-
Radix astragali is the dried root of the legume Mongolian Astragalus or Astragalus membranaceus[1]. Modern research has found that the main active ingredients in astragalus are astragalus polysaccharides, astragaloside, and astragalus isoflavones. Astragaloside IV (AS-IV) has been used internationally as a standard for evaluating the quality of astragalus medicinal materials. Clinical and experimental studies have found that AS-IV has a wide range of biological activities. However, the drug mechanism of AS-IV has not yet been clearly elucidated; this restricts AS-IV from being applied in practical and clinical use.
Small ubiquitin-related modifier (SUMO)-mediated modification (SUMOylation) is a dynamically reversible post-translational modification of proteins and plays a key role in regulating various cellular processes. Studies have shown that the regulation of SUMOylation is essential for maintaining blood glucose levels. For example, the glucose transporter GLUT4 accumulates on the surface cell membrane directly through SUMO modification[2]. SUMO modification can inhibit the expression and catalytic activity of protein tyrosine phosphatase 1B (PTP1B), which has a negative regulatory effect on insulin receptor function[3]; therefore, SUMOylation may promote the transmission of the insulin signaling pathway.
We screened a variety of traditional Chinese medicine monomers and found that these monomers can promote or inhibit covalent modification of different SUMO members. Among the monomers, AS-IV can significantly promote the expression of SUMO1 and promote SUMO1 to covalently modify target proteins; this greatly increased our interest in elucidating the mechanism of AS-IV in treating diabetes and its related complications through protein SUMOylation regulation. In the present study, we explored whether AS-IV promotes angiogenesis and improves skin healing in diabetic rats by regulating SUMOylation modification pathways.
Human umbilical vein endothelial cells (HUVECs) were cultured at 37 °C under 5% CO2 humidified atmospheric conditions. We defined the cells treated under high glucose conditions as those that were cultured in 25 mmol/L glucose (G8150, ≥ 99.8% purity, Solarbio Life Sciences, Beijing, China). The cells that we cultured in 5.5 mmol/L glucose, which we defined as normal conditions, were used as the controls.
AS-IV (≥ 98%) (CAS No.: 84687-43-4; Cat. No.: CS-4272, ≥ 99.15% purity) was purchased from the Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). AS-IV was dissolved in a solvent consisting of anhydrous ethanol and propylene glycol in a 2:1 proportion (invention patent, China, 200510014971.2), sonicated for 30 min, to plane solubilis, and diluted with sterilized water to a final concentration of 5 ng/mL. For the evaluation of the time-dependent effects of AS-IV treatment, cells were cultured in fresh media supplemented with 6 µmol/L AS-IV for 0, 24, 48, and 72 h.
HUVECs cultured in high glucose or normal conditions were treated with AS-IV at a final concentration of 6 µmol/L for 3 days. Proteins were extracted from the cultured cells by using RIPA buffer (Solarbio, Beijing, China) supplemented with 1 mmol/L phenylmethanesulfonyl fluoride and 20 mmol/L N-ethylmaleimide. The protein extracts were resolved in 7.5% or 10% running gel and electrophoretically transferred onto polyvinylidene fluoride membranes (EMD Millipore, Inc.). The membranes were incubated with antibodies that are specific to target proteins; β-Actin (ab8227, 1:2000, Abcam, Cambridge, MA) was used as the internal control. Information on all antibodies is shown in Supplementary Table S1, available in www.besjournal.com. Densitometric quantification analysis of the immunoblot bands was performed by using an image analysis software (ImageJ version 1.48; National Institutes of Health, Bethesda, MD, USA). Each experiment was repeated at least 4 times.
Table S1. Information of all the related antibodies in this study
Antibody SUMO1 Ubc9 SAE1 SAE2 SENP1 PCNA Ras HIF-1α PPARγ VEGF2 Company Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Code ab11672 ab75854 ab185552 aAb185955 ab108981 ab92552 ab52939 ab1 ab209350 ab39378 Dilution 1:1,000/1:400 1:2,000/1:200 1:5,000/1:200 1:1,000/1:200 1:5,000/1:500 1:2,000 1:5,000 1:1,000 1:1,000 1:1,000 HUVECs were cultured in 25 mmol/L glucose or 6 µmol/L AS-IV for 3 days. Then, the subcellular localization of proteins such as SUMO1 and SAE1 were determined by using immunofluorescence analysis. The cells were incubated with primary antibodies (Supplementary Table S1) at 4 ℃ overnight, with Alexa Fluor 488-conjugated anti-rabbit IgG H&L (ab150077, Abcam; 1:500) to detect SENP1 and Ubc9, and with Alexa Fluor 594-conjugated anti-rabbit IgG H&L (ab150080, Abcam; 1:500) to detect SUMO1, SAE1, and SAE2. Specimens were mounted onto glass slides in VECTASHIELD mounting medium containing 3 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen; D1306). Fluorescence intensities were quantified by using ImageJ software (National Institutes of Health).
Cell proliferation was evaluated using Cell Counting Kit-8 (CCK-8; Yeasen, Shanghai, China). HUVECs cultured in 96-well plates under high glucose or normal conditions were treated with AS-IV for 24 h. Absorbance values at 450 nm were used to determine the cell number according to a standard curve. The HUVECs were serum-starved for 12 h and then wounded by manual scraping with a pipette tip. The plates were washed 2 times to remove nonadherent cells, and the cells were cultured under high glucose or normal conditions with or without AS-IV. The migration of cells into the wounded areas was assessed under a light microscope after an additional 72 h of incubation. Afterwards, 2-mm thick semi-solid gel was pre-coated onto the bottom of the 96-well plate with Matrigel (1:3; BD Biosciences, San Jose, CA) overnight. Then, the HUVECs were transferred onto the surface of the gel and inoculated in 0.1 mL growth medium with high glucose or normal glucose concentration with or without AS-IV. After 7 days, the formation of blood vessels was observed, and the tube length was quantified under an inverted microscope (Olympus cellSens Entry 1.16). HUVECs were seeded into 96-well plates and incubated in growth medium with high glucose or normal glucose concentrations with or without AS-IV for 48 h. LDH content in the conditioned medium was measured by using an enzyme-linked immunosorbent assay and ELISA-based LDH Activity Assay Kit (Yuanmu Biotechnology Co., Ltd., Shanghai, China).
A total of 20 8-week-old female diabetic Sprague-Dawley (SD) rats were used according to a previous report[4]. The rats were purchased from Tianjin Elan Biotechnology Co., Ltd. and were housed in the Experimental Animal Center of The Fifth Central Hospital of Tianjin. Full-thickness circular wounds (15 mm in diameter) were created on the dorsal skin of the rats. Then, the rats were randomly divided into two groups (n = 10). (1) The AS-IV group contained the rats that were intraperitonially injected daily with 4 mg/kg AS-IV for 15 days. (2) The Control group contained the rats that were administered saline at volumes same as that administered in the AS-IV group for 15 days. The wounded areas of the rats were photographed by using a camera at days 5, 10, and 15 post-operation. Wound healing rates were calculated by using the formula: [1 – (nonhealing area)/original area] × 100%. This experiment was performed according to the regulations and guidelines approved by the Animal Ethics Committee of the Fifth Central Hospital of Tianjin (Tianjin, China). Five days after the operation (Day 5), skin samples (7 mm × 7 mm) from the wound tissues and adjacent normal tissues were obtained under general anesthesia. Then, the skin samples were embedded in paraffin for hematoxylin and eosin (H&E) staining, and the degree of neovascularization was identified using rabbit polyclonal CD31 antibody (ab24590, 1:50, Abcam, Cambridge, MA) labeling. The capillaries were manually counted across 6 random fields under a microscope (200× magnification).
Each experiment was performed at least three times. The statistical data were plotted as the mean ± standard deviation (SD). A P value of < 0.05 was considered statistically significant. We tested the differences among the data obtained from the experimental and control group by using an unpaired t-test. For the comparison of the differences among four experimental groups, the means were compared using Tukey-Kramer test.
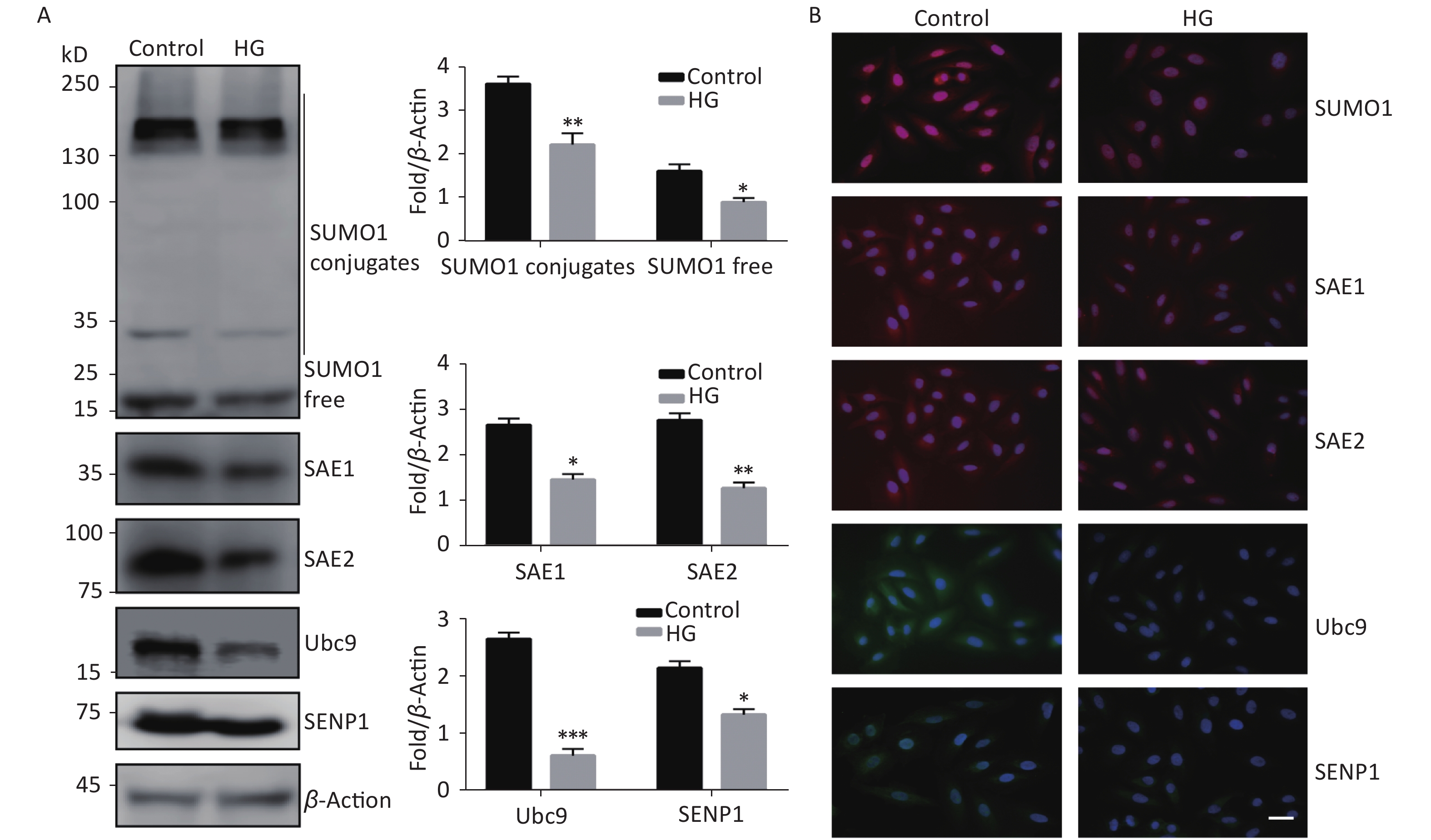
To investigate the effect of high glucose on the SUMO1 pathway, we first examined the expression levels of SUMO1, SAE1, SAE2, Ubc9, and SENP1 protein in HUVECs cultured under high glucose conditions. We found that the expression levels of all the tested proteins showed varying degrees of decline, with SUMO1 and Ubc9 showing the most significant reduction (Figure 1A). In addition, we confirmed through immunofluorescence experiments that the reduction of these proteins occurs in both the cytoplasm and the nucleus (Figure 1B), indicating that the inhibition of the SUMO1 pathway by high glucose conditions is profound and extensive.
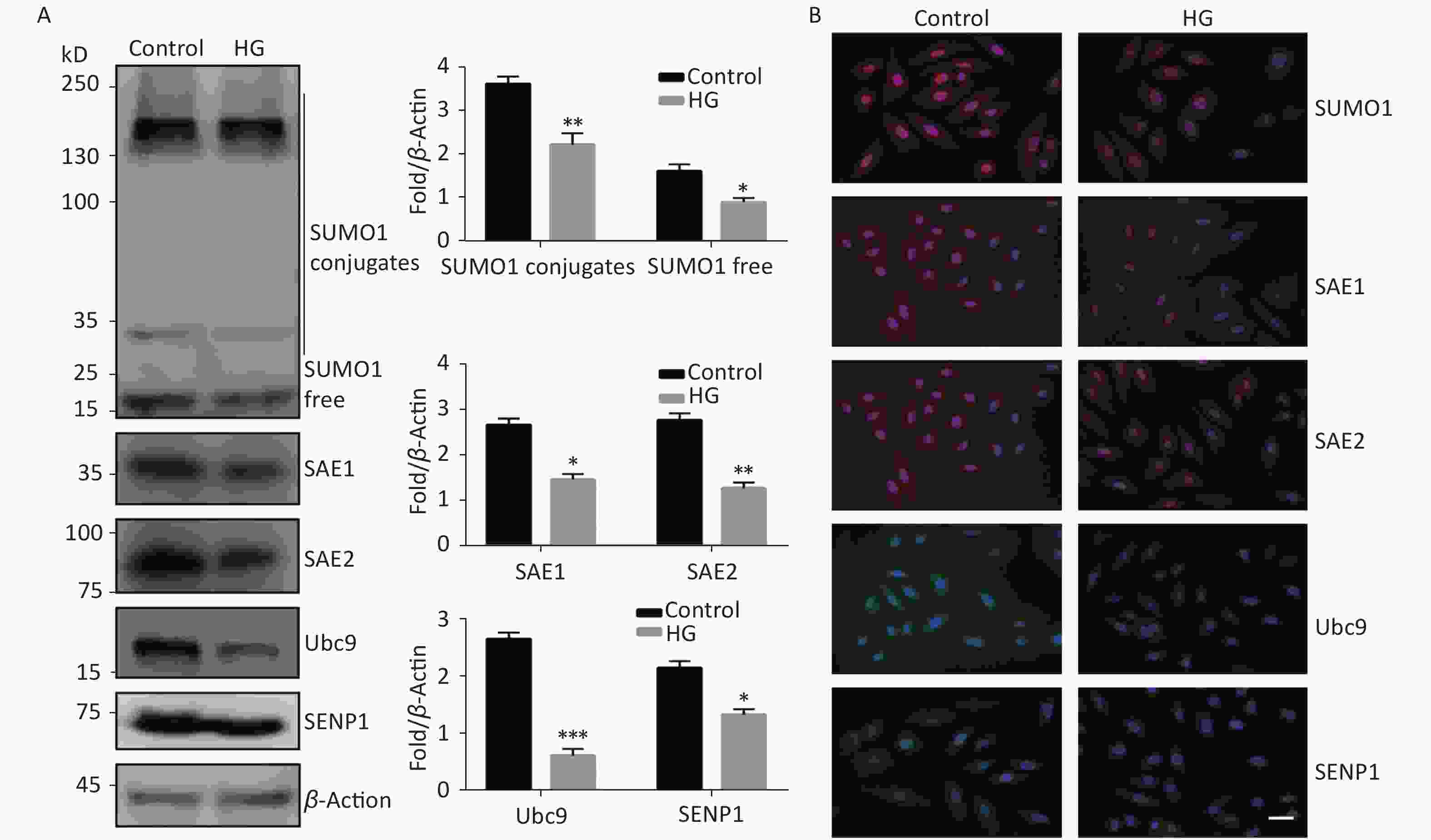
Figure 1. High glucose reduced SUMOylation and protein level of SAE1, SAE2, Ubc9, and SENP1 in HUVECs. (A) SUMO1, SAE1, SAE2, Ubc9, and SENP1 protein expression were examined by Western blotting. Data are shown as means ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group. (B) The subcellular localization of SUMO1 pathway members in HUVECs were examined by immunofluorescence (IF) (scale bar, 20 µm). DAPI was used to stain cell nuclei. SUMO, small ubiquitin-related modifier; DAPI, 4′,6-diamidino-2-phenylindole.
Next, we examined the effect of AS-IV on the SUMO1 pathway in HUVECs. The results indicated that, contrary to the effects of high glucose conditions, AS-IV was able to increase expression of SUMO1 pathway effector molecules in multiple cascades, including SUMO1, E1-activating enzymes (SAE1 and SAE2), E2 binding enzyme Ubc9, and de-SUMOase SENP1. In particular, the concentration levels of SUMO1, SAE1, and SAE2 proteins were significantly increased (Figure 2A). Furthermore, we determined that the activation of the SUMO1 pathway by AS-IV both occurs in the cytoplasm and nucleus (Figure 2B). The effect of AS-IV in HUVECs is extensive and similar to that of high glucose.
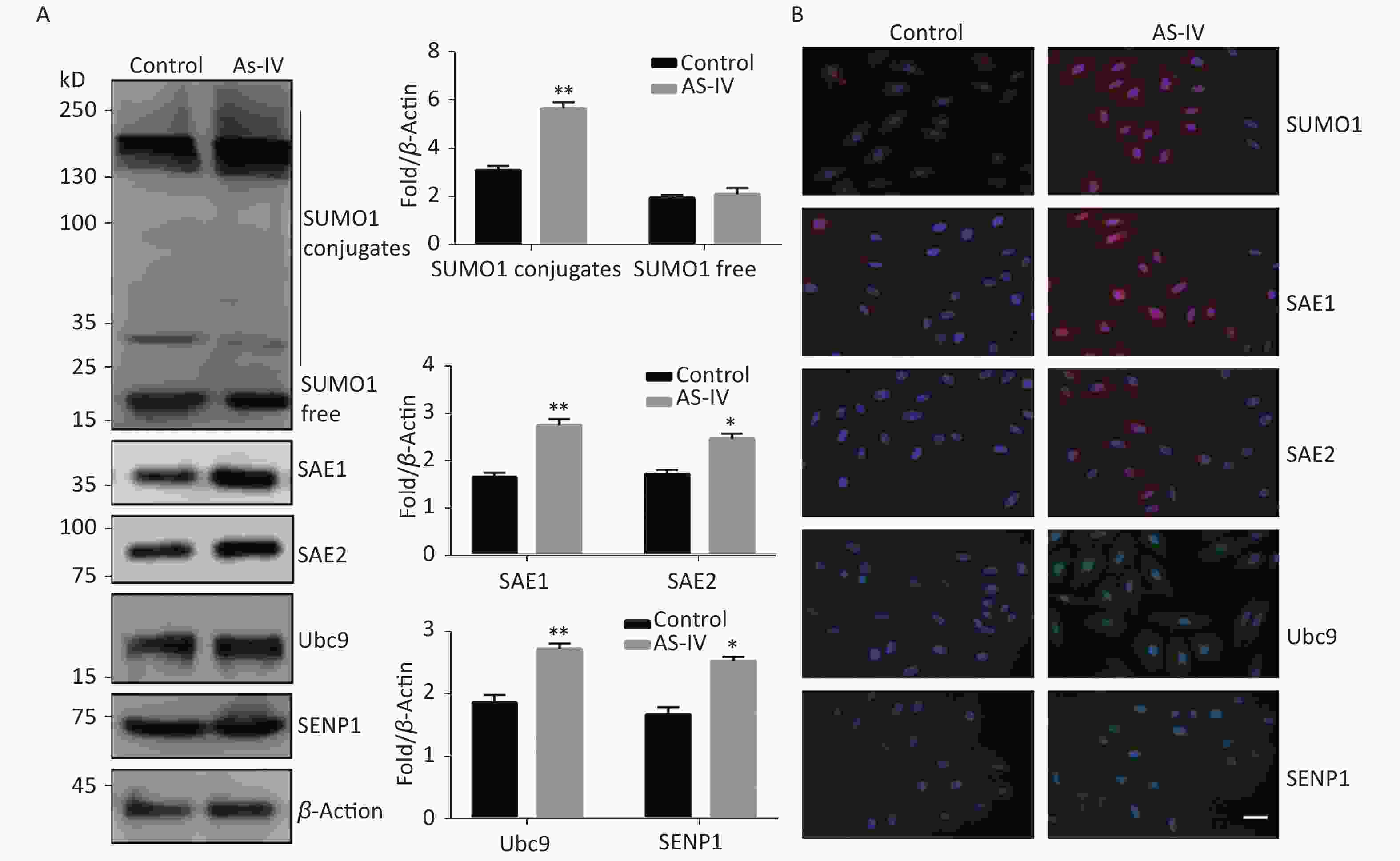
Figure 2. AS-IV activated the SUMO1 pathway in HUVECs. (A) SUMO1, SAE1, SAE2, Ubc9, and SENP1 protein expression were examined by Western blotting. Data are shown as means ± SD (n = 4); *P < 0.05 and **P < 0.01 compared with control group. (B) The subcellular localization of SUMO1 pathway members in HUVECs were examined by immunofluorescence (IF) (scale bar, 20 µm). DAPI was used to stain cell nuclei. SUMO, small ubiquitin-related modifier; DAPI, 4′,6-diamidino-2-phenylindole.
Some proteins have been identified as the target proteins of SUMO1; these proteins are involved in endothelial cell proliferation, migration, hypoxia, and high glucose tolerance. Therefore, in this study we tested the protein expression levels of PCNA, Ras, HIF-1α, PPARγ, and VEGFR2. The results indicated that high glucose conditions can reduce the protein concentration levels of Ras, HIF-1α, PPARγ, and VEGFR2 but has no significant effect on the protein level of PCNA (Figure 3A). However, in contrast to high glucose conditions, AS-IV can increase the concentration levels of all the tested proteins (PCNA, Ras, HIF-1α, PPARγ, and VEGFR2) (Figure 3A).
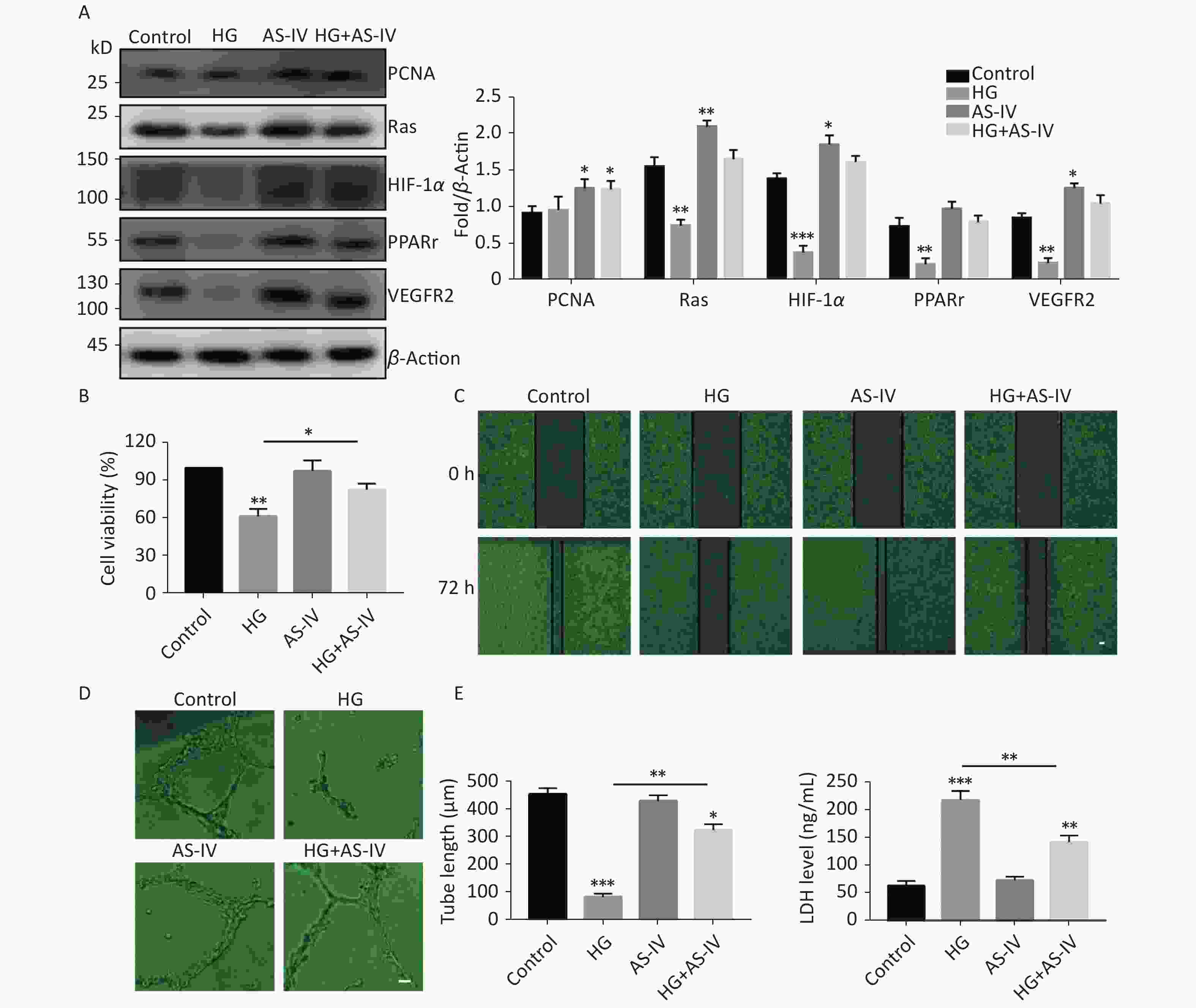
Figure 3. AS-IV antagonized high glucose conditions and improved angiogenesis and wound healing of HUVECs. (A) PCNA, Ras, HIF-1α, PPARγ, and VEGFR2 protein expression were examined by Western blotting. (B) The viability of HUVECs treated with HG or/and with AS-IV were examined using the CCK-8 assay. (C) The migration of HUVECs treated with HG or/and with AS-IV were examined over 72 h by wound healing assay (scale bar, 100 µm). (D) The angiogenic potential was detected by simulated angiogenesis experiments when HUVECs were exposed to high glucose medium with or without AS-IV (scale bar, 50 µm) and the tube length of blood vessels was quantified. (E) LDH content was measured using an ELISA kit after HUVECs were exposed to high glucose medium (25 mmol/L) with or without AS-IV (µmol/L) for 48 h. Data are shown as mean ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control or the specific group.
We observed the effects of high glucose conditions and treatment with AS-IV on the behavioral characteristics of vascular endothelial cells. We found that high glucose conditions can inhibit wound healing (Figure 3C) and angiogenesis (Figure 3D) and increase endothelial cell damage (Figure 3E). On the other hand, we found that AS-IV can promote the proliferation of HUVECs (Figure 3B), improve the ability of wound healing (Figure 3C) and angiogenesis (Figure 3D), and reduce endothelial cell damage under high glucose conditions (Figure 3E).
To further determine whether AS-IV can promote skin healing in vivo, we established skin defect models in diabetic rats and regularly administered AS-IV or saline to the rats. The results indicated that the administration of AS-IV can shorten healing duration (Supplementary Figure S1A, available in www.besjournal.com) as the skin injury in rats treated with AS-IV were smaller in diameter than those of rats that did not receive AS-IV treatment at certain elapsed times (Supplementary Figure S1B). Furthermore, H&E staining results indicated that there was a richer vascular network and less inflammatory cell infiltration at the skin resection margin in the AS-IV group; this was consistent with the immunohistochemical results of the CD31 labeled blood vessels (Supplementary Figure S1 C, D).
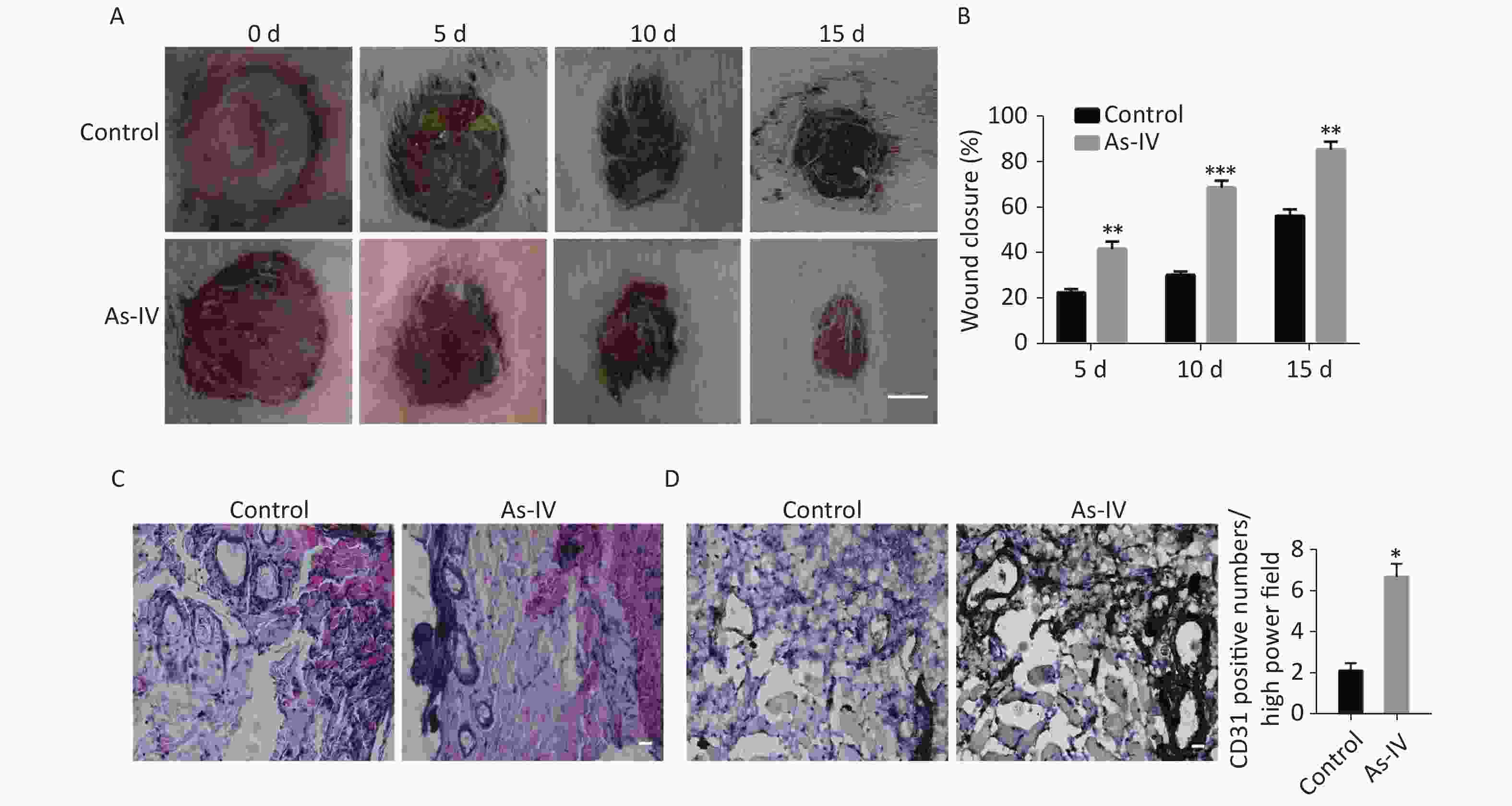
Figure S1. AS-IV increases neovascularization and shortens wound healing time in diabetic rats. (A) The appearance of skin defects in diabetic rats showed the extent of wound healing (scale bar, 5 mm). (B) Wound closure rate of defective skin in diabetic rats. (C) HE staining showed infiltration of inflammatory cells and vascular morphology at the edge of the defective skin (scale bar, 50 µm). (D) CD31 immunostaining showed new blood vessels at the edge of the defective skin (scale bar, 50 µm). (E) Quantitative analysis of relative CD31 positivity. Data are shown as mean ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control.
SUMO protein is widely distributed and expressed in nematodes, fruit flies, budding yeast, and cultured vertebrate cells[5]. The human genome mainly encodes four SUMO protein subtypes, namely SUMO1, SUMO2, SUMO3, and SUMO4. Among them, SUMO1, SUMO2, and SUMO3 play a major role in regulating various cellular processes and are expressed in various tissues, whereas SUMO4 is mainly expressed in the kidney, spleen, and lymph nodes[6,7]. Theoretically, SUMO1 mainly mediates delayed intracellular response and plays a role in the progression of chronic diseases such as diabetes, hypertension, and tumors, whereas SUMO2 and SUMO3 are mainly involved in the rapid stress response of cells to internal and external environmental changes[5,7]. Therefore, in this study, we focused on the cell response and function regulated by the SUMO1 protein.
In this study, we first examined the effect of high glucose conditions on SUMO1 pathway effector molecules in HUVECs. The results indicated that high glucose conditions resulted in the decreased expression of several key members of the SUMO1 pathway, including SUMO1, SAE1, SAE2, Ubc9, and the de-SUMO enzymes SENP1; this indicates that high glucose conditions profoundly inhibit the protein expression of SUMO pathway effectors. Next, we examined the effect of AS-IV on SUMO1 pathway and its effectors; the results indicated that the administration of AS-IV resulted in the increased expression of SUMO1 pathway members, suggesting that AS-IV activated the SUMO1 pathway in full.
At present, the research on SUMO1 is the most abundant compared with SUMO2, SUMO3, and SUMO4. PCNA, Ras, HIF-1α, PPARγ, and VEGFR2 are among the target proteins of SUMO1 that have been identified. Ubiquitin and SUMO compete for the modification of PCNA, which is an essential processivity factor for DNA replication and repair. The RAS/RAF/MEK/ERK2 signaling pathway plays a central role in mediating cell survival, proliferation, and differentiation[8]. Choi BH et al. reported that Ras proteins are modified by SUMOylation and that Lys-42 plays an important role in mediating the SUMOylation[9]. HIF-1 is closely involved in various biological processes, including angiogenesis, energy metabolism, and cell survival. Zhang et al revealed that AS-IV regulates HIF-1α and angiogenesis through the PI3K/Akt pathway in HUVECs that are exposed to hypoxia[10]. Moreover, the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) has been reported to be a master regulator of adipocyte differentiation and the target for the insulin-sensitizing thiazolidinedione (TZD) drugs used to treat type 2 diabetes.
In this study, we examined the effect of AS-IV on the expression of PCNA, Ras, HIF-1α, PPARγ, and VEGFR2 proteins in vascular endothelial cells; our results indicated that AS-IV can increase the expression of these proteins in HUVECs by activating the SUMO pathway; this may explain how AS-IV promotes the proliferation and migration of vascular endothelial cells, antagonizes the adverse microenvironment of hypoxia and high glucose, and improves angiogenesis and wound healing in vitro and in vivo. However, as these results are limited to primary observations and analyses, the in-depth molecular mechanisms and network regulatory relationships wherein AS-IV could be involved remain subjects of further study. In addition, other post-translational modifications also regulate SUMO conjugation, suggesting that SUMO signaling is integrated with other signal transduction pathways. A better understanding of SUMO regulatory mechanisms would lead to improved approaches for analyzing the function of SUMO and substrate conjugation in distinct cellular pathways and help us identify the effective components of Chinese herbal medicine.
WANG Bao Shen performed the experiments and analyzed the data. MA Xiao Fang wrote the paper. ZHANG Chun Yan prepared the Figures. LI Yan Xia collected the data. LIU Xiao Zhi and HU Chang Qing designed the experiments.
The authors declare that they have no conflicts of interests.
doi: 10.3967/bes2021.018
Astragaloside IV Improves Angiogenesis and Promotes Wound Healing in Diabetic Rats via the Activation of the SUMOylation Pathway
-
-
Figure 1. High glucose reduced SUMOylation and protein level of SAE1, SAE2, Ubc9, and SENP1 in HUVECs. (A) SUMO1, SAE1, SAE2, Ubc9, and SENP1 protein expression were examined by Western blotting. Data are shown as means ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control group. (B) The subcellular localization of SUMO1 pathway members in HUVECs were examined by immunofluorescence (IF) (scale bar, 20 µm). DAPI was used to stain cell nuclei. SUMO, small ubiquitin-related modifier; DAPI, 4′,6-diamidino-2-phenylindole.
Figure 2. AS-IV activated the SUMO1 pathway in HUVECs. (A) SUMO1, SAE1, SAE2, Ubc9, and SENP1 protein expression were examined by Western blotting. Data are shown as means ± SD (n = 4); *P < 0.05 and **P < 0.01 compared with control group. (B) The subcellular localization of SUMO1 pathway members in HUVECs were examined by immunofluorescence (IF) (scale bar, 20 µm). DAPI was used to stain cell nuclei. SUMO, small ubiquitin-related modifier; DAPI, 4′,6-diamidino-2-phenylindole.
Figure 3. AS-IV antagonized high glucose conditions and improved angiogenesis and wound healing of HUVECs. (A) PCNA, Ras, HIF-1α, PPARγ, and VEGFR2 protein expression were examined by Western blotting. (B) The viability of HUVECs treated with HG or/and with AS-IV were examined using the CCK-8 assay. (C) The migration of HUVECs treated with HG or/and with AS-IV were examined over 72 h by wound healing assay (scale bar, 100 µm). (D) The angiogenic potential was detected by simulated angiogenesis experiments when HUVECs were exposed to high glucose medium with or without AS-IV (scale bar, 50 µm) and the tube length of blood vessels was quantified. (E) LDH content was measured using an ELISA kit after HUVECs were exposed to high glucose medium (25 mmol/L) with or without AS-IV (µmol/L) for 48 h. Data are shown as mean ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control or the specific group.
S1. AS-IV increases neovascularization and shortens wound healing time in diabetic rats. (A) The appearance of skin defects in diabetic rats showed the extent of wound healing (scale bar, 5 mm). (B) Wound closure rate of defective skin in diabetic rats. (C) HE staining showed infiltration of inflammatory cells and vascular morphology at the edge of the defective skin (scale bar, 50 µm). (D) CD31 immunostaining showed new blood vessels at the edge of the defective skin (scale bar, 50 µm). (E) Quantitative analysis of relative CD31 positivity. Data are shown as mean ± SD (n = 4); *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control.
S1. Information of all the related antibodies in this study
Antibody SUMO1 Ubc9 SAE1 SAE2 SENP1 PCNA Ras HIF-1α PPARγ VEGF2 Company Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Abcam Code ab11672 ab75854 ab185552 aAb185955 ab108981 ab92552 ab52939 ab1 ab209350 ab39378 Dilution 1:1,000/1:400 1:2,000/1:200 1:5,000/1:200 1:1,000/1:200 1:5,000/1:500 1:2,000 1:5,000 1:1,000 1:1,000 1:1,000 -
[1] Li Y, Guo S, Zhu Y, et al. Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS. J Pharm Anal, 2019; 9, 392−9. doi: 10.1016/j.jpha.2019.06.002 [2] Giorgino F, de Robertis O, Laviola L, et al. The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc Natl Acad Sci USA, 2000; 97, 1125−30. doi: 10.1073/pnas.97.3.1125 [3] Saha S, Chernoff J. Analysis of PTP1B sumoylation. Methods, 2014; 65, 201−6. doi: 10.1016/j.ymeth.2013.09.012 [4] Qasem MA, Noordin MI, Arya A, et al. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ, 2018; 6, e4788. doi: 10.7717/peerj.4788 [5] Rosa MTG, Abreu IA. Exploring the regulatory levels of SUMOylation to increase crop productivity. Curr Opin Plant Biol, 2019; 49, 43−51. doi: 10.1016/j.pbi.2019.04.009 [6] Han ZJ, Feng YH, Gu BH, et al. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol, 2018; 52, 1081−94. [7] Lin HY, Wang CL, Hsiao PJ, et al. SUMO4 M55V variant is associated with diabetic nephropathy in type 2 diabetes. Diabetes, 2007; 56, 1177−80. doi: 10.2337/db06-1283 [8] Zou Y, Liu FY, Wu J, et al. Mutational analysis of the RAS/RAF/MEK/ERK signaling pathway in 260 Han Chinese patients with cervical carcinoma. Oncol Lett, 2017; 14, 2427−31. doi: 10.3892/ol.2017.6435 [9] Choi BH, Philips MR, Chen Y, et al. K-Ras Lys-42 is crucial for its signaling, cell migration, and invasion. J Biol Chem, 2018; 293, 17574−81. doi: 10.1074/jbc.RA118.003723 [10] Zhang L, Liu Q, Lu L, et al. Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1α accumulation via phosphatidylinositol 3-kinase/Akt pathway. J Pharmacol Exp Ther, 2011; 338, 485−91. doi: 10.1124/jpet.111.180992 -




 下载:
下载:



 Quick Links
Quick Links