufficient to elicit this decrease. No studies have reported the relationship between bone iron levels and diabetes. However, Simcox and McClain[10] have shown that iron overload was a risk factor for diabetes. In fact, iron plays an important role in the pathology of diabetes, mediated by insulin resistance and β-cell failure. According to these authors, the increase in iron levels in bone can be assumed to be a complication due to diabetes. This hypothesis will be the subject of future work.
Our study revealed the effectiveness of ULPS treatment on OS (TBARS), antioxidant enzyme activity in various crucial organ tissues, and the essential mineral content in bone in alloxan-induced diabetic rats after 30 days of treatment. Globally, our results showed that ULPS treatment of diabetes had a protective effect against OS, and elicited improvements in SOD, CAT, and GPx activity and bone mineral levels. Future work will be focused on molecular analyses to determine the mechanisms through which ULPS improve OS and to stimulate skeletal strength.
The authors declare that they have no conflicts of interest.
The authors thank Pr. Eliane Stubert (Associate Professor at the Strasbourg Military School) for kind assistance in English proofreading.
doi: 10.3967/bes2021.088
Polysaccharides from the Green Alga Ulva lactuca Improve Antioxidant Balance and Bone Mineral Density in Diabetic Rats
-
&These authors contributed equally to this work.
注释: -
Table 1. Effect of Ulva lactuca polysaccharides (ULPS) administration on SOD, CAT, and GPx activity and TBARS levels in the liver, kidney, pancreas and heart in Ctr, Diab, Diab + ULPS, and Diab + Gly rats
Parameters Ctr Diab Diab + ULPS Diab + Gly Liver SOD (U/mg protein) 10.84 ± 0.39 4.51 ± 0.12** 18.33 ± 0.74**$$$ 11.84 ± 0.16$$ CAT (moles of H2O2 consumed/min/mg protein) 37.04 ± 0.50 21.77 ± 0.08** 22.62 ± 0.14$$ 20.95 ± 0.33$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 1.20 ± 0.07 0.62 ± 0.08** 2.08 ± 0.12***$$$ 1.02 ± 0.07$ TBARS (nmol/mg protein) 0.45 ± 0.03 0.66 ± 0.03** 0.47 ± 0.02$$ 0.47 ± 0.01$$ Kidney SOD (U/mg protein) 13.33 ± 0.99 4.90 ± 0.28*** 9.91 ± 0.18*$$ 11.67 ± 0.37$$$ CAT (moles of H2O2 consumed/min/mg protein) 20.15 ± 2.16 6.70 ± 1.41** 22.73 ± 1.71$$$ 18.02 ± 0.27$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 0.83 ± 0.05 0.42 ± 0.05*** 0.69 ± 0.02*$$ 0.67 ± 0.01$$ TBARS (nmol/mg protein) 0.71 ± 0.02 1.11 ± 0.02*** 0.70 ± 0.01$$$ 0.74 ± 0.03$$$ Pancreas SOD (U/mg protein) 12.58 ± 0.17 5.50 ± 0.19*** 11.51 ± 0.19**$$$ 11.28 ± 0.26*$$$ CAT (moles of H2O2 consumed/min/mg protein) 19.76 ± 1.75 6.80 ± 0.55** 25.97 ± 2.29$$$ 15.06 ± 1.35$ GPx (nmoles of glutathione (GSH) oxidized/min/mg protein) 0.57 ± 0.02 0.20 ± 0.02*** 0.66 ± 0.01$$$ 0.51 ± 0.03$$$ TBARS (nmol/mg protein) 0.47 ± 0.02 1.31 ± 0.07*** 0.45 ± 0.03$$$ 0.50 ± 0.07$$$ Heart SOD (U/mg protein) 11.81 ± 0.84 7.21 ± 0.06*** 11.57 ± 0.16$$ 10.88 ± 0.47$$ CAT (moles of H2O2 consumed/min/mg protein) 33.38 ± 2.77 15.46 ± 1.46** 32.62 ± 1.36$$$ 30.13 ± 2.55$$$ GPx [nmoles of glutathione (GSH) oxidized/min/mg protein] 0.88 ± 0.06 0.72 ± 0.01* 1.07 ± 0.14$ 0.81 ± 0.03 TBARS (nmol/mg protein) 0.34 ± 0.00 0.79 ± 0.02*** 0.23 ± 0.02**$$$ 0.35 ± 0.00$$$ Note. Ctr: normal rats; Diab: alloxan-induced diabetic rats; Diab + Gly: diabetic rats treated with Glimepiride; Diab + ULPS: diabetic rats treated with ULPS. SOD: Superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase; TBARS: Thiobarbituric acid reactive substances. Values are means ± S.E.M of six samples per group. One-way analysis of variance (ANOVA) followed by Fisher’s test for comparison between groups was performed. *Comparison with Ctr group. $Comparison with Diab group. Statistically significant at *,$P-value < 0.05, **,$$P-value < 0.01, ***,$$$P-value < 0.001. -
[1] Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International diabetes federation diabetes atlas. 9th edition. Diabetes Res Clin Pract, 2019; 157, 107843. doi: 10.1016/j.diabres.2019.107843 [2] Sellmeyer DE, Civitelli R, Hofbauer LC, et al. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes, 2016; 65, 1757−66. doi: 10.2337/db16-0063 [3] Wu DS, Chen YH, Wan XZ, et al. Structural characterization and hypoglycemic effect of green alga Ulva lactuca oligosaccharide by regulating microRNAs in Caenorhabditis elegans. Algal Res, 2020; 51, 102083. doi: 10.1016/j.algal.2020.102083 [4] BelHadj S, Hentati O, Elfeki A, et al. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch Physiol Biochem, 2013; 119, 81−7. doi: 10.3109/13813455.2013.775159 [5] Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol, 1984; 105, 114−20. [6] Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr, 1993; 57, 779S−86S. doi: 10.1093/ajcn/57.5.779S [7] Hussein UK, Mahmoud HM, Farrag AG, et al. Chemoprevention of diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis in rats by sulfated polysaccharides and aqueous extract of Ulva lactuca. Integr Cancer Ther, 2015; 14, 525−45. doi: 10.1177/1534735415590157 [8] Ferreira ECS, Bortolin RH, Freire-Neto FP, et al. Zinc supplementation reduces RANKL/OPG ratio and prevents bone architecture alterations in ovariectomized and type 1 diabetic rats. Nutr Res, 2017; 40, 48−56. doi: 10.1016/j.nutres.2017.03.004 [9] Leão AAP, Fritz CK, Dias MRMG, et al. Bone mass and dietary intake in children and adolescents with type 1 diabetes mellitus. J Diabetes Complications, 2020; 34, 107573. doi: 10.1016/j.jdiacomp.2020.107573 [10] Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab, 2013; 17, 329−41. doi: 10.1016/j.cmet.2013.02.007 -




 下载:
下载:



 Quick Links
Quick Links