-
The destruction of the skin barrier and the presence of necrotic tissue in large burns increase the risk of multiple infections, often leading to sepsis, bacteremia, and other complications. Infective endocarditis (IE) is a severe manifestation of organ damage, and if conservative medical treatment fails to control the infection, irreversible pathological changes may occur, including valvular redundancy. This can ultimately result in heart failure and may even be life-threatening. Currently, few reports describe infective endocarditis complicating heart valve abnormalities after burns, and no cases have been reported in China in which heart failure was relieved by surgical treatment. This paper presents a case of extensive burns managed through an interprofessional and multidisciplinary approach. Treatment included multiple skin grafting procedures to close the burn wound, prolonged antibiotic therapy to control bacteremia, cardiac surgery to treat heart failure, and negative-pressure wound therapy to prevent infection and promote healing. This case represents a successful multidisciplinary collaborative diagnosis and treatment (MDT), which is systematically explored alongside a review of the literature.
The global incidence of IE ranges from 3 to 10 cases per 100,000 individuals, with mortality rates reaching 48.5% in some regions. The incidence of endocarditis among patients in burn units is four to six times higher than in other hospital populations, and most cases are diagnosed postmortem[1].
The patient was a 44-year-old man admitted on August 26, 2021, after sustaining burns from hot salt material and hydrochloric acid on multiple areas of the body. The total burn area was 98% of the total body surface area (TBSA), with burn depths of second to third degree (II°–III°). He was initially treated at a local hospital, where he underwent a tracheotomy, rehydration therapy, antiresorptive and anti-infective treatment, multiple sessions of debridement and eschar excision, skin grafting surgery, and symptomatic supportive therapy. On November 22, 2021, he developed chills and high fever, with a body temperature reaching 39.5 °C. Blood culture results indicated Acinetobacter baumannii infection. Laboratory tests showed a white blood cell (WBC) count of 16.3 × 10⁹/L, neutrophil percentage (NEUT%) of 89.7%, and procalcitonin (PCT) level of 4.2 µg/L. The patient was administered meropenem (1.0 g, every 8 hours) via intravenous infusion, which was discontinued after three days. However, the antibiotic was readministered on November 24, 2021, and subsequently on December 1, 8, and 15, 2022, following debridement and skin grafting procedures. Each postoperative course included three consecutive days of meropenem therapy (1.0 g, every 8 hours). During these periods, the patient’s temperature fluctuated between 37.3 °C and 38.3 °C. By February 15, 2022, the burn wounds had clinically healed; however, his body temperature remained at 38.3 °C, his heart rate fluctuated between 100 and 130 beats per minute, and bone exposure was noted on the outer left calf. Osteomyelitis was suspected, and he was administered vancomycin hydrochloride (1.0 g, every 12 hours) via intravenous injection for four weeks. His body temperature gradually normalized. On March 6, 2022, after referral to the local orthopedic department, the patient reported occasional panic, chest tightness, and breathlessness. Cardiac ultrasonography revealed heart valve abnormalities, though specific details were unavailable. On March 17, 2022, positron emission tomography (PET) of the left ankle suggested osteomyelitis. On March 23, 2022, he again developed hyperpyrexia, with a body temperature reaching 39 °C. Blood culture and high-throughput sequencing identified Enterococcus faecalis infection. Antibiotic therapy was adjusted to vancomycin hydrochloride (1.0 g) combined with ciprofloxacin (0.4 g, every 12 hours) via intravenous administration. Left ankle trauma debridement and continuous negative-pressure drainage were initiated. The patient’s temperature gradually normalized; however, his heart rate continued to fluctuate between 100 and 120 beats per minute. On April 15, 2022, blood cultures showed no bacterial growth, and antibiotic therapy was discontinued. The patient had a history of diabetes mellitus for more than three years but had no known cardiac disease before the injury and no risk factor for infective endocarditis (IE). The disease course was characterized by recurrent chills and high fever. Blood cultures detected Acinetobacter baumannii and Enterococcus faecalis infections. The prolonged retention of a central venous catheter for infusion therapy was identified as the most likely risk factor for the development of IE. With the rapid advancement of intensive care technology, invasive monitoring and treatment methods have increased. However, there remains a lack of standardized guidelines for the indication and management of invasive procedures, contributing to an increasing incidence of catheter-related infections and other complications[2]. While Streptococcus oxysporus is the most common causative organism associated with heart valve redundancy, Enterococcus, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii infections have been increasingly reported in recent years[3] (Table 1).
Table 1. Case reports of patients with extensive burns and heart valve redundancy
Publication date Author Journal Patient information Blood cultures Antibiotic regimen Auxiliary tests Surgical timing and modality Outcome 1998 Carlotto RC[4] Burns Canada, 39-year-old man, 40% total body surface area (TBSA) burns Staphylococcus aureus, Escherichia coli Unknown Autopsy at 12.5 days showed mitral valve redundancy Trauma debridement and skin grafting, skin removal from thighs at 12 days Death 2002 Apple J[5] J Trauma USA, 23-year-old woman, 44% TBSA burns Escherichia coli Ampicillin, vancomycin, gentamicin Aortic valve regurgitation, thickening No surgery Death 2002 Apple J[5] J Trauma USA, 50-year-old man, 35% TBSA burns Pseudomonas aeruginosa Ampicillin, tobramycin Aortic valve perforation (12 × 10 mm) Ross procedure on day 30 Survival 2008 Smith MA[6] Ann Thorac Surg USA, 15-year-old man, 90% TBSA burns with inhalation injury Methicillin-resistant Staphylococcus aureus (MRSA) Unknown Mitral valve redundancy (23 mm) Mitral valve replacement on day 21 Survival 2009 Gianesello L[7] Minerva Anestesiol Italy, 40-year-old woman, 85% TBSA burns Pseudomonas aeruginosa, Klebsiella pneumoniae, MRSA, Candida Penicillin, β-lactamase inhibitor, amikacin, carbapenem, vancomycin, fluconazole, rifampicin, amikacin, pefloxacin Transesophageal echocardiography (TEE) at 2.5 months showed mitral valve redundancy Bovine pericardial patch for mitral valve replacement at 3 months Death 38 days postoperatively 2015 Mohebali J[8] Burns USA, 33-year-old man, 90% TBSA burns Gram-negative rods, Pseudomonas aeruginosa Amikacin, tobramycin, polymyxin, aminoglycoside Transthoracic echocardiography (TTE) at 4 months showed mitral valve redundancy Valve repair at 5 months Survival Note. TEE, transesophageal echocardiography; TTE, transthoracic echocardiography. The patient was transferred to our department on May 9, 2022, due to an increased heart rate, chest tightness, breathing difficulty, and other symptoms of unclear cause. Upon admission, his oxygen saturation was 99%, body temperature was 36.5 °C, respiratory rate was 26 breaths/min, heart rate was 116 beats/min, and blood pressure was 106/78 mmHg. The patient was conscious but displayed poor mental status and anemic sitting breathing. Auscultation of the left lung revealed thick respiratory sounds. A murmur was heard in the mitral and aortic valve auscultation areas. No edema was observed in either lower limb. Specialized examination showed keloid scar proliferation and contracture in multiple areas of the body, leading to functional limitations. The anterior thoracic keloid scar was protruding and had a hard texture. Residual trauma was observed in approximately 1% of the TBSA on the left lower limb, with minor exudation from the basal granulation tissue. The left anterior aspect of the lower leg had a 1 × 2 cm exposed tibia with a visible portion of necrotic tendon tissue. Laboratory test results were as follows: HB, 109 g/L; PLT, 73 × 10⁹/L; BNP, 10,974 pg/mL. Chest radiography showed inflammation in both lungs, more pronounced in the left lung, along with left-sided pleural effusion. Chest color Doppler ultrasound revealed liquid dark areas measuring approximately 2.1 cm in the left thoracic cavity and 2.3 cm in the right thoracic cavity. Electrocardiography showed sinus tachycardia with inverted T waves in leads V1–V4. Echocardiography revealed aortic valve redundancy, severe aortic valve regurgitation, and perforation of the anterior mitral valve leaflet (Table 2 and Figure 1C). He was admitted to the hospital with the following diagnoses: (1) multiple scar hyperplasia throughout the body following burns; (2) residual trauma affecting 2% of both lower limbs; (3) osteomyelitis of the left calf; (4) infective endocarditis with fluctuating organisms; and (5) heart failure. On the second day after admission, the patient’s chest tightness and breath-holding worsened without an obvious trigger. Central venous pressure (CVP) was measured at 17 cm H₂O through the internal jugular vein. Given the patient’s history and symptoms, the following treatments were administered: (1) immobilization in a semi-recumbent position to prevent valvular vegetation embolization; (2) active regulation of fluid intake and output to maintain a slightly negative fluid balance; and (3) temporary administration of 0.25 mg dyphylline and 20 mg furosemide. One hour after emergency treatment, the patient urinated 200 mL and reported relief from chest tightness and suffocation. CVP decreased to 13 cm H₂O. Considering that the valvular vegetations were likely associated with infections from extensive burns in the past, blood cultures from both arms were performed using double bottles for three consecutive days, all of which showed sterile growth. Since the patient had relatively large valvular vegetations and medical management alone was insufficient to resolve the organic lesions, surgical intervention was considered as soon as possible.
Table 2. Preoperative and postoperative echocardiographic parameters
Date Structural indicators Functional indicators Left atrial diameter (mm) Left ventricular end-systolic diameter (mm) Left ventricular end-diastolic diameter (mm) Right atrial diameter (mm) Right Ventricular diameter (mm) Aortic sinus (mm) Pulmonary artery (mm) Inferior vena cava (mm) Left atrial diameter (mm) Nterventricular septum thickness (mm) Ejection fraction (%) Left ventricular end-diastolic volume (mL) Left ventricular end-systolic volume (mL) Stroke volume (mL) Mitral valve (E/A) Tricuspid valve (E/A) Aortic valve (flow rate/differential pressure) 05.10 49 64 47 40 42 34 26 25 9.8 50 210 107 103 1.1 0.5 2.9/34 1.3/7 05.11 47 58 46 39 41 26 25 20 9.6 53 162 76 86 1.3 0.7 1.4/8 1.3/7 06.14 36 42 27 34 34 29 20 20 10.9 51 101 50 51 1.0/1.1 0.7/0.6 1.8/13 0.9/3 07.06 35 44 35 34 34 32 24 19 11.8 52 94 46 48 1.2/1.6 0.6/0.9 1.7/13 0.8/3 08.09 40 41 31 33 33 36 23 13 11.3 52 79 38 41 1.2/1.3 0.5/0.6 1.4/8 0.7/2 09.01 42 45 30 33 32 34 23 − 11.4 51 85 42 43 0.9/1.1 0.6/0.5 1.3/7 0.7/2 10.27 40 40 26 32 33 32 24 − 11.3 52 85 39 43 1.0/1.3 0.6/0.5 1.3/7 0.9/3 10.31 39 41 27 34 34 35 19 13 10.6 55 70 31 39 1.1/1.2 0.7/0.4 1.8/13 0.7/2 Due to the patient’s heart failure with mitral valve perforation and heart valve redundancy, surgical valve replacement was indicated. Therefore, he was transferred to Fuwai Hospital for cardiac surgery. A coronary artery CT examination on May 12, 2022, showed a calcification score of 179.11 points. The coronary arteries were of the right dominant type, with approximately 50% middle stenosis in the anterior descending branch and left ventricular enlargement. One week before surgery, the patient received the following preoperative treatments: metoprolol tartrate tablets (12.5 mg, every 12 hours) orally and vancomycin hydrochloride (1 g, every 12 hours) intravenously. On May 17, 2022, under general anesthesia with hypothermic extracorporeal circulation, the patient underwent aortic and mitral valve replacement, coronary artery bypass grafting, and anastomosis of the left internal mammary artery to the anterior descending branch (Figure 2A, B and Figure 1A, B). Due to surgical access through the anterior chest scar, the postoperative incision edges exhibited poor blood flow and scar ulceration. Following a departmental consultation, negative pressure wound therapy (vacuum-assisted closure [VSD]) was used to manage the incision infection, resulting in wound healing within two weeks (Figure 2C, D, E). Postoperative pathology revealed mucoid degeneration of the aortic and mitral valves. Postoperatively, the patient was treated with Sulperazon (3 g, every 8 hours) and vancomycin hydrochloride (1 g, every 12 hours) via intravenous injection. Dopamine and norepinephrine were administered as continuous low-dose infusions to stabilize circulation, and systemic anticoagulation therapy was initiated. Additional postoperative management included strategies to improve cardiac remodeling and protect gastrointestinal function, along with other symptomatic treatments. Echocardiography confirmed mechanical valve replacement at the mitral and aortic valve positions, with no apparent abnormalities in mechanical valve function (Table 2 and Figure 1D). In this patient’s case, extensive burns had led to severe anterior thoracic scarring, which posed challenges during open thoracic surgery. The incision could not be aligned properly, and suture tension was excessive. Additionally, poor blood flow at the incision edges increased the risk of wound infection. To address these potential complications, a comprehensive preoperative assessment and preparation were conducted. A 2009 report in Surgical Intensive Care Medicine (Florence, Italy) described a woman with 85% TBSA burns, including severe anterior chest burns. She underwent heart valve replacement surgery but died 38 days postoperatively due to bacterial infections originating from the sternal wound, which progressed to mediastinitis[7]. At the corresponding author’s institution, negative pressure wound therapy has been widely used to manage infected sternal wounds following open-heart surgery. This approach was applied in the present case, similar to the surgical cases described in the 2009 report. Vacuum sealing drainage (VSD) involves using microporous materials to cover or fill the wound, creating point-to-point contact between the material and the wound. This promotes wound healing through shear forces and negative pressure suction[9]. The main benefits of negative pressure suction include: (1) reducing incisional tension, (2) promoting drainage of incisional exudate, (3) improving incision alignment, (4) enhancing incisional healing, (5) preventing incisional infection, and (6) minimizing reactions to suture knots. The patient was transferred back to our department on June 13, 2022, for continued post-burn generalized scar rehabilitation.
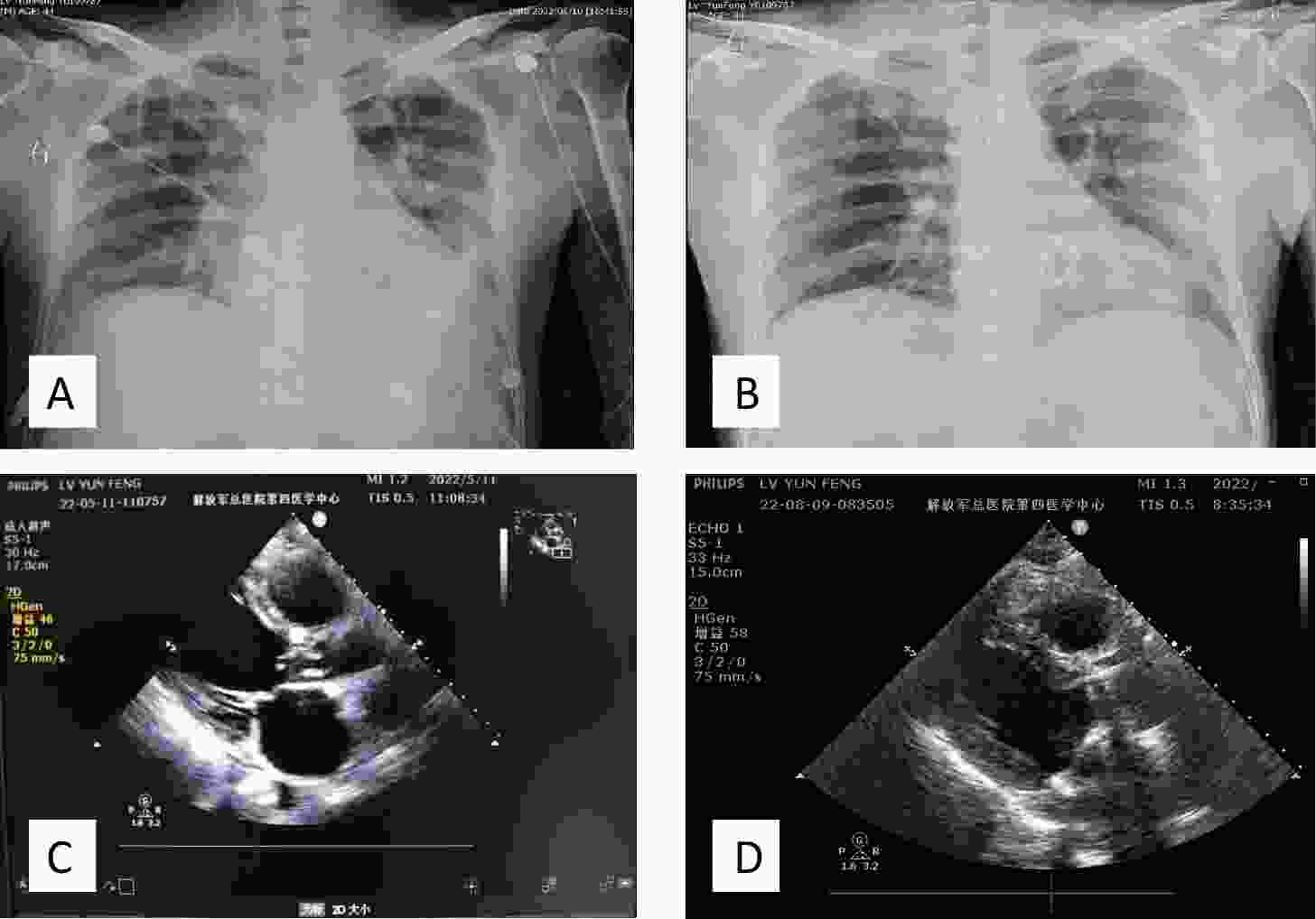
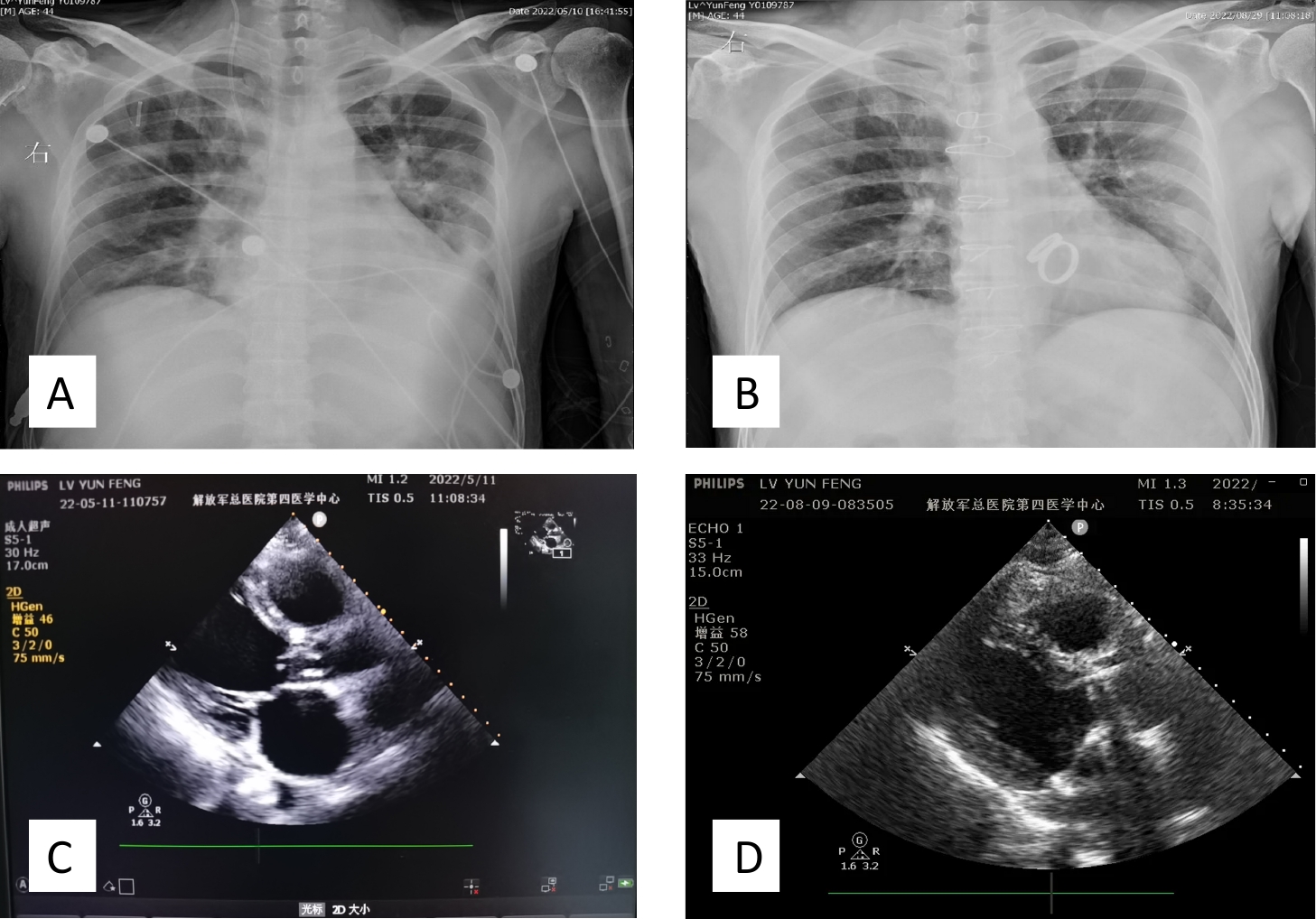
Figure 1. Imaging of a patient with heart valve redundancy. Chest X-ray: (A) Preoperative chest X-ray showing heart valve redundancy and left pleural effusion. (B) Postoperative chest X-ray following heart valve replacement surgery, demonstrating removal of redundant valve tissue and presence of a prosthetic heart valve. Echocardiographic examination: (C) Preoperative echocardiography: left atrium (LA), 49 mm; left ventricle (LV), 62 mm; left ventricular ejection fraction (LVEF), 50%. Findings included infective endocarditis, aortic valve redundancy (19 × 4 mm), massive aortic and mitral valve regurgitation, thickening of the mitral valve with perforation of the anterior leaflet (3 mm), moderate tricuspid valve regurgitation, and mild pulmonary valve regurgitation. The left heart was markedly enlarged, while the right heart was mildly enlarged. Left ventricular diastolic function was reduced, and systolic function was at the lower limit of normal. A small pericardial effusion and tachycardia were also noted. (D) Postoperative echocardiography: LA, 38 mm; LV, 42 mm; LVEF, 57%. Mechanical valve replacement was observed at the mitral and aortic valve positions, with no obvious functional abnormalities. Coronary artery bypass grafting was also performed. Thickening of the interventricular septum was noted.
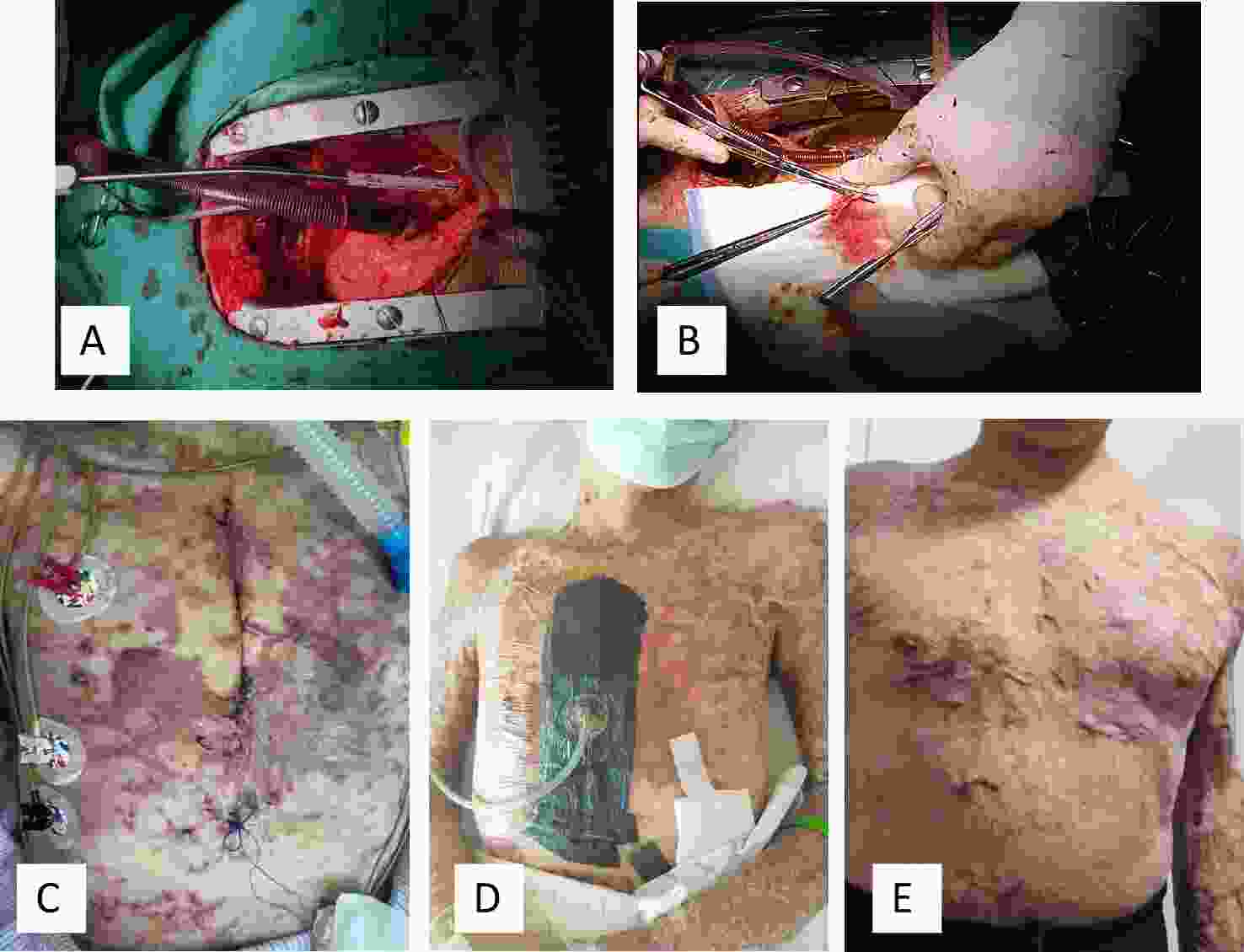
Figure 2. Prosthetic heart valve replacement and surgical incision healing. (A) Open chest exposure for extracorporeal circulation. (B) Excision of redundant heart valve tissue. (C) Postoperative day 1 dressing change. Due to the poor elasticity of scarred skin and excessive tension at the sutured incision, the incision edges appeared pale. Additionally, the lack of a dermal vascular reticulation layer resulted in impaired microcirculation at the incision margins compared with normal skin. Scar rupture at the incision edges was observed, and bacterial colonization of the scar tissue could not be excluded as a potential infectious cause. (D) Postoperative day 1, VSD was applied to the operative area, effectively relieving wound tension, improving microcirculation at the incision margins, reducing microbial infection, and preventing wound non-healing. (E) Six months postoperatively, the surgical incision appeared flat and had healed well.
Patients with extensive burns rarely develop cardiac failure due to heart valve redundancy during the functional recovery period, and no case treated with early open-heart valve replacement surgery has been reported in China. Based on the treatment outcomes of this patient with infective endocarditis and redundant valvular tissue, it was confirmed that despite scar-related skin ulceration, incision infection, and osteomyelitis of the left calf, there was no re-infection of the incision after open-heart surgery. This outcome was achieved through an MDT approach involving the burns and cardiac surgery departments. The initial wound healing was favorable, and adequate protective measures were implemented to facilitate open-heart surgery, ensuring that collaborating departments could proceed with treatment without concerns.
doi: 10.3967/bes2025.023
The Role of VSD in Enhancing the Biological Environment of Scarred Skin Incisions in Valve Replacement: A Clinical Study on Postoperative Outcomes in Patients with Extensive Burns and Cardiac Valve Neoplasms
-
The authors declare no competing interests.
This study is exempt from ethical review.
注释:1) Competing Interests: 2) Ethics: -
Figure 1. Imaging of a patient with heart valve redundancy. Chest X-ray: (A) Preoperative chest X-ray showing heart valve redundancy and left pleural effusion. (B) Postoperative chest X-ray following heart valve replacement surgery, demonstrating removal of redundant valve tissue and presence of a prosthetic heart valve. Echocardiographic examination: (C) Preoperative echocardiography: left atrium (LA), 49 mm; left ventricle (LV), 62 mm; left ventricular ejection fraction (LVEF), 50%. Findings included infective endocarditis, aortic valve redundancy (19 × 4 mm), massive aortic and mitral valve regurgitation, thickening of the mitral valve with perforation of the anterior leaflet (3 mm), moderate tricuspid valve regurgitation, and mild pulmonary valve regurgitation. The left heart was markedly enlarged, while the right heart was mildly enlarged. Left ventricular diastolic function was reduced, and systolic function was at the lower limit of normal. A small pericardial effusion and tachycardia were also noted. (D) Postoperative echocardiography: LA, 38 mm; LV, 42 mm; LVEF, 57%. Mechanical valve replacement was observed at the mitral and aortic valve positions, with no obvious functional abnormalities. Coronary artery bypass grafting was also performed. Thickening of the interventricular septum was noted.
Figure 2. Prosthetic heart valve replacement and surgical incision healing. (A) Open chest exposure for extracorporeal circulation. (B) Excision of redundant heart valve tissue. (C) Postoperative day 1 dressing change. Due to the poor elasticity of scarred skin and excessive tension at the sutured incision, the incision edges appeared pale. Additionally, the lack of a dermal vascular reticulation layer resulted in impaired microcirculation at the incision margins compared with normal skin. Scar rupture at the incision edges was observed, and bacterial colonization of the scar tissue could not be excluded as a potential infectious cause. (D) Postoperative day 1, VSD was applied to the operative area, effectively relieving wound tension, improving microcirculation at the incision margins, reducing microbial infection, and preventing wound non-healing. (E) Six months postoperatively, the surgical incision appeared flat and had healed well.
Table 1. Case reports of patients with extensive burns and heart valve redundancy
Publication date Author Journal Patient information Blood cultures Antibiotic regimen Auxiliary tests Surgical timing and modality Outcome 1998 Carlotto RC[4] Burns Canada, 39-year-old man, 40% total body surface area (TBSA) burns Staphylococcus aureus, Escherichia coli Unknown Autopsy at 12.5 days showed mitral valve redundancy Trauma debridement and skin grafting, skin removal from thighs at 12 days Death 2002 Apple J[5] J Trauma USA, 23-year-old woman, 44% TBSA burns Escherichia coli Ampicillin, vancomycin, gentamicin Aortic valve regurgitation, thickening No surgery Death 2002 Apple J[5] J Trauma USA, 50-year-old man, 35% TBSA burns Pseudomonas aeruginosa Ampicillin, tobramycin Aortic valve perforation (12 × 10 mm) Ross procedure on day 30 Survival 2008 Smith MA[6] Ann Thorac Surg USA, 15-year-old man, 90% TBSA burns with inhalation injury Methicillin-resistant Staphylococcus aureus (MRSA) Unknown Mitral valve redundancy (23 mm) Mitral valve replacement on day 21 Survival 2009 Gianesello L[7] Minerva Anestesiol Italy, 40-year-old woman, 85% TBSA burns Pseudomonas aeruginosa, Klebsiella pneumoniae, MRSA, Candida Penicillin, β-lactamase inhibitor, amikacin, carbapenem, vancomycin, fluconazole, rifampicin, amikacin, pefloxacin Transesophageal echocardiography (TEE) at 2.5 months showed mitral valve redundancy Bovine pericardial patch for mitral valve replacement at 3 months Death 38 days postoperatively 2015 Mohebali J[8] Burns USA, 33-year-old man, 90% TBSA burns Gram-negative rods, Pseudomonas aeruginosa Amikacin, tobramycin, polymyxin, aminoglycoside Transthoracic echocardiography (TTE) at 4 months showed mitral valve redundancy Valve repair at 5 months Survival Note. TEE, transesophageal echocardiography; TTE, transthoracic echocardiography. Table 2. Preoperative and postoperative echocardiographic parameters
Date Structural indicators Functional indicators Left atrial diameter (mm) Left ventricular end-systolic diameter (mm) Left ventricular end-diastolic diameter (mm) Right atrial diameter (mm) Right Ventricular diameter (mm) Aortic sinus (mm) Pulmonary artery (mm) Inferior vena cava (mm) Left atrial diameter (mm) Nterventricular septum thickness (mm) Ejection fraction (%) Left ventricular end-diastolic volume (mL) Left ventricular end-systolic volume (mL) Stroke volume (mL) Mitral valve (E/A) Tricuspid valve (E/A) Aortic valve (flow rate/differential pressure) 05.10 49 64 47 40 42 34 26 25 9.8 50 210 107 103 1.1 0.5 2.9/34 1.3/7 05.11 47 58 46 39 41 26 25 20 9.6 53 162 76 86 1.3 0.7 1.4/8 1.3/7 06.14 36 42 27 34 34 29 20 20 10.9 51 101 50 51 1.0/1.1 0.7/0.6 1.8/13 0.9/3 07.06 35 44 35 34 34 32 24 19 11.8 52 94 46 48 1.2/1.6 0.6/0.9 1.7/13 0.8/3 08.09 40 41 31 33 33 36 23 13 11.3 52 79 38 41 1.2/1.3 0.5/0.6 1.4/8 0.7/2 09.01 42 45 30 33 32 34 23 − 11.4 51 85 42 43 0.9/1.1 0.6/0.5 1.3/7 0.7/2 10.27 40 40 26 32 33 32 24 − 11.3 52 85 39 43 1.0/1.3 0.6/0.5 1.3/7 0.9/3 10.31 39 41 27 34 34 35 19 13 10.6 55 70 31 39 1.1/1.2 0.7/0.4 1.8/13 0.7/2 -
[1] Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med (Lond), 2020; 20, 31−5. [2] Sasaki TM, Panke TW, Dorethy JF, et al. The relationship of central venous and pulmonary artery catheter position to acute right-sided endocarditis in severe thermal injury. J Trauma, 1979; 19, 740−3. doi: 10.1097/00005373-197910000-00005 [3] Afeke I, Adu-Amankwaah J, Nyarko M, et al. Acinetobacter baumannii-induced infective endocarditis: new insights into pathophysiology and antibiotic resistance mechanisms. Future Microbiol, 2022; 17, 1335−44. doi: 10.2217/fmb-2021-0279 [4] Cartotto RC, Macdonald DB, Wasan SM. Acute bacterial endocarditis following burns: case report and review. Burns, 1998; 24, 369−73. doi: 10.1016/S0305-4179(98)00027-8 [5] Apple J, Hunt JL, Wait M, et al. Delayed presentations of aortic valve endocarditis in patients with thermal injury. J Trauma, 2002; 52, 406−9. [6] Smith MA, Yuh DD. Bioprosthetic mitral valve implantation for active mitral valve endocarditis in third degree thorax burn. Ann Thorac Surg, 2008; 85, 1791−2. doi: 10.1016/j.athoracsur.2007.10.056 [7] Gianesello L, Pavoni V, Paparella L, et al. Mitral valve endocarditis in a severe burn patient. Minerva Anestesiol, 2009; 75, 530−2. [8] Mohebali J, Ibrahim AE, MacGillivray TE, et al. Mitral valve repair via right thoracotomy for multidrug resistant pseudomonal endocarditis in a burn patient: case report and review of the literature. Burns, 2015; 41, e47−50. doi: 10.1016/j.burns.2015.01.002 [9] Shen CA, Hao DF. Vacuum sealing drainage in burn and trauma. People’s Medical Publishing House. 2016. (In Chinese) -




 下载:
下载:



 Quick Links
Quick Links