-
Endometriosis (EMs) is a hormone-dependent chronic inflammatory disease[1]. EMs is more common in women of childbearing age and is closely associated with infertility. Infertility is experienced by 30%–50% of patients with EMs, and 25%–50% of infertile women have varying degrees of EMs. The incidence of infertility in patients with EMs is 20 times higher than that in patients without EMs[2,3]. The decline in fertility caused by EMs has escalated from a health issue to a social problem and has had a significant impact on the sustainable development of the population. Endometrial polyps (EP) are protrusions formed by excessive local proliferation of the endometrium, which is locally in a high estrogen state. EP is the most common endometrial lesion in women with infertility and is a risk factor for early spontaneous abortion. Infertile women undergoing hysteroscopy have an incidence of EP as high as 32%–35%[4]. The prevalence of EP in patients with recurrent spontaneous abortion ranges from 15%–50%[4].
Both EMs and EP can reduce endometrial receptivity and lead to infertility and may have the same physiological and pathological basis[5,6]. As early as 1996, McBean reported a correlation between EMs and EP, and the risk of EMs complicated by EP was as high as 47.6%–68.35%[7]. This risk was more pronounced in patients with infertility and EMs. Nan Z reported that the probability of EP in infertile women with EMs was 47.83%, which is significantly higher than that in women without EMs[8]. The combination of EMs and EP can lead to more complex treatment strategies for infertile women while increasing their psychological burden and reducing their quality of life[9]. Hysteroscopy combined with laparoscopic surgery can not only accurately diagnose and evaluate the severity of the condition but also clear the lesion. The report states that surgical treatment can improve the pregnancy rate of infertile patients with endometriosis and that 12 months after surgery is the optimal time for fertility[10]. Therefore, it is crucial to identify the factors that affect postoperative pregnancy and the choice of postoperative assisted reproductive methods. Studies have suggested that EP may have a negative impact on pregnancy outcomes in infertile women with EMs[11]. The current clinical issues are: (1) hysteroscopy is a recognized method for treating EP[12], but it is not clear whether hysteroscopic EP resection still has a negative impact on pregnancy and pregnancy outcomes; and (2) there is no consensus on whether necessary pregnancy assistance plans should still be given to patients based on their other conditions after hysteroscopic EP resection. This meta-analysis aimed to compare the pregnancy outcomes of infertile women with EMs and EP and those with Ems and without EP after hysteroscopic and laparoscopic surgery and to clarify the impact of EP on the pregnancy outcomes of infertile women with EMs after surgical treatment.
-
We registered the systematic review and meta-analysis of PROSPERO (CRD42020149636).
-
Randomized controlled trials, cohort studies, and case-control studies. Subjects: Women with endometriosis diagnosed by pathology; infertility duration ≥ 1 year. Intervention: All patients underwent laparoscopic surgery and those with endometrial polyps underwent hysteroscopic surgery. Outcomes: Cumulative pregnancy, natural pregnancy, clinical pregnancy, embryonic arrest, and live birth rates.
Pregnancy is defined as menstrual cessation and serum β-hCG higher than normal; Clinical pregnancy is defined as the presence of a gestational sac and fetal heartbeat in the uterus in ultrasound. Embryo abortion is defined as abnormal morphology of the embryo or fetus in the uterus, absence of fetal heartbeat, or withered sac visible in ultrasound.
-
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) extension statement for the network for conducting and reporting the study. An extensive electronic search of Pubmed, Embase, Cochrane Library, Wanfang, CNKI, VIP, and SinoMed databases was undertaken for RCTs, cohort studies, and case-control studies without language restriction. Searched keywords included “endometriosis” OR “endometrioma” AND “endometrial polyps” OR “polyps” AND “infertility” OR “pregnancy”. We opted for these broad inclusive search concepts with subsequent limitations in studies of patients undergoing surgery placed during the title/abstract screening. The most recent search was conducted on October 30, 2020.
-
(1) Repeated publication; (2) Unclear or controversial outcome index; (3) The data has obvious errors; (4) The literature is not in Chinese or English.
-
Two reviewers independently assessed the eligibility of all identified citations and extracted data from original trial reports using a specifically designed form that captured information on study design, patient characteristics (including inclusion criteria, age, duration of infertility), sample sizes, follow-up duration, and details of endometriosis treatment options and outcomes. If indicated, additional trial details or protocols were obtained by contacting the original authors. Disagreements were resolved by a third reviewer to reach a consensus.
Two researchers independently evaluated the risk of bias in the included studies and crosschecked the results. The Cochrane Collaboration tool for assessing the risk of bias was used to evaluate the quality of randomized controlled clinical studies, and the Newcastle-Ottawa quality scale (NOS) was used to assess the risk of bias in observational studies[13]. The tool used to assess the risk of bias addressed eight specific domains: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, outcome of the interest not present at the start of the study, comparability of the cohort, assessment of outcome, follow-up duration, and adequacy of follow-up of cohorts. Studies that scored 7–10 were identified as high quality, those with scores of 3–6 were considered to be of moderate quality, and the others were of low quality. Only studies with a score ≥ 6 were included in this study.
-
Data analysis was performed using Review Manager (v5.3; Nordic Cochrane Center, The Cochrane Collaboration). We presented a summary of pregnancy outcomes (odds ratios [ORs]) with corresponding 95% confidence intervals (CIs) of patients with EMs and infertility and EP and compared with the non-EP group. χ2 analysis (test level α = 0.1) and I2 statistics were used to evaluate statistical heterogeneity between the included studies. If I2 was < 25%, there was no heterogeneity among the studies; 25%–50% indicated low heterogeneity; and > 50% indicated moderate-to-high heterogeneity among the studies. A fixed-effects model meta-analysis was applied in the absence of substantial heterogeneity (chi-squared test, P > 0.10, I2 index < 50%). Otherwise, we selected a random effects model to pool the data.
By comparing the differences between the aggregate indices of the fixed effects model and the random effects model, and by eliminating a single study individually, a sensitivity analysis was conducted to evaluate the robustness of the results of our meta-analysis and explore the sources of heterogeneity. If the results changed slightly after excluding a single study, the sensitivity was considered low, indicating that the results obtained were robust and reliable. Otherwise, these conclusions should be interpreted with caution. Funnel plots were used to qualitatively assess whether the included studies had publication bias. The level of statistical significance was set at α = 0.05.
-
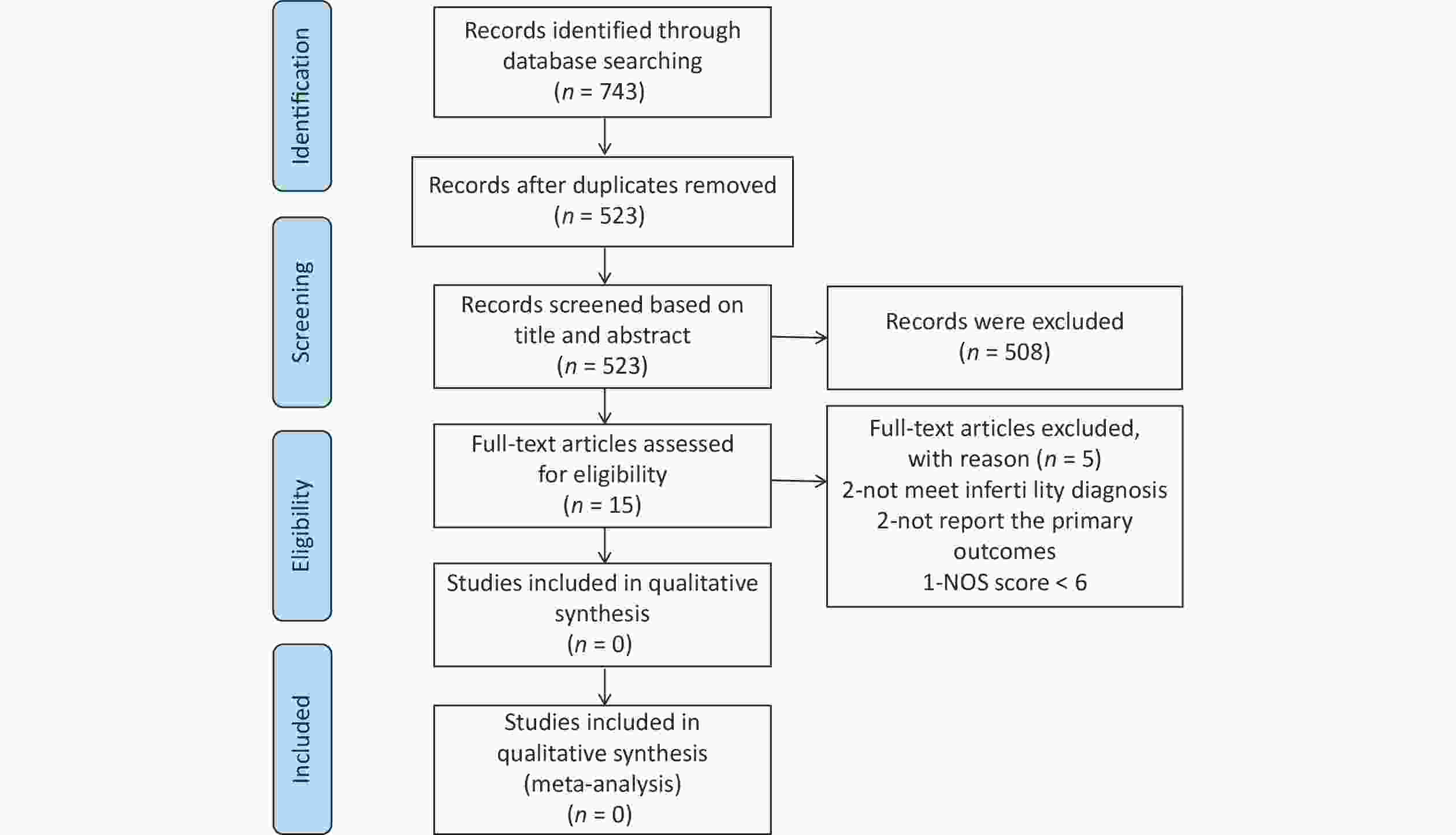
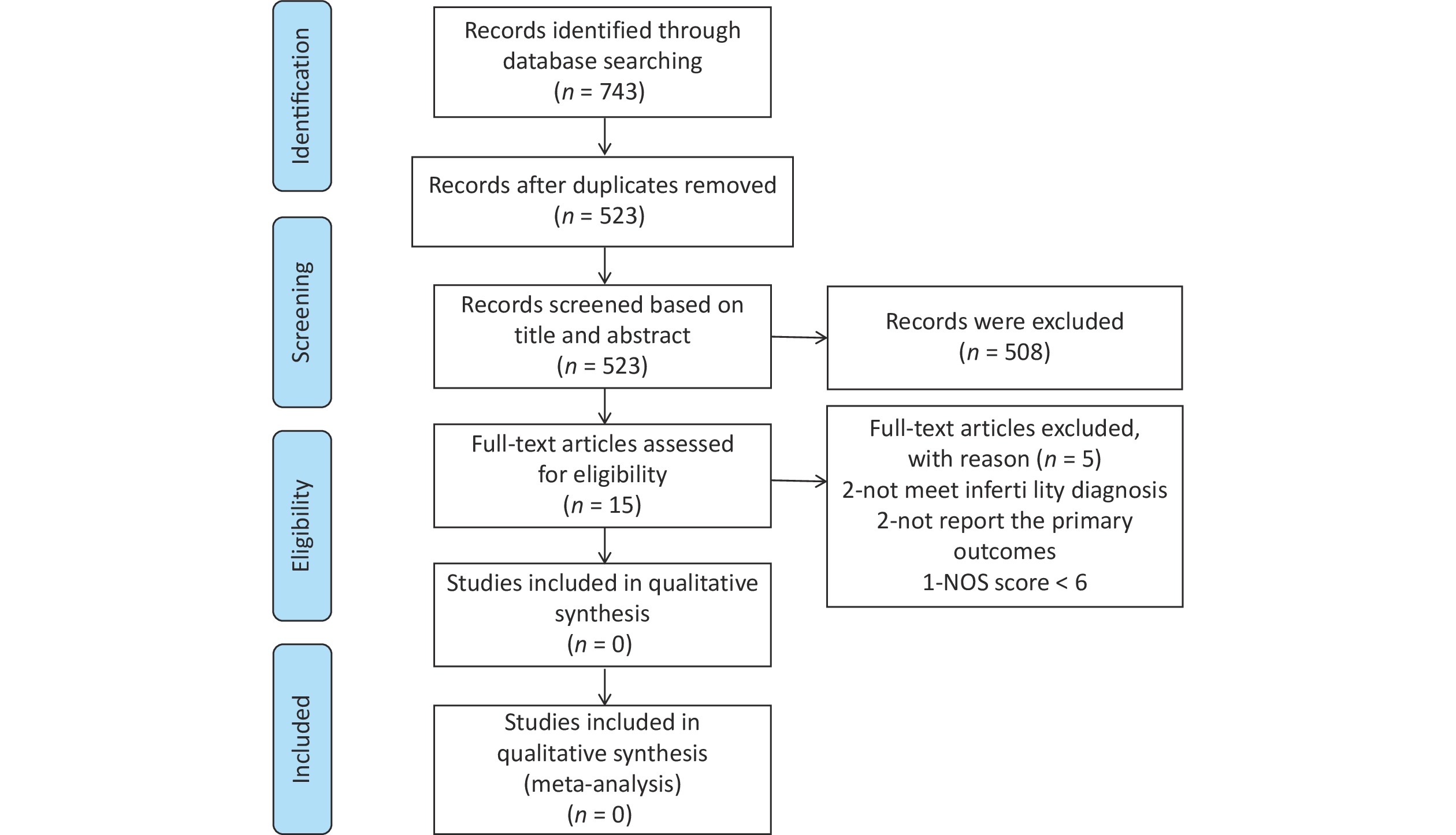
Figure 1 shows the selection process for the articles included in the meta-analysis. In total, 743 citations were retrieved from the database. After removing duplicate articles, 523 citations were screened. Subsequently, 508 articles were excluded after screening titles and abstracts. Finally, we carefully reviewed the full text of the remaining 15 articles, 10 of which were included in the meta-analysis. NOS was used to assess the quality of the included studies.
-
The characteristics of the eligible studies are shown in Table 1. Of the 10 articles included in our meta-analysis, eight were cohort studies[11,14-20] and the remaining two were case-control studies[21,22], including a total of 1,691 patients with EMs and infertility, of whom 651 patients had EP and 1040 did not have EP. All the studies were conducted in China. NOS was used to assess the quality of the included studies. All the included studies were of high quality, as shown in Table 2.
Table 1. Study characteristics
Author, Year Study design NO. of sample Follow-up duration (month) Outcomes NOS EMs+EP EMs Lin SH 2018[14] Cohort 129 172 24–36 ①②③④ 8 Wang JL 2016[15] Cohort 60 60 9–44 ①②③④⑤ 6 Chen YH 2018[16] Cohort 33 33 24 ①④⑤ 6 Xu GX 2014[17] Cohort 77 42 18 ① 6 Wang Y 2014[11] Cohort 71 181 9–44 ①②③④⑤ 8 Feng X 2018[18] Cohort 105 143 12–45 ①② 8 Wang JL 2019[19] Cohort 37 65 24 ①②③④⑤ 6 Xu WN 2020[20] Cohort 30 34 36 ①② 8 Li SZ 2017[21] Case-control 50 197 18–24 ① 6 Han Q 2019[22] Case-control 59 113 12 ①②③④⑤ 6 Note. ① , ②, ③, ④ and ⑤ represent follow-up outcomes:Cumulative pregnancy rates; Natural pregnancy rates; Clinical pregnancy rates; Embryonic arrest rates; Live birth rates. EP, endometrial polyps; EMs, endometriosis. Table 2. Risk of bias for included studies (score)
Author, Year Selection Comparability Outcome Total score Representativeness of the exposed cohort Selection of non-exposed cohort Ascertainment of exposure Outcome of interest not present at the start of the study Study controls for the most important factor Study controls for any additional factor Assessment of outcome Was follow-up long enough for outcomes to occur Adequacy of follow-up of cohorts Lin SH 2018[14] 1 1 1 1 1 0 1 1 1 8 Wang JL 2016[15] 1 1 1 1 1 0 0 1 0 6 Chen YH 2018[16] 1 1 1 1 1 0 0 1 0 6 Xu GX 2014[17] 1 1 1 1 1 0 0 1 0 6 Wang Y 2014[11] 1 1 1 1 1 0 1 1 1 8 Feng X 2018[18] 1 1 1 1 1 0 1 1 1 8 Wang JL 2019[19] 1 1 1 1 1 0 0 1 0 6 Xu WN 2020[20] 1 1 1 1 1 0 1 1 1 8 Li SZ 2017[21] 1 1 1 1 0 0 1 1 0 6 Han Q 2019[22] 1 1 1 1 0 0 0 1 1 6 -
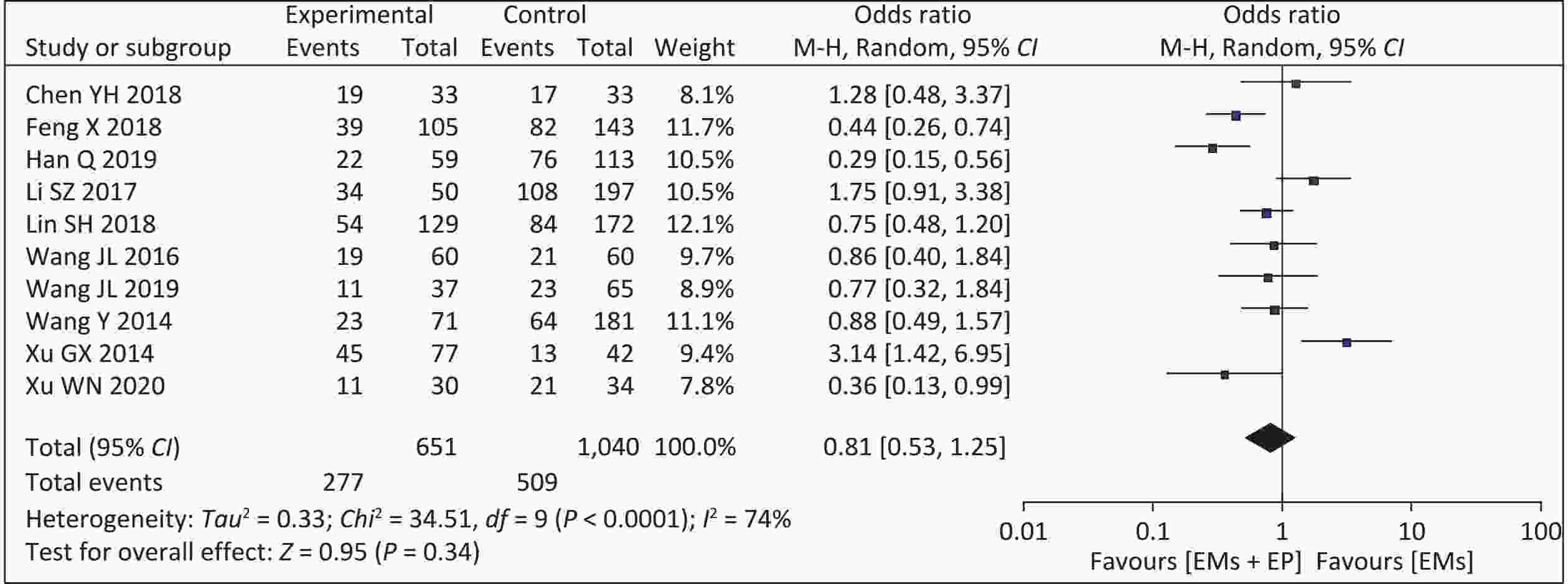
Ten studies used cumulative pregnancy rates as an outcome. Our meta-analysis of the 10 articles showed a higher cumulative pregnancy rate in patients with EMs and infertility in the non-EP group (509/1,040, 48.94%) than those in the EP group (277/651, 42.55%). Moderate heterogeneity was observed among the studies (I2 = 74%, P < 0.001). The analysis result using the random effect model found that the combined effect size was OR = 0.81 (95% CI: 0.53–1.25, P = 0.340) (Figure 2). After excluding the study with the smallest sample size, the inter-study heterogeneity was reduced, but the results showed no change.
-
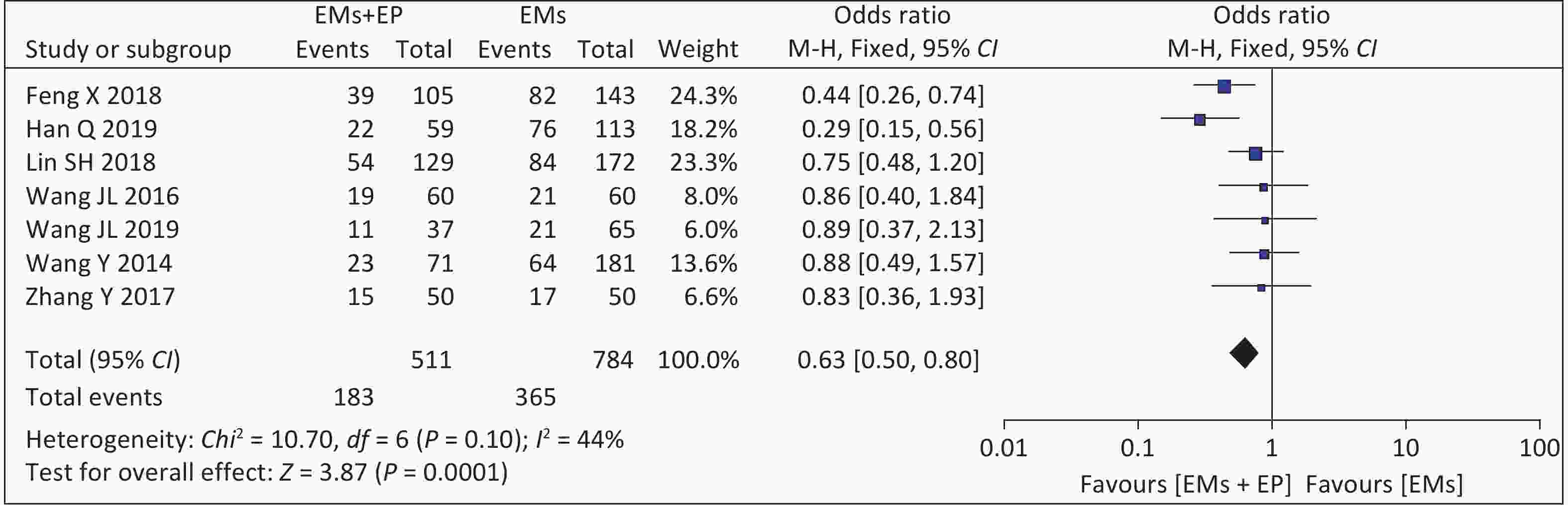
Seven studies reported natural pregnancy rates. The meta-analysis of these seven articles found that the combined effect size was OR = 1.34 (95% CI: 0.94-1.90, P < 0.001) (Figure 3). There was low heterogeneity among the studies (I2 = 44%, P = 0.100), and the analysis results using the fixed-effect model showed that EP can significantly reduce the natural pregnancy rates in patients with EMs and infertility (46.56% vs. 35.81%).
-
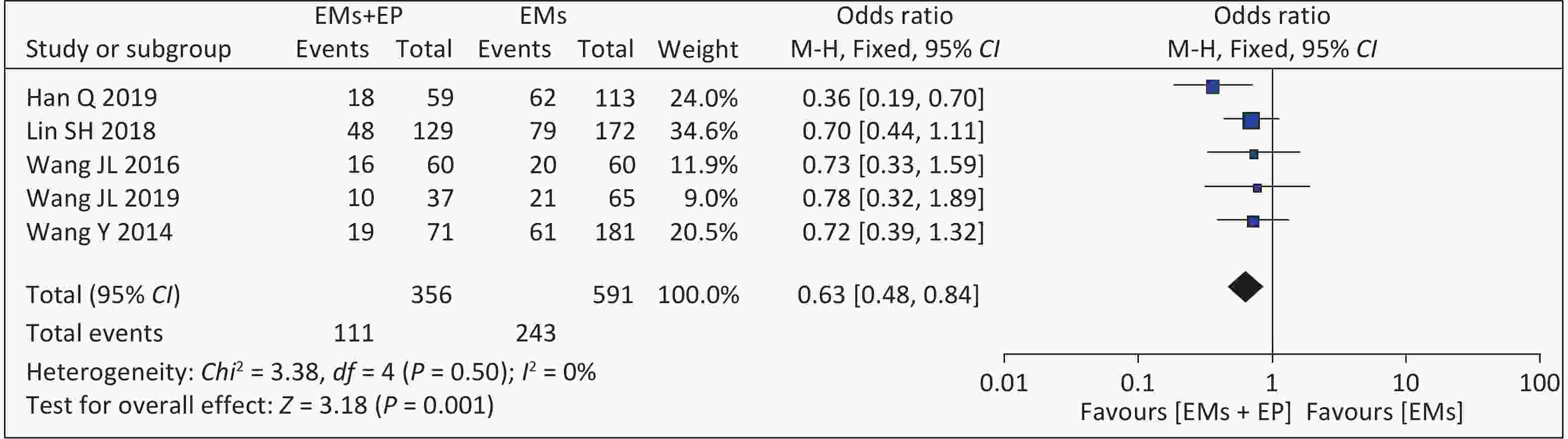
Five studies reported clinical pregnancy rates. The meta-analysis of these five articles found that the combined effect size was OR = 0.63 (95% CI: 0.48–0.84, P = 0.001) (Figure 4). There was low heterogeneity among the studies (I2 = 0%, P = 0.500), and the analysis results using the fixed-effect model showed that patients with EMs and infertility with EP had lower clinical pregnancy rates (111/356, 31.18%) than those in the non-EP group (243/591, 41.12%). Among them, the definition of clinical pregnancy rates was not explicitly mentioned in Wang JL 2016 and Wang JL 2019 articles[15,19].
-
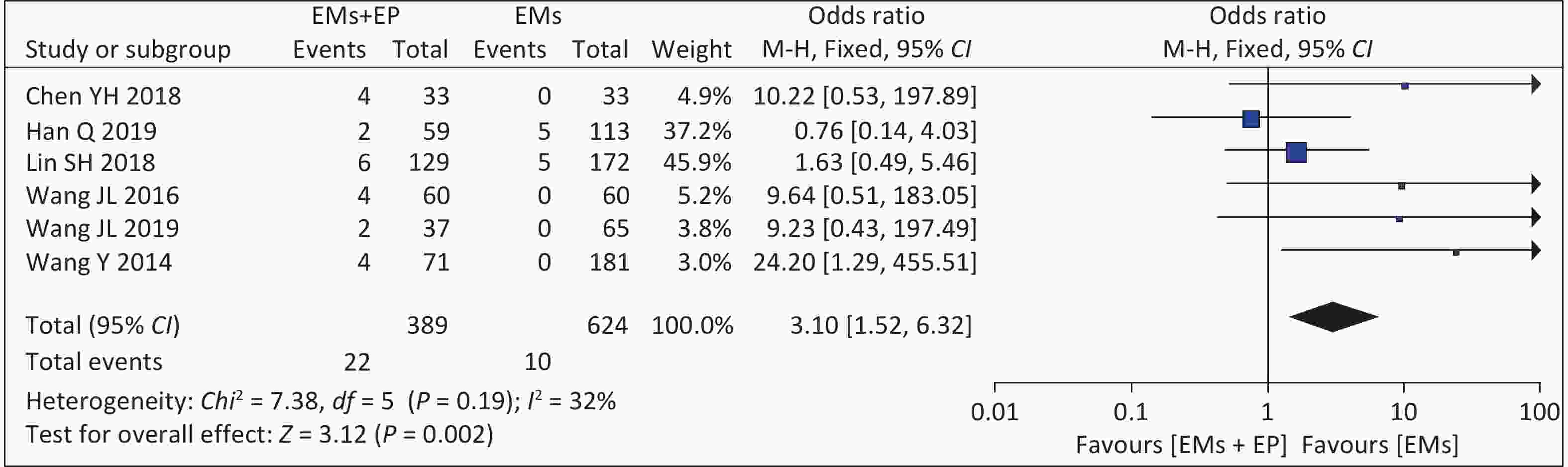
Six studies assessed embryonic arrest rates. The meta-analysis of these six articles found that the combined effect size was OR = 3.10 (95% CI: 1.52–6.32, P = 0.002) (Figure 5). There was low heterogeneity among the studies (I2 = 32%, P = 0.190), and the analysis results using the fixed-effects model showed that the embryonic arrest rates of patients with EMs and infertility in the EP group were higher than those in the non-EP group (5.66% vs. 1.60%).
-
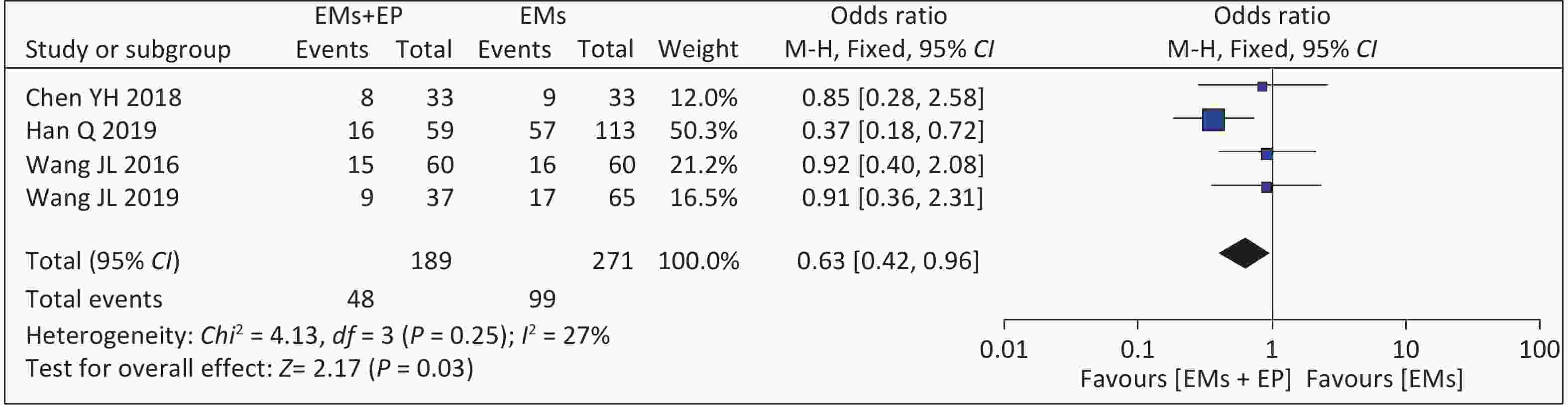
Four studies reported live birth rates. The meta-analysis of these four articles found that the combined effect size was OR = 0.63 (95% CI: 0.42–0.96, P = 0.03) (Figure 6). There was low heterogeneity among the studies (I2 = 27%, P = 0.25), and the analysis results using the fixed effects model showed that the live birth rate of patients with EMs and infertility in the EP group was 25.40% (48/189), which was lower than that of the non-EP group (99/271, 36.53%). In Wang Y’s article[11], 13 patients were still pregnant, and the pregnancy outcomes were not yet followed up. We attempted to contact the corresponding author by email but failed to reach the author.
-
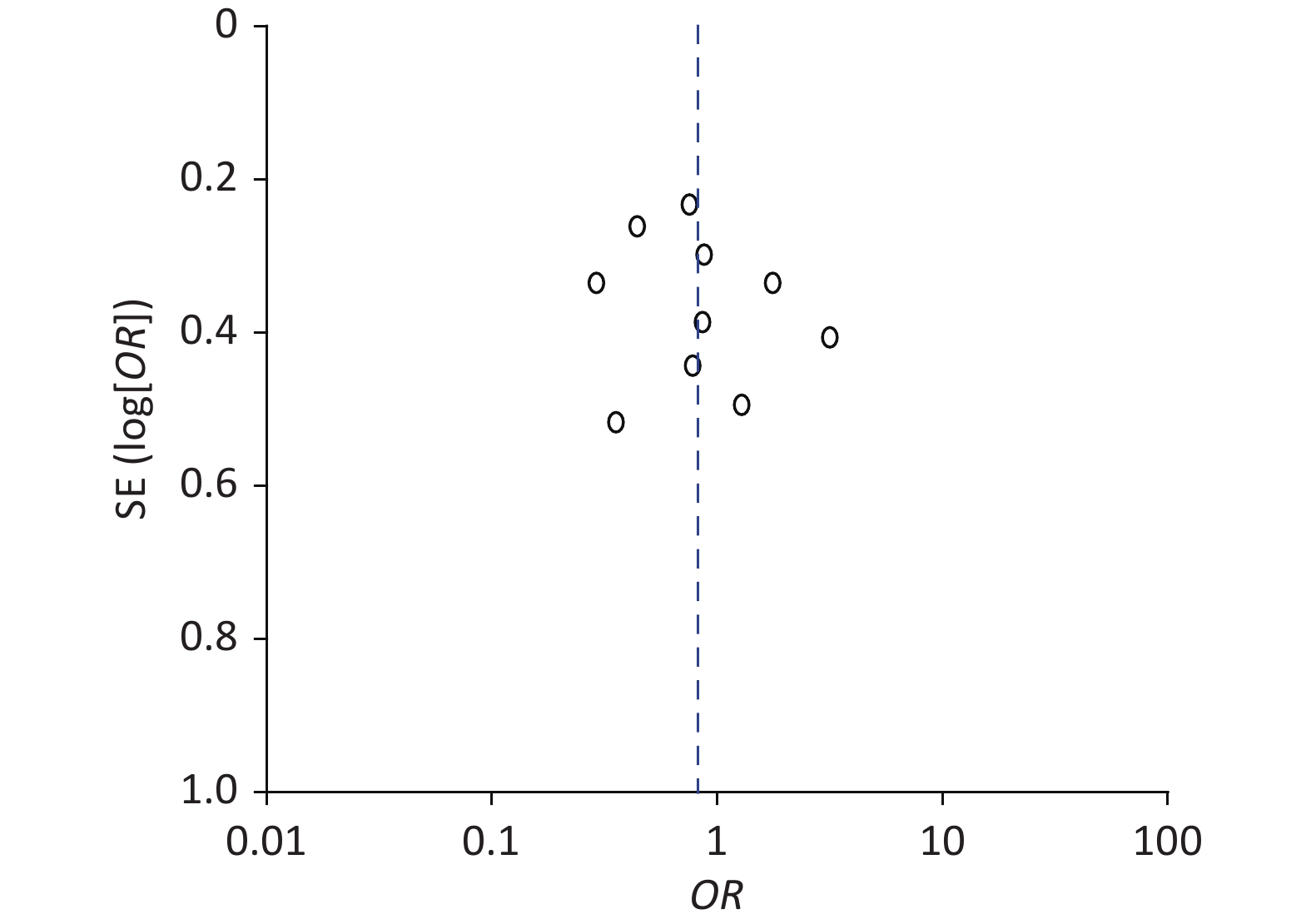
The funnel plot exploring the cumulative pregnancy rate difference between patients with EMs and infertility in the EP and non-EP groups was not symmetrical, indicating that the meta-analysis may have had publication bias (Figure 7). As other outcomes were not studied extensively, we did not draw additional funnel plots.
-
After excluding a single study, there was no obvious change in the outcomes, indicating that the sensitivity is low, and the results of our meta-analysis are robust and reliable.
-
EMs and EP are both estrogen-dependent and chronic inflammatory diseases, and they seem to be related. The abnormally high expression of estrogen receptors and aromatase in endometriosis suggests that it is an estrogen-dependent disease[23,24], and the high expression of inflammatory factors and high risk of endometritis suggest that it is a chronic inflammatory disease[25,26]. EP is an estrogen-dependent inflammatory disease. EP formation is closely related to local estrogen stimulation, long-term surface mechanical stimulation, and inflammatory factor infiltration[6]. The accuracy of EP in the diagnosis of endometritis is as high as 90%[27].
There was a significant correlation between EMs and EP, especially in infertile patients[28]. The mechanism of infertility caused by EMs is complex, and EP can further reduce the fertility of patients; both can affect the eutopic endometrial function. Increasing evidence has shown that EMs affects the normal position of the endometrium and causes implantation failure, and high expression of aromatase promotes an increase in estradiol and inhibits the progesterone required for endometrial receptivity[29]. However, the mechanism of EP-induced infertility is not only related to the destruction of uterine anatomy but also to the abnormal secretion of local proteins and cytokines. Abnormal uterine cavity morphology can hinder sperm and egg transport and binding, and thus interfere with pregnancy. For example, EP in the uterine horn is a high-risk factor for tubal obstruction, and the secretion of cytokines such as interferon and placental protein in the endometrium can also poison sperm and inhibit sperm-egg binding[30].
Laparoscopic and hysteroscopic surgeries are the gold standards for the diagnosis and treatment of EMs and EP. It has the advantages of reduced trauma, faster recovery, and fewer complications. Laparoscopic surgery can remove visible lesions, separate adhesions, restore anatomical structures, and remove harmful cytokines and inflammatory factors from the peritoneal fluid. Hysteroscopy can directly examine the shape of the uterine cavity, remove the EP, restore the shape and volume of the uterine cavity, and avoid damage to the surrounding normal endometrial tissue. Hysteroscopy combined with laparoscopy can significantly improve pregnancy rates. It has been suggested that EP can increase the natural pregnancy rate in infertile patients from 43% to 80%[31]. Our results showed that the natural pregnancy rates of patients with EMs and infertility with EP were 35.81% (183/511) and 46.56% (365/784) without EP, suggesting that the natural pregnancy rates in the EP group is significantly lower than that in non-EP group [OR = 0.63, 95% CI: 0.50–0.80, P = 0.0001]. It was also significantly lower than the 80% natural pregnancy rate after hysteroscopy in patients with infertility and EP. This may be associated with the change in the structure of the uterine cavity after hysteroscopy; however, it does not completely change the endometrial microenvironment caused by EP. When EMs and EP coexist, they have a greater effect on the endometrial microenvironment. However, it is difficult to determine whether the EP is the primary lesion or concurrent after EMs. Many studies have shown that EMs and EP have a common pathological mechanism, such as an imbalance of estrogen and progesterone receptors, imbalance of cell proliferation and apoptosis, secretion of inflammatory cytokines, and influence of the endometrial blood supply[6,29].
Therefore, the effect of EMs combined with EP on the endometrium is dual; the endometrial microenvironment is not completely improved by hysteroscopic surgery, and the natural pregnancy rates are lower than expected. Natural pregnancy rates in the EP group were significantly lower than those in the non-EP group, suggesting that patients with EMs and infertility with EP should be actively treated after surgery.
The effect of EP on endometrial receptivity is a key factor in the failure and loss of embryo implantation. The main mechanisms are as follows: the expression of vascular endothelial growth factor (VEGF) in EP tissue and surrounding tissue is decreased[32], subendometrial blood flow is decreased, the receptivity of vascularization is decreased, the expression of homeobox gene HOXA10 is down-regulated during the implantation window[33], the abnormal signal pathway of endometrial decidualization affects embryo implantation, trophoblast invasion, decidualization, and subsequent functional placental formation[34], produces placental protein to initiate immunosuppression, and interferes with sperm-egg binding and implantation[35,36]. Additionally, EP can cause local chronic inflammatory stimulation of the endometrium, eventually leading to early abortions[6]. It has been reported that the activity of nuclear factor kB (NF-KB) in infertile patients with EP is significantly higher than that in unexplained infertility patients and fertile healthy women. High activity of NF-KB may affect endometrial receptivity by mediating certain signaling pathways and regulating implantation-related protein transcription[37]. The results of our study showed that the rates of embryo termination in patients with EMs and infertility with EP was significantly higher than that in the non-EP group [OR = 3.10, 95% CI: 1.52–6.32, P = 0.002], and the live birth rate was significantly lower than that in the non EP group [OR = 0.63, 95% CI: 0.42–0.96, P = 0.03]. Although endometrial polyps are removed, they still have a negative effect on the endometrium. Owing to the lack of research on second exploration after EP, there is insufficient evidence to support the relevant assumptions. However, it should be noted that EMs and EP are often associated with pregnancy complications, such as premature rupture of membranes, preeclampsia, postpartum hemorrhage, fetal distress, placenta previa, and increased perinatal adverse outcomes, such as full-term low birth weight infants, perinatal asphyxia, and perinatal death[38,39]. There were few studies involving pregnancy complications in the included literature; therefore, this study did not carry out a statistical analysis of the outcome.
When comparing the postoperative cumulative pregnancy rates, there was high heterogeneity among the studies, and there was no significant difference in the cumulative pregnancy rates between the two groups. Due to the many factors that affect postoperative pregnancy in endometriosis[40,41], follow-up time is a very important factor. Some studies did not record follow-up time in detail; therefore, we could not further stratify the analysis based on follow-up time.
-
(1) Most of the included studies are retrospective cohort studies and case-control studies, and the included studies are only published in Chinese and English. The included literature is not comprehensive enough and may have publication bias; (2) The included studies are all single-center studies, which also have an impact on the results; (3) The research institutes are concentrated in China, and most of them are single-center studies, and only Han's research is multi-center research, which is not representative enough; it has great reference significance and has selection deviation; (4) Due to incomplete research data, we have not obtained complete data after contacting the corresponding authors. We failed to perform a subgroup analysis of the characteristics, location of the EP, and surgical stage of the EMs, which may have caused bias in the results.
-
EP may have a negative impact on the pregnancy outcomes of patients with EMs and infertility after surgery. It is necessary to expand the study area in the future and further verify this finding using a prospective cohort test.
-
EP has a high incidence rate among infertile women with EMs and negatively impacts their pregnancy. Even after surgery, EP reduces the natural pregnancy rate in patients with EMs and increases the rate of embryo loss. Therefore, attention must be paid to the negative effects of EP on patients with EMs, and accurate fertility guidance should be provided according to the actual situation of patients to promote fertility.
doi: 10.3967/bes2024.175
Impact of Endometrial Polyps on Pregnancy Outcomes in Patients with Endometriosis and Infertility: A Systematic Review and Meta-analysis
-
Abstract:
Objective To evaluate the impact of endometrial polyps (EP) on postoperative pregnancy outcomes in infertile women with endometriosis (EMs). Methods PubMed, Embase, The Cochrane Library, CNKI, VIP, SinoMed, and WanFang Data databases were searched to include clinical studies on the effect of EP on pregnancy outcomes in patients with EMs, published before August 31, 2020. A meta-analysis was performed using Rev Man 5.3 software after two investigators independently screened the literature, extracted information, and evaluated the risk of bias of the included studies. Results The meta-analysis included ten studies (651 and 1,040 in the combined EP and uncomplicated EP groups, respectively). The spontaneous pregnancy rate, clinical pregnancy rate, and live birth rate were significantly lower in the group with combined EPs than in the group without combined EPs [Odd’s ratio (OR) = 0.63, 95% confidence interval (CI): 0.50–0.80, P = 0.0001; OR = 0.63, 95% CI: 0.48–0.84, P = 0.001; OR = 0.63, 95% CI: 0.42–0.96, P = 0.03], and the rate of embryonic abortion was significantly higher than that in the uncomplicated EP group [OR = 3.10, 95% CI: 1.52–6.32, P = 0.002]. Conclusion EP may adversely affect pregnancy outcomes in patients with infertility and EMs. Even after surgical treatment, EP can still reduce natural pregnancy, clinical pregnancy, and live birth rates in infertile women with EMs and increase the risk of embryo arrest in these women. -
Key words:
- Endometriosis /
- Endometrial polyps /
- Infertility /
- Pregnancy outcome /
- Meta-analysis
The authors declare that they have no competing interests.
This research is based on publicly available data and computational analysis, which does not involve any ethical concerns.
&These authors contributed equally to this work.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Table 1. Study characteristics
Author, Year Study design NO. of sample Follow-up duration (month) Outcomes NOS EMs+EP EMs Lin SH 2018[14] Cohort 129 172 24–36 ①②③④ 8 Wang JL 2016[15] Cohort 60 60 9–44 ①②③④⑤ 6 Chen YH 2018[16] Cohort 33 33 24 ①④⑤ 6 Xu GX 2014[17] Cohort 77 42 18 ① 6 Wang Y 2014[11] Cohort 71 181 9–44 ①②③④⑤ 8 Feng X 2018[18] Cohort 105 143 12–45 ①② 8 Wang JL 2019[19] Cohort 37 65 24 ①②③④⑤ 6 Xu WN 2020[20] Cohort 30 34 36 ①② 8 Li SZ 2017[21] Case-control 50 197 18–24 ① 6 Han Q 2019[22] Case-control 59 113 12 ①②③④⑤ 6 Note. ① , ②, ③, ④ and ⑤ represent follow-up outcomes:Cumulative pregnancy rates; Natural pregnancy rates; Clinical pregnancy rates; Embryonic arrest rates; Live birth rates. EP, endometrial polyps; EMs, endometriosis. Table 2. Risk of bias for included studies (score)
Author, Year Selection Comparability Outcome Total score Representativeness of the exposed cohort Selection of non-exposed cohort Ascertainment of exposure Outcome of interest not present at the start of the study Study controls for the most important factor Study controls for any additional factor Assessment of outcome Was follow-up long enough for outcomes to occur Adequacy of follow-up of cohorts Lin SH 2018[14] 1 1 1 1 1 0 1 1 1 8 Wang JL 2016[15] 1 1 1 1 1 0 0 1 0 6 Chen YH 2018[16] 1 1 1 1 1 0 0 1 0 6 Xu GX 2014[17] 1 1 1 1 1 0 0 1 0 6 Wang Y 2014[11] 1 1 1 1 1 0 1 1 1 8 Feng X 2018[18] 1 1 1 1 1 0 1 1 1 8 Wang JL 2019[19] 1 1 1 1 1 0 0 1 0 6 Xu WN 2020[20] 1 1 1 1 1 0 1 1 1 8 Li SZ 2017[21] 1 1 1 1 0 0 1 1 0 6 Han Q 2019[22] 1 1 1 1 0 0 0 1 1 6 -
[1] Leone Roberti Maggiore U, Chiappa V, Ceccaroni M, et al. Epidemiology of infertility in women with endometriosis. Best Pract Res Clin Obstet Gynaecol, 2024; 92, 102454. doi: 10.1016/j.bpobgyn.2023.102454 [2] Bulletti C, Coccia ME, Battistoni S, et al. Endometriosis and infertility. J Assist Reprod Genet, 2010; 27, 441−7. doi: 10.1007/s10815-010-9436-1 [3] The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril, 2012; 98, 591−8. doi: 10.1016/j.fertnstert.2012.05.031 [4] Varasteh NN, Neuwirth RS, Levin B, et al. Pregnancy rates after hysteroscopic polypectomy and myomectomy in infertile women. Obstet Gynecol, 1999; 94, 168−71. [5] Shan J, Li DJ, Wang XQ. Towards a better understanding of endometriosis-related infertility: a review on how endometriosis affects endometrial receptivity. Biomolecules, 2023; 13, 430. doi: 10.3390/biom13030430 [6] Al Chami A, Saridogan E. Endometrial polyps and subfertility. J Obstet Gynaecol India, 2017; 67, 9−14. doi: 10.1007/s13224-016-0929-4 [7] McBean JH, Gibson M, Brumsted JR. The association of intrauterine filling defects on hysterosalpingogram with endometriosis. Fertil Steril, 1996; 66, 522−6. doi: 10.1016/S0015-0282(16)58562-8 [8] Zhang YN, Zhang YS, Yu Q, et al. Higher prevalence of endometrial polyps in infertile patients with endometriosis. Gynecol Obstet Invest, 2018; 83, 558−63. doi: 10.1159/000487946 [9] Mori LP, Zaia V, Montagna E, et al. Endometriosis in infertile women: an observational and comparative study of quality of life, anxiety, and depression. BMC Womens Health, 2024; 24, 251. doi: 10.1186/s12905-024-03080-5 [10] Tahmasbi Rad M, Akpinar-Isci D, Nobs T, et al. Pregnancy after laparoscopic surgery for endometriosis: how long should we wait? A retrospective study involving a long-term follow up at a university endometriosis center. Int J Gynaecol Obstet, 2023; 163, 108−14. doi: 10.1002/ijgo.14849 [11] Wang Y, Ma CH, Qiao J, et al. Natural pregnancy outcomes of hysteroscopy combined with laparoscopic surgery for infertile patients with endometriosis and endometrial polyps. Chin J Min Inv Surg, 2014; 14, 207−11. (In Chinese) [12] Bosteels J, Van Wessel S, Weyers S, et al. Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev, 2018; 12, CD009461. [13] Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol, 2010; 25, 603−5. doi: 10.1007/s10654-010-9491-z [14] Lin SH, Xie X, Liu CB, et al. Effect of laparoscopy and hysteroscopy in infertility patients with endometriosis and endometrial polyps. Chin J Reprod Contracept, 2018; 38, 542−7. (In Chinese) [15] Wang JL, Luo SH. Effect of hysteroscopy combined with laparoscopy on the pregnancy outcome of patients with endometriosis and endometrial polyps. Chin J Hum Sexol, 2016; 25, 118−20. (In Chinese) [16] Chen YH. Curative effect of uterine laparoscopic combined surgery on endometriosis complicated with endometrial polyps. Primary Medicine Forum, 2018; 22, 3955−3956. (In Chinese) [17] Xu GX, Wang BJ, Shen AR, et al. Correlation between endometriosis-associated infertility and endometrial polyps. J Chin Pract Diagn Ther, 2014; 28, 355−7. (In Chinese) [18] Feng X, Hao LJ, Lin Y, et al. Predictive value of endometriosis fertility index in natural pregnancy outcome of patients with endometriosis and endometrial polyps after hysteroscopy combined with laparoscopic surgery. Chin J Reprod Contracept, 2018; 38, 887−92. (In Chinese) [19] Wang JL. Effect of combined laparoscopic surgery on pregnancy outcomes in patients with endometriosis complicated with endometrial polyps. Qingdao Medicine and Health, 2019; 51, 422−424. (In Chinese) [20] Xu WN, Wu XJ, Chen XD, et al. The clinical value of endometriosis fertility index score in patients with endometriosis with polyps. Maternal and Child Health Care in China, 2020; 35, 296−299. (In Chinese) [21] Li SZ, Deng XH. Analysis of postoperative pregnancy related factors in patients with endometriosis complicated with infertility. J Int Reprod Health/Fam Plann, 2017; 36, 291−4. (In Chinese) [22] Han Q. Effect of sequential treatment of traditional Chinese medicine on endometrial receptivity of infertility patients with endometriosis. Beijing University of Chinese Medicine. 2019. (In Chinese) [23] Han SJ, Lee JE, Cho YJ, et al. Genomic function of estrogen receptor β in endometriosis. Endocrinology, 2019; 160, 2495−516. doi: 10.1210/en.2019-00442 [24] Zhou Y, Zeng C, Li X, et al. IGF-I stimulates ERβ and aromatase expression via IGF1R/PI3K/AKT-mediated transcriptional activation in endometriosis. J Mol Med, 2016; 94, 887−97. doi: 10.1007/s00109-016-1396-1 [25] Lee MY, Kim SH, Oh YS, et al. Role of interleukin-32 in the pathogenesis of endometriosis: in vitro, human and transgenic mouse data. Hum Reprod, 2018; 33, 807−16. doi: 10.1093/humrep/dey055 [26] Cicinelli E, Trojano G, Mastromauro M, et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil Steril, 2017; 108, 289-95. e1. [27] Cicinelli E, Resta L, Nicoletti R, et al. Endometrial micropolyps at fluid hysteroscopy suggest the existence of chronic endometritis. Hum Reprod, 2005; 20, 1386−9. doi: 10.1093/humrep/deh779 [28] Yang XH, Liu LW, Liu YH, et al. Relationship between endometriosis syndrome and endometrial polyps of infertility patients. Chin J Fam Plann, 2020; 28, 1101−3,1107. (In Chinese) [29] Ata B, Somigliana E. Endometriosis, staging, infertility and assisted reproductive technology: time for a rethink. Reprod Biomed Online, 2024; 49, 103943. doi: 10.1016/j.rbmo.2024.103943 [30] Sun Y, Zhang J, Bai WP. Higher prevalence of endometrial polyps in patients with Fallopian tube obstruction: a case-control study. J Minim Invasive Gynecol, 2019; 26, 935−40. doi: 10.1016/j.jmig.2018.07.024 [31] Spiewankiewicz B, Stelmachów J, Sawicki W, et al. The effectiveness of hysteroscopic polypectomy in cases of female infertility. Clin Exp Obstet Gynecol, 2003; 30, 23−5. [32] Feng M, Han LW, Wu SM, et al. Expression changes of VEGF and Ki-67 in endometrial tissues of infertility patients with endometrial polyps during proliferation period and implantation window period. Shandong Medicine, 2019; 59, 58−60. (In Chinese) [33] Du HL, Taylor HS. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med, 2015; 6, a023002. [34] Rackow BW, Jorgensen E, Taylor HS. Endometrial polyps affect uterine receptivity. Fertil Steril, 2011; 95, 2690−2. doi: 10.1016/j.fertnstert.2010.12.034 [35] Richlin SS, Ramachandran S, Shanti A, et al. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Hum Reprod, 2002; 17, 2742−7. doi: 10.1093/humrep/17.10.2742 [36] Sorak M, Devic A. Analysis of Glycodelin levels before and after hysteroscopic polypectomy in infertile patients. Serb J Exp Clin Res, 2018; 19, 247−53. doi: 10.1515/sjecr-2017-0001 [37] Bozkurt M, Şahin L, Ulaş M. Hysteroscopic polypectomy decreases NF-κB1 expression in the mid-secretory endometrium of women with endometrial polyp. Eur J Obstet Gynecol Reprod Biol, 2015; 189, 96−100. doi: 10.1016/j.ejogrb.2015.03.032 [38] Zullo F, Spagnolo E, Saccone G, et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil Steril, 2017; 108, 667-72. e5. [39] Kobayashi H, Kawahara N, Ogawa K, et al. A relationship between endometriosis and obstetric complications. Reprod Sci, 2020; 27, 771−8. doi: 10.1007/s43032-019-00118-0 [40] Grigoriadis G, Daniilidis A, Merlot B, et al. Surgical treatment of deep endometriosis: Impact on spontaneous conception. Best Pract Res Clin Obstet Gynaecol, 2024; 93, 102455. doi: 10.1016/j.bpobgyn.2024.102455 [41] Morcel K, Merviel P, Bouée S, et al. What is the impact of endometriosis and the AFS stage on cumulative pregnancy rates in IVF programs? Reprod Health, 2024; 21, 13. -




 下载:
下载:



 Quick Links
Quick Links