-
Cervical cancer, a leading malignancy globally, poses a significant threat to women’s health, with an estimated 604,000 new cases and 342,000 deaths reported in 2020[1]. As cervical cancer is closely linked to human papilloma virus (HPV) infection, early detection relies on HPV screening; however, late-stage prognosis remains poor, underscoring the need for novel diagnostic and therapeutic targets[2]. Most cervical cancers have poor outcomes for female reproduction and poor prognosis in the later stages. Surgical treatment, including hysterectomy and cervical conization, remains the main treatment modality for early-stage cervical cancer, and the surgical methods are constantly evolving towards minimally invasive approaches to reduce patient trauma and postoperative complications[3]. The discovery of new diagnostic markers for monitoring and early screening of cervical cancer is currently a major issue in the development of women’s health and disease prevention.
Isocitrate dehydrogenase 2 (IDH2), a mitochondrial enzyme critical for metabolic regulation (e.g., isocitrate-α-ketoglutarate conversion), has been implicated in tumorigenesis through metabolic reprogramming and epigenetic alterations[4-6]. IDH2 mutations drive oncogenesis in acute myeloid leukemia, gliomas, and other cancers, highlighting it as a promising therapeutic target. Despite these advances, the role of IDH2 in cervical squamous cell carcinoma (CESC) remains unclear. This study investigated the expression, immune interactions, and clinical relevance of IDH2 in CESC to identify its potential as a biomarker for diagnosis, prognosis, and targeted therapy.
Transcriptome data for 34 types of cancer and CESC were obtained from The Cancer Genome Atlas (TCGA), Genotype-Tissue Expression, and other databases. Wilcoxon’s test was used to analyze the difference in IDH2 expression levels between cancer tissue and normal tissue. The infiltration level of 22 types of immune cells was evaluated using the CIBERSORT[7] algorithm, and the association between IDH2 levels and immune cells was analyzed using Spearman’s correlation. Single-cell data analysis from the Broad Institute and Cancer SCEM databases was used to identify the distribution of IDH2 in CESC immune cells. Gene Ontology/Kyoto Encyclopedia of Genes and Genomes enrichment analysis was used to explore IDH2-related signaling pathways, and receiver operating characteristic (ROC) curves and the Kaplan-Meier method were used to evaluate their diagnostic and prognostic value.
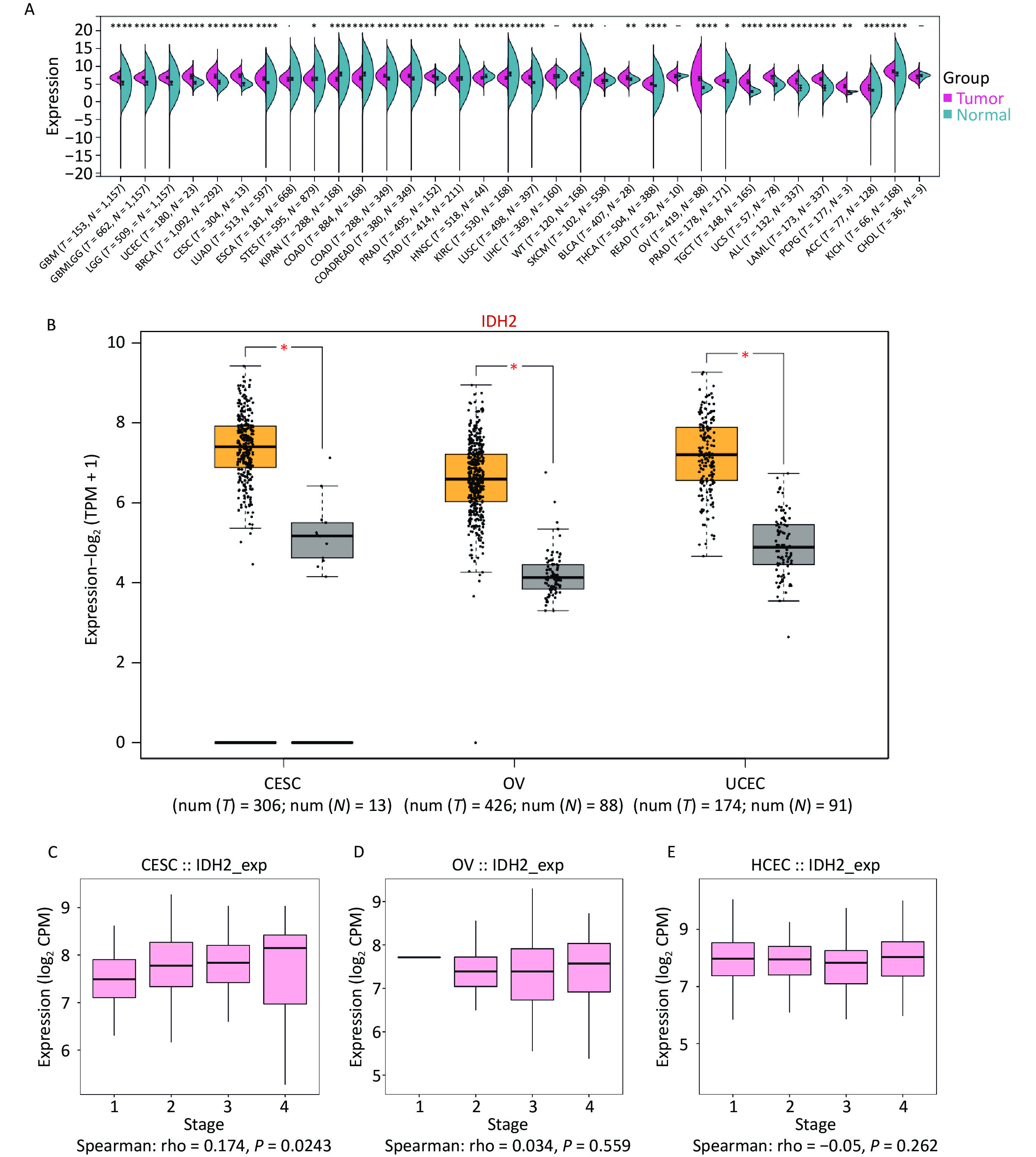
To investigate IDH2 expression in cancer, we analyzed 34 cancer types, with a focus on female reproductive tumors. IDH2 was found to be upregulated in most cancer tissues compared to normal tissues, with significant overexpression in CESC, ovarian cancer (OV), and uterine corpus endometrial carcinoma (UCEC). CESC showed the highest IDH2 levels (Figure 1A–B). Correlation analysis revealed a significant positive association between IDH2 expression levels and the clinical stage of CESC (rho = 0.174, P = 0.0243), whereas no significant association was found for OV (rho = 0.034, P = 0.559) or UCEC (rho = −0.05, P = 0.262) (Figure 1C–D). These findings prompted us to prioritize CESC for further functional analysis of IDH2 (Figure 1E). Using TCGA-CESC data, we found that IDH2 mRNA levels were significantly upregulated in CESC tissues compared to adjacent normal tissues (Supplementary Figure S1A). Survival analysis revealed that a high IDH2 expression level correlated with poorer overall survival in CESC (log-rank P = 0.012, Figure 1B). ROC curve analysis demonstrated strong diagnostic value for IDH2 levels in CESC, with an area under the curve of 0.985 (Supplementary Figure S1C). Immunohistochemical staining data from the Human Protein Atlas revealed elevated IDH2 protein levels in CESC tissues, with normal cervical tissues exhibiting higher glandular cell expression (Supplementary Figure S1D–E). Subcellular localization analysis indicated that IDH2 was primarily localized in the cytoplasm, which is consistent with its mitochondrial function (Supplementary Figure S1F). By constructing an IDH2 interaction network, we found that the expression patterns of ACO2, IDH1, IDH3A, IDH3B, and IDH3G were aggregated in samples with high IDH2 expression levels, which may indicate that these genes are more directly related to IDH2 (Supplementary Figure S2A–F). Functional enrichment analysis showed that IDH2-related genes were significantly enriched in energy metabolic pathways, such as the TCA cycle and oxidative phosphorylation, suggesting that IDH2 may promote tumor cell proliferation by regulating cell energy metabolism (Supplementary Figure S3A–F). Immune infiltration analysis showed that the expression level of IDH2 was positively correlated with the infiltration of naïve B cells (R = 0.159, P = 0.005) and resting mast cells (P < 0.05), which were negatively correlated with the infiltration of eosinophils (R = −0.166, P = 0.003), resting NK cells (R = −0.160, P = 0.005), macrophages (P < 0.05), and infiltration of memory-activated CD4+ T cells (P < 0.05) were negatively correlated (Figure 2A–E). Violin plots suggested that the infiltration scores of initial B cells and resting mast cells were significantly higher (P < 0.05) than those of the low-expression group, while the infiltration scores of eosinophils (P < 0.05) and activated memory CD4+ T cells (P < 0.01) were significantly lower than those of the low-expression group, which suggests that the high expression of IDH2 and the level of infiltration of specific immune cells are significantly correlation, and that this correlation showed variability across cell types (Figure 2F). Remarkably, in the event of deletion of the gene encoding IDH2, the proliferation and effector functions of T cells remain unimpaired, but memory CD8+ T cell differentiation was notably facilitated[8]. Single-cell sequencing further showed that IDH2 was mainly distributed in B cells, macrophages, T cells, and NK cells and was expressed at higher levels in B cells and macrophages (Figure 3A–D), suggesting that IDH2 may participate in tumor immune escape by regulating immune cell infiltration. Since IDH2 is mainly distributed in macrophages among cervical cancer immune cells, we conducted cell interaction analysis of macrophages in cervical cancer and found that they interacted most closely with dendritic cells and monocytes (Figure 3E–F). B cells can differentiate into regulatory B cells with immunosuppressive functions that inhibit the antitumor immune response and create a favorable microenvironment for tumor growth and spread[9]. In addition, in the tumor microenvironment, macrophages can be polarized into tumor-associated macrophages (TAMs). TAMs usually exhibit the characteristics of M2-type macrophages, promoting the proliferation and invasion of tumor cells. TAMs can also inhibit the activity of immune cells, ultimately leading to a poor prognosis in patients with tumors[10]. We conclude that IDH2 promotes immune cell infiltration and assists tumor cell immune escape through multiple pathways, thereby exacerbating CESC progression. In future, we will conduct in vitro and in vivo studies to elucidate how IDH2 regulates the proliferation and migration of CESC and its interaction with immune cells. IDH2 expression levels were significantly and positively correlated with the expression levels of most immune checkpoint genes (such as PD-L1 and CTLA4; P < 0.05), indicating that IDH2 may promote tumor immune escape by regulating immune checkpoint molecules (Supplementary Figure S4A–B). In addition, somatic copy number variation analysis showed that IDH2 hyperamplification was associated with increased infiltration of CD8+ T cells and dendritic cells (P < 0.05), suggesting that gene copy number changes may affect the immune cell composition in the tumor microenvironment (Supplementary Figure S4C).
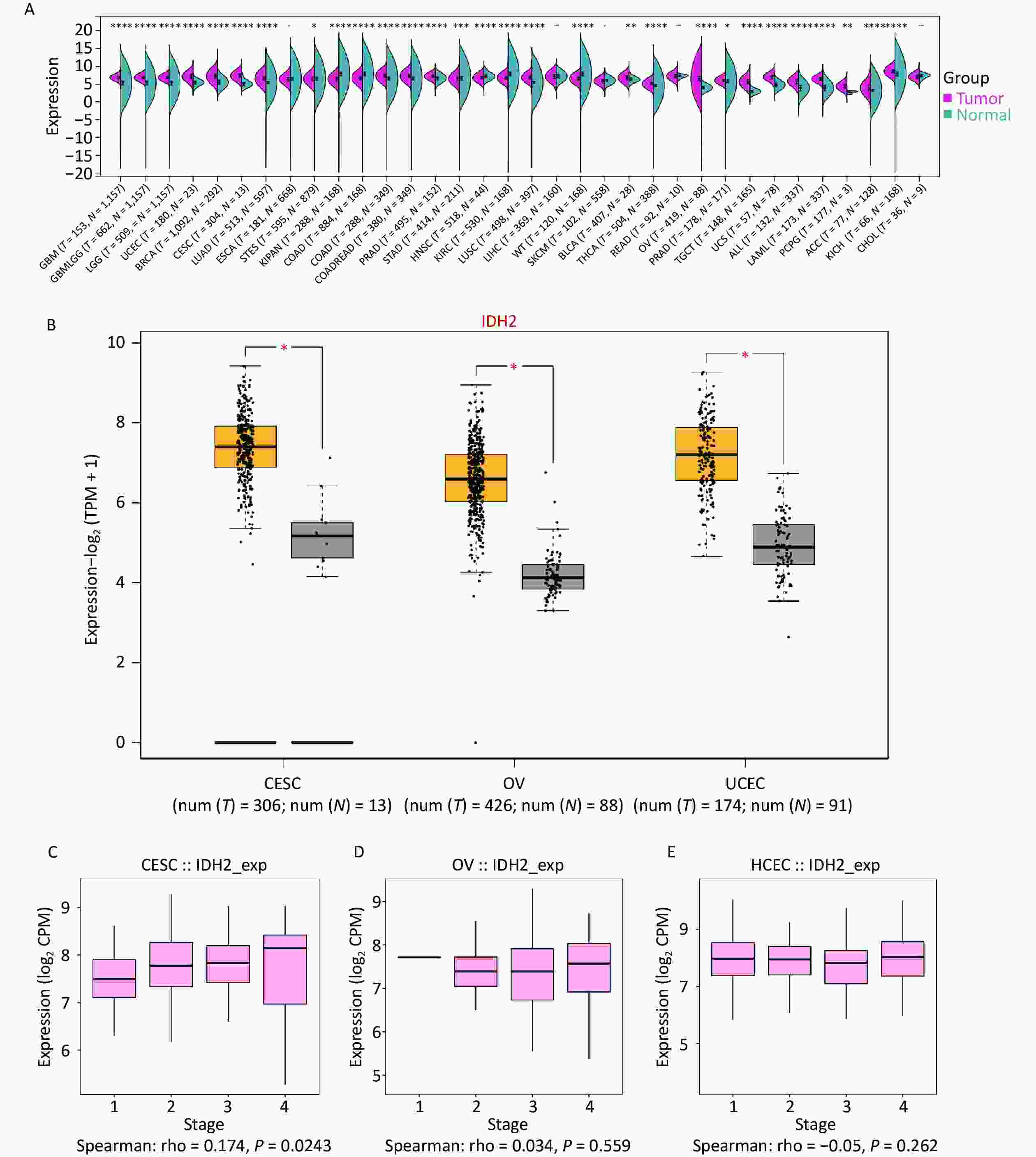
Figure 1. Expression of isocitrate dehydrogenase 2 (IDH2) in cancer. (A) Comparative expression status of IDH2 in 34 different types of cancer tissues versus normal tissues from The Cancer Genome Atlas (TCGA) database. (B) Expression profile of IDH2 in gynecological tumors. (C) Correlation between IDH2 expression level and stage in cervical squamous cell carcinoma (CESC) from the TISIDB database (Spearman’s correlation: rho = 0.174, P = 0.0243). (D) Correlation between IDH2 expression level and stage of ovarian cancer (OV) from the TISIDB database (Spearman’s correlation: rho = 0.034, P = 0.559). (E) Correlation between IDH2 expression level and stage of uterine carcinosarcoma (UCES) from the TISIDB database (Spearman’s correlation: rho = −0.05, P = 0.262).
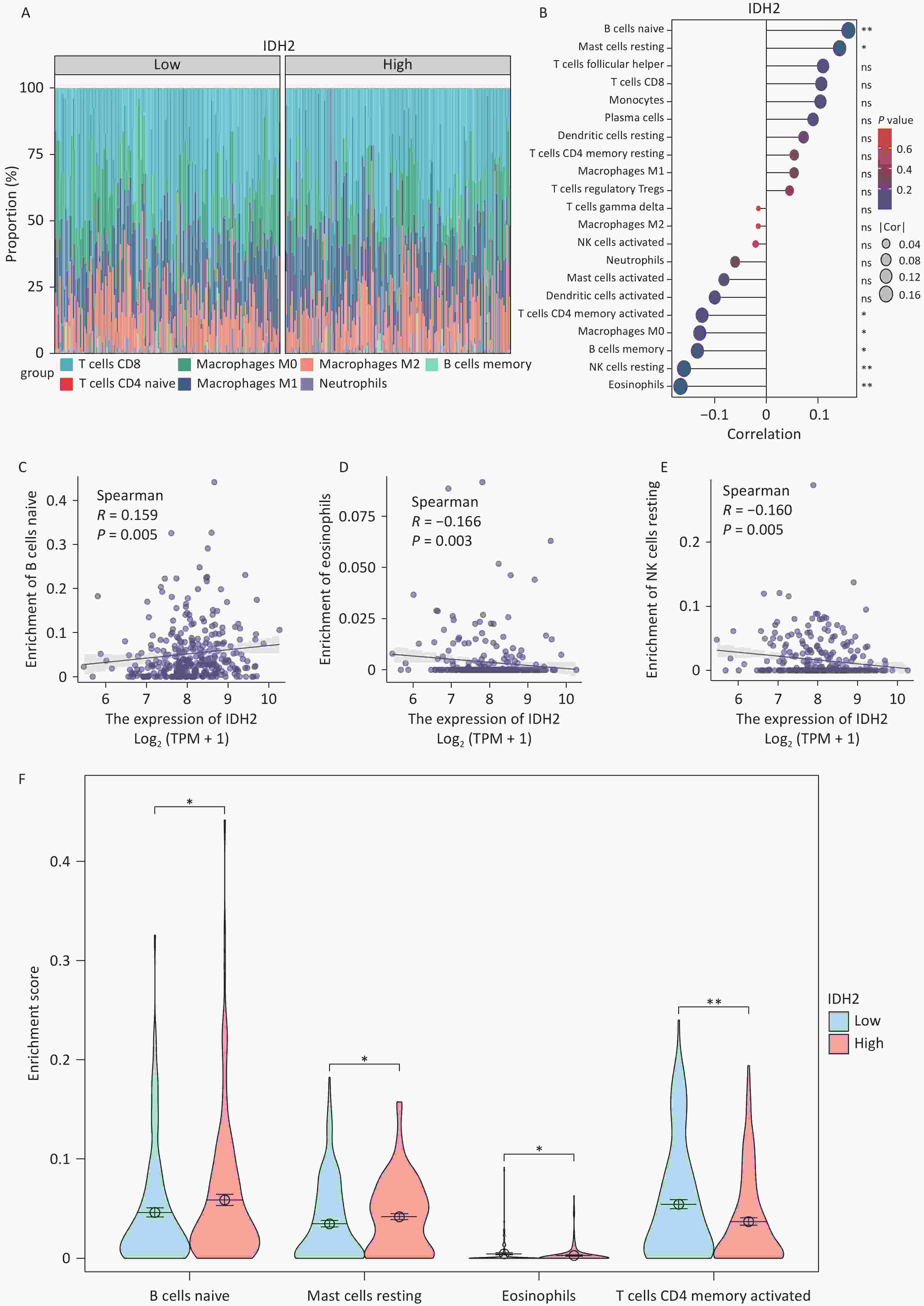
Figure 2. Immune Infiltration Analysis of isocitrate dehydrogenase 2 (IDH2). (A) Overlay map of immune cells with IDH2 in cervical squamous cell carcinoma (CESC). (B) Relationship between the expression level of IDH2 and immune infiltrating cells. Correlation analysis between (C) the number of naive B cells (R = 0.159, P = 0.005); (D) the number of eosinophils (R = −0.166, P = 0.003); and (E) the number of resting natural killer (NK) cells (R = −0.160, P = 0.005) and IDH2 expression levels. (F) Correlation analysis among the numbers of naive B cells, resting mast cells, eosinophils, and activated CD4+ memory T cells between the IDH2-high- and low-expression groups in CESC.
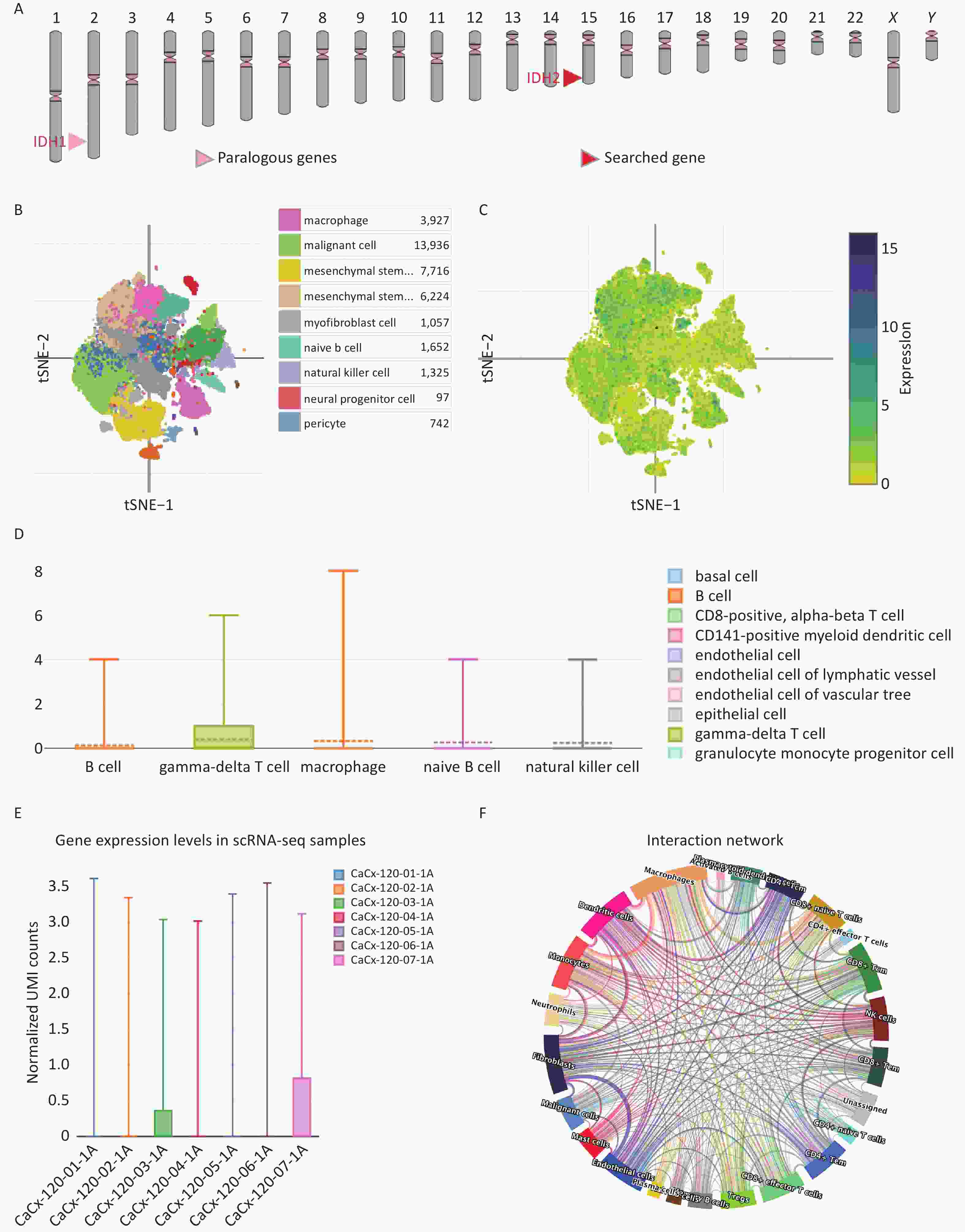
Figure 3. Single-Cell Analysis of isocitrate dehydrogenase 2 (IDH2) in Cervical Squamous Cell Carcinoma. (A) The positional distribution of the IDH2 gene on the chromosome and its interacting genes. (B) The distribution of all cells in cervical squamous cell carcinoma (CESC). (C) The distribution of IDH2 in all CESC cells. (D) Violin plot of IDH2 expression in immune cells of CESC. (E) Violin plot of IDH2 expression in multiple CESC samples. (F) “Circos” plot of the interaction between macrophages and other cells in CESC.
In conclusion, IDH2, a mitochondrial enzyme critical for metabolic regulation, was significantly overexpressed in CESC and exhibited strong diagnostic and prognostic value. Mechanistically, IDH2 may promote tumor progression by regulating cell energy metabolism and facilitating immune cell infiltration, thereby contributing to tumor immune escape. Specifically, IDH2 expression levels were positively correlated with the infiltration of certain immune cells and the expression levels of immune checkpoint genes, suggesting its role in modulating the tumor microenvironment. In addition, the expression level of IDH2 had a certain correlation with prognosis and diagnosis. Thus, IDH2 may serve as a promising biomarker for the diagnosis and prognosis of CESC and as a potential therapeutic target for cervical cancer treatment. However, this study has some limitations. These findings were primarily based on bioinformatic analyses and require further validation through in vitro and in vivo experiments. Additionally, the exact molecular mechanisms by which IDH2 regulates tumor cell proliferation and migration and interactions with immune cells in CESC remain to be elucidated. Future research should incorporate experimental studies to clarify these mechanisms and to explore the therapeutic potential of IDH2 in cervical cancer.
doi: 10.3967/bes2025.067
Single-Cell and Multi-Dimensional Data Analysis of the Key Role of IDH2 in Cervical Squamous Cell Carcinoma Progression
-
Conceptualization and funding acquisition: Huiying Zhang. Supervision and funding acquisition: Lisha Shu. Data curation and writing–original draft preparation: Xiaojuan Liu. Methodology and software: Zhenpeng Zhu. Writing, review, and editing: Chenyang Hou. Software validation and funding acquisition: Hui Ma and Chunxing Ma. Supervision: Xiaoyan Li. All the authors reviewed the results and approved the final version of the manuscript.
The authors declare that they have no competing interests.
This study did not require ethics board approval as it did not include human or animal trials.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. Expression of isocitrate dehydrogenase 2 (IDH2) in cancer. (A) Comparative expression status of IDH2 in 34 different types of cancer tissues versus normal tissues from The Cancer Genome Atlas (TCGA) database. (B) Expression profile of IDH2 in gynecological tumors. (C) Correlation between IDH2 expression level and stage in cervical squamous cell carcinoma (CESC) from the TISIDB database (Spearman’s correlation: rho = 0.174, P = 0.0243). (D) Correlation between IDH2 expression level and stage of ovarian cancer (OV) from the TISIDB database (Spearman’s correlation: rho = 0.034, P = 0.559). (E) Correlation between IDH2 expression level and stage of uterine carcinosarcoma (UCES) from the TISIDB database (Spearman’s correlation: rho = −0.05, P = 0.262).
Figure 2. Immune Infiltration Analysis of isocitrate dehydrogenase 2 (IDH2). (A) Overlay map of immune cells with IDH2 in cervical squamous cell carcinoma (CESC). (B) Relationship between the expression level of IDH2 and immune infiltrating cells. Correlation analysis between (C) the number of naive B cells (R = 0.159, P = 0.005); (D) the number of eosinophils (R = −0.166, P = 0.003); and (E) the number of resting natural killer (NK) cells (R = −0.160, P = 0.005) and IDH2 expression levels. (F) Correlation analysis among the numbers of naive B cells, resting mast cells, eosinophils, and activated CD4+ memory T cells between the IDH2-high- and low-expression groups in CESC.
Figure 3. Single-Cell Analysis of isocitrate dehydrogenase 2 (IDH2) in Cervical Squamous Cell Carcinoma. (A) The positional distribution of the IDH2 gene on the chromosome and its interacting genes. (B) The distribution of all cells in cervical squamous cell carcinoma (CESC). (C) The distribution of IDH2 in all CESC cells. (D) Violin plot of IDH2 expression in immune cells of CESC. (E) Violin plot of IDH2 expression in multiple CESC samples. (F) “Circos” plot of the interaction between macrophages and other cells in CESC.
-
[1] Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2021; 71, 209−49. doi: 10.3322/caac.21660 [2] Perkins RB, Wentzensen N, Guido RS, et al. Cervical cancer screening: a review. JAMA, 2023; 330, 547−58. doi: 10.1001/jama.2023.13174 [3] Kyrgiou M, Mitra A, Paraskevaidis E. Fertility and early pregnancy outcomes following conservative treatment for cervical intraepithelial neoplasia and early cervical cancer. JAMA Oncol, 2016; 2, 1496−8. doi: 10.1001/jamaoncol.2016.1839 [4] Xu X, Zhao JY, Xu Z, et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem, 2004; 279, 33946−57. doi: 10.1074/jbc.M404298200 [5] Joseph JW, Jensen MV, Ilkayeva O, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem, 2006; 281, 35624−32. doi: 10.1074/jbc.M602606200 [6] Koh HJ, Lee SM, Son BG, et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J Biol Chem, 2004; 279, 39968−74. doi: 10.1074/jbc.M402260200 [7] Chen BB, Khodadoust MS, Liu CL, et al. Profiling tumor infiltrating immune cells with CIBERSORT. In: von Stechow L. Cancer Systems Biology: Methods and Protocols. Humana Press. 2018, 243-59. [8] Jaccard A, Wyss T, Maldonado-Pérez N, et al. Reductive carboxylation epigenetically instructs T cell differentiation. Nature, 2023; 621, 849−56. doi: 10.1038/s41586-023-06546-y [9] Rawlings DJ, Metzler G, Wray-Dutra M, et al. Altered B cell signalling in autoimmunity. Nat Rev Immunol, 2017; 17, 421−36. doi: 10.1038/nri.2017.24 [10] Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol, 2010; 11, 889−96. doi: 10.1038/ni.1937 -




 下载:
下载:



 Quick Links
Quick Links