-
Air pollution is an environmental problem as it poses an ongoing threat to people’s health and is a leading cause of global mortality worldwide, causing up to 8.8 million deaths globally (11.6% of all global deaths)[1,2]. Particulate matter (PM), especially ultrafine PM (UPM, aerodynamic diameter < 0.1 μm), is the most harmful component of air pollution. Owing to their small grain diameter and large specific surface area, UPM can be easily transported through the respiratory system, penetrating the pulmonary alveoli, and even more easily enter the circulation and distribute to various organs[3]. However, the biological processes by which UPM is inhaled through the lungs, leading to cardiovascular disease, remain unclear. Although the pathogenesis of UPM-induced cardiovascular disease is still not fully understood, systemic inflammatory response is considered to be the most important mechanism in diseases development. Miller et al.[4] hypothesized that the inhalation of air pollution could activate inflammatory cells in the lung, leading to the release of inflammatory mediators that pass into the circulation to influence cardiovascular function. This hypothesis was later studied by several researchers. Epidemiological evidence has shown that air pollution is associated with increased levels of hs-CRP, IL-6, sTNF-RII, and white blood cells, especially among urban populations[5], and can regulate different inflammation-related signaling pathways to elevate the levels of interleukin (IL)-4, IL-5, and IL-13; increase the expression of IL-8, histamines, and leukotrienes; and promote the infiltration of eosinophils[6]. Similarly, in animal studies, UPM also regulates inflammatory signaling pathways by activating the MAP kinase[7], NF-κB[8], NLRP3/caspase-1[9], TLR4/p38/NF-κB[10], MAPK/NF-κB/STAT1[11], AMPK-Nrf2/KEAP, and MAPK-NLRP3/caspase-1 signaling pathways[12]. PM2.5 (i.e., PM ≤ 2.5 μm in diameter) has been shown to induce apoptosis in macrophages through a mitochondrial-mediated pathway[13], decrease neutrophil apoptosis to exacerbate inflammation[14], and promote cardiomyocyte apoptosis by aromatic hydrocarbon receptor[13,15] or NF-κB activation[16].
Shenlian (SL) extract, a traditional Chinese medicine (TCM), exhibits the effects of clearing heat-toxin and promoting blood circulation for removing blood stasis, and it has been used to treat cardiovascular disease and atherosclerosis for a long time. Previous studies have found that SL possesses anti-inflammatory and antiapoptotic properties[17,18] and has a promising protective effect at all stages of the atherosclerotic disease process[19]. Additionally, our previous study also demonstrated that SL could inhibit inflammation by activating the NLRP3 inflammasome and had a protective effect on UPM-aggravated myocardial ischemic injury[20]. However, it remains unclear if SL can ameliorate UPM-induced apoptosis. In the present study, through a series of in vitro and in vivo experiments, we found that SL improved UPM-aggravated myocardial ischemic injury by inhibiting apoptosis and the inflammatory response. The mechanisms were related to the downregulation of macrophages infiltrating heart tissues.
-
Male 6-week-old C57BL/6 J wild-type mice (6–8 weeks, 22 ± 2 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The mice were housed under controlled temperature (22 °C) and relative humidity (40% ± 5%) conditions with a 12-h light/dark cycle. This study was conducted in accordance with the principles of the Declaration of Helsinki and recommendations of the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China.
-
The preparation process of Shenlian extract is as follows: SL contained 3% tanshinone II A, 38% salvianolic acid B, and 20% andrographolide, which were provided by the Chemical Laboratory of Institute of Chinese Materia Medica, Chinese Academy of Sciences (Beijing, China). Briefly, Salvia miltiorrhiza Bunge and Andrographis paniculata (Burm. f.) Nees were mixed at the ratio of 15:9, which were first extracted using ethanol followed by water.
-
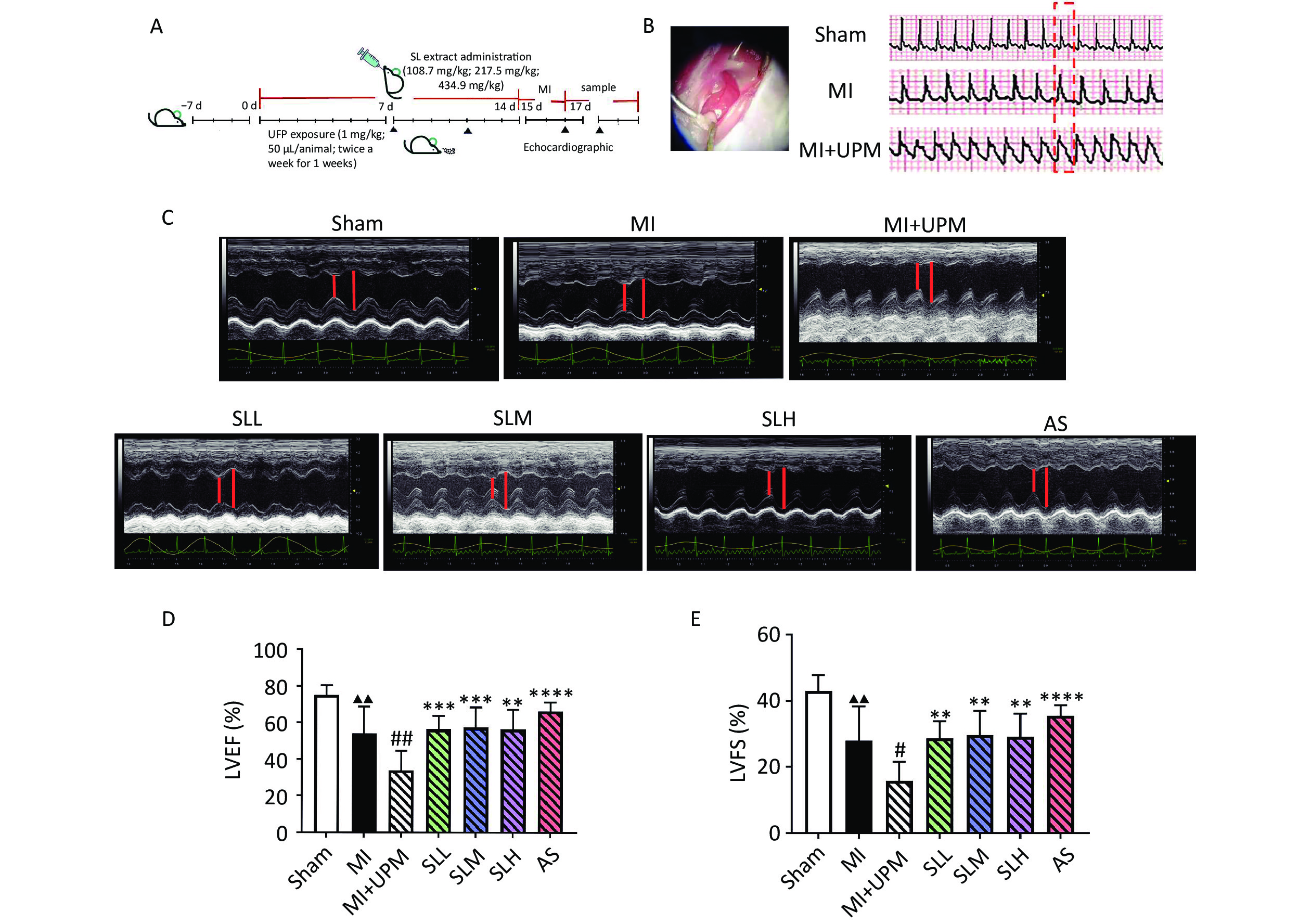
The experiment was initiated after 1 week of adaptive feeding. Mice were pretreated with SL, which was administered via intragastric gavage for 7 days, and categorized into the high-dose (434.9 mg/kg; SLH), medium-dose (217.5 mg/kg; SLM), and low-dose (108.7 mg/kg; SLL) groups; the positive control group (AS) was administered aspirin (13 mg/kg). Simultaneously, the sham and Myocardial Ischemia (MI) group mice were treated with 50 μL of phosphate-buffered saline (PBS) and MI+UPM and drug-treated groups were treated with 1 mg/mL UPM (NIST, SRM1650b) twice a week for 1 week via intratracheal instillation (drug administration was continued during this period), followed by ligation of the left anterior descending (LAD) coronary artery to induce 24 h of ischemia, as previously described[21]. The same operation was used in the Sham group, but with only threading without ligation. The experimental design is shown in Figure 1A.
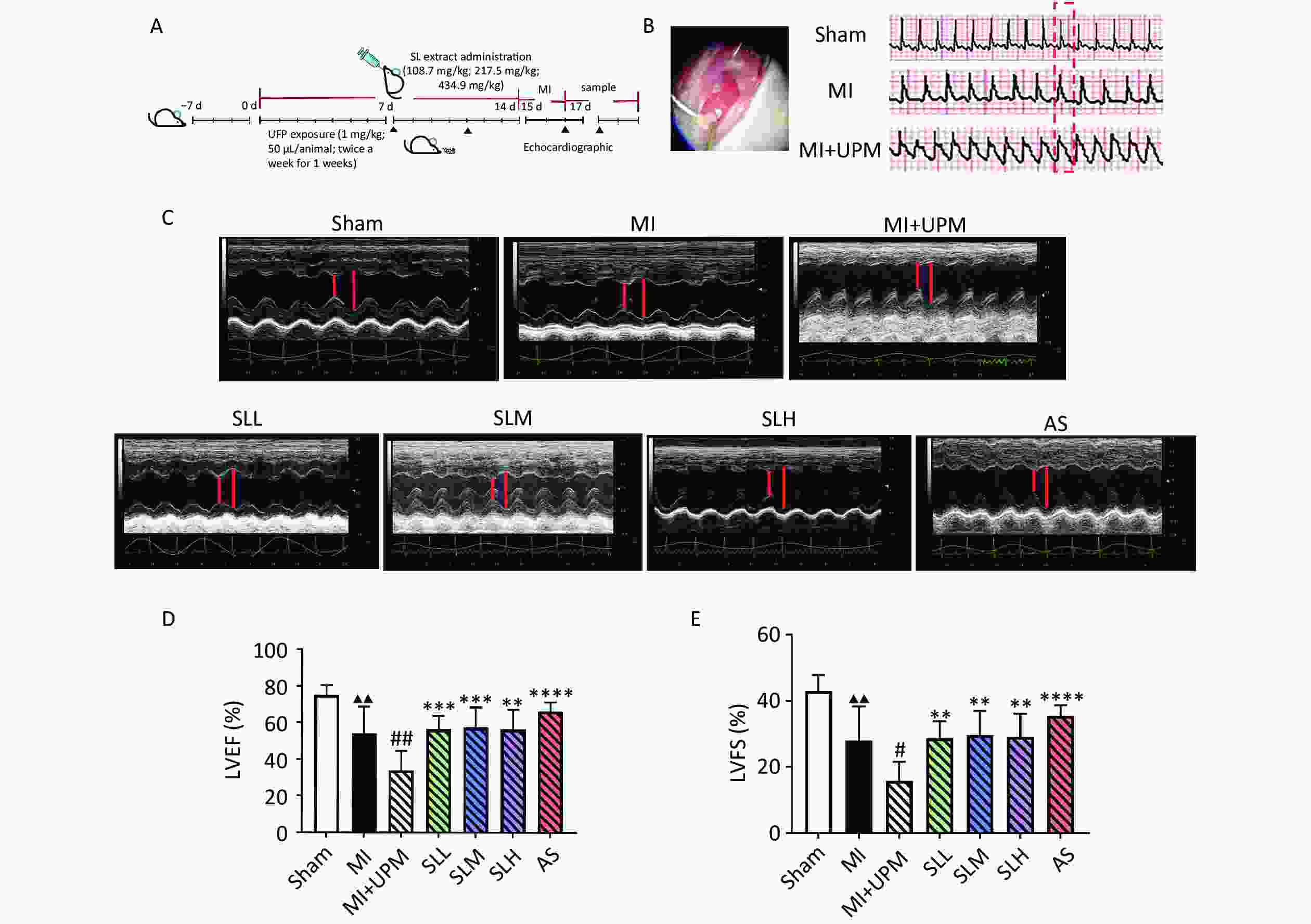
Figure 1. SL extract can relieve UPM-induced cardiac dysfunction. (A) Flowchart of experiments. (B) Effects of UPM on the ST segment. (C) M-mode ultrasound of the heart to evaluate cardiac function. (D, E) An ultrahigh-resolution small animal ultrasound imaging system was used to obtain left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS); all values are mean ± SD (n = 6/group). ▲▲P < 0.01, comparing with the Sham group; #P < 0.05, ##P < 0.01 comparing with the MI group; **P < 0.01, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia + ultrafine particulate matter exposure group; SLL: Shenlian low-dose group; SLM: Shenlian medium-dose group; SLH: Shenlian high-dose group; AS: Aspirin group.
-
Shenlian formula, developed by researcher Liu Shuzhi of the Chemistry Department at the Institute of Chinese Medicine, China Academy of Chinese Medicine, involves alcohol extraction and macroporous adsorption resin enrichment and purification to control more than 60% of its components. The formula, identified with batch number 201609, consists of a ratio of Salvia miltiorrhiza to Andrographis at 15:9, with an extraction rate of 2.436%. The extraction yield of tanshinone IIA is 1.1525%, while the extraction rate of salvianolic acid B is 1.99%. The fat-soluble extract of Salvia miltiorrhiza is measured at 12.52 mg, and the water-soluble extract of salvia Miltiorrhiza weighing 21.61 mg, and the fat soluble extract of Andrographis andrographis weighing 15.87 mg were accurately measured. The fat-soluble extract of Salvia miltiorrhiza was dissolved in 600 μL of DMSO, fully dissolved, mixed, centrifuged, and then transferred to a sterile EP tube containing the fat-soluble extract of Andrographolia andrographolis, fully dissolved and mixed. Repeat this twice, adding 200 μL of DMSO each time. Finally, it was thoroughly mixed with the solution in the sterile EP tube containing the water-soluble extract of salvia miltiorrhiae until there was no precipitation, and 50 mg/mL of SL extract reserve liquid was obtained. Following filtration by a 0.22 μm sterile filter membrane, the bacteria were aliquoted into 10 μL volumes in sterile EP tubes and stored at −20 °C. Prior to use, the medium was diluted to the desired concentration.
-
The H9c2 rat myocardial cell line was purchased from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai), and cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. The cells were incubated under 5% CO2 at 37 °C. After culturing for 24 h, the cells were preprocessed with 0.1% dimethyl sulfoxide or 1.25, 2.5, 5, 10, and 20 μg/mL SL for 24 h. Then cell viability was measured using the CCK8 kit (Dojindo Laboratories, Japan) to determine the optimum administration concentration. Subsequently, the cells were exposed to freshly dispersed UPM preparations for 24 h. After being placed in serum-free medium, the cells were cultured in a hypoxia or normoxia incubator for 12 h.
-
After mice were anesthetized via intraperitoneal injection of tribromoethanol, the hair in the chest area was removed using a depilatory cream. Left ventricular (LV) function and the peak velocities of aortic flow in the heart were noninvasively assessed using an ultrahigh-resolution small animal ultrasound imaging system (Vevo 2100, Visual Sonics, Toronto, ON, Canada) equipped with a 30-MHz transducer.
-
Myocardial infarct size was measured by staining with 2% 2,3,5-triphenyl tetrazolium chloride (TTC, Solarbio, Beijing, China). Mouse hearts were harvested and dissected into 2-mm-thick slices, which were then incubated in the TTC solution at 37 °C for 15 min. The infarct area was stained as a pale color and was analyzed using ImageJ software. The infarct size was indicated as a ratio to the LV area (= infarct area/LV size).
-
Serum levels of lactate dehydrogenase (LDH) were measured using the Hitachi 7600 automatic biochemistry analyzer (Hitachi Co. Ibaraki, Japan).
-
Blood samples were extracted at the endpoints of experiments and injected into tubes, followed by centrifugation at 900 ×g (3,000 rpm) for 10 min at 4 °C to collect the supernatant. Cardiac troponin T (cTnT), IL-6, tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) levels were quantified using an ELISA kit (Hcusabio, Wuhan, China).
-
To assess morphological changes, myocardial tissue samples were immediately fixed in 10% formalin and embedded in paraffin. Subsequently, 5-μm slices cut from the paraffin blocks were stained with hematoxylin-eosin. After staining, changes in the heart tissues were observed and photographed using a light microscope (Eclipse E100, Nikon, Japan).
-
Myocardial tissue sections were prepared as described in section 2.8. The tissue sections were incubated in 20 μg/mL Proteinase K for 15–30 min at 21–37 °C, followed by immersing them twice in PBS at room temperature (5 min each time). The tissue sections were then incubated with the TUNEL reaction solution (GDP1041; Servicebio, Wuhan, China) for 1 h at 37 °C. The specific methods were performed with reference to the literature[22]. Images were acquired using a light microscope (Eclipse E100, Nikon, Japan).
-
The H9c2 cells were seeded in a 6-well plate, after culturing for 24 h, H9c2 preprocessed with 0.1% dimethyl sulfoxide or 5 μg/mL SL for 24 h. Subsequently, the cells were exposed to freshly dispersed UPM preparations for 24 h. After being placed in serum-free medium, the cells were cultured in a hypoxia or normoxia incubator for 12 h. Then cells were double-stained by Annexin V-FITC/PI, incubating at room temperature for 20 min in the dark, followed by observing with a fluorescence microscope. Green fluorescence represents Annexin V-FITC staining positive, and red fluorescence represents PI staining positive. Apoptotic cells were single-stained by green fluorescence, late apoptotic or necrotic cells were double-stained by green and red fluorescence, and normal cells were not stained by fluorescence.
-
Expression of caspase-3 in heart tissues was determined via immunohistochemical staining. Briefly, heart tissue sections were first blocked with goat serum for 30 min at room temperature and incubated with caspase-3 (ProteinTech, Wuhan, China, 1:300 dilution) rabbit polyclonal antibody overnight at 4 °C, followed by incubation with biotinylated goat anti-rabbit immunoglobulin at a concentration of 1:100 at 37 °C for 30 min. Positive staining was reflected as brown staining and quantified in terms of average optical density (AOD) in high-power vision fields using the Image-Pro Plus 6.0 software (AOD = integrated optical density SUM/Area SUM).
-
Western blotting and quantitative real-time PCR were performed according to standard protocols. Total proteins were extracted from murine hearts or cell lysates, and the protein concentration was determined using a bicinchoninic acid protein assay kit. Subsequently, the proteins were separated via 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk for 2 h at room temperature and then incubated with primary antibodies at 4 °C overnight, followed by incubation with secondary antibodies for an additional 2 h. Images were acquired using the FluorChem R imaging system (ProteinSimple, Santa Clara, CA) and analyzed using Image-Pro Plus 6.0 software.
Total RNA was extracted using TRIzol reagent. A PrimeScript RT Master Mix (Gene Protein Link, Beijing, China) was then used for reverse transcription, and qPCR was performed using the SYBR Green qPCR Mix kit (TIANGEN Biotech (Beijing) Co., Ltd) according to the manufacturer’s instructions.
-
Experimental data are presented as the mean ± standard deviation. GraphPad Prism 8 software (La Jolla, CA, United States) was used for statistical analysis. Multiple group comparisons were performed using one-way analysis of variance with the Sidak post-hoc test to determine differences among the groups. A P-value of < 0.05 was considered statistically significant.
-
ST segment elevation on an electrocardiogram monitor represented a successful myocardial infarction (MI) model surgery. The sinus rhythm and heart rate of mice in the sham group were normal. However, after MI surgery, the ST segment significantly increased, and exposure to UPM exacerbated ST segment elevation (Figure 1B). Echocardiographic measurements of ejection fraction (EF), fractional shortening (FS), and the peak velocities of aortic flow in the heart are shown in Figure 1 and Table 1. Ligation of the LAD coronary artery (MI group) led to significantly lower LVEF and LVFS compared with the sham group (P < 0.01, Figure 1C–E). UPM exposure further reduced LVEF and LVFS (MI vs. MI+UPM, P < 0.01 and P < 0.05, respectively). SLL, SLM, and SLH groups all exhibited significantly increased LVEF (P < 0.001, P < 0.001, and P < 0.01, respectively) and LVFS (P < 0.01) as well as improved cardiac systolic function. The effects of SL on the peak velocities of aortic flow were examined using color Doppler mode at 24 h after MI and UPM exposure. As shown in Table 1, the aortic valve (AV) peak velocity decreased markedly in the MI group compared with the sham group (P < 0.05). When exposed to UPM, the AV peak velocity exhibited a persistent decline. After treatment, each dose of SL increased blood flow in the aorta outflow tract, especially the medium and high doses (P < 0.01 and P < 0.05, respectively). These results suggest that SL improves cardiac dysfunction activity comparable to that of the positive drug aspirin group.
Table 1. Effects of UPM exposure and SL intervention on aortic valve peak flow velocity after myocardial ischemia
Group n AV peak vel (mm/s) Sham 6 1,189.12 ± 256.85 MI 6 965.36 ± 131.21▲ MI+UPM 6 795.90 ± 120.58 SLL 6 972.60 ± 185.15 SLM 6 1,039.69 ± 332.54** SLH 6 1,002.02 ± 343.94* AS 6 1,017.10 ± 187.43* Note. Compared with Sham group, ▲P < 0.05; Compared with the MI+UPM group,*P < 0.05,**P< 0.01. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLL: Shenlianlow-dose group; SLM: Shenlianmedium-dose group; SLH: Shenlianhigh-dose group; AS: Aspiringroup. -
To further confirm myocardial damage, TTC staining was performed to measure infarct sizes (Figure 2A, B). We observed an increase in myocardial infarct size in the MI group compared with the sham group (P < 0.001). After UPM exposure, the infarct size increased further. Compared with the MI+UPM group, the infarct size decreased significantly in response to all doses of SL (P < 0.05, P < 0.0001, P < 0.05, respectively). The results of hematoxylin–eosin staining are shown in Figure 2C. In the sham group, myocardial cells were neatly arranged, myocardial fibers were regularly arranged, and there was no evident inflammatory cell infiltration. In the MI group, the arrangement of myocardial fibers was evidently disordered. Part of the tissue exhibited fibrous edema, and the number of myocardial fibers between the gaps increased, accompanied by inflammatory cell infiltration. Compared with the MI group, the deformation of myocardial fibers, swelling and rupture of myocardial cells, and infiltration of inflammatory cells were more severe in the MI+UPM group. After SL or aspirin treatment, the structural disorder of cardiac muscle fibers improved, and the arrangement of cell nuclei was orderly. Elevated levels of cTnT and LDH in the blood can reflect the degree of myocardial tissue injury after MI. As shown in Figure 2D–2E, the serum levels of cTnT and LDH were higher in the MI group than in the sham group; additionally, we found that UPM further increased the levels of cTnT and LDH, which decreased after SL and aspirin treatment.

Figure 2. SL extract relieve UPM-induced cardiac injury. (A, B) Effect of SL on infarct size in the experimental mice and the infarct rate as a percentage of the total size. Heart sections of the experimental mice stained with 2, 3, 5-triphenyltetrazolium chloride. Normal tissue was stained red, and infarcted tissue was pale. (C) Histological micrograph of sof hematoxylin & eosin staining myocardial sections, (hematoxylin & eosin: ×400) (D, E) LDH and cTnT levels in mice;All values are mean ± SD (n = 6/group). ▲▲▲P < 0.001, ▲▲▲▲P < 0.0001, comparing with the Sham group; ####P < 0.0001 comparing with the MI group; *P < 0.05, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLL: Shenlian low-dose group; SLM: Shenlian medium-dose group; SLH: Shenlian high-dose group; AS: Aspirin group.
-
Inflammatory cytokines in the serum of mice were assayed via ELISA to determine the anti-inflammatory effects of SL. The results are illustrated in Figure 3; the expression of IL-6 and MCP-1 was markedly increased in response to the MI surgery (P < 0.0001). Additionally, expression of the inflammatory cytokines IL-6, TNF-α, and MCP-1 markedly increased after tracheal instillation of UPM (P < 0.0001, P < 0.0001, P < 0.01, respectively) and then significantly decreased upon treatment with SL (P < 0.0001) (Figure 3A–3C). Subsequently, we used immunofluorescence to detect the expression of the macrophage cell marker CD68 in myocardial tissue (Figure 3D). After permanent ligation of the LAD coronary artery, CD68 protein staining intensity in ischemic areas of mice heart was significantly increased, indicating the infiltration of macrophages in the ischemic areas of the heart. Cardiac macrophage infiltration was further exacerbated by tracheal instillation of UPM, and SL administration reduced the infiltration of macrophages.

Figure 3. SL extract alleviated UPM-induced inflammation. (A–C) The changes in inflammatory cytokine interleukin (IL)-6 (IL-6), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) levels in the serum of mice and the effects of SL; (D) CD68 immunofluorescence staining for macrophages; values are mean ± SD (n = 6/group). ▲▲▲▲P < 0.0001 , comparing with the Sham group; ####P < 0.0001 comparing with the MI group; **P < 0.01, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLM: Shenlian medium-dose group.
-
Apoptotic death was examined using TUNEL assay and by determining the proportion of caspase-3-positive cells in the myocardial tissue. Compared with Sham, the ratio of apoptotic-positive cells in myocardial cells of the MI group significantly increased (P < 0.01); compared with MI, there was no statistical difference in MI+UPM; compared with MI+UPM, the myocardial cell apoptotic-positive cell rate in SL (P < 0.01). (Figure 4A, B). Subsequently, the expression of caspase-3 protein in the myocardial tissue was detected via immunohistochemical analysis; the results are shown in Figure 4C, D. The level of caspase-3 protein in the heart tissue of the MI group was increased (but not significantly) compared with the sham group. Compared with the MI group, UPM exposure significantly increased the expression of caspase-3 in the myocardium tissue. Additionally, we found that UPM triggered myocardial apoptosis, which was markedly attenuated by SL treatment.
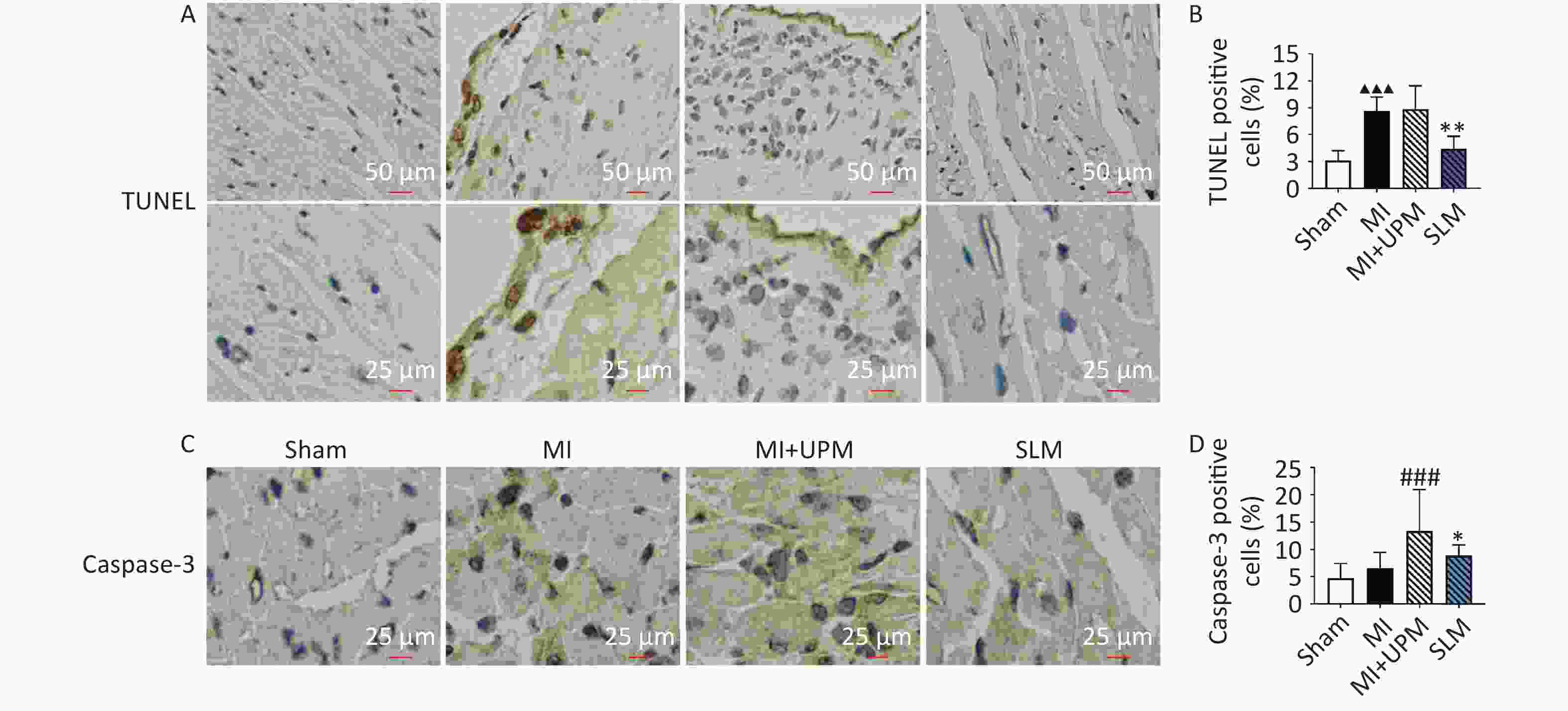
Figure 4. SL extract alleviated UPM-induced H9c2 cells apoptosis. (A, B) TUNEL-positive cells and quantification. (C, D) Caspase 3 immunohistochemical staining of apoptosis and quantification; values are mean ± SD (n = 6/group). ▲▲▲P < 0.001 , comparing with the Sham group; ###P < 0.001 comparing with the MI group; *P < 0.05, **P < 0.01 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLM: Shenlian medium-dose group.
-
H9c2 cells were incubated with various concentrations of SL (0, 1.25, 2.5, 5, 10, 20 μg/mL) for 24 h, and cell viability was assessed via the CCK8 assay. The results revealed that cell viability did not decrease among the SL-treated groups compared with the control group. Interestingly, cell viability increased by 8.47% in the 5 μg/mL SL-treated group (Figure 5A). Changes in morphological characteristics of 5 μg/mL SL-treated H9c2 cells were assessed microscopically. The microscopic images demonstrated that SL was not cytotoxic and was able to promote cell growth (Figure 5B). Furthermore, the morphological changes of H9c2 cells were observed after UPM exposure combined with hypoxia treatment. The H9c2 cells in the control group were fusiform with a full cytoplasm and clear edges. Conversely, after 12 h of hypoxia, the cells showed shrinkage and inconspicuous edges; however, with UPM exposure, the number of H9c2 cells reduced, and many cells dislodged from the bottom of the cell culture dish. Morphological observation of H9c2 cells revealed that the cells were shrunken and broken, with blurred edges and large deposits of black particles (Figure 5C). This phenotype could be rescued by SL (5 μg/mL) (Figure 5C). Therefore, SLE not only exhibited no toxicity toward H9c2 cells but also improved cell viability and prevented cell damage in H9c2 cells exposed to UPM under hypoxia.
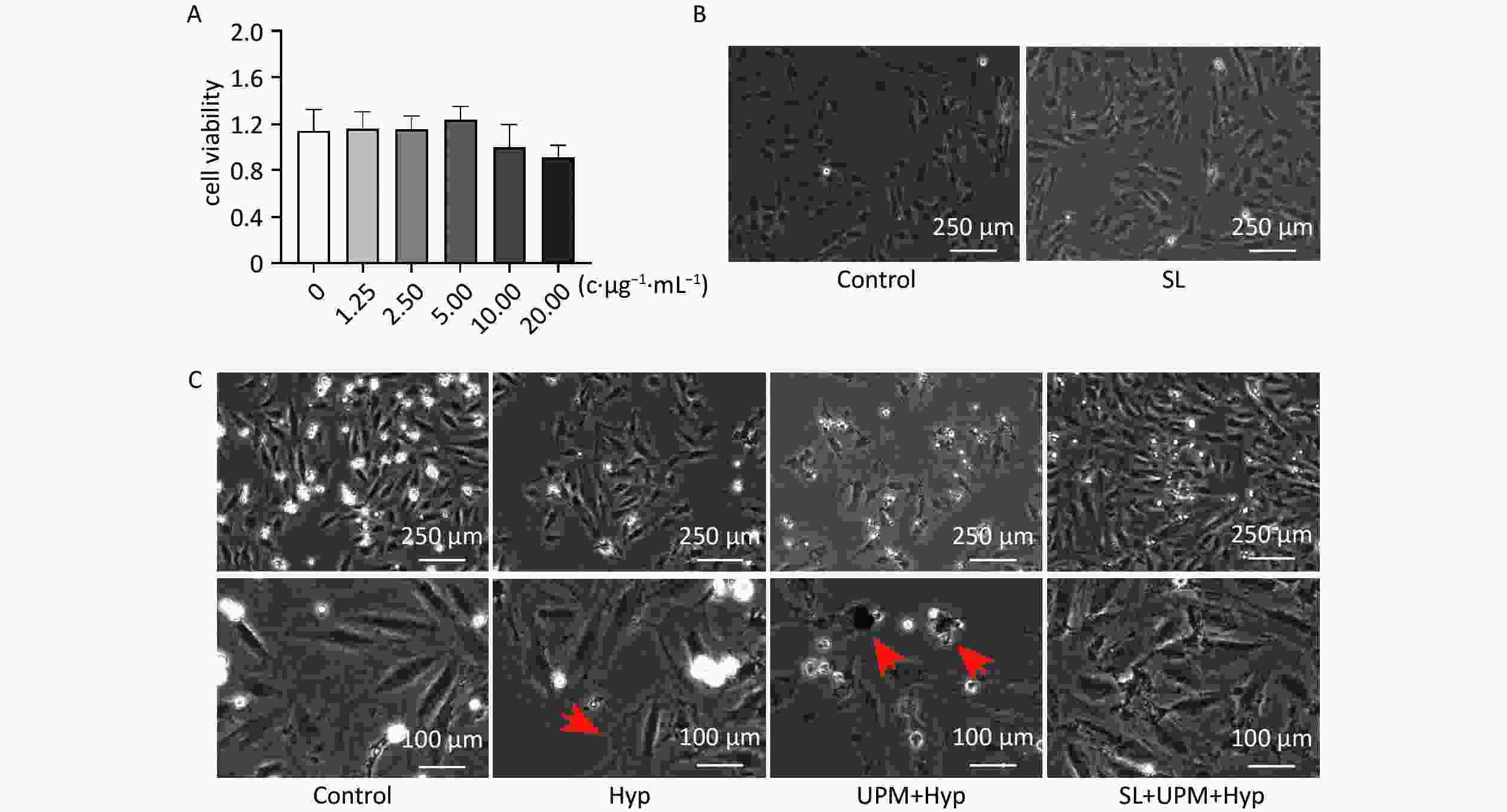
Figure 5. SL extract prevented cell damage in H9c2 cells with UPM exposure. (A) H9c2 cells were incubated with various concentrations of SL (0, 1.25, 2.5, 5, 10, 20 μg/mL) for 24 h and cell viability was measured by CCK8 assay. (B) The microscopy images of cell state after 24 h incubation of SL; (C) Images of cell state were taken at the end point of the experiment. SL: Shenlian group; Hyp: hypoxia group; UPM+Hyp: Ultrafine particulate matter exposure+hypoxia group; SL+UPM+Hyp: Shenlian+Ultrafine particulate matter exposure+hypoxia group.
-
To detect the effects of SL on UPM exposure combined with hypoxia-induced H9c2 cell apoptosis, we investigated cell apoptosis and the expression of apoptosis-associated proteins in H9c2 cells. As shown in Figure 6A–6D, we found that hypoxia treatment of H9c2 cells induced a significant decrease in Bcl-2 expression (P < 0.0001) but had no effect on Bax expression. Exposure to UPM led to the downregulation of Bcl-2 (P < 0.0001) and the upregulation of Bax (P < 0.01), which, in turn, led to an increased ratio of Bax/Bcl-2 compared with the Hyp group (P < 0.0001). SL treatment increased the expression of the antiapoptotic protein Bcl-2 and inhibited that of the proapoptotic marker Bax, resulting in an overall decrease in the Bax/Bcl-2 ratio (all P < 0.0001). Subsequently, apoptosis was examined after Annexin V-FITC/PI fluorescence staining. The results confirmed that UPM exposure promoted cell apoptosis and that SL treatment reversed apoptosis (Figure 6F). Additionally, PCR was performed to determine the gene expression level of the inflammatory mediator NFκB (Figure 6E), and the results showed that NFκB expression was slightly increased under UPM exposure but not significantly. After SL treatment, NFκB expression was significantly decreased (P < 0.01).
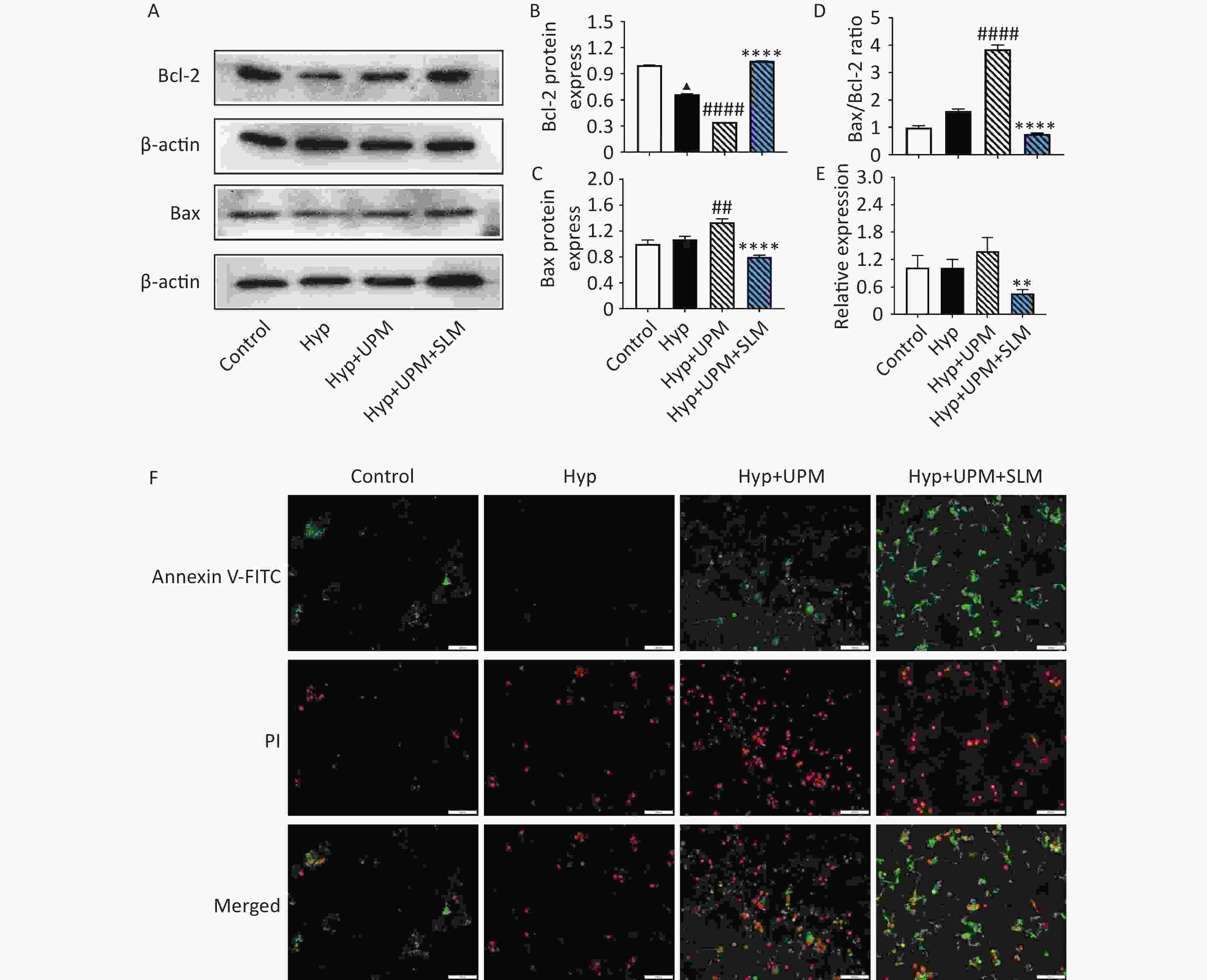
Figure 6. SL extract prevented cell apoptosis and decreased expression of NFκB in H9c2 cells with UPM exposure. (A–D) The expression of Bax and Bcl-2 in the H9c2 cells; quantitative analysis of Bax, Bcl-2 and Bax/Bcl-2. (E) Real-time PCR analysis shows changes in the relative expression of NFκB genes relative to the housekeeping β-actin gene; (F) Fluorescence microscope were used to examine the results of staining of FITC and PI. values are mean ± SD (n = 3/group). ▲▲▲P < 0.001, ▲▲▲▲P < 0.0001 comparing with the Control group; ##P < 0.01, ####P < 0.0001 comparing with the Hyp group; **P < 0.01, ****P < 0.0001 comparing with the Hyp+UPM group. Hyp: hypoxia group; Hyp+UPM: hypoxia+Ultrafine particulate matter exposure group; Hyp+UPM+SL: hypoxia+Ultrafine particulate matter exposure+Shenlian group.
-
The World Health Organization (WHO) reports that almost the entire global population (99%) breathes air that exceeds WHO air quality limits, posing a threat to their health. Despite considerable air pollution prevention and control measures being implemented in recent years, with some success, air pollution remains one of the most significant risk factors for health outcomes[23]. Air pollution is responsible for approximately seven million deaths per year worldwide[24]. The reduction of air pollution has become a top priority globally. The evidence base for the harm caused by air pollution has been growing rapidly, pointing to significant harm even at low levels of many air pollutants[25-28]. In 2021, the WHO revised its Air Quality Guidelines, recommending an annual mean PM2.5 concentration of 5 μg/m3[29]. Air pollution should receive our attention, especially regarding sensitive groups, including elderly people[26], men[30], postmenopausal women[31], patients with prior MI[32,33], lower socioeconomic and minority populations[34], and individuals with pre-existing chronic pulmonary or cardiometabolic diseases[35].
Although many studies have attempted to elucidate the mechanism of PM2.5-induced cardiovascular diseases, a definitive molecular mechanism remains elusive. Evidence to date implicates mechanisms involving oxidative stress, inflammation, metabolic dysfunction, cell apoptosis, dyslipidemia, autonomic dysfunction, and hypertension[36]. Based on these previous studies and our experimental data in both acute and subacute exposure models in vivo and in vitro, all verify that oxidative stress and inflammation represent crucial pathological mechanisms[20,37]. In recent years, apoptosis has been considered one of the most important mechanisms for PM2.5-induced heart damage[38-40]. Researchers are also trying to identify effective therapeutic drugs; however, unfortunately, there is no recommended first-line medication. In the context of the present study, the pathogenic mechanism of PM2.5 is complex and diverse. It can cause damage to multiple organs and lead to the disorder of body function by being involved in different physiological processes. TCM emphasizes the “holistic treatment concept” and focuses on systemic functional adjustments. Additionally, from the perspective of substance nature, it is a complex mixed system with multiple ingredients and multiple targets, making it more suitable for diseases with complex pathogenesis. Many researchers are exploring potential therapeutic drugs from TCM and have found that numerous Chinese formulas can treat lung damage caused by PM. Examples include Ma xing shi gan decoction[41], San’ao decoction[42], Wu’ao decoction[43], modified qianjin weijing decoction[44], Qingzao runfei huazhuo xingxue decoction[45], YG-1 extract[46], YiQiFuMai lyophilized injection powder[47], and Shengmai formula[37]. However, only few studies have demonstrated whether these herbs can ameliorate heart damage caused by PM. Our previous study demonstrated that SL could reduce the release of cytokines and protect against UPM-aggravated myocardial ischemic injury by inhibiting the activation of NLRP3 inflammasomes[20]. Research has also shown that SL can reduce inflammation and apoptosis by activating the PI3K/AKT and JAK2/STAT3 pathways[48], as well as intervening in inflammasome activation by regulating mitochondrial autophagy[49], thereby exerting a protective effect on myocardial infarction. The main active ingredient in the single herb medicine Salvia miltiorrhiza, namely Tanshinone IIA, has been confirmed to be widely used for its anti-inflammatory, antioxidant, anti-apoptotic, and neuroprotective properties in cerebral ischemic stroke and ischemia-reperfusion injury[50]. The main active component of Prunella vulgaris, Andrographolide, has been found to possess significant anti-inflammatory activity by inhibiting the expression of NFκB and STAT3, thereby reducing the synthesis of inflammatory factors[51]. Additionally, it has been discovered that its protective effect against myocardial ischemic injury is closely associated with its anti-inflammatory and anti-apoptotic properties. Amelioratory effect of neoandrographolide on myocardial ischemic-reperfusion injury by its anti-inflammatory and anti-apoptotic activities
The present study, based on the mouse model of myocardial ischemia combined with UPM subacute exposure, revealed that SL treatment increased the expression of antiapoptotic protein Bcl-2 and inhibited that of the proapoptotic marker Bax, improving myocardial cell apoptosis. Additionally, we found infarcted myocardium with many macrophage infiltrates, and exposure to UPM exacerbated macrophage infiltration. Interestingly, SL treatment markedly decreased infarcted myocardium macrophage infiltration. Therefore, we speculate that macrophages may be the key cells mediating cross-talk between the lung and heart, a hypothesis that requires further investigation. A previous study showed that PM2.5 subverts macrophage phagocytic activity and decreases proinflammatory cytokine production in response to pneumococcal infection[52]. PM2.5 is associated with macrophage polarization via the PP2A-mTOR-p70S6K/4E-BP1 axis, thereby regulating pulmonary injury[53]. However, few studies have reported on the role of macrophages in PM-induced heart damage. In recent years, with the increasing research focus on extracellular vesicles, there is another idea for elaborating the mechanism of PM2.5-induced cardiopulmonary injury. One study indicated that the extracellular vesicles secreted by macrophages can induce lung inflammation in mice after PM2.5 treatment[54]. Another study showed that TGF β-enriched small extracellular vesicles, released from macrophages after PM2.5 exposure, could promote the process of cardiac fibrosis[55]. A recent study also confirmed that macrophages are associated with myocardial inflammation, fibrosis, and diastolic dysfunction[56]. These findings confirm that macrophages play an important role in PM-induced heart damage.
In conclusion, inflammation and cell apoptosis emerge as major outcomes of UPM exposure. Our findings confirm that SL exhibits highly promising anti-inflammatory and antiapoptotic activities, indicating that SL may be a promising medication for the treatment of UPM-aggravated cardiopulmonary injury. In this study, we primarily focused on drug efficacy, and we did not explore the related biological mechanisms extensively. Therefore, in future studies, we can further explore the role of macrophages in this process. Subsequently, we plan to extract myocardial macrophages for transcriptome sequencing to elucidate the downstream signaling pathways.
doi: 10.3967/bes2024.137
Shenlian Extract Protects against Ultrafine Particulate Matter-Aggravated Myocardial Ischemic Injury by Inhibiting Inflammation and Cell Apoptosis
-
Abstract:
Objective Emerging evidence suggests that exposure to ultrafine particulate matter (UPM, aerodynamic diameter < 0.1 µm) is associated with adverse cardiovascular events. Previous studies have found that Shenlian (SL) extract possesses anti-inflammatory and antiapoptotic properties and has a promising protective effect at all stages of the atherosclerotic disease process. In this study, we aimed to investigated whether SL improves UPM-aggravated myocardial ischemic injury by inhibiting inflammation and cell apoptosis. Methods We established a mouse model of MI+UPM. Echocardiographic measurement, measurement of myocardialinfarct size, biochemical analysis, enzyme-linked immunosorbent assay (ELISA), histopathological analysis, Transferase dUTP Nick End Labeling (TUNEL), Western blotting (WB), Polymerase Chain Reaction (PCR) and so on were used to explore the anti-inflammatory and anti-apoptotic effects of SL in vivo and in vitro. Results SL treatment can attenuate UPM-induced cardiac dysfunction by improving left ventricular ejection fraction, fractional shortening, and decreasing cardiac infarction area. SL significantly reduced the levels of myocardial enzymes and attenuated UPM-induced morphological alterations. Moreover, SL significantly reduced expression levels of the inflammatory cytokines IL-6, TNF-α, and MCP-1. UPM further increased the infiltration of macrophages in myocardial tissue, whereas SL intervention reversed this phenomenon. UPM also triggered myocardial apoptosis, which was markedly attenuated by SL treatment. The results of in vitro experiments revealed that SL prevented cell damage caused by exposure to UPM combined with hypoxia by reducing the expression of the inflammatory factor NF-κB and inhibiting apoptosis in H9c2 cells. Conclusion Overall, both in vivo and in vitro experiments demonstrated that SL attenuated UPM-aggravated myocardial ischemic injury by inhibiting inflammation and cell apoptosis. The mechanisms were related to the downregulation of macrophages infiltrating heart tissues. -
Key words:
- Ultrafine particulate matter /
- Shenlian extract /
- Inflammation /
- Apoptosis /
- Macrophage
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study protocol was approved by the Laboratory Animal Ethics Committee of China Academy of Chinese Medical Sciences (ethics approval number: 2022B087).
&These authors contributed equally to this work.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. SL extract can relieve UPM-induced cardiac dysfunction. (A) Flowchart of experiments. (B) Effects of UPM on the ST segment. (C) M-mode ultrasound of the heart to evaluate cardiac function. (D, E) An ultrahigh-resolution small animal ultrasound imaging system was used to obtain left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS); all values are mean ± SD (n = 6/group). ▲▲P < 0.01, comparing with the Sham group; #P < 0.05, ##P < 0.01 comparing with the MI group; **P < 0.01, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia + ultrafine particulate matter exposure group; SLL: Shenlian low-dose group; SLM: Shenlian medium-dose group; SLH: Shenlian high-dose group; AS: Aspirin group.
Figure 2. SL extract relieve UPM-induced cardiac injury. (A, B) Effect of SL on infarct size in the experimental mice and the infarct rate as a percentage of the total size. Heart sections of the experimental mice stained with 2, 3, 5-triphenyltetrazolium chloride. Normal tissue was stained red, and infarcted tissue was pale. (C) Histological micrograph of sof hematoxylin & eosin staining myocardial sections, (hematoxylin & eosin: ×400) (D, E) LDH and cTnT levels in mice;All values are mean ± SD (n = 6/group). ▲▲▲P < 0.001, ▲▲▲▲P < 0.0001, comparing with the Sham group; ####P < 0.0001 comparing with the MI group; *P < 0.05, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLL: Shenlian low-dose group; SLM: Shenlian medium-dose group; SLH: Shenlian high-dose group; AS: Aspirin group.
Figure 3. SL extract alleviated UPM-induced inflammation. (A–C) The changes in inflammatory cytokine interleukin (IL)-6 (IL-6), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) levels in the serum of mice and the effects of SL; (D) CD68 immunofluorescence staining for macrophages; values are mean ± SD (n = 6/group). ▲▲▲▲P < 0.0001 , comparing with the Sham group; ####P < 0.0001 comparing with the MI group; **P < 0.01, ****P < 0.0001 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLM: Shenlian medium-dose group.
Figure 4. SL extract alleviated UPM-induced H9c2 cells apoptosis. (A, B) TUNEL-positive cells and quantification. (C, D) Caspase 3 immunohistochemical staining of apoptosis and quantification; values are mean ± SD (n = 6/group). ▲▲▲P < 0.001 , comparing with the Sham group; ###P < 0.001 comparing with the MI group; *P < 0.05, **P < 0.01 comparing with the MI+UPM group. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLM: Shenlian medium-dose group.
Figure 5. SL extract prevented cell damage in H9c2 cells with UPM exposure. (A) H9c2 cells were incubated with various concentrations of SL (0, 1.25, 2.5, 5, 10, 20 μg/mL) for 24 h and cell viability was measured by CCK8 assay. (B) The microscopy images of cell state after 24 h incubation of SL; (C) Images of cell state were taken at the end point of the experiment. SL: Shenlian group; Hyp: hypoxia group; UPM+Hyp: Ultrafine particulate matter exposure+hypoxia group; SL+UPM+Hyp: Shenlian+Ultrafine particulate matter exposure+hypoxia group.
Figure 6. SL extract prevented cell apoptosis and decreased expression of NFκB in H9c2 cells with UPM exposure. (A–D) The expression of Bax and Bcl-2 in the H9c2 cells; quantitative analysis of Bax, Bcl-2 and Bax/Bcl-2. (E) Real-time PCR analysis shows changes in the relative expression of NFκB genes relative to the housekeeping β-actin gene; (F) Fluorescence microscope were used to examine the results of staining of FITC and PI. values are mean ± SD (n = 3/group). ▲▲▲P < 0.001, ▲▲▲▲P < 0.0001 comparing with the Control group; ##P < 0.01, ####P < 0.0001 comparing with the Hyp group; **P < 0.01, ****P < 0.0001 comparing with the Hyp+UPM group. Hyp: hypoxia group; Hyp+UPM: hypoxia+Ultrafine particulate matter exposure group; Hyp+UPM+SL: hypoxia+Ultrafine particulate matter exposure+Shenlian group.
Table 1. Effects of UPM exposure and SL intervention on aortic valve peak flow velocity after myocardial ischemia
Group n AV peak vel (mm/s) Sham 6 1,189.12 ± 256.85 MI 6 965.36 ± 131.21▲ MI+UPM 6 795.90 ± 120.58 SLL 6 972.60 ± 185.15 SLM 6 1,039.69 ± 332.54** SLH 6 1,002.02 ± 343.94* AS 6 1,017.10 ± 187.43* Note. Compared with Sham group, ▲P < 0.05; Compared with the MI+UPM group,*P < 0.05,**P< 0.01. MI: Myocardial Ischemia group; MI+UPM: Myocardial Ischemia+ultrafine particulate matter exposure group; SLL: Shenlianlow-dose group; SLM: Shenlianmedium-dose group; SLH: Shenlianhigh-dose group; AS: Aspiringroup. -
[1] Joshi SS, Miller MR, Newby DE. Air pollution and cardiovascular disease: the Paul Wood Lecture, British Cardiovascular Society 2021. Heart, 2022; 108, 1267−73. doi: 10.1136/heartjnl-2021-319844 [2] Lelieveld J, Pozzer A, Pöschl U, et al. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res, 2020; 116, 1910−7. doi: 10.1093/cvr/cvaa025 [3] Zhang JW, Chen Z, Shan D, et al. Adverse effects of exposure to fine particles and ultrafine particles in the environment on different organs of organisms. J Environ Sci (China), 2024; 135, 449−73. doi: 10.1016/j.jes.2022.08.013 [4] Miller MR, Newby DE. Air pollution and cardiovascular disease: car sick. Cardiovasc Res, 2020; 116, 279−94. [5] Jaafari J, Naddafi K, Yunesian M, et al. Associations between short term exposure to ambient particulate matter from dust storm and anthropogenic sources and inflammatory biomarkers in healthy young adults. Sci Total Environ, 2021; 761, 144503. doi: 10.1016/j.scitotenv.2020.144503 [6] Lu X, Li RQ, Yan XX. Airway hyperresponsiveness development and the toxicity of PM2.5. Environ Sci Pollut Res Int, 2021; 28, 6374−91. doi: 10.1007/s11356-020-12051-w [7] Kleinman MT, Araujo JA, Nel A, et al. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett, 2008; 178, 127−30. doi: 10.1016/j.toxlet.2008.03.001 [8] Bhargava A, Shukla A, Bunkar N, et al. Exposure to ultrafine particulate matter induces NF-κβ mediated epigenetic modifications. Environ Pollut, 2019; 252, 39−50. doi: 10.1016/j.envpol.2019.05.065 [9] Li J, An Z, Song J, et al. Fine particulate matter-induced lung inflammation is mediated by pyroptosis in mice. Ecotoxicol Environ Saf, 2021; 219, 112351. doi: 10.1016/j.ecoenv.2021.112351 [10] Li RJ, Zhao LF, Tong JL, et al. Fine particulate matter and sulfur dioxide coexposures induce rat lung pathological injury and inflammatory responses via TLR4/p38/NF-κB pathway. Int J Toxicol, 2017; 36, 165−73. doi: 10.1177/1091581816682225 [11] Dou CM, Zhang J, Qi CC. Cooking oil fume-derived PM2.5 induces apoptosis in A549 cells and MAPK/NF-кB/STAT1 pathway activation. Environ Sci Pollut Res Int, 2018; 25, 9940−8. doi: 10.1007/s11356-018-1262-5 [12] Badamjav R, Zhang L, Sonom D, et al. Thalictrum minus L. ameliorates particulate matter-induced acute lung injury in mice. J Ethnopharmacol, 2021; 264, 113379. doi: 10.1016/j.jep.2020.113379 [13] Wei HY, Yuan WJ, Yu H, et al. Cytotoxicity induced by fine particulate matter (PM2.5) via mitochondria-mediated apoptosis pathway in rat alveolar macrophages. Environ Sci Pollut Res Int, 2021; 28, 25819−29. doi: 10.1007/s11356-021-12431-w [14] Geng XW, Wang XH, Luo M, et al. Induction of neutrophil apoptosis by a Bcl-2 inhibitor reduces particulate matter-induced lung inflammation. Aging (Albany NY), 2018; 10, 1415−23. [15] Ren F, Ji C, Huang YJ, et al. AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Sci Total Environ, 2020; 719, 135097. doi: 10.1016/j.scitotenv.2019.135097 [16] Li XL, Geng J, Chen YH, et al. Exposure to particulate matter induces cardiomyocytes apoptosis after myocardial infarction through NFκB activation. Biochem Biophys Res Commun, 2017; 488, 224−31. doi: 10.1016/j.bbrc.2017.05.047 [17] Guo Y, Yang Q, Weng XG, et al. Shenlian extract against myocardial injury induced by ischemia through the regulation of NF-κB/IκB signaling axis. Front Pharmacol, 2020; 11, 134. doi: 10.3389/fphar.2020.00134 [18] Li YJ, Chen Y, You Y, et al. Effects of Shenlian extracts on atherosclerosis by inhibition of the inflammatory response. J Tradit Chin Med, 2011; 31, 344-8. [19] Liu L, Li Q, Yin J, et al. ShenLian extract enhances TGF-β functions in the macrophage-SMC unit and stabilizes atherosclerotic plaques. Front Pharmacol, 2021; 12, 669730. doi: 10.3389/fphar.2021.669730 [20] Qu SQ, Li K, Yang T, et al. Shenlian extract protects against ultrafine particulate matter-aggravated myocardial ischemic injury by inhibiting inflammation response via the activation of NLRP3 inflammasomes. Environ Toxicol, 2021; 36, 1349−61. doi: 10.1002/tox.23131 [21] Qu SQ, Deng SQ, Yang T, et al. Shengmai Yin alleviated plaque vulnerability and ischemic myocardial damage in diesel exhaust particle-aggravated atherosclerosis with myocardial ischemia. Ecotoxicol Environ Saf, 2022; 234, 113379. doi: 10.1016/j.ecoenv.2022.113379 [22] Hou Y, Fan FH, Xie N, et al. Rhodiola crenulata alleviates hypobaric hypoxia-induced brain injury by maintaining BBB integrity and balancing energy metabolism dysfunction. Phytomedicine, 2024; 128, 155529. doi: 10.1016/j.phymed.2024.155529 [23] Zeng YY, Cao YF, Qiao X, et al. Air pollution reduction in China: recent success but great challenge for the future. Sci Total Environ, 2019; 663, 329−37. doi: 10.1016/j.scitotenv.2019.01.262 [24] Mannucci PM, Harari S, Franchini M. Novel evidence for a greater burden of ambient air pollution on cardiovascular disease. Haematologica, 2019; 104, 2349−57. doi: 10.3324/haematol.2019.225086 [25] Peters A, Dockery DW, Muller JE, et a yocardial infarction. Circulation, 2001; 103, 2810-5. [26] Chen C, Zhu PF, Lan L, et al. Short-term exposures to PM2.5 and cause-specific l. Increased particulate air pollution and the triggering of m mortality of cardiovascular health in China. Environ Res, 2018; 161, 188−94. doi: 10.1016/j.envres.2017.10.046 [27] Shi LH, Zanobetti A, Kloog I, et al. Low-concentration PM2.5 and mortality: estimating acute and chronic effects in a population-based study. Environ Health Perspect, 2016; 124, 46−52. doi: 10.1289/ehp.1409111 [28] Beber LCC, da Silva MOAF, dos Santos AB, et al. The association of subchronic exposure to low concentration of PM2.5 and high-fat diet potentiates glucose intolerance development, by impairing adipose tissue antioxidant defense and eHSP72 levels. Environ Sci Pollut Res Int, 2020; 27, 32006−16. doi: 10.1007/s11356-020-09581-8 [29] Hoffmann B, Boogaard H, de Nazelle A, et al. WHO air quality guidelines 2021-aiming for healthier air for all: a joint statement by medical, public health, scientific societies and patient representative organisations. Int J Public Health, 2021; 66, 1604465. doi: 10.3389/ijph.2021.1604465 [30] Chang ET, Lau EC, Moolgavkar SH. Smoking, air pollution, and lung cancer risk in the Nurses' Health Study cohort: time-dependent confounding and effect modification. Crit Rev Toxicol, 2020; 50, 189−200. doi: 10.1080/10408444.2020.1727410 [31] Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med, 2007; 356, 447−58. doi: 10.1056/NEJMoa054409 [32] Hung CS, Huang CC, Pan SC, et al. Acute particulate matter exposure is associated with disturbances in heart rate complexity in patients with prior myocardial infarction. Sci Total Environ, 2020; 733, 138842. doi: 10.1016/j.scitotenv.2020.138842 [33] Chen H, Burnett RT, Copes R, et al. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population-based cohort study. Environ Health Perspect, 2016; 124, 1421−8. doi: 10.1289/EHP185 [34] Tibuakuu M, Michos ED, Navas-Acien A, et al. Air pollution and cardiovascular disease: a focus on vulnerable populations worldwide. Curr Epidemiol Rep, 2018; 5, 370−8. doi: 10.1007/s40471-018-0166-8 [35] Pope III CA, Turner MC, Burnett RT, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res, 2015; 116, 108−15. doi: 10.1161/CIRCRESAHA.116.305060 [36] Aryal A, Harmon AC, Dugas TR. Particulate matter air pollutants and cardiovascular disease: strategies for intervention. Pharmacol Ther, 2021; 223, 107890. doi: 10.1016/j.pharmthera.2021.107890 [37] Chen LN, Guo Y, Qu SQ, et al. The protective effects of Shengmai formula against myocardial injury induced by ultrafine particulate matter exposure and myocardial ischemia are mediated by the PI3K/AKT/p38 MAPK/Nrf2 pathway. Front Pharmacol, 2021; 12, 619311. doi: 10.3389/fphar.2021.619311 [38] Wang Y, Tang M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci Total Environ, 2020; 710, 136397. doi: 10.1016/j.scitotenv.2019.136397 [39] Pei YH, Chen J, Wu X, et al. LncRNA PEAMIR inhibits apoptosis and inflammatory response in PM2.5 exposure aggravated myocardial ischemia/reperfusion injury as a competing endogenous RNA of miR-29b-3p. Nanotoxicology, 2020; 14, 638−53. doi: 10.1080/17435390.2020.1731857 [40] Jiang Y, Zhao XH, Chen J, et al. PM2.5 induces cardiac malformations via PI3K/akt2/mTORC1 signaling pathway in zebrafish larvae. Environ Pollut, 2023; 323, 121306. doi: 10.1016/j.envpol.2023.121306 [41] Wang YF, Fei YX, Zhao B, et al. Ma Xing Shi Gan decoction protects against PM2.5-induced lung injury through suppression of epithelial-to-mesenchymal transition (EMT) and epithelial barrier disruption. Evid Based Complement Alternat Med, 2020; 2020, 7176589. doi: 10.1155/2020/7176589 [42] Wang PL, Liu H, Fan XS, et al. Effect of San'ao decoction on aggravated asthma mice model induced by PM2.5 and TRPA1/TRPV1 expressions. J Ethnopharmacol, 2019; 236, 82−90. doi: 10.1016/j.jep.2019.02.043 [43] Liu H, Fan XS, Zhu Y. Effects of Wu'ao decoction on the expressions of TRPA1 and TRPV1 in lung in PM2.5 and OVA induced severe asthma mice model. Chin J Integr Tradit West Med, 2019; 39, 997−1003. (In Chinese) [44] Shen ZY, Fu SG, Yang AD, et al. Effects of modified Qianjin Weijing decoction on TNF-α and NF-κB in rats with lung injury induced by particulate matter. Chin J Inf Tradit Chin Med, 2018; 25, 38−42. (In Chinese) [45] Zhang JB, Sun L, Li SQ, et al. Therapeutic effect of Qingzao Runfei Huazhuo Xingxue decoction on PM2.5-induced respiratory disease in mice. Chin Crit Care Med, 2016; 28, 916−20. (In Chinese) [46] Kim HY, Yoon JJ, Kim DS, et al. YG-1 extract improves acute pulmonary inflammation by inducing bronchodilation and inhibiting inflammatory cytokines. Nutrients, 2021; 13, 3414. doi: 10.3390/nu13103414 [47] Xia YL, Dolgor S, Jiang SY, et al. YiQiFuMai lyophilized injection attenuates particulate matter-induced acute lung injury in mice via TLR4-mTOR-autophagy pathway. Biomed Pharmacother, 2018; 108, 906−13. doi: 10.1016/j.biopha.2018.09.088 [48] Li JJ, Wang CM, Wang YJ, et al. Network pharmacology analysis and experimental validation to explore the mechanism of Shenlian extract on myocardial ischemia. J Ethnopharmacol, 2022; 288, 114973. doi: 10.1016/j.jep.2022.114973 [49] Wang CM. Protective effects of Shenlian extract on myocardial infarction of rats by regulating mitochondrial autophagy and intervening inflammasomes activation. China Academy of Chinese Medical Sciences. 2021. (In Chinese) [50] Arefnezhad R, Nejabat A, Behjati F, et al. Tanshinone IIA against cerebral ischemic stroke and ischemia-reperfusion injury: a review of the current documents. Mini Rev Med Chem, 2024; 24, 1701−9. doi: 10.2174/0113895575299721240227070032 [51] Gupta S, Mishra KP, Kumar B, et al. Andrographolide attenuates complete Freund's adjuvant induced arthritis via suppression of inflammatory mediators and pro-inflammatory cytokines. J Ethnopharmacol, 2020; 261, 113022. doi: 10.1016/j.jep.2020.113022 [52] Chen YW, Huang MZ, Chen CL, et al. PM2.5 impairs macrophage functions to exacerbate pneumococcus-induced pulmonary pathogenesis. Part Fibre Toxicol, 2020; 17, 37. doi: 10.1186/s12989-020-00362-2 [53] Chen S, Chen LP, Ye LZ, et al. PP2A-mTOR-p70S6K/4E-BP1 axis regulates M1 polarization of pulmonary macrophages and promotes ambient particulate matter induced mouse lung injury. J Hazard Mater, 2022; 424, 127624. doi: 10.1016/j.jhazmat.2021.127624 [54] Cui XJ, Lai WJ, Zhao Y, et al. The exosome-mediated cascade reactions for the transfer and inflammatory responses of fine atmospheric particulate matter in macrophages. Environ Sci Technol, 2023; 57, 7891−901. doi: 10.1021/acs.est.3c01436 [55] Hu XQ, Chen M, Cao X, et al. TGF-β-containing small extracellular vesicles from PM2.5-activated macrophages induces cardiotoxicity. Front Cardiovasc Med, 2022; 9, 917719. doi: 10.3389/fcvm.2022.917719 [56] Vendrov AE, Xiao H, Lozhkin A, et al. Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy. Redox Biol, 2023; 67, 102937. doi: 10.1016/j.redox.2023.102937 -




 下载:
下载:



 Quick Links
Quick Links