-
With the rapid development of industrialization and urbanization, air pollution is becoming a serious global problem and a threat to humanity. As one of the most important air pollutants, fine particulate matter (PM2.5) has a small diameter (≤ 2.5 μm), with large specific surface area, strong toxin adsorption capacity, and long-term suspension in the air[1]. The World Health Organization (WHO, 2018) identified PM2.5 as class I carcinogen. Accumulating studies showed that exposure to high levels of PM2.5 result in respiratory [chronic obstructive pulmonary disease (COPD), asthma, and lung cancer][2-6] and heart diseases[7,8].
The lung is an important organ that controls the exchange of oxygen and carbon dioxide in the blood. PM2.5 can affect pulmonary artery access and aggravate COPD, pneumonia, and asthma[4,5,9]. Nevertheless, the mechanisms of PM2.5-induced lung injury are not yet entirely clear. Animal studies have found that PM2.5 can increase the production of reactive oxygen species (ROS) in mice’s peripheral blood supernatant, lung and heart tissues[10]. Excessive ROS can cause a series of damage to airway epithelial cells and alveolar cells, including structural damage, activation of inflammation-related transcription factors, and release of inflammatory mediators[11,12].
Mitochondria is susceptible to oxidative stress and is also target of many toxic substances such as PM2.5[3]. Abnormal changes in mitochondrial structure and function caused by PM2.5 are considered to be the main factors of respiratory diseases[13,14]. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), as a transcriptional coactivator, is widely considered the main regulator of mitochondrial biogenesis[3]. Previous studies showed that changes in sirtuin 1 (SIRT 1), a member of the sirtuin family that regulates mitochondrial biogenesis and participates in antioxidant defense under mitochondrial stress[14,15], can lead to the disorder of PGC-1α, which induces oxidative stress and apoptosis[3]. PM2.5 exposure can inhibit SIRT1-PGC-1α and then cause apoptosis of human bronchial epithelial cells[14].
Salidroside is the main medicinal component of Rhodiola. Previous studies have shown that salidroside has anti-hypoxia and anti-aging effects, and can also enhance antioxidant activity, anti-fibrosis activity, and immunity[14,16,17]. Some studies suggested that salidroside can stimulate the SIRT1-PGC-1α axis and improve diabetes nephropathy in mice[18]. A study suggested that salidroside could activate SIRT1 and improve the damage to human bronchial epithelial cells caused by PM2.5[14]. The purpose of this study was to clarify the intervention effect of salidroside on PM2.5-induced lung injury in mice, explore the relevant mechanism, and examine the key role of SIRT1-PGC-1 in the protective effect of salidroside on PM2.5-induced lung injury of mice.
-
PM2.5 were prepared following a previously described approach[14]. Atmospheric PM2.5 was collected from October 2019 to April 2020 in Weifang city (China). Fine particles were then ultrasonicated, filtered, and lyophilized. Then, PM2.5 dry powder was obtained, weighed, and stored at −20 °C. PM2.5 stock suspension was prepared using sterile normal saline and stored at −20 °C. The stock suspension was thawed naturally and then mixed again with ultrasonic vibration before the experiment.
-
Specific pathogen-free (SPF) male C57BL/6 mice (6–8 weeks old, 18–22 g, 40 mice in total) were purchased from the Medical Experimental Animal Center of Weifang Medical University (Weifang, Shandong, China). All mice were maintained in an SPF environment with a 12 h light/dark cycle, room temperature of 22 ± 2 °C, relative humidity of 55% ± 5%, and had free access to food and water. All experimental animal protocols were conducted following the Guide for Care and Use of Laboratory Animals, and this study was performed under the approval of the Animal Experimental Ethics Committee of Weifang Medical University. Mice were randomly divided into four groups (10 mice per group), namely Control group, SAL group, PM2.5 group, and SAL+ PM2.5 group. An animal model was constructed as follows, Day 1: mice in the SAL group and SAL + PM2.5 group were given 60 mg/kg SAL by intragastric administration, while mice in the Control group and PM2.5 group were given sterile normal saline by intragastric administration. Day 2: mice in the PM2.5 group and SAL + PM2.5 group were intratracheally instilled with 8 mg/kg PM2.5 suspension, while mice in the Control group and the SAL group were given sterile normal saline by intratracheal instillation. Day 1 + Day 2 constituted a complete experimental cycle, a total of 10 cycles were included in this study, lasting 20 days. After the last administration, the mice fasted overnight and were anesthetized with pentobarbital sodium (1%, 50 mg/kg). Blood and bronchoalveolar lavage fluid (BALF) were collected, and lung tissue was extracted (part of lung tissue was used for pathological sections, while the other part was stored at –80 °C for future use).
-
Mice were placed in the supine position on the operating table and fixed. Then, the chest was open, the ribs were cut along the left sternal sideline, the heart was exposed, and the left lung was ligated. Consequently, we separated the exposed neck trachea, cut an incision with scissors at the tracheal eminence, and threaded the surgical suture under the opening for standby. Next, the irrigation needle was slowly inserted with a polyethylene tube ligated and fixed with a surgical suture. Finally, the syringe containing 0.8 mL of precooled sterile normal saline was slowly injected into the trachea, the alveolar lavage fluid was slowly drawn back (recovery rate is about 80%) after 5 seconds, and put into the precooled 1.5 mL EP tube.
-
BALF was centrifuged at 3,000 rpm for 10 minutes at 4 °C and the supernatant was retained. The total protein concentration in BALF was detected using the Bicitrine (BCA) Protein Quantification Kit (Beyotime Biotechnology). LDH activity in BALF was determined using an LDH assay kit (Jian Cheng Bioengineering Research Institute, Nanjing, China).
-
Lung tissue (1 cm2) was taken from the left lung that had not been irrigated with sterile normal saline and fixed with 4% paraformaldehyde. Next, the fixed tissues were dehydrated, paraffin-embedded, and cut into 3 µm-thick slices. The sections were then stained with hematoxylin-eosin (HE), and the pathological changes in lung tissue were observed with a microscope (IX73, Olympus, Japan).
-
Part of the non-lavaged lung tissue was cut into 1 × 1 × 1 cubic millimeter, wrapped with gauze, immediately fixed in 4% glutaraldehyde solution, and left at 4 °C overnight. After fixation with 1% (w/v) osmic acid for 2 h, the tissues were washed with 0.1 M PBS buffer and dehydrated with ethanol and acetone before subsequent embedding and sectioning. After staining with uranyl acetate and lead citrate, the mitochondrial structure of lung tissue cells was observed using a transmission electron microscope (HT 7700, Hitachi, Tokyo, Japan).
-
The lower lobe of the right lung was isolated and weighed to obtain the wet weight (W). Then, these lung tissues were desiccated in an incubator at 60 °C for drying for 48 h until a constant dry weight (D) was achieved. The lung W/D ratios were used to evaluate the degree of pulmonary edema.
-
Lung tissue and precooled normal saline were mixed in a ratio of 1:9 and homogenized with a tissue grinder. The whole process was carried out on ice. Then, the homogenate was centrifuged at 3,000 rpm for 15 minutes at 4 °C, and the supernatant was collected. A 10% lung tissue homogenate was used to detect the level of related antioxidant enzymes. Levels of malondialdehyde (MDA), superoxide dismutases (SOD), and glutathione (GSH) were measured using corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
-
The prepared 10% tissue homogenate was diluted to 5% concentration. Then, MPO activity was determined using an MPO activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions. The absorbance value at an absorption wavelength of 460 nm was detected using a Multiskan FC microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
-
The remaining tissues of the right lung were homogenized in Ripa lysate supplemented with PMSF, then lysed on ice for 30 minutes, centrifuged at 12,000 g for 5 minutes, after which the supernatant was aspirated and preserved. Protein concentrations were determined using the BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). Protein samples were mixed with dithiothreitol (100 mm) and loading buffer and boiled at 100 °C for 5 min. After cooling, protein expression was detected, and the remaining samples were stored at –80 °C.
Protein samples were separated by 6%–15% SDS-PAGE electrophoresis and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Subsequently, membranes were incubated with 5% skim milk dissolved in TBST for 2 h. Thereafter, the membranes were incubated with primary antibodies in a shaker at 4 °C for 16 h. After washing the membranes three times, samples were incubated with a second antibody for 1 h at room temperature. The visualization was performed by chemiluminescent reagents according to the manufacturer’s instructions, and the protein bands were quantified by ImageJ software (National Institutes of Health, MD, USA).
The following antibodies were used: anti-nuclear factor-erythroid 2-related factor 2 (anti-Nrf2) rabbit antibody (Novus Biologicals, CO, USA); anti-SOD1 rabbit antibody and anti-heme oxygenase-1 (anti-HO-1) rabbit antibody (Beyotime Biotechnology, Shanghai, China); anti-IL1β rabbit antibody (Abcam, Cambridge, MA, UK); anti-BAX rabbit antibody (Beyotime Biotechnology, Shanghai, China); anti-Caspase-9 rabbit antibody, anti-Cleaved Caspase-3 rabbit antibody (Cell Signaling Technology, MA, USA); anti-β-actin rabbit antibody, anti-SIRT1 rabbit antibody and anti-PGC-1ɑ rabbit antibody (Beyotime Biotechnology, Shanghai, China); anti-IL-6 mouse antibody (Abcam, Cambridge, MA, UK).
-
SPSS 20.0 (SPSS Inc, IL, USA) was used for statistical analysis. All data are presented as mean ± SEM. Statistical significance was analyzed by the one-way ANOVA, followed by Tukey’s post hoc test for comparison between multiple groups, and P < 0.05 was regarded as statistically significant.
-
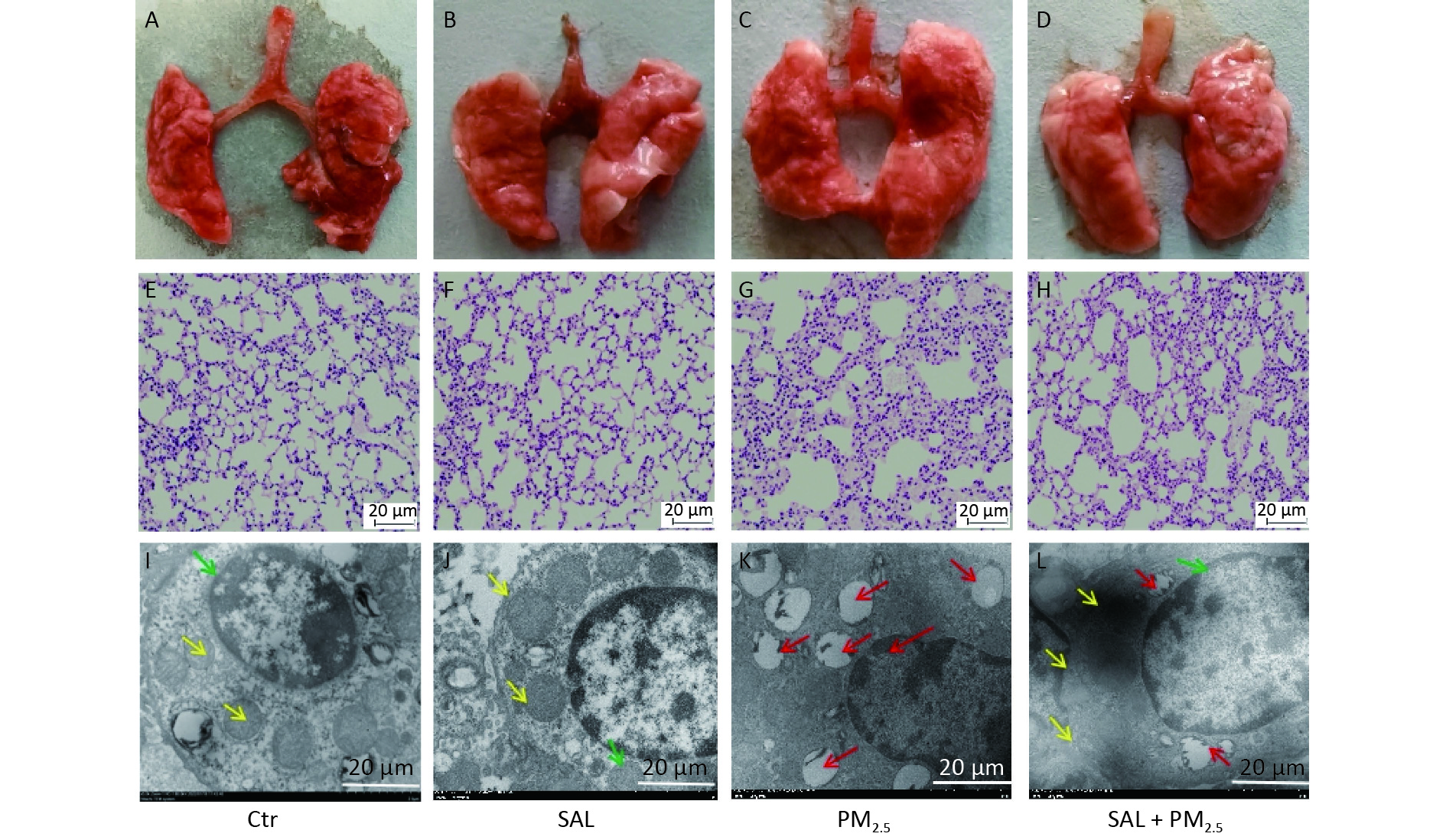
Visual observation showed that the lungs of the mice in the control group were light red, bright, and shiny, with sharp edges, soft texture, good elasticity, no ecchymosis, and no swelling and discoloration (Figure 1A). Similar findings were found in the SAL group, the lungs of mice were red, shiny, elastic, and soft (Figure 1B). On the other hand, the lungs of mice in the PM2.5 group were swollen and discolored, with increased volume, poor gloss, rough and hard texture, blunt lung edges, and poor elasticity (Figure 1C). In the SAL+PM2.5 group, the volume of both lungs of the mice was slightly expanded, the degree of edema was reduced, the texture was slightly rough, the blunt edge of the lungs was weakened, and the elasticity was better than that of the injury group (Figure 1D). 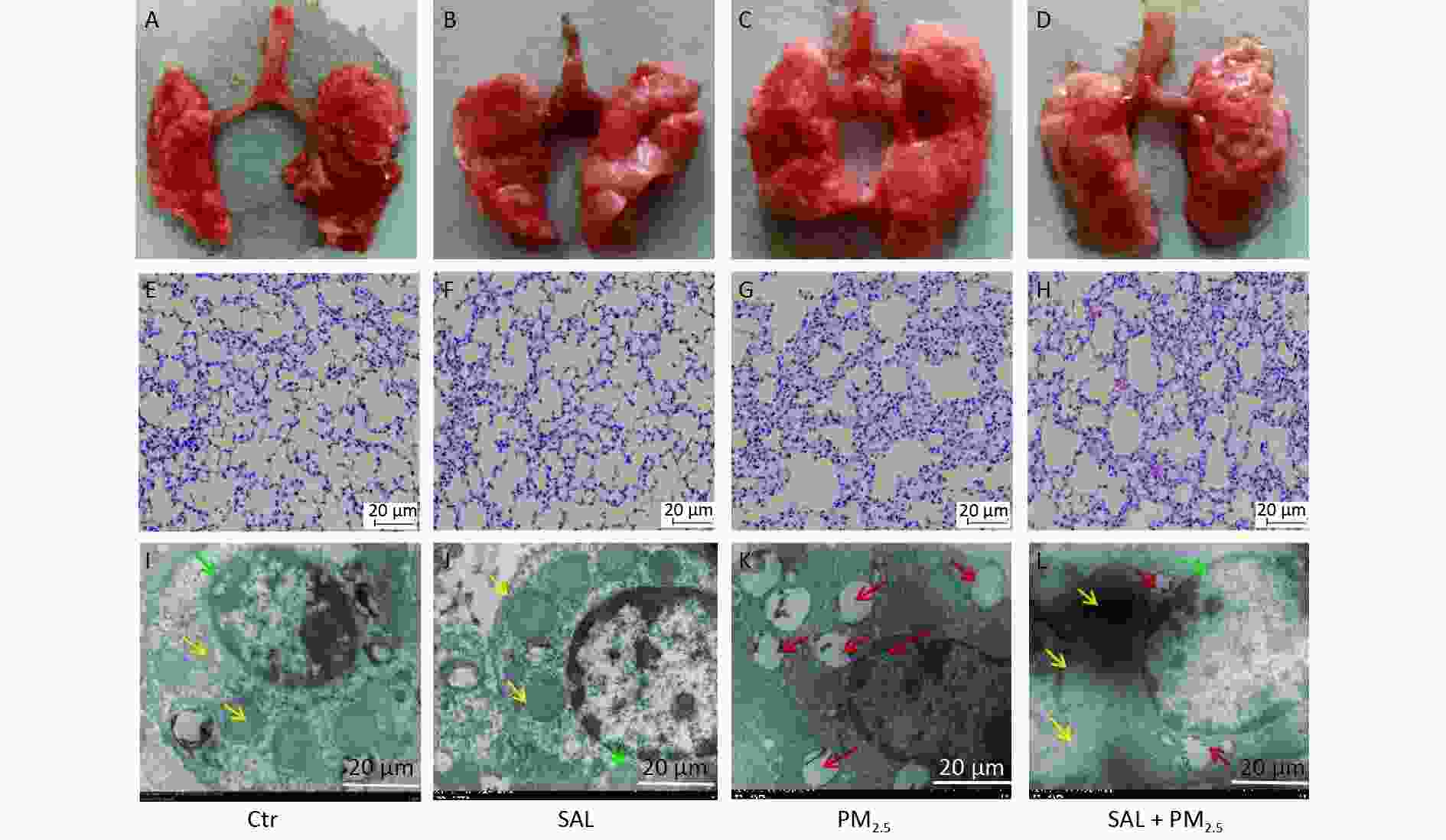
Figure 1. Effect of SAL on lung injury induced by PM2.5. (A–D) Morphological changes of mouse lung tissue. (E–H) Pathological changes of mouse lung tissues assessed by hematoxylin and eosin (HE) staining (200× magnification, scale bars, 20 μm). (I–L) Ultrastructure changes in lung tissues assessed by transmission electron microscopy (4,000× magnification, scale bars, 20 μm). Green arrow indicate nuclear collapse and deformation; red arrow indicates cavitated mitochondria.
In order to further understand the changes in lung tissue, we observed the pathological changes. The lung tissue structure of mice in the SAL group was similar to that of the control group (Figure 1E–F). On the other hand, in the PM2.5 group, the alveolar septum was widened, some alveolar structures were destroyed, the alveolar cavity was enlarged, and inflammatory cell infiltration was seen in the pulmonary interstitium. Erythrocytes and a large amount of protein exudate were seen in the alveolar cavity and pulmonary interstitium (Figure 1G). Compared with the PM2.5 group, the alveolar structure of mice in the SAL+PM2.5 group was relatively intact, the proliferation of the alveolar septum was relieved, and the degree of inflammatory infiltration in the pulmonary interstitium was reduced. At the same time, there were only a small number of red blood cells and occasional protein exudate in the alveolar cavity and pulmonary interstitium (Figure 1H). These findings suggest that SAL may partially alleviate damage to the lungs caused by PM2.5 exposure.
Furthermore, we observed the ultrastructural changes in lung tissues using transmission electron microscopy. In the control and SAL groups, there were no abnormal changes in the alveolar epithelium's structure, nucleus, and mitochondria (Figure 1I–J). After PM2.5 treatment, alveolar epithelial cells and mitochondria were destroyed, the nucleus was deformed, mitochondrial vacuolation became highly obvious, and some mitochondrial cristae disappeared (Figure 1K). Salidroside pretreatment significantly reduced the deterioration of alveolar epithelial cells and mitochondria (Figure 1L).
-
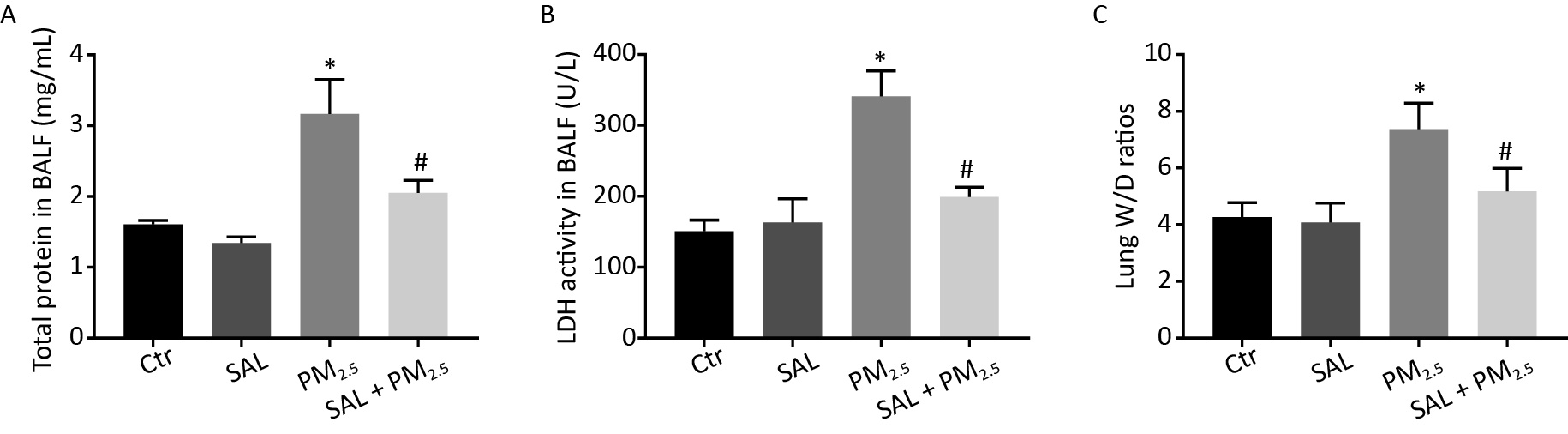
PM2.5 can damage pulmonary endothelial and epithelial cells, increase the permeability of blood vessels, and infiltrate proteins into the lung stroma, leading to pulmonary edema. Therefore, we detected the total protein content and LDH activity in BALF and the ratio of wet weight to dry weight (W/D) of lung tissue to observe the changes in pulmonary vascular permeability, the degree of lung injury and the severity of pulmonary edema. The total protein level, LDH activity in BALF, and W/D ratio of lung tissue in the PM2.5 group were higher than those in the control group (Figure 2A–C, all P < 0.05). Compared with the PM2.5 group, SAL pretreatment reduced the levels of the above indexes, and the difference was statistically significant (Figure 2A–C, all P < 0.05).
-
The level of MDA reflects the degree of lipid peroxidation and represents the level of oxidative stress. T-SOD and GSH are two important antioxidant in the body, which can effectively remove oxygen free radicals and H2O2 and protect the body against oxidative stress damage. Compared with the control group, the level of MDA in lung tissue in the PM2.5 group increased (Figure 3A, P < 0.05), while the levels of T-SOD and GSH decreased (Figure 3B–C, P < 0.05). Compared with the PM2.5 group, SAL pretreatment reduced the level of MDA and increased T-SOD and GSH in the lung tissue of mice (Figure 3A–C). Moreover, the protein expression levels of HO-1, Nrf2, and SOD1 in the lung tissue of the PM2.5 group decreased compared with the control group (Figure 3D–G, P < 0.05). After SAL pretreatment, the protein expression levels of HO-1 and SOD1 were significantly higher than those in the PM2.5 group (Figure 3E, G, P < 0.05).
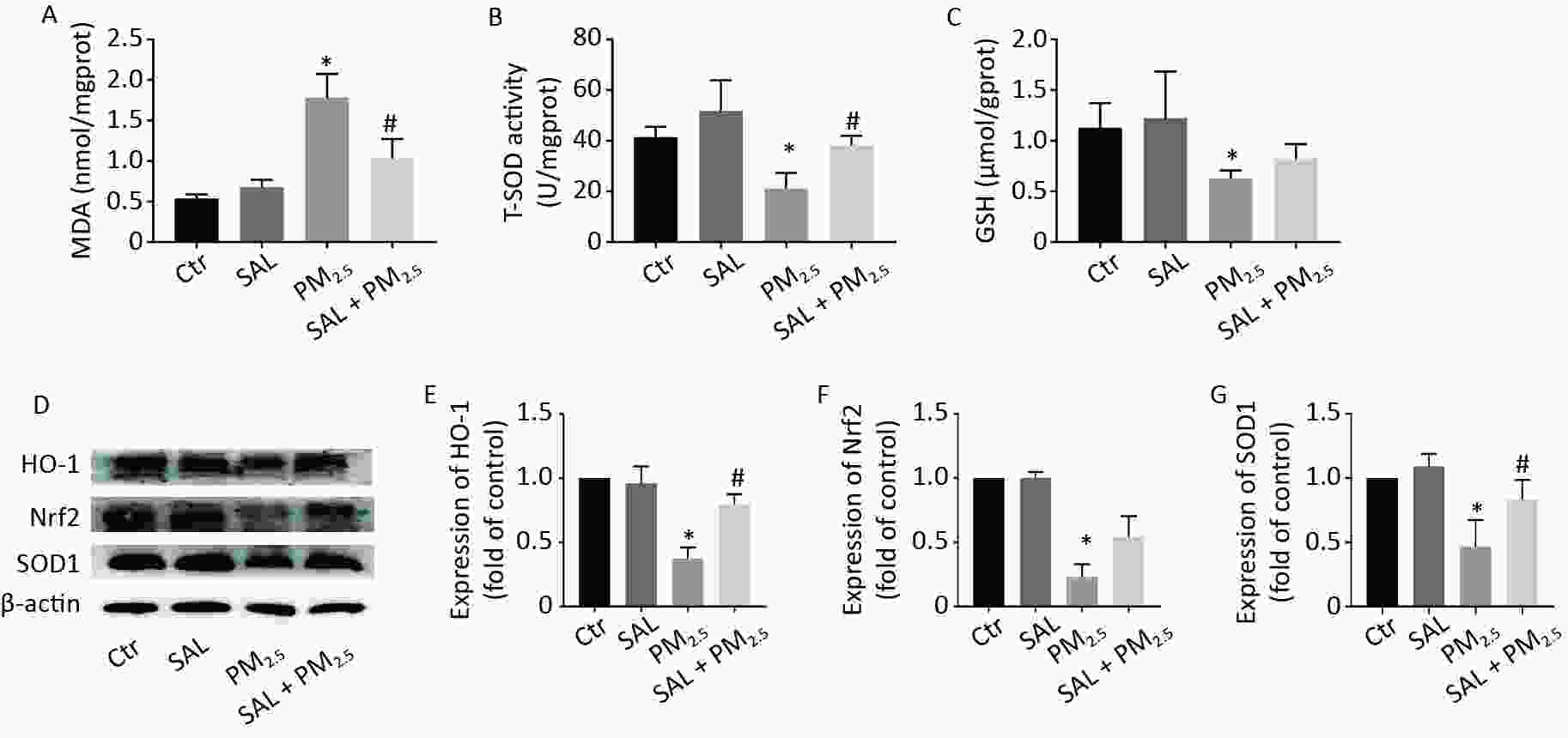
Figure 3. Effect of SAL on oxidative damage index level in lung tissue caused by PM2.5. (A) The level of MDA in lung tissue; (B) The level of T-SOD in lung tissue; (C) The level of GSH in lung tissue. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group. (D) expression levels of HO-1, Nrf2 and SOD1 in lung tissue; (E) HO-1 gray analysis results; (F) Nrf2 gray analysis results; (G) SOD1 gray analysis results. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group.
-
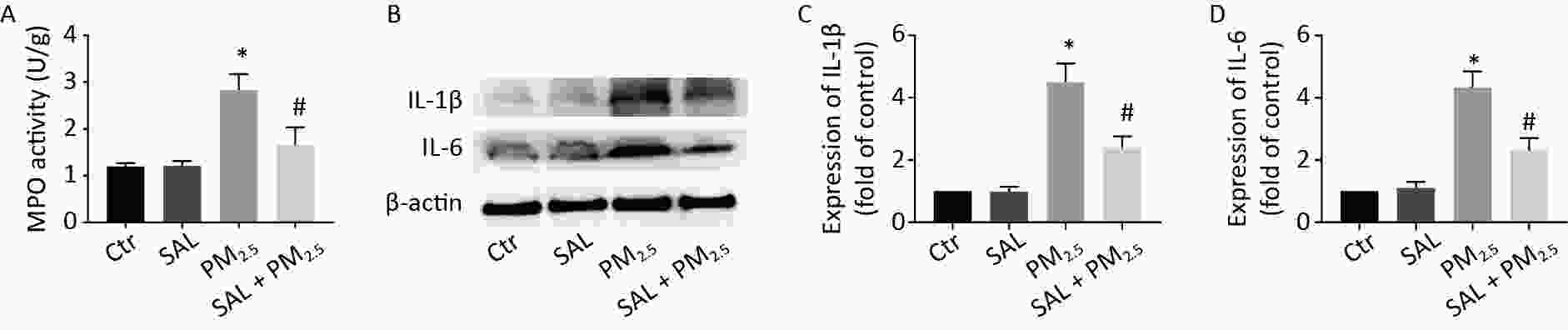
MPO is a specific and stable peroxidase in neutrophils, which directly reflects the level of neutrophils in a certain tissue and indirectly reflects the degree of inflammatory damage to the tissue. The MPO activity of lung tissue in the PM2.5 group was higher than that in the control group (Figure 4A, P < 0.05). Compared with the PM2.5 group, pretreatment with SAL counteracted the increased MPO activity of lung tissue caused by PM2.5 (Figure 4A, P < 0.05). Moreover, protein expression levels of IL-6 and IL-1β of lung tissue in the PM2.5 group were significantly higher than those in the control group (Figure 4B–D, P < 0.05). Nevertheless, pretreatment with SAL significantly ameliorated the higher expression levels of IL-6 and IL-1β induced by PM2.5 (all P < 0.05).
-
To investigate the effect of PM2.5 on apoptosis in lung tissue and the protective effect of salidroside, we detected the expression level of apoptosis-related proteins (Figure 5). The expression levels of BAX, caspase-9, and cleaved caspase-3 did not change between control group and SAL group. However, the expression levels of the above proteins were significantly higher in the PM2.5 group than control group (Figure 5, P < 0.05). However, SAL pretreatment reduced the protein expressions of BAX, Caspase-9, and Cleaved Caspase-3.
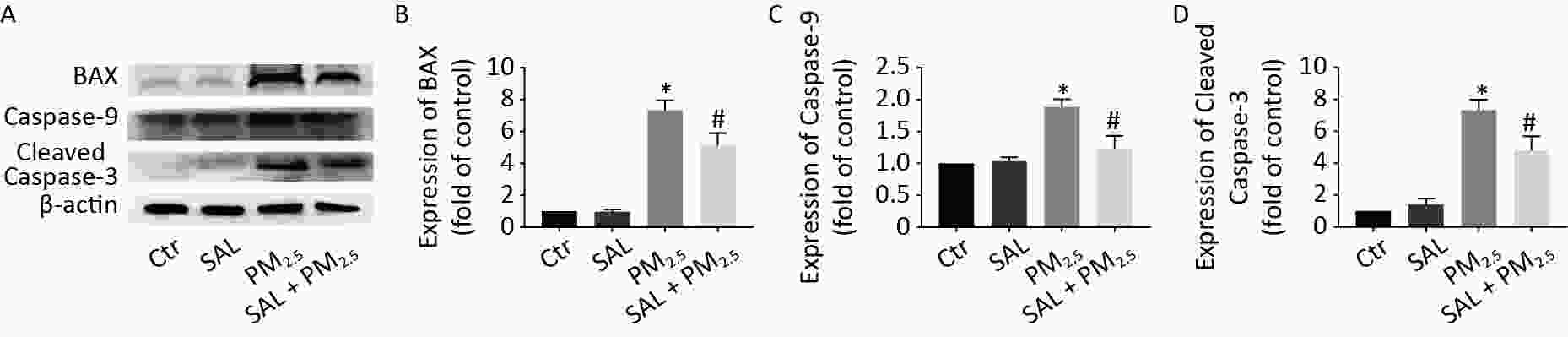
Figure 5. Effect of SAL on the changes of apoptosis related protein level in lung tissue caused by PM2.5. (A) Expression level of BAX, Caspase-9 and Cleaved Caspase-3 in lung tissue; (B) BAX gray analysis results; (C) Caspase-9 gray analysis results; (D) Cleared caspase-3 gray analysis results. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group.
-
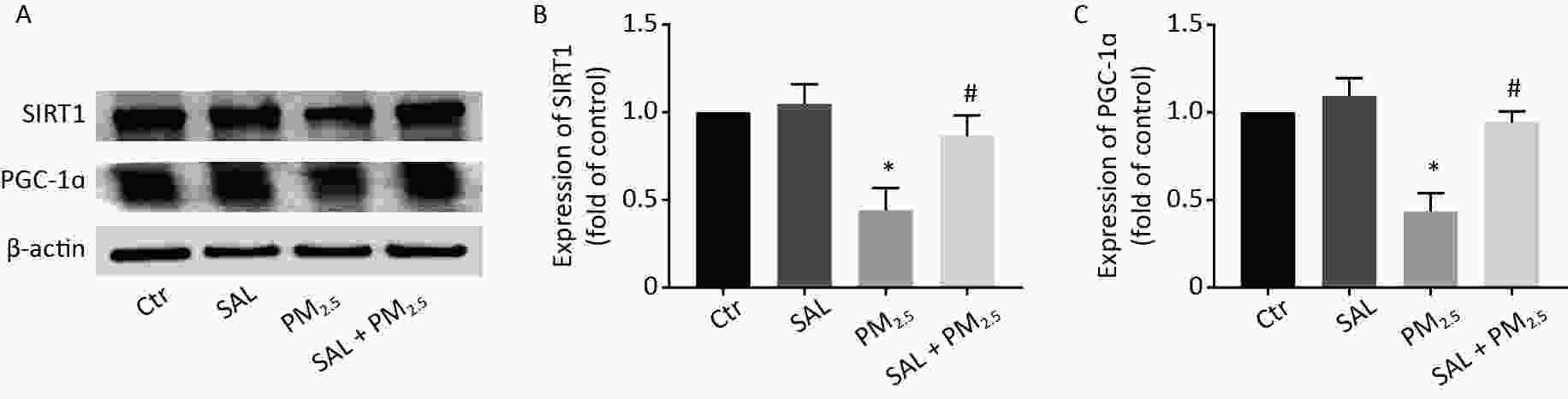
SIRT1/PGC-1α pathways have an important role in defense against oxidative stress. To investigate whether SIRT1/PGC-1α axis plays a role in PM2.5-induced lung injury in mice, we evaluated protein expression levels of SIRT1 and PGC-1α by Western blotting. PM2.5 exposure resulted in a significant decrease in the protein expression of SIRT1 and PGC-1α in lung tissue (Figure 6A–C, P < 0.05). Our result implicated that SIRT1/PGC-1α signal participates in PM2.5-induced lung injury. As expected, SAL pretreatment significantly increased the expression levels of SIRT1 and PGC-1α (Figure 6A–C, all P < 0.05 vs. PM2.5 group), suggesting that SAL may improve the lung injury caused by PM2.5 exposure through SIRT1/PGC-1α signal pathway.
-
Lung is the direct target organ for PM2.5 respiratory exposure. In the present study, we found that the PM2.5-induced lung injury in mice may be involved with oxidative damage, apoptosis, inflammation, and changes in the SIRT1-PGC-1α signaling pathway. Salidroside pretreatment significantly improved the oxidative stress, apoptosis, and inflammation induced by PM2.5 exposure. Moreover, salidroside can alleviate the lung injury caused by PM2.5 to a certain extent via activating SIRT1/PGC-1α signal pathway.
In this study, we carefully collected the intact lungs of mice and observed that PM2.5 exposure resulted in swelling, discoloration, and volume increase in both lungs of mice. In order to further observe the pulmonary edema of mice after PM2.5 exposure, we measured the total protein content and LDH activity in BALF. The results showed that the total protein content and LDH activity increased, suggesting an increase in pulmonary vascular permeability[19], which ultimately led to pulmonary edema. W/D ratio is an indicator of pulmonary edema[20,21]. In our study, we found a significantly increased W/D ratio in the PM2.5 exposure group, indicating that the blood and interstitial fluid in the lung leaked after PM2.5 exposure.
In order to examine the lung injury more comprehensively after PM2.5 exposure, we evaluated the histological changes of lung tissues by HE staining and transmission electron microscopy. HE staining showed that PM2.5 exposure lead to the widening of the alveolar septum, the destruction of some alveolar structures, the enlargement of the alveolar cavity, the infiltration of inflammatory cells in the lung interstitium, the increase of red blood cells and a large number of protein exudates in the alveolar cavity[22]. As shown in Figure 1K, the ultrastructure of rat lung revealed that parts of alveolar epithelial cells and mitochondria were injured, the nucleus was deformed, mitochondrial vacuolation became highly obvious, and some mitochondrial cristae disappeared[3,22].
To examine the mechanism of PM2.5-induced lung injury, we investigated the role of the redox system in lungs of mice. Previous studies have shown that PM2.5 exposure can lead to an increase in ROS levels in the lung, heart, and testis tissues of mice[10,23]. A study showed that PM2.5 could reduce antioxidant enzymes such as CAT, GSH, GSH-Px, and T-SOD[24]. In this study, the level of lipid peroxidation in lung tissue increased in mice exposed to PM2.5. In order to maintain the redox balance, endogenous antioxidant enzymes were consumed, resulting in a decrease in T-SOD and GSH levels in the lung tissue of mice.
As a redox-sensitive transcription factor, Nrf2 can mediate a series of cytoprotective factors, including heme oxygenase-1 (HO-1) and superoxide dismutase (SOD)[25]. Therefore, we further detected the expression levels of the above-mentioned related proteins and found that the expression levels of Nrf2, HO-1, and SOD1 in the lung tissue of mice were decreased in the PM2.5 group. Our results showed that PM2.5-induced lung injury in mice is closely related to the oxidative stress induction of PM2.5, which is consistent with previous studies. These results seem to implicate that intervention with substances with antioxidant functions may alleviate the lung injury caused by PM2.5.
Salidroside is a kind of bioactive ingredient contained in traditional chinese herb-medicine Rhodiola rosea and with multiple biological activities, such as anti-inflammatory, antioxidant, and anti-apoptotic activities[26,27]. Some studies have shown that Sal can inhibit oxidative stress-induced pulmonary fibrosis and cardiomyocyte damage by promoting the transcription of Nrf2 regulatory genes (HO-1 and NQO1), thereby reducing the excessive production of ROS and improving mitochondrial function[26,28,29]. In our study, salidroside pretreatment could reduce the consumption of endogenous antioxidant enzymes, inhibit the reduction of expression levels of antioxidant-related proteins, such as Nrf2, HO-1, and SOD1, and maintain the balance of redox to a certain extent.
On the other hand, a large number of reactive oxygen species produced by PM2.5 exposure also led to the activation of inflammation-related transcription factors and the release of inflammatory mediators in mouse airway epithelial cells and alveolar cells[11,12]. Rhodiola extract and compounds (mainly salidroside) have been confirmed in vitro and in vivo experiments to exert immune regulatory effects through various inflammatory mediators and signaling pathways[30,31]. It has been reported that salidroside improved sepsis induced lung injury to some extent by inhibiting NF-κB. via activating SIRT1[31]. Our previous study showed that the large production of ROS induced by PM2.5 could damage the mitochondria of bronchial epithelial cells and lead to the release of cytochrome c and apoptosis[14]. Salidroside also inhibited inflammation induced chondrocyte apoptosis through phosphatidylinositol 3-kinase (PI3K)/Akt signaling[32] In the present study, we further confirmed that PM2.5 exposure results in an increase in the levels of IL-1B and IL-6. Moreover, our findings also showed that PM2.5 exposure increased the expression level of apoptosis-related proteins in the lung tissue of mice. Take together, our findings suggest that PM2.5 might induce inflammatory response and apoptosis by inducing a large number of ROS production. Conversely, salidroside pretreatment can inhibit PM2.5-induced inflammation and apoptosis[33], indicating that oxidative stress has an important role in PM2.5-induced lung injury.
Furthermore, we focused on sirtuin 1 (SIRT1), a member of the sirtuin family, has a key role in cell physiology and biochemical processes and is closely involved in the regulation of biological processes of oxidative stress[34,35]. In this study, PM2.5 exposure reduced the expression of SIRT1 and PGC-1α. Existing study have found that salidroside have an antioxidant role as an activator of SIRT1[34]. Our results further indicated that salidroside alone increases the expression level of SIRT1 and PGC-1α, and salidroside pretreatment significantly inhibits the reduction of SIRT1 and PGC-1α protein expression caused by PM2.5.
Conclusively, our results indicated that SIRT1/PGC-1α regulated oxidative stress, and has an important role in PM2.5-induced lung injury. Aditionally, salidroside improves the state of oxidative stress by activating SIRT1-PGC-1α, thus alleviating the lung injury caused by PM2.5. Our research provides an insights into the use of active ingredients in Chinese herbal medicine to prevent and alleviate damage caused by PM2.5 exposure.
doi: 10.3967/bes2024.041
Salidroside Ameliorates Lung Injury Induced by PM2.5 by Regulating SIRT1-PGC-1α in Mice
-
Abstract:
Objective This study aimed to clarify the intervention effect of salidroside (SAL) on lung injury caused by PM2.5 in mice and illuminate the function of SIRT1-PGC-1ɑ axis. Methods Specific pathogen-free (SPF) grade male C57BL/6 mice were randomly assigned to the following groups: control group, SAL group, PM2.5 group, SAL+PM2.5 group. On the first day, SAL was given by gavage, and on the second day, PM2.5 suspension was given by intratracheal instillation. The whole experiment consist of a total of 10 cycles, lasting 20 days. At the end of treatment, blood samples and lung tissues were collected and analyzed. Observation of pathological changes in lung tissue using inverted microscopy and transmission electron microscopy. The expression of inflammatory, antioxidants, apoptosis, and SIRT1-PGC-1ɑ proteins were detected by Western blotting. Results Exposure to PM2.5 leads to obvious morphological and pathologica changes in the lung of mice. PM2.5 caused a decline in levels of antioxidant-related enzymes and protein expressions of HO-1, Nrf2, SOD2, SIRT1 and PGC-1ɑ, and an increase in the protein expressions of IL-6, IL-1β, Bax, caspase-9 and cleaved caspase-3. However, SAL reversed the aforementioned changes caused by PM2.5 by activating the SIRT1-PGC-1α pathway. Conclusion SAL can activate SIRT1-PGC-1ɑ to ameliorate PM2.5-induced lung injury. -
Key words:
- PM2.5 /
- Salidroside /
- Oxidative stress /
- Inflammation /
- Apoptosis /
- SIRT1-PGC-1α
The authors declare that they have no conflicts of interest.
&These authors contributed equally to this work.
注释:1) AUTHOR CONTRIBUTIONS: 2) CONFLICTS OF INTEREST: -
Figure 1. Effect of SAL on lung injury induced by PM2.5. (A–D) Morphological changes of mouse lung tissue. (E–H) Pathological changes of mouse lung tissues assessed by hematoxylin and eosin (HE) staining (200× magnification, scale bars, 20 μm). (I–L) Ultrastructure changes in lung tissues assessed by transmission electron microscopy (4,000× magnification, scale bars, 20 μm). Green arrow indicate nuclear collapse and deformation; red arrow indicates cavitated mitochondria.
Figure 3. Effect of SAL on oxidative damage index level in lung tissue caused by PM2.5. (A) The level of MDA in lung tissue; (B) The level of T-SOD in lung tissue; (C) The level of GSH in lung tissue. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group. (D) expression levels of HO-1, Nrf2 and SOD1 in lung tissue; (E) HO-1 gray analysis results; (F) Nrf2 gray analysis results; (G) SOD1 gray analysis results. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group.
Figure 5. Effect of SAL on the changes of apoptosis related protein level in lung tissue caused by PM2.5. (A) Expression level of BAX, Caspase-9 and Cleaved Caspase-3 in lung tissue; (B) BAX gray analysis results; (C) Caspase-9 gray analysis results; (D) Cleared caspase-3 gray analysis results. *P < 0.05 vs. control, #P < 0.05 vs. PM2.5 group.
-
[1] Kim Y, Seo J, Kim JY, et al. Characterization of PM2.5 and identification of transported secondary and biomass burning contribution in Seoul, Korea. Environ Sci Pollut Res Int, 2018; 25, 4330−3. doi: 10.1007/s11356-017-0772-x [2] Clofent D, Culebras M, Loor K, et al. Environmental pollution and lung cancer: the carcinogenic power of the air we breathe. Arch Bronconeumol (Engl Ed), 2021; 57, 317−8. doi: 10.1016/j.arbr.2021.03.002 [3] Zhang N, Li P, Lin H, et al. IL-10 ameliorates PM2.5-induced lung injury by activating the AMPK/SIRT1/PGC-1α pathway. Environ Toxicol Pharmacol, 2021; 86, 103659. doi: 10.1016/j.etap.2021.103659 [4] Wang L, Xu JY, Liu H, et al. PM2.5 inhibits SOD1 expression by up-regulating microRNA-206 and promotes ROS accumulation and disease progression in asthmatic mice. Int Immunopharmacol, 2019; 76, 105871. doi: 10.1016/j.intimp.2019.105871 [5] Deng QH, Deng LJ, Lu C, et al. Parental stress and air pollution increase childhood asthma in China. Environ Res, 2018; 165, 23−31. doi: 10.1016/j.envres.2018.04.003 [6] Guo XJ, Li ZY, Ling WJ, et al. Epidemiology of childhood asthma in mainland China (1988-2014): a meta-analysis. Allergy Asthma Proc, 2018; 39, 15−29. doi: 10.2500/aap.2018.39.4131 [7] Abrams JY, Weber RJ, Klein M, et al. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ Health Perspect, 2017; 125, 107008. doi: 10.1289/EHP1545 [8] Bhatnagar A. Cardiovascular effects of particulate air pollution. Annu Rev Med, 2022; 73, 393−406. doi: 10.1146/annurev-med-042220-011549 [9] Lamichhane DK, Kim HC, Choi CM, et al. Lung cancer risk and residential exposure to air pollution: a Korean population-based case-control study. Yonsei Med J, 2017; 6, 1111−8. [10] Tseng CY, Chung MC, Wang JS, et al. Potent in vitro protection against PM2.5-caused ROS generation and vascular permeability by long-term pretreatment with Ganoderma tsugae. Am J Chin Med, 2016; 44, 355−76. doi: 10.1142/S0192415X16500208 [11] Abuelezz SA. Nebivolol attenuates oxidative stress and inflammation in a guinea pig model of ovalbumin-induced asthma: a possible mechanism for its favorable respiratory effects. Can J Physiol Pharmacol, 2018; 96, 258−65. doi: 10.1139/cjpp-2017-0230 [12] Zhang N, Deng CW, Zhang XX, et al. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract, 2018; 4, 3. doi: 10.1186/s40733-018-0040-y [13] Hall AR, Burke N, Dongworth RK, et al. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol, 2014; 171, 1890−906. doi: 10.1111/bph.12516 [14] Shan H, Li XH, Ouyang C, et al. Salidroside prevents PM2.5-induced BEAS-2B cell apoptosis via SIRT1-dependent regulation of ROS and mitochondrial function. Ecotoxicol Environ Saf, 2022; 231, 113170. doi: 10.1016/j.ecoenv.2022.113170 [15] Lin SZ, Xing HP, Zang TT, et al. Sirtuins in mitochondrial stress: indispensable helpers behind the scenes. Ageing Res Rev, 2018; 44, 22−32. doi: 10.1016/j.arr.2018.03.006 [16] Zhu Y, Zhang YJ, Liu WW, et al. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules, 2016; 21, 1033. doi: 10.3390/molecules21081033 [17] Rong L, Li ZD, Leng X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother, 2020; 122, 109726. doi: 10.1016/j.biopha.2019.109726 [18] Xue HY, Li PP, Luo YS, et al. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine, 2019; 54, 240−7. doi: 10.1016/j.phymed.2018.10.031 [19] Sun Q, Zhang GQ, Chen RC, et al. Central IKK2 inhibition ameliorates air pollution-mediated hepatic glucose and lipid metabolism dysfunction in mice with type II diabetes. Toxicol Sci, 2018; 164, 240−9. doi: 10.1093/toxsci/kfy079 [20] Liu Y, Chen W, Wang F. Fine-particulate matter (PM2.5), a risk factor for rat gestational diabetes with altered blood glucose and pancreatic GLUT2 expression. Gynecol Endocrinol, 2017; 33, 611−6. doi: 10.1080/09513590.2017.1301923 [21] Yang B, Guo J, Xiao CL. Effect of PM2.5 environmental pollution on rat lung. Environ Sci Pollut Res Int, 2018; 25, 36136−46. doi: 10.1007/s11356-018-3492-y [22] Tang WT, Du LL, Sun W, et al. Maternal exposure to fine particulate air pollution induces epithelial-to-mesenchymal transition resulting in postnatal pulmonary dysfunction mediated by transforming growth factor-β/Smad3 signaling. Toxicol Lett, 2017; 267, 11−20. doi: 10.1016/j.toxlet.2016.12.016 [23] Wei Y, Cao XN, Tang LX, et al. Urban fine particulate matter (PM2.5) exposure destroys blood-testis barrier (BTB) integrity through excessive ROS-mediated autophagy. Toxicol Mech Methods, 2018; 28, 302−19. doi: 10.1080/15376516.2017.1410743 [24] Xiong R, Jiang WY, Li N, et al. PM2.5-induced lung injury is attenuated in macrophage-specific NLRP3 deficient mice. Ecotoxicol Environ Saf, 2021; 221, 112433. doi: 10.1016/j.ecoenv.2021.112433 [25] Shin IS, Hong J, Jeon CM, et al. Diallyl‐disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf‐2/HO‐1 pathway and NF‐kappaB suppression. Food Chem Toxicol, 2013; 62, 506−13. doi: 10.1016/j.fct.2013.09.012 [26] Xu N, Huang F, Jian CD, et al. Neuroprotective effect of salidroside against central nervous system inflammation‐induced cognitive deficits: a pivotal role of sirtuin 1-dependent Nrf-2/HO-1/NF-κB pathway. Phytother Res, 2019; 33, 1438−47. doi: 10.1002/ptr.6335 [27] Zhu LP, Wei TT, Gao J, et al. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis, 2015; 20, 1433−43. doi: 10.1007/s10495-015-1174-5 [28] Tang HY, Gao LL, Mao JW, et al. Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones, 2016; 21, 239−49. doi: 10.1007/s12192-015-0654-4 [29] Han J, Xiao Q, Lin YH, et al. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen Res, 2015; 10, 1989−96. doi: 10.4103/1673-5374.172317 [30] Pu WL, Zhang MY, Bai RY, et al. Anti-inflammatory effects of Rhodiola rosea L. : a review. Biomed Pharmacother, 2020; 121, 109552. doi: 10.1016/j.biopha.2019.109552 [31] Lan KC, Chao SC, Wu HY, et al. Salidroside ameliorates sepsis-induced acute lung injury and mortality via downregulating NF-κB and HMGB1 pathways through the upregulation of SIRT1. Sci Rep, 2017; 20; 12026. [32] Wu MZ, Hu R, Wang JW, et al. Salidroside suppresses IL-1β-induced apoptosis in chondrocytes via phosphatidylinositol 3-Kinases (PI3K)/Akt signaling inhibition. Med Sci Monit, 2019; 25, 5833−40. doi: 10.12659/MSM.917851 [33] Do MT, Kim HG, Choi JH, et al. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med, 2014; 74, 21−34. doi: 10.1016/j.freeradbiomed.2014.06.010 [34] Gao XY, Wang SN, Yang XH, et al. Gartanin protects neurons against glutamate-induced cell death in HT22 Cells: independence of Nrf-2 but involvement of HO-1 and AMPK. Neurochem Res, 2016; 41, 2267−77. doi: 10.1007/s11064-016-1941-x [35] Xu FL, Xu JX, Xiong X, et al. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep, 2019; 24, 70−4. doi: 10.1080/13510002.2019.1658377 -




 下载:
下载:



 Quick Links
Quick Links