-
Squamous cell carcinoma (SCC) is the most common in oral and maxillofacial regions and occupy more than 90% of the malignant lesions in the oral cavity[1]. The complex anatomy of the head and neck region gives rise to intricate patterns of local invasion and regional spread, which makes primary tumors more difficult to eradicate. Surgical resection combined with chemotherapy using cytotoxic drugs or radiation therapy are routine means for the treatment of SCC. However, drug resistance, severe side effects, and high recurrence rate markedly limit the effectiveness of clinical treatments. Consequently, the search for novel anti-cancer strategies with low toxicity has acquired the interest of many researchers.
Ginsenoside Rh2 (G-Rh2) is a biologically active phytochemical extracted from ginseng, which is used as a traditional herbal medicine. It has been reported that G-Rh2 exhibits a variety of biological effects, such as reducing blood glucose and delaying aging along with anti-inflammatory, anti-tumor, anti-bacterial, and anti-fatigue activity[2-3]. Previous studies had demonstrated that G-Rh2 was found to have an inhibitory effect on the growth of many kinds of cancer cells, which could be attributed to this compound being capable of arresting cell cycle at G1 phase by modulating cell cycle-related proteins, and inducing cell death through the activation of death-associated signaling pathways, such as JNK and caspase as well as via the promotion of death receptor expression[4]. However, there are only a few reports about the effects of G-Rh2 on SCC cells. This study was aimed at investigating the potential anti-tumor effect of G-Rh2 on KB cell line, and to explore the underlying anti-tumor mechanisms involved, such as the regulation of the expression of MMP-2 and VEGF proteins as well as the production of reactive oxygen species (ROS). This could provide a strategy for the development of low-toxicity drugs against cancer.
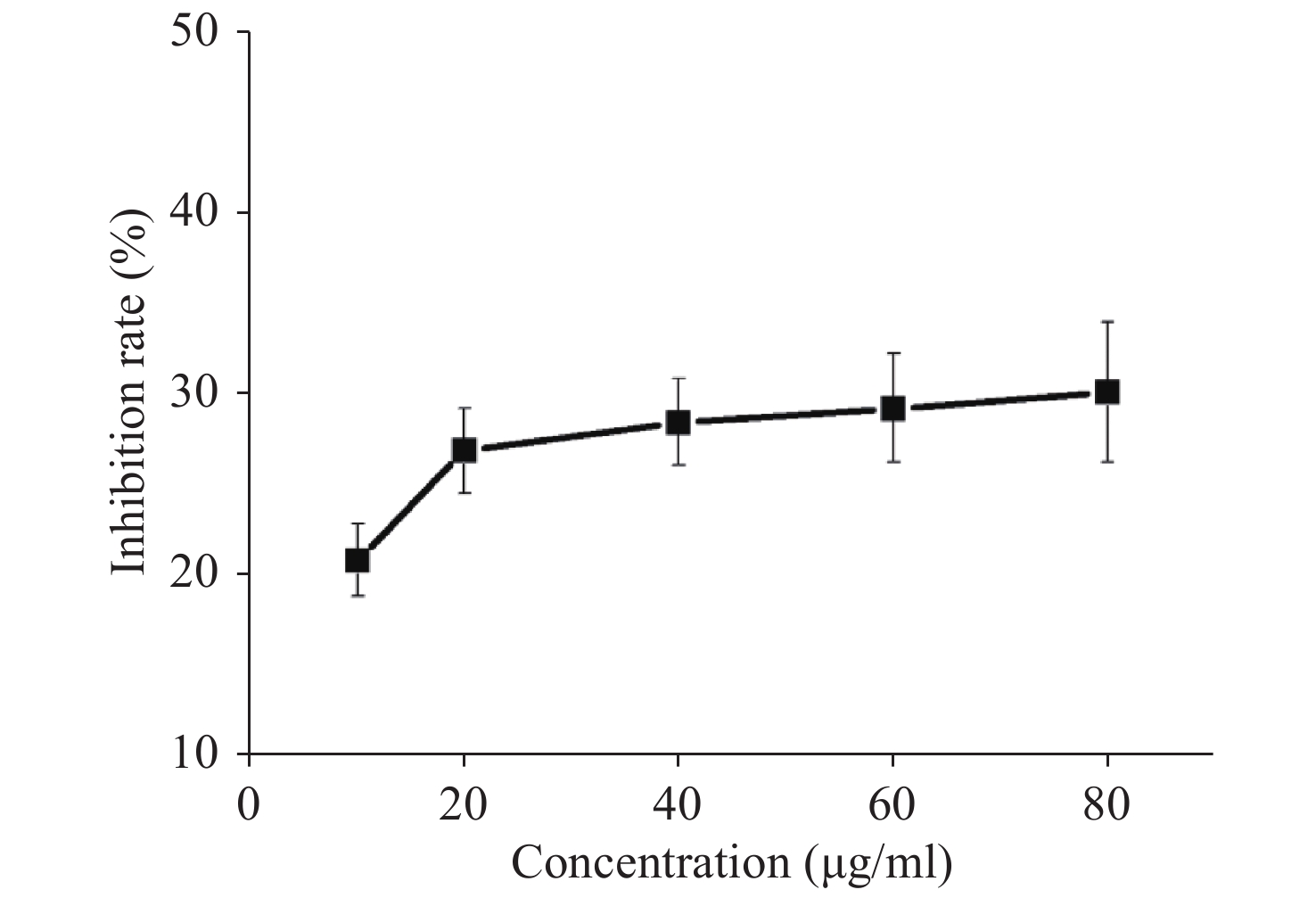
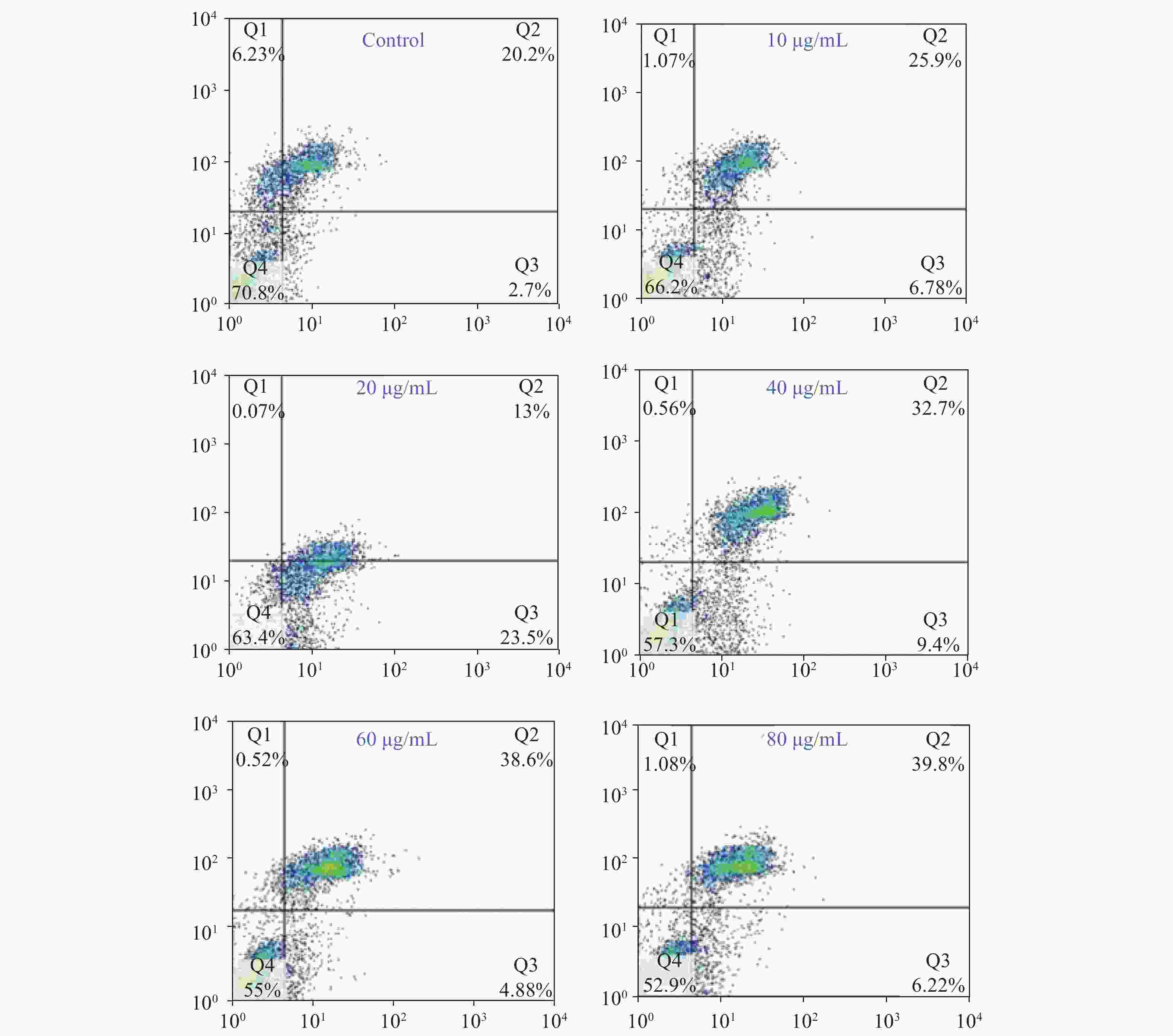
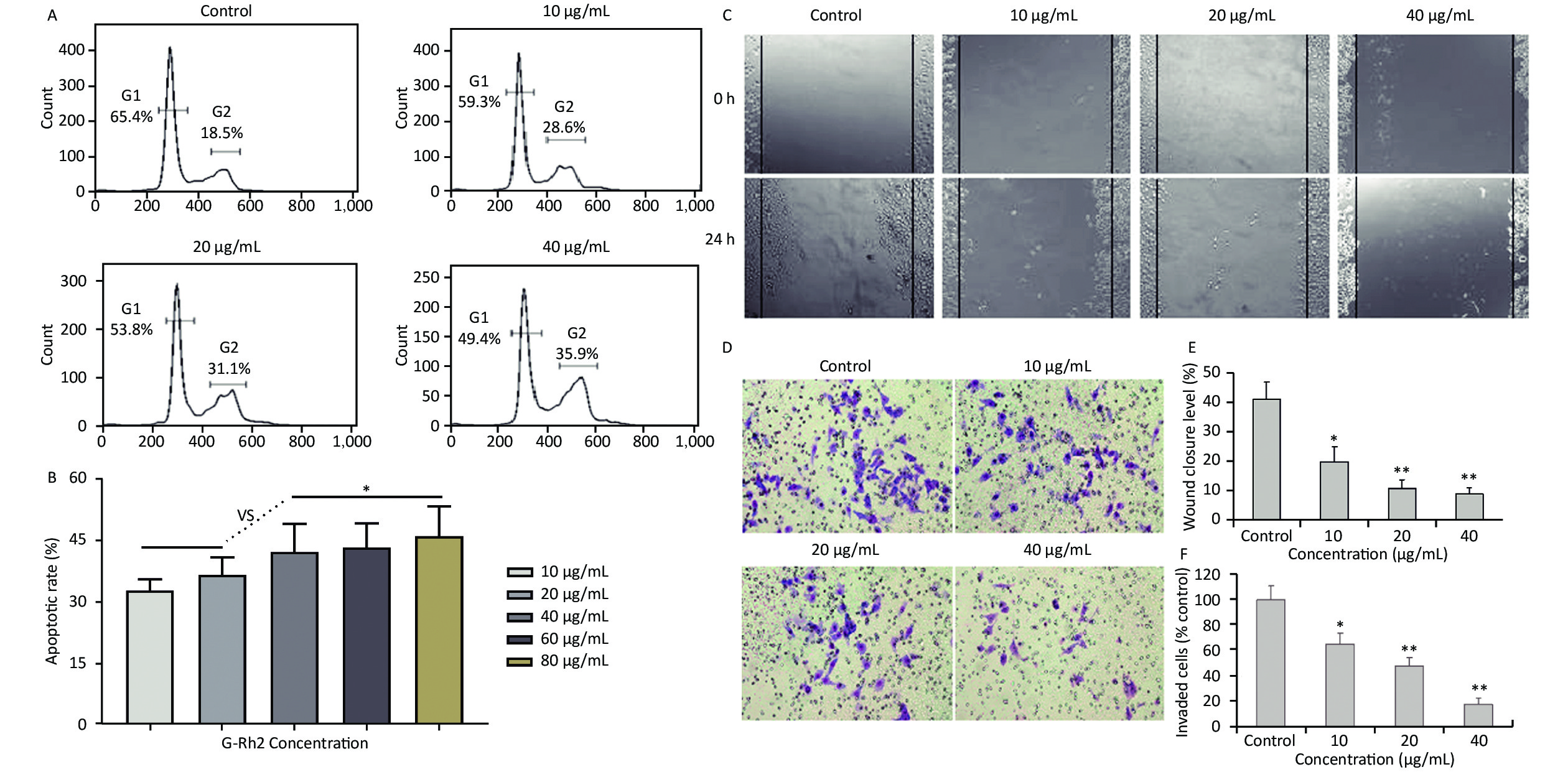
Acquired experimental results showed that different concentrations of G-Rh2 inhibited the viability of KB cells in a dose-dependent manner (Supplementary Figures S1–S2 available in www.besjournal.com). The treatment of KB cells with different concentrations of G-Rh2 resulted in a significant reduction in the IC50 values compared to that observed in the control group. Subsequently, we chose the concentrations of 10, 20, 40 μg/mL, which had low cytotoxic effect on control cells and inhibitory effect on KB cells for the follow-up experiments. On the basis of quantitative analysis of the cell cycle, the KB cell cycle was found to be arrested at the G0/G1 phase after G-Rh2 induction. After 24 h treatment of KB cells with different concentrations of G-Rh2 (10, 20, 40 µg/mL) showed a significant increase in the percentage of KB cells in the G2/M phase accompanied by a decrease in their percentage in the G0/G1 phase (Figure 1A). These results indicated that the cell cycle was arrested at the G0/G1 phase by G-Rh2. The growth of KB cells was inhibited by reducing the synthesis of DNA and mitosis to induce apoptosis. Based on the results of Annexin V/PI staining, the apoptotic rate of the cells treated with different concentrations of G-Rh2 (10, 20, 40, 60, 80 µg/mL) after 24 h was found to be 32.7%, 36.5%, 42.1%, 43.5%, and 46.0%, respectively (Figure 1B and Supplementary Figure S3 available in www.besjournal.com). These findings suggest that G-Rh2 may regulate cell cycle transition, thereby suppressing cancer cell proliferation. Obviously, G-Rh2 could inhibit the proliferation and promote the generation of apoptotic bodies in KB cells in a dose-dependent manner.
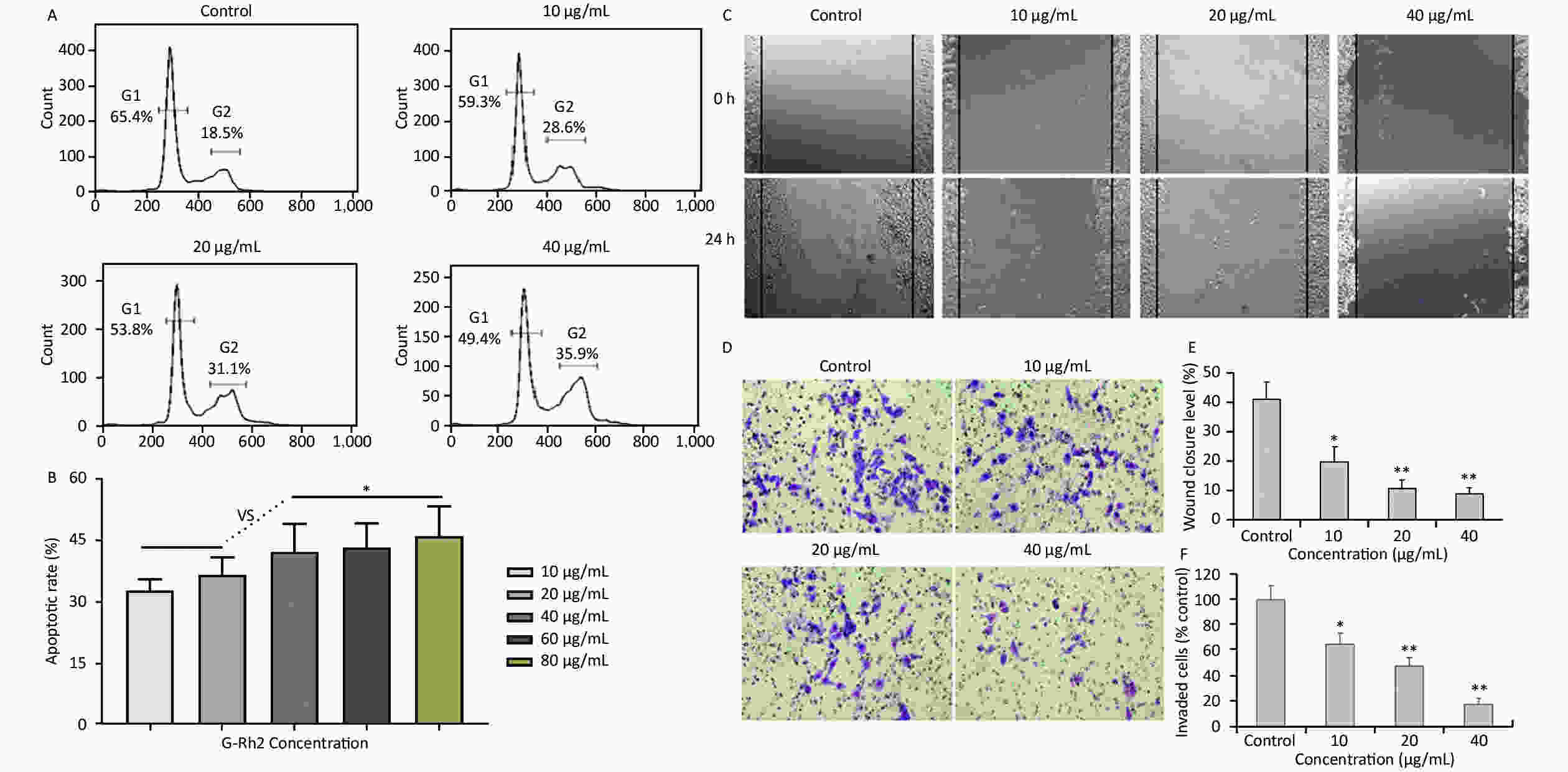
Figure 1. (A) Effects of G-Rh2 on KB cells cycle distribution. (B) The rate of KB cell apoptosis. *P < 0.05. (C) Inhibitory effect of G-Rh2 on KB cells migration. (D) Inhibitory effect of G-Rh2 on KB cells invasion. (E) Quantitative results. *P < 0.05, **P < 0.01. (F) Quantitative results. *P < 0.05, **P < 0.01.
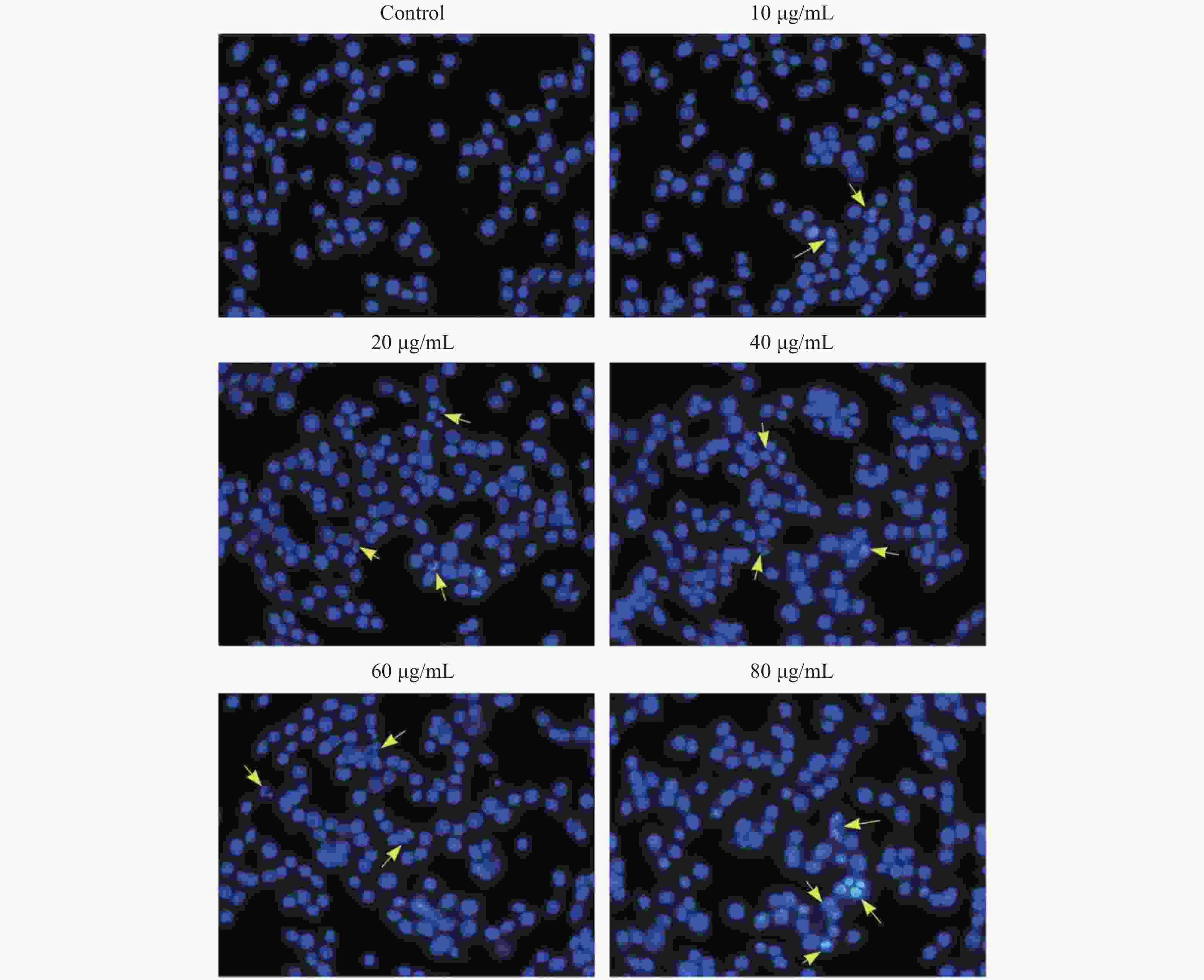
Figure S2. The morphology of KB Cell by Hoechst 33342 staining. The yellow arrow points to the apoptotic cells.
One typical characteristic of oral cancer is the ability of metastasis, including local invasion and metastasis to lymph nodes in early stages. Hence, scratch test and matrigel invasion assay were used to detect the ability of cell migration and invasion. The rate of scratch wound healing after 24 h for different concentrations of G-Rh2 (from 0 to 40 µg/mL) were 40.9%, 19.5%, 10.5%, and 8.8% (vs. control group, P < 0.01 = 0.0098), respectively (Figure 1C and 1E). Especially, treating with G-Rh2 at a concentration of 40 μg/mL for 24 h clearly inhibited the migration ability of KB cells as compared to that of the control group (Figure 1D and 1F). The number of cells invading through the matrigel and filtering into the lower surface in the group treated with G-Rh2 at various concentrations (10, 20, and 40 µg/mL) were as follows, 65.4%, 43.2%, 16.9% (vs. control, P < 0.01 = 0.0081). These data suggest that G-Rh2 inhibits the invasion and migration ability of KB cells in a dose-dependent manner.
It is common knowledge that tumor cell invasion is largely regulated by the activity of matrix metalloproteinases (MMPs) as it plays a major role in extracellular matrix degradation related to cancer cell invasion, tumor metastasis, and angiogenesis. Among them, MMP-2 expression in cancer cells is considered to be most closely associated with metastatic potential[5]. G-Rh2 prevented the invasion and migration of human pancreatic cancer cell line, Bxpc-3, through the downregulation of MMP-2 and MMP-9[6]. Herein, the expression of MMP-2 was examined by gelatin zymography. Compared to the control group, G-Rh2 downregulated the expression of MMP-2 in KB cells in a dose-dependent manner (Figure 2A and 2B). Protein expression levels of MMP-2 (among the concentrations of 10, 20, 40 µg/mL) were 78.9%, 52.1%, and 50.2%, respectively (P < 0.01 = 0.0092) (Figure 2B). Average optical density value of MMP-2 expression in the control group was 0.37 ± 0.04, while it was 0.15 ± 0.03 in 40 µg/mL group (Figure 2C and 2D). These results reveal that G-Rh2 inhibits MMP-2 in a dose-dependent manner to affect KB cell invasion.
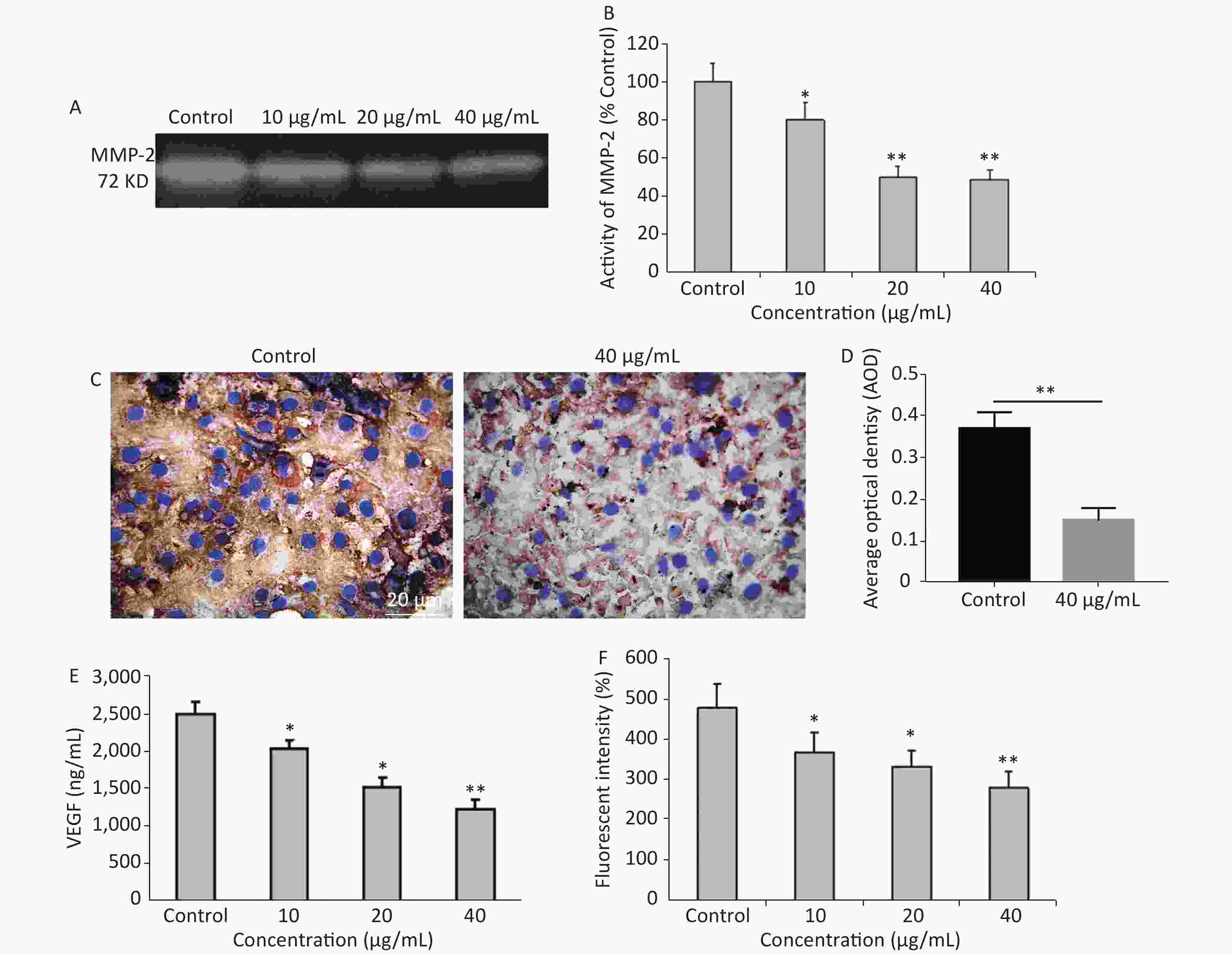
Figure 2. (A) Representative images of MMP-2 expression. (B) Quantitative results. *P < 0.05, **P < 0.01. (C, D) The expression of MMP-2 protein in KB cells. **P < 0.01. (E) Quantitative results of VEGFlevels after G-Rh2 treatment. *P < 0.05, **P < 0.01. (F) Effects of G-Rh2 on the intracellular ROS formation of KB cells. *P < 0.05, **P < 0.01.
In addition, angiogenesis is required for tumor progression and metastasis, and VEGF is one of the most important inducers of angiogenesis[7]. Currently, the overexpression of VEGF is observed in pre-cancerous conditions[8]. The results showed that G-Rh2 was capable of decreasing the expression of VEGF, thereby inhibiting tumor angiogenesis. Figure 2E reveals that compared to the control group, the expression level of VEGF was decreased in the group treated with G-Rh2 (10, 20, 40 µg/mL), thereby indicating that G-Rh2 could downregulate the expression of VEGF in KB cells and inhibit the angiogenesis.
More significantly, the mechanisms of signal transduction by VEGF receptors have received significant attention. The role of ROS as a mediator in signal transduction by human VEGFR-2 has been reported[9]. ROS stimulates induction of VEGF secretion, which in turn increases ROS through the activation of NADPH oxidases involved in VEGFR-2 autophosphorylation[10]. DCFH-DA is a fluorescent dye that diffuses through cell membranes, it is hydrolyzed by intracellular esterases to dichlorodihydrofluorescein (DCF). The increase of cellular fluorescence is often interpreted as an increase in intracellular levels of ROS. After being treated with different concentrations of G-Rh2 (10, 20, and 40 µg/mL), the level of DCF fluorescence was found to decrease in KB cells as compared to that in the control (Figure 2F). Apparently, the ROS in KB cells were eliminated by treatment with G-Rh2. In this study, G-Rh2 can remove the ROS of KB cells in a dose-dependent manner. By decreasing the production of ROS, the signal of angiogenesis is blocked subsequently by inhibition of tumor progression and metastasis.
Cytoskeletal remodeling in response to drug stimuli is a fundamental process in dying apoptotic cells. In this scenario, cell mechanical properties depend on the conversion of extracellular cues into intracellular cytoskeletal responses, whereas the changes in cell stiffness are largely related to cytoskeleton depolymerization and remodeling. Hence, a biomechanical perspective of analysis was further employed to verify the influence of G-Rh2 on the mechanical phenotype signatures involved in the anti-tumor effect. This study focused on the single-cell topography and cellular mechanical properties based on cytoskeletal alterations during mechanical remodeling caused by G-Rh2. Figure 3A and 3B showed that the non-treated control cells were characterized by a polygonal shape on cell profiling. The microstructure of cell surface was homogeneous and revealed that the cell membrane was relatively smooth and intact, whereas the nucleus displayed continuous upheaval and plumping. Treated G-Rh2-taken, the KB cells shrank and became round. The cell membrane was severely damaged, exhibiting larger surface fluctuations and appearance of crimp on plasma membrane. Furthermore, actin filament arrangement of the cytoskeleton is also used as an important factor to evaluate the micromorphological changes at a single cell level. In contrast to the normal cells, the actin filament arrangement of the cancer cells was chaotic and irregular. When exposed to different concentrations (from 10 µg/mL to 40 µg/mL) of G-Rh2, the fine cytoskeletal structures/units of all the cells were disassembled and indistinguishable, and the actin filaments were almost dissolved. With an increasing concentration of G-Rh2, the elastic modulus of the treated group exhibited a downward trend.
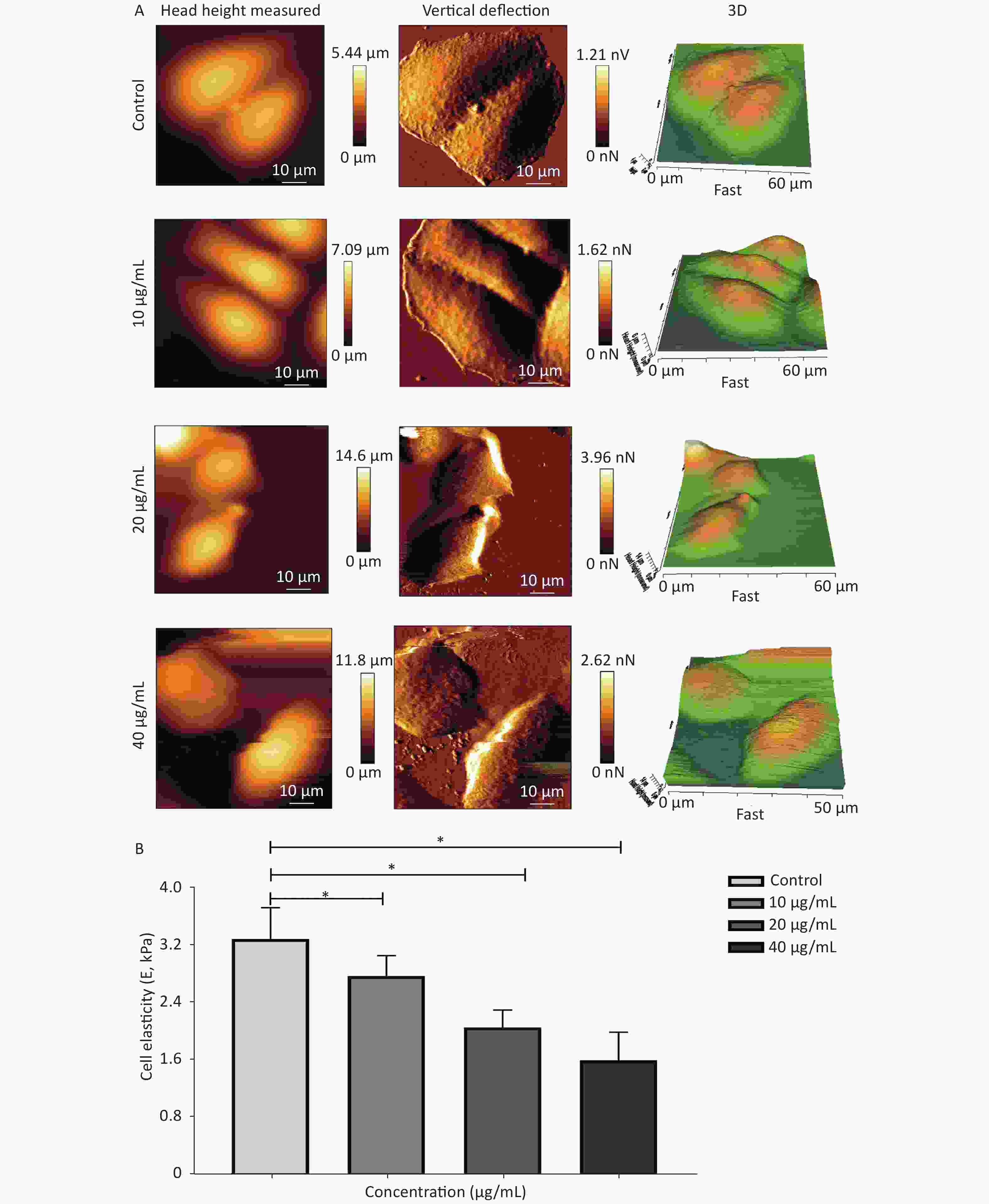
Figure 3. (A) Cellular micromorphology and (B) mechanical properties (elastic modulus, E) of KB cells under the influence of G-Rh2. *P < 0.05
Substantial obtained evidences on the effects of G-Rh2 in SCC reveal that it inhibits cell proliferation and induces cell apoptosis in a dose-dependent manner as well as arrests cells in the G0/G1 phase, while changing the mechanical phenotype signatures (such as single-cell topography and cell stiffness) in this process. Importantly, G-Rh2 inhibits KB cell invasion and metastasis by downregulating MMP-2 and VEGF expression, and decreasing the ROS level, thereby exerting a high antioxidant activity and low toxicity. This study provides a novel experimental basis for the potential utility of G-Rh2 in clinical application against SCC.
No potential conflicts of interest were disclosed.
The authors would like to thank Dr GAN Lu (Department of Heavy Ion Radiation Medicine, Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China) and Prof. YU Zhan Hai (School of Stomatology, Lanzhou University, Lanzhou, China) for their kind assistance in proofreading the manuscript and discussion. This study is supported by grants from the Fundamental Research Funds for the Central Universities of Lanzhou University (lzujbky-2017-17, lzujbky-2017-143 and lzujbky-2020-cd03).
ZHANG Bao Ping conceived the design, LIU Bin and LI Zhi Ge supervised the study. LI Bo performed single cell mechanics experiments. GAO Shu Ting, HUANG Chun Juan, LI Rui Ping performed the molecular biology experiments. ZHANG Bao Ping and NING Jing wrote the manuscript. LI Bo, CHENG Jing Yang and CAO Rui analyzed the data. LIU Bin and LI Zhi Ge reviewed the manuscript.
doi: 10.3967/bes2020.093
Anti-cancer Effect of 20(S)-Ginsenoside-Rh2 on Oral Squamous Cell Carcinoma Cells via the Decrease in ROS and Downregulation of MMP-2 and VEGF
-
&These authors contributed equally to this work
注释: -
Figure 1. (A) Effects of G-Rh2 on KB cells cycle distribution. (B) The rate of KB cell apoptosis. *P < 0.05. (C) Inhibitory effect of G-Rh2 on KB cells migration. (D) Inhibitory effect of G-Rh2 on KB cells invasion. (E) Quantitative results. *P < 0.05, **P < 0.01. (F) Quantitative results. *P < 0.05, **P < 0.01.
Figure 2. (A) Representative images of MMP-2 expression. (B) Quantitative results. *P < 0.05, **P < 0.01. (C, D) The expression of MMP-2 protein in KB cells. **P < 0.01. (E) Quantitative results of VEGFlevels after G-Rh2 treatment. *P < 0.05, **P < 0.01. (F) Effects of G-Rh2 on the intracellular ROS formation of KB cells. *P < 0.05, **P < 0.01.
-
[1] Feller L, Lemmer J. Oral Squamous Cell Carcinoma: Epidemiology, Clinical Presentation and Treatment. J Cancer Ther, 2012; 3, 263−8. doi: 10.4236/jct.2012.34037 [2] Leung KW and Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med, 2010; 5, 20. doi: 10.1186/1749-8546-5-20 [3] Kim JH, Yi YS, Kim MY, et al. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res, 2017; 41, 435−43. doi: 10.1016/j.jgr.2016.08.004 [4] Ham YM, Lim JH, Na HK, et al. Ginsenoside-Rh2-induced mitochondrial depolarization and apoptosis are associated with reactive oxygen species- and Ca2+- mediated c-Jun NH2-terminal kinase 1 activation in HeLa cells. J Pharmacol Exp Ther, 2006; 319, 1276−85. doi: 10.1124/jpet.106.109926 [5] Shia CS, Suresh G, Hou YC, et al. Suppression on metastasis by rhubarb through modulation on MMP-2 and uPA in human A549 lung adenocarcinoma: an ex vivo approach. J Ethnopharmacol, 2011; 133, 426−33. doi: 10.1016/j.jep.2010.10.020 [6] Tang X P, Tang G D, Fang C Y, et al. Effects of ginsenoside Rh2 on growth and migration of pancreatic cancer cells. World Journal of Gastroenterology, 2013; 19, 1582−92. doi: 10.3748/wjg.v19.i10.1582 [7] Rivera LB and Bergers G. Tumor angiogenesis, from foe to friend. Science, 2015; 349, 694−5. doi: 10.1126/science.aad0862 [8] Costache MI, Ioana M, Iordache S, et al. VEGF Expression in Pancreatic Cancer and Other Malignancies: A Review of the Literature. Rom J Intern Med, 2015; 53, 199−208. [9] Ge G, Yan Y and Cai H. Ginsenoside Rh2 Inhibited Proliferation by Inducing ROS Mediated ER Stress Dependent Apoptosis in Lung Cancer Cells. Biol Pharm Bull, 2017; 40, 2117−24. doi: 10.1248/bpb.b17-00463 [10] Kim YM, Kim SJ, Tatsunami R, et al. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol, 2017; 312, 749−64. doi: 10.1152/ajpcell.00346.2016 -




 下载:
下载:



 Quick Links
Quick Links