-
Hyperlipidemia is a major risk factor for cardiovascular disease, which is the leading cause of death worldwide[1]. Hyperlipidemia is characterized by an elevated plasma concentration of low-density lipoprotein cholesterol (LDL-C). One of the main treatments for hyperlipidemia and cardiovascular disease is a reduction in LDL-C levels[2]. A 1.0 mmol/L reduction in LDL-C levels is associated with a 21% reduction in major vascular events in both women and men[3,4]. Although statins are the first-line therapy for lowering LDL-C levels[5], some patients are unable to achieve sufficient treatment effects because of treatment resistance, insufficient response, or adverse events[6]. The risk of liver damage, myositis, rhabdomyolysis, and modest but significant increases in the development of diabetes mellitus have been recognized[7].
Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors are human monoclonal antibodies that bind to PCSK9, a serine protease crucial for cholesterol homeostasis. PCSK9 is the ninth member of the pro-protein convertase family and is an important regulator of LDL-C metabolism. It prevents the recycling of the receptor back to the cell surface by binding to LDL receptor (LDLR), initiating endocytosis and lysosomal degradation of LDLR[8]. This causes a decline in LDL-C clearance and elevates serum LDL-C levels[9]. PCSK9 inhibitors, in turn, can reduce serum LDL-C levels by preventing LDLR degradation. They can lower LDL-C levels by approximately 50%–60% on top of conventional lipid lowering treatments[10], reverse plaque formation, and significantly improve the prognosis of patients with stable coronary arteriosclerotic vessels[11]. Monoclonal antibodies against PCSK9 have been the most common PCSK9 inhibitors since they were first described in 2009[12], and they have been dubbed the greatest advancement in lipid therapy over the past 30 years[13]. At present, the two PCSK9 inhibitors approved by the Food and Drug Administration (FDA) in 2015 that are fully human monoclonal antibodies that bind to extracellular PCSK9 are alirocumab and evolocumab. In February 2021, the International Lipid Expert Panel proposed that if LDL-C levels remain > 1.0 mmol/L after 4–6 weeks of dual lipid-lowering therapy (high-intensity statin combined with ezetimibe), a PCSK9 inhibitor should be added[14]. Many studies have evaluated the LDL-C-lowering efficacy of PCSK9 inhibitors in patients with cardiovascular disease risk, heterozygous and homozygous familial hyperlipidemia, and statin intolerance[15,16]. PCSK9 inhibitors have demonstrated therapeutic efficacy in reducing LDL-C levels and significantly improving cardiovascular outcomes, with favorable clinical tolerability and safety[17]. However, because statin therapy can increase the risk of diabetes in a dose-dependent manner, concerns have been raised regarding whether PCSK9 inhibitors might also increase this risk[18-20].
PCSK9 is expressed in multiple tissues, including the liver and extrahepatic tissues such as the small intestine, kidney, and pancreas[8,17]. PCSK9 inhibition can increase LDLR and cholesterol concentrations in pancreatic β-cells, which may impair glucose metabolism and reduce insulin secretion[21]. However, there is increasing evidence that the loss of circulating PCSK9 does not worsen glycemia because it is compensated for by local PCSK9 expression in β-cells and other islet cells[19]. Furthermore, some Mendelian randomization studies of genetic polymorphisms that mimic the effects of PCSK9 inhibitors have suggested that lifelong reductions in LDL-C levels may be associated with an increased risk of diabetes[13,20,22-25], whereas others have not reported this[26]. Moreover, clinical studies have also yielded conflicting results. Some studies have shown that PCSK9 inhibitors do not affect glucose levels[27]. Plasma PCSK9 levels are not significantly associated with new-onset diabetes, suggesting that inhibition of the PCSK9 extracellular pathway is not deleterious to glucose homeostasis[28]. However, an increased risk of new-onset diabetes may be observed[29]. A small but significant increase in fasting glucose levels was reported with bococizumab compared with placebo over a median exposure period of approximately 1 year[30]. Therefore, PCSK9 inhibitors may have unexpected effects.
However, there is a lack of randomized controlled trials on whether PCSK9 inhibitors affect glucose levels in Chinese patients with hyperlipidemia. This study aimed to evaluate the effects of treatment on glycemia in patients with hyperlipidemia following a 24-week management in China.
-
This was a secondary analysis of a randomized, double-blind, placebo-controlled phase 1b/2 clinical trial. The original trial aimed to evaluate the safety, tolerability, and efficacy of multiple subcutaneous injections of recaticimab (SHR-1209) in hyperlipidemia patients administered stable doses of statins. Recaticimab (SHR-1209) is a humanized immunoglobulin monoclonal antibody that binds to PCSK9 with high affinity and causes robust LDL-C reduction in healthy volunteers[6]. The main study was conducted at 12 sites in China. A total of 110 participants were enrolled in the study. All participants with hyperlipidemia received stable statin treatment for > 28 days. Eligible participants were aged 18–65 years, had hyperlipidemia with LDL-C levels ≥ 2.6 mmol/L (on statin at screening) or ≥ 3.4 mmol/L (not on statin at screening and still ≥ 2.6 mmol/L before randomization), had body mass index (BMI) of 18–35 kg/m2, and could receive stable-dose statin for over 28 days before randomization and throughout the study. Patients with homozygous familial hypercholesterolemia, type 1 diabetes, poorly controlled type 2 diabetes (glycated hemoglobin [HbA1c] > 8.5%), type 2 diabetes treated with insulin or GLP-1 injections, a history of drug or atopic allergic diseases (asthma, urticaria, eczema dermatitis), heart failure (NYHA II-IV), and acute coronary syndrome were excluded. The brief design and full inclusion and exclusion criteria are available elsewhere[6]. The study was approved by the local ethics committees of all hospitals in accordance with the ethical standards of the national research committees. Written informed consent was obtained from all patients. The study was registered at ClinicalTrials.gov (NCT03944109). In the current study, we specifically focused on the effect of recaticimab on glycemic parameters. The entire study population was included in this analysis.
-
This study included a screening and 4-week statin lead-in period (D-30–D-2, View1–View3), treatment period, and follow-up period. All patients received stable atorvastatin treatment for at least 28 days before randomization and this treatment was continued throughout the study period. If the patients met the inclusion criteria but did not meet the exclusion criteria, they entered the baseline period and were randomized to receive recaticimab or placebo treatment in a 5:1 ratio using a centralized interactive web response system with no stratification factor (D-1, View4). Recaticimab or placebo was administered, and physical examination, fasting plasma glucose (FPG), HbA1c, and other relevant investigations were performed according to the study protocol (24 weeks, View 5–View 24). Details of the procedure, outcomes, and assessments have been previously published[6]. The hypoglycemic and lipid-lowering therapy regimens did not change throughout the study period. We analyzed the data of 110 patients with hyperlipidemia who completed the 24-week study period. We measured FPG levels (EKF Biosen C-Line, Beijing Neckar Healthcare Co., Ltd., Beijing, China) 12 times, i.e., at baseline (D-30–D-1, 4 times before the first recaticimab treatment) and at weeks 1, 3, 5, 8, 12, 16, 20, and 24. HbA1c levels (The ADAMS™ A1c HA-8180T, ARKRAY Inc., Kyoto, Japan) were measured 3 times, i.e., at baseline, week 12, and week 24. We aimed to assess the effects of recaticimab on glycemic control by comparing the changes in the glycemic parameters between the recaticimab and placebo groups over time.
-
We calculated the average of the four pretreatment FPG measurements to obtain the baseline value. Based on patient history, medical records, and baseline FPG or HbA1c levels, the study population was divided into normal (FPG = 3.9–6.1 mmol/L, HbA1c = 4%–6%) and abnormal (FPG > 6.1 mmol/L, HbA1c > 6%) glucose metabolism groups. The baseline characteristics were reported separately for the recaticimab and placebo groups. Distributional characteristics, including the normality of each variable, were assessed using the Kolmogorov–Smirnov or Shapiro–Wilk tests. All values are expressed as mean ± standard deviation for continuous parametric variables, median (interquartile range) for continuous nonparametric variables, or frequency (percentage) for categorical variables. Demographic characteristics were compared using the independent t-test or Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables (nationality and sex). To fit a normal-distribution curve, a reciprocal transformation was used for non-normal variables (FPG and HbA1c levels).
In both the normal and abnormal glucose metabolism populations, we compared on-trial FPG and HbA1c levels between the recaticimab and placebo groups. Repeated-measures mixed-effects models, assuming an autoregressive order 1 covariance structure[31], were used to determine longitudinal associations between PCSK9 inhibitors and FPG and HbA1c levels. Two mixed-effects models were constructed: one for the association between FPG levels and the interaction of time and group, and the other for HbA1c. Time, group, and their interactions were treated as fixed effects, while study participants were placed in the model as random effects. Sociodemographic characteristics (age, sex, and BMI) were included as covariates. Interactions between treatment effects and subgroups were assessed using a likelihood-ratio test with Bonferroni-corrected P-values[32]. All statistical analyses were performed using IBM SPSS Statistics for Windows version 13.0. Statistical significance was set at P < 0.05.
-
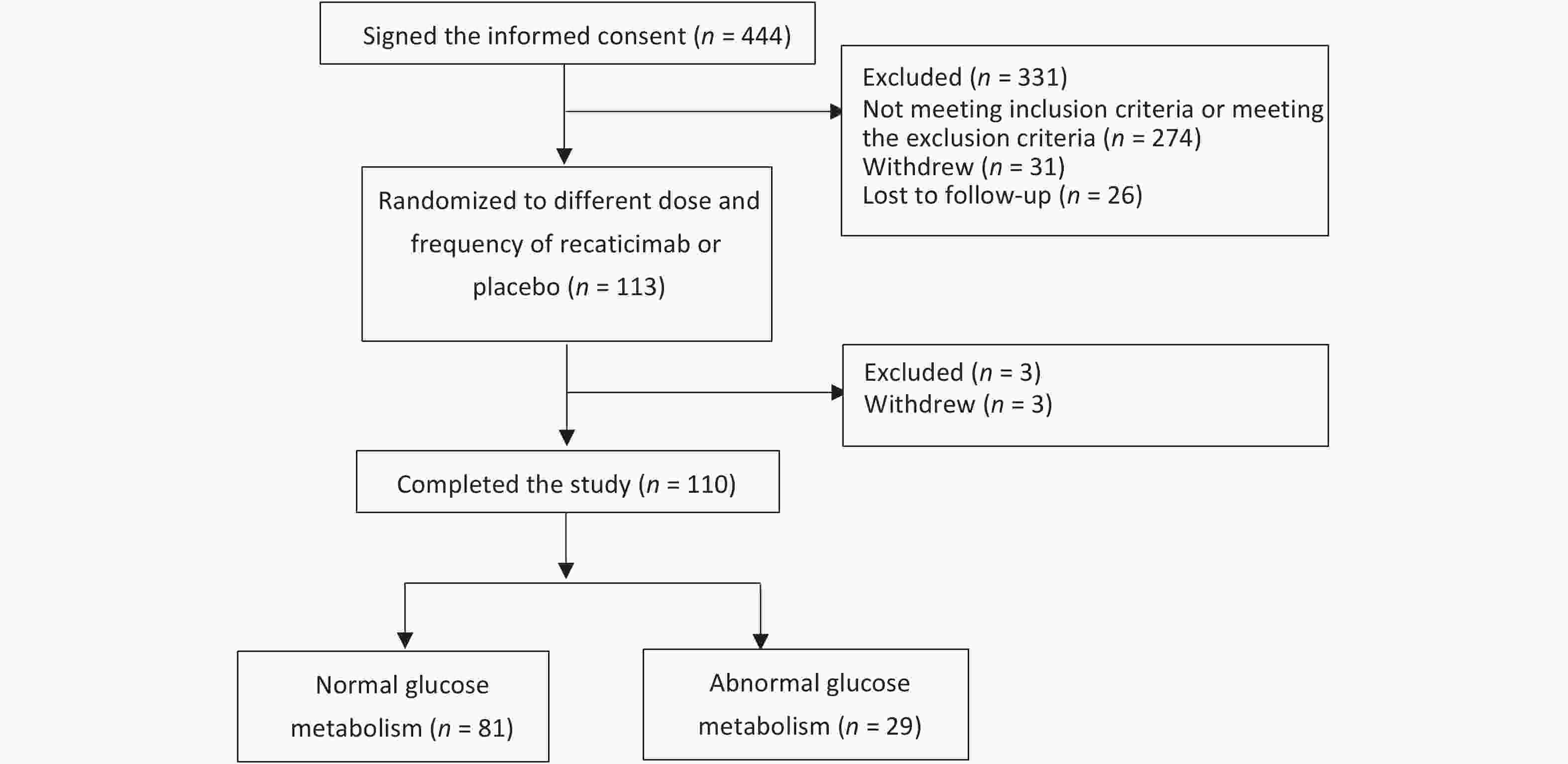
A total of 444 participants provided informed consent at baseline data collection. Of these, 274 failed to meet the inclusion and exclusion criteria, 31 withdrew, and 26 were lost to follow-up. A total of 113 participants who provided baseline data were randomized into different doses and frequencies of recaticimab or placebo groups on D-1. Three participants withdrew from the study after randomization. Finally, 110 participants completed the study: 81 with normal glucose metabolism and 29 with abnormal glucose metabolism. The recruitment of participants is shown in Figure 1.
-
Table 1 shows the sociodemographic characteristics of participants at the baseline. The mean duration of hyperlipidemia was 6.5 years. Approximately 27% of the enrolled participants had glycometabolic abnormalities at the time of randomization. The median age for participants with normal and abnormal glucose metabolism was 48.0 and 56.0 years, respectively (P = 0.039). No sex, BMI, or FPG level differences were found between participants with normal and abnormal glucose metabolism. A statistically significant relationship was observed between HbA1c levels (P = 0.002).
Table 1. Clinical characteristics of recaticimab and placebo in normal and abnormal glucose metabolism participants at baseline
Characteristic Normal (n = 81) Abnormal (n = 29) P3 Total Recaticimab
(n = 69)Placebo
(n = 12)P1 Total Recaticimab
(n = 22)Placebo
(n = 7)P2 Age (years) 48.0 (43.0, 56.0) 48.0 (43.0, 56.0) 53.5 (42.3, 60.5) 0.423 56.0(53.0,59.0) 55.0 (53.0,58.3) 60.0 (55.0,64.0) 0.098 0.039* Sex, n (%) 0.305 1.000 0.127 Male 48 (59.26) 43 (53.09) 5 (6.17) 10 (34.48) 8 (27.59) 2 (6.90) Female 33 (40.74) 26 (32.10) 7 (8.64) 19 (65.52) 14 (48.28) 5 (17.24) National 1.000 1.000 1.000 Han 77 (95.06) 66 (81.48) 11 (13.58) 27 (93.10) 20 (68.97) 7 (24.14) Others 4 (4.94) 3 (3.70) 1 (1.23) 2 (6.90) 2 (6.90) 0 (0.00) BMI (kg/m2) 25.00
(23.25, 26.80)25.20
(23.40, 26.95)23.90
(21.60, 26.08)0.336 25.40
(24.80, 28.00)25.50
(24.80, 27.18)24.90
(22.20,30.00)0.636 0.209 FPG (mmol/L) 5.17 (4.87, 5.41) 5.17 (4.88, 5.44) 5.14 (4.78, 5.40) 0.994 6.30 (5.98, 6.99) 6.40 (6.04, 6.88) 6.26 (5.70,8.16) 0.396 0.260 HbA1c (%) 5.60 (5.40, 5.80) 5.50 (5.40, 5.80) 5.85 (5.55, 5.98) 0.008* 6.40 (5.95, 7.15) 6.30 (5.80, 7.10) 6.50 (6.30,7.90) 0.072 0.002* Note. Data were represented as median (Q25, Q75). BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; P1: P value between recaticimab and placebo group in normal glucose metabolism participants; P2: P value between recaticimab and placebo group in abnormal glucose metabolism participants; P3: P value between normal and abnormal glucose metabolism participants; *P < 0.05. Among the participants with normal glucose metabolism, 69 were in the recaticimab group (43 men and 26 women, median age: 48.0 years) and 12 were in the placebo group (5 men and 7 women, median age: 53.5 years), showing no sex, age, or national differences. BMI and FPG levels did not differ between the two groups. FPG levels ranged from 5.17 to 5.14 mmol/L and HbA1c levels ranged from 5.50% to 5.85%, showing good control for all patients. A statistically significant relationship was observed between HbA1c levels (P = 0.008).
Among the participants with abnormal glucose metabolism, 22 were in the recaticimab group (8 men and 14 women, median age: 55.0 years) and 7 were in the placebo group (2 men and 5 women, median age: 60.0 years), showing no age, sex, national status, BMI, FPG level, or HbA1c level differences. FPG levels ranged from 6.40 to 6.26 mmol/L and HbA1c levels ranged from 6.30% to 6.50%.
-
Changes in FPG and HbA1c levels in the recaticimab and placebo groups in participants with normal and abnormal glucose metabolism are shown in Table 2 and Figure 2. In participants with normal glucose metabolism, recaticimab led to a significant decrease in HbA1c levels compared with those in the placebo group (F = 4.568, P = 0.036), whereas FPG levels showed no significant changes. In participants with abnormal glucose metabolism, FPG levels had a significant time effect (F = 2.492, P = 0.016), but recaticimab did not affect HbA1c levels compared with those in the placebo group. These data are presented in Table 3.
Table 2. Changes in FPG and HbA1c between baseline and follow-up time points in normal and abnormal glucose metabolism participants
Glucose
metabolismNormal glucose metabolism (n = 81) Abnormal glucose metabolism (n = 29) FPG (mmol/L) HbA1c (%) FPG (mmol/L) HbA1c (%) Recaticimab
(n = 69)Placebo
(n = 12)Recaticimab
(n = 69)Placebo
(n = 12)Recaticimab
(n = 22)Placebo
(n = 7)Recaticimab
(n = 22)Placebo
(n = 7)Baseline 5.17 (4.88, 5.44) 5.14 (4.78, 5.40) 5.50 (5.40, 5.80) 5.85 (5.55, 5.98) 6.40 (6.04, 6.88) 6.26 (5.70, 8.16) 6.30 (5.80, 7.10) 6.50 (6.30, 7.90) W1 5.26 (4.82, 5.64) 5.05 (4.79, 5.51) / / 6.13 (5.68, 6.81) 6.00 (5.08, 8.79) / / W3 5.27 (4.88, 5.63) 5.12 (4.85, 5.67) / / 6.55 (5.59, 7.18) 6.10 (6.04, 8.83) / / W5 5.10 (4.72, 5.35) 5.26 (4.86, 6.11) / / 6.76 (5.20, 7.30) 6.27 (5.31, 7.28) / / W8 5.15 (4.86, 5.55) 5.32 (4.95, 5.58) / / 6.43 (5.74, 7.77) 6.26 (5.56, 7.56) / / W12 5.40 (5.01, 5.78) 5.20 (4.94, 5.53) 5.50 (5.30, 5.70) 5.65 (5.53, 5.90) 6.51 (5.75, 7.63) 6.97 (6.32, 7.83) 6.55 (5.70, 7.25) 6.80 (6.60, 7.90) W16 5.23 (4.91, 5.63) 5.32 (4.90, 5.88) / / 6.85 (6.02, 7.40) 6.94 (6.60, 9.74) / / W20 5.20 (5.00, 5.59) 5.26 (5.00, 5.70) / / 6.54 (5.90, 7.99) 6.97 (6.10, 9.27) / / W24 5.26 (4.98, 5.47) 5.26 (4.88, 5.59) 5.50 (5.40, 5.85) 5.70 (5.28, 6.13) 6.60 (5.82, 8.40) 5.88 (4.78, 7.01) 6.50 (5.78, 7.38) 6.60 (6.50, 7.20) Note. Data were represented as median (Q25, Q75). FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c. 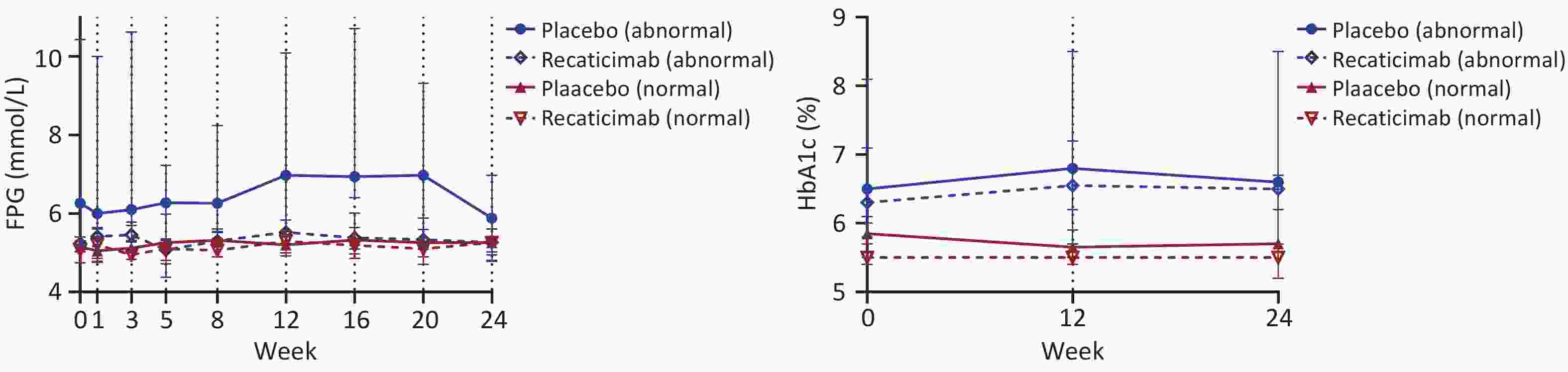
Figure 2. Change in FPG and HbAlc after recaticimab or placebo administered over 24 weeks follow-up. Data presented as median and 95% confidence interval (Cl), FpG, fasting plasma glucose, HbAlc, glycated haemoglobin Alc.
Table 3. Results of repeated-measures mixed-effects model for FPG and HbA1c level in normal and abnormal glucose metabolism participants
Items Group effect Time effect Group*time interaction F P F P F P FPG (normal) 0.138 0.711 0.858 0.552 0.500 0.856 HbA1c (normal) 4.568 0.036* 2.243 0.112 0.488 0.615 FPG (abnormal) 0.171 0.683 2.492 0.016* 1.090 0.376 HbA1c (abnormal) 1.594 0.218 1.176 0.321 0.068 0.935 Note. *P < 0.05. -
Two major concerns regarding PCSK9 inhibitors are their potential effects on glycemic deterioration in diabetes patients and the development of diabetes in normoglycemia or pre-diabetes patients. The goal of our study was to evaluate the glycemic safety of recatimib in Chinese patients, regardless of their baseline glucose and HbA1c levels. This study extends the findings of previous studies on PCSK9 inhibitors by providing evidence of the lack of effects of recaticimab on glucose in participants with normal glucose metabolism in a multicenter sample. Measured by changes in FPG levels from baseline to the 24-week endpoint, the results demonstrated that regular intake of PCSK9 inhibitors showed no significant difference in FPG compared with placebo. However, a significant group effect was observed for HbA1c levels; HbA1c levels in the placebo group were slightly higher than those in the recaticimab group. Recaticimab appeared to have a favorable effect on HbA1c levels. The inconsistencies in FPG and HbA1c levels may be due to several reasons. First, perhaps due to the small sample size of the placebo group, the recaticimab and placebo groups showed a significant difference at the baseline. Second, recaticimab may not affect FPG levels, but could affect postprandial blood glucose levels. This resulted in a significant difference in HbA1c levels between the two groups. The effect did not change over time, and there was no time-group interaction. In addition, FPG is a single-point value, whereas HbA1c reflects blood glucose levels over 3 months. Their distinct nature in representing glucose control likely contributes to the divergent results between the two indices. In participants with abnormal glucose metabolism, the results demonstrated that regular intake of PCSK9 inhibitors was associated with no significant difference in HbA1c levels compared with regular intake of placebo. However, a significant time effect was observed for FPG levels (W1 vs. W16), possibly due to the small sample size of the placebo group. The effect did not change between the groups, indicating that recaticimab did not increase glucose levels compared with placebo, and there was no time-group interaction. Overall, our study concluded that recaticimab did not impair glucose metabolism, regardless of the glucose metabolism status.
Similar findings were reported in the FOURIER and ODYSSEY clinical trials. FOURIER was a randomized trial of evolocumab vs. placebo in 27,564 patients with atherosclerotic disease and was followed up for a median of 2.2 years. Prespecified analysis[29] showed that evolocumab neither increased the risk of new-onset diabetes (HR: 1.05, 95% CI: 0.94–1.17 in non-diabetes patients; HR: 1.00, 95% CI: 0.89–1.13 in pre-diabetes patients) nor worsened glycemia. HbA1c and FPG levels were similar between the evolocumab and placebo groups over time in patients with diabetes, pre-diabetes, and normoglycemia. These results suggest that the PCSK9 inhibitor evolocumab is efficacious and safe for patients with and without diabetes. In smaller open-label extension studies of evolocumab performed for up to 4 years, no excess new-onset diabetes was observed[33]. Moreover, adding evolocumab to patients’ treatment regimens can reduce lipid levels and improve cardiovascular prognosis without increasing the incidence of adverse reactions[11]. Similar results were obtained using another PCSK9 inhibitor, alirocumab, in the ODYSSEY OUTCOMES trial[34]. ODYSSEY OUTCOMES was a randomized, double-blind, placebo-controlled trial, conducted at 1315 sites in 57 countries, that compared alirocumab with placebo in patients with acute coronary syndrome for a median of 2.8 years. The study showed that alirocumab did not increase the risk of new-onset diabetes (HR: 1.00, 95% CI: 0.89–1.11), pre-diabetes (HR: 0.97, 95% CI: 0.87–1.09), and normoglycemia (HR: 1.30, 95% CI: 0.93–1.81). Alirocumab treatment did not increase the risk of new-onset diabetes. Two pooled analyses from the ODYSSEY trial also showed no association between alirocumab and impaired glycemic control. No changes in HbA1c or FPG levels were observed from 8 to 104 weeks, regardless of the presence or absence of alirocumab[35]. There was no evidence of an effect of alirocumab on the transition to new-onset diabetes without diabetes at the baseline compared with either placebo or ezetimibe[36,37].
In line with this conclusion, a systematic review and meta-analysis of 38 trials comparing PCSK9 inhibitors with a placebo or active drugs in patients with diabetes showed that PCSK9 inhibitors did not affect glucose metabolism. Their efficacy on LDL-C and MACE in patients with diabetes did not seem to be dissimilar to that observed in non-diabetes participants[38]. A recent meta-analysis of all available data investigated the efficacy and safety of PCSK9 inhibitors in patients with diabetes. The mean weighted follow-up time was 39.1 weeks. Treatment with PCSK9 inhibitors did not increase FPG or HbA1c levels in patients with diabetes[39].
Some genetic studies have arrived at similar conclusions. Marie-Line Peyot et al.[40] generated the first β-cell-specific KO of PCSK9 (βKO). Using both whole body KO and βKO models, the data demonstrated that PCSK9 deletion in mice does not have a toxic effect on β-cell function and glucose homeostasis. Another study assessed whether PCSK9 or its inhibition modulates β-cell function and reached a similar conclusion. Although PCSK9 regulates LDLR abundance in β-cells, inhibition of exogenous or endogenous PCSK9 does not appear to significantly affect insulin secretion. This confirms the safety of PCSK9 inhibitors with respect to β-cell function[41].
However,in addition to LDL-C-lowering therapy, whether PCSK9 inhibitors can affect plasma glucose levels remains unclear. Although some recent meta-analyses of clinical trials on PCSK9 inhibitors have indicated no effect on plasma glucose levels in diabetes and non-diabetes patients, the risk may be more pronounced after prolonged treatment with statins[42,43]. A systematic review and meta-analysis of 68,123 participants (20 RCTs) with a median follow-up of 78 weeks showed that PCSK9 inhibitors increased FPG (P < 0.001) and HbA1c levels (P < 0.001) levels compared with placebo but did not increase the incidence of diabetes (P = 0.427). There was an association between the increased risk of diabetes and the potency (P = 0.029) and duration (P = 0.026) of PCSK9 inhibitor treatment. In the short term, PCSK9 inhibitor therapy favors a small but significant increase in plasma glycemia and HbA1c levels[44]. According to the FDA Adverse Event Reporting System, PCSK9 inhibitor treatment has been associated with increased reports of mild hyperglycemia, but not diabetes[45]. By assessing the T2D risk among carriers of PCSK9 variants, which are proxies for PCSK9 inhibitors, the long-term effects of PCSK9 inhibitors can be determined[43]. However, previous studies have reported conflicting results. Recent Mendelian randomization studies have suggested that genetic variants of PCSK9 are associated with an increased risk of diabetes[13,20,22-24]. However, they cannot replace randomized trials, but instead provide complementary information[46]. Some data suggest an association between the risk of diabetes and variants of several genes that affect LDL-C levels[24]. Investigators have extrapolated to estimate the odds ratio for new-onset diabetes per 1 mmol/L lower LDL-Cmediated through PCSK9 to be 1.19 to 1.29[20,24]. Therefore, the long-term effects of PCSK9 inhibitors on glycemic status remain unknown and may result in unexpected outcomes.
The real-world experience with PCSK9 inhibitors is still in its infancy. Although this is the first trial to assess the potential effects of the PCSK9 inhibitor recaticimab on blood glucose levels in Chinese adults with hyperlipidemia, some limitations should be acknowledged. The follow-up duration was only 24 weeks. New-onset diabetes treated with statins has been confirmed for many years after regulatory approval through meta-analyses of multiple trials[34]. We cannot rule out whether long-term exposure to PCSK9 inhibitors leads to an increased risk of diabetes; therefore, studies with longer durations are required. These data were also limited by the sample size. The sample size was not large, and the impact of PCSK9 inhibitors on plasma glucose levels needs to be improved by expanding the sample size. In addition, the present analysis did not include individuals with type 1 diabetes, who may differ in terms of clinical characteristics and demographics. Therefore, future longitudinal studies with a larger number of patients treated with PCSK9 inhibitors should validate the glycometabolic safety of PCSK9 inhibitors. Moreover, background lipid-lowering therapy, especially the use of statins, may affect the results of efficacy and the effect on glucose metabolism.
In conclusion, at the follow-up of 4 months, the PCSK9 inhibitor recaticimab did not adversely affect glycemia in participants with normal and abnormal glucose metabolism. These data suggest that recaticimab treatment is equally safe in patients with hyperlipidemia, with and without diabetes.
doi: 10.3967/bes2025.123
Impact of PCSK9 Inhibitor Recaticimab on Hyperlipidemia and Plasma Glucose: A Randomized, Double-blind, Placebo-controlled Phase 1b/2 Study
-
Abstract:
Objective Recaticimab (SHR-1209) significantly reduces low-density lipoprotein cholesterol levels. However, its effect on glucose metabolism remains unclear. This study aimed to evaluate its effect on glycemic parameters in a Chinese population. Methods Recaticimab versus placebo was administered in a 5:1 ratio to 110 hyperlipidemia patients who were followed up for 24 weeks. Glycated hemoglobin (HbA1c) levels were measured at baseline every 12 weeks. Fasting plasma glucose (FPG) levels were measured at baseline at week 1, 3, 5, 8, 12, 16, 20, and 24. Repeated-measures mixed-effects models were used to determine the longitudinal association between reacticimab and FPG and HbA1c levels. Results Among the 81 participants with normal glucose metabolism, HbA1c levels significantly decreased (F = 4.568, P = 0.036). In the 29 participants with abnormal glucose metabolism, a significant time effect was observed for FPG levels (F = 2.492, P = 0.016). For participants with normal and abnormal glucose metabolism, no significant group × time interaction effects on FPG or HbA1c levels were identified. Conclusion Recaticimab showed no adverse glycemic effects in participants with normal or abnormal glucose metabolism, indicating its safety in patients with or without diabetes. -
Key words:
- Hyperlipidemia /
- PCSK9 inhibitor /
- Glycemia /
- HbA1c /
- Fasting plasma glucose
The authors have no competing interests to declare.
The study was approved by the ethics committee at each study site and was conducted in accordance with the Declaration of Helsinki, Guidelines for Good Clinical Practice, and local laws and regulations. Written informed consent was obtained from all patients.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Table 1. Clinical characteristics of recaticimab and placebo in normal and abnormal glucose metabolism participants at baseline
Characteristic Normal (n = 81) Abnormal (n = 29) P3 Total Recaticimab
(n = 69)Placebo
(n = 12)P1 Total Recaticimab
(n = 22)Placebo
(n = 7)P2 Age (years) 48.0 (43.0, 56.0) 48.0 (43.0, 56.0) 53.5 (42.3, 60.5) 0.423 56.0(53.0,59.0) 55.0 (53.0,58.3) 60.0 (55.0,64.0) 0.098 0.039* Sex, n (%) 0.305 1.000 0.127 Male 48 (59.26) 43 (53.09) 5 (6.17) 10 (34.48) 8 (27.59) 2 (6.90) Female 33 (40.74) 26 (32.10) 7 (8.64) 19 (65.52) 14 (48.28) 5 (17.24) National 1.000 1.000 1.000 Han 77 (95.06) 66 (81.48) 11 (13.58) 27 (93.10) 20 (68.97) 7 (24.14) Others 4 (4.94) 3 (3.70) 1 (1.23) 2 (6.90) 2 (6.90) 0 (0.00) BMI (kg/m2) 25.00
(23.25, 26.80)25.20
(23.40, 26.95)23.90
(21.60, 26.08)0.336 25.40
(24.80, 28.00)25.50
(24.80, 27.18)24.90
(22.20,30.00)0.636 0.209 FPG (mmol/L) 5.17 (4.87, 5.41) 5.17 (4.88, 5.44) 5.14 (4.78, 5.40) 0.994 6.30 (5.98, 6.99) 6.40 (6.04, 6.88) 6.26 (5.70,8.16) 0.396 0.260 HbA1c (%) 5.60 (5.40, 5.80) 5.50 (5.40, 5.80) 5.85 (5.55, 5.98) 0.008* 6.40 (5.95, 7.15) 6.30 (5.80, 7.10) 6.50 (6.30,7.90) 0.072 0.002* Note. Data were represented as median (Q25, Q75). BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c; P1: P value between recaticimab and placebo group in normal glucose metabolism participants; P2: P value between recaticimab and placebo group in abnormal glucose metabolism participants; P3: P value between normal and abnormal glucose metabolism participants; *P < 0.05. Table 2. Changes in FPG and HbA1c between baseline and follow-up time points in normal and abnormal glucose metabolism participants
Glucose
metabolismNormal glucose metabolism (n = 81) Abnormal glucose metabolism (n = 29) FPG (mmol/L) HbA1c (%) FPG (mmol/L) HbA1c (%) Recaticimab
(n = 69)Placebo
(n = 12)Recaticimab
(n = 69)Placebo
(n = 12)Recaticimab
(n = 22)Placebo
(n = 7)Recaticimab
(n = 22)Placebo
(n = 7)Baseline 5.17 (4.88, 5.44) 5.14 (4.78, 5.40) 5.50 (5.40, 5.80) 5.85 (5.55, 5.98) 6.40 (6.04, 6.88) 6.26 (5.70, 8.16) 6.30 (5.80, 7.10) 6.50 (6.30, 7.90) W1 5.26 (4.82, 5.64) 5.05 (4.79, 5.51) / / 6.13 (5.68, 6.81) 6.00 (5.08, 8.79) / / W3 5.27 (4.88, 5.63) 5.12 (4.85, 5.67) / / 6.55 (5.59, 7.18) 6.10 (6.04, 8.83) / / W5 5.10 (4.72, 5.35) 5.26 (4.86, 6.11) / / 6.76 (5.20, 7.30) 6.27 (5.31, 7.28) / / W8 5.15 (4.86, 5.55) 5.32 (4.95, 5.58) / / 6.43 (5.74, 7.77) 6.26 (5.56, 7.56) / / W12 5.40 (5.01, 5.78) 5.20 (4.94, 5.53) 5.50 (5.30, 5.70) 5.65 (5.53, 5.90) 6.51 (5.75, 7.63) 6.97 (6.32, 7.83) 6.55 (5.70, 7.25) 6.80 (6.60, 7.90) W16 5.23 (4.91, 5.63) 5.32 (4.90, 5.88) / / 6.85 (6.02, 7.40) 6.94 (6.60, 9.74) / / W20 5.20 (5.00, 5.59) 5.26 (5.00, 5.70) / / 6.54 (5.90, 7.99) 6.97 (6.10, 9.27) / / W24 5.26 (4.98, 5.47) 5.26 (4.88, 5.59) 5.50 (5.40, 5.85) 5.70 (5.28, 6.13) 6.60 (5.82, 8.40) 5.88 (4.78, 7.01) 6.50 (5.78, 7.38) 6.60 (6.50, 7.20) Note. Data were represented as median (Q25, Q75). FPG, fasting plasma glucose; HbA1c, glycated haemoglobin A1c. Table 3. Results of repeated-measures mixed-effects model for FPG and HbA1c level in normal and abnormal glucose metabolism participants
Items Group effect Time effect Group*time interaction F P F P F P FPG (normal) 0.138 0.711 0.858 0.552 0.500 0.856 HbA1c (normal) 4.568 0.036* 2.243 0.112 0.488 0.615 FPG (abnormal) 0.171 0.683 2.492 0.016* 1.090 0.376 HbA1c (abnormal) 1.594 0.218 1.176 0.321 0.068 0.935 Note. *P < 0.05. -
[1] Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol, 2020; 76, 2982−3021. doi: 10.1016/j.jacc.2020.11.010 [2] Coppinger C, Pomales B, Movahed MR, et al. Berberine: a multi-target natural PCSK9 inhibitor with the potential to treat diabetes, alzheimer's, cancer and cardiovascular disease. Curr Rev Clin Exp Pharmacol, 2024; 19, 312−26. doi: 10.2174/0127724328250471231222094648 [3] Cholesterol Treatment Trialists' (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet, 2015; 385, 1397−405. doi: 10.1016/S0140-6736(14)61368-4 [4] Baumgartner S, Bruckert E, Gallo A, et al. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis, 2020; 311, 116−23. doi: 10.1016/j.atherosclerosis.2020.07.019 [5] Yu Q, Zheng HD, Zhang YP. Inducible degrader of LDLR: a potential novel therapeutic target and emerging treatment for hyperlipidemia. Vascul Pharmacol, 2021; 140, 106878. doi: 10.1016/j.vph.2021.106878 [6] Xu MT, Zhu XX, Wu JY, et al. PCSK9 inhibitor recaticimab for hypercholesterolemia on stable statin dose: a randomized, double-blind, placebo-controlled phase 1b/2 study. BMC Med, 2022; 20, 13. doi: 10.1186/s12916-021-02208-w [7] Mansi IA, Chansard M, Lingvay I, et al. Association of statin therapy initiation with diabetes progression: a retrospective matched-cohort study. JAMA Intern Med, 2021; 181, 1562−74. doi: 10.1001/jamainternmed.2021.5714 [8] Kockx M, Kritharides L. Pancreatic PCSK9 and its involvement in diabetes. J Thorac Dis, 2019; 11, S2018−22. doi: 10.21037/jtd.2019.06.37 [9] Kosmas CE, Silverio D, Sourlas A, et al. Impact of lipid-lowering therapy on glycemic control and the risk for new-onset diabetes mellitus. Drugs Context, 2018; 7, 212562. [10] Seidah NG, Prat A, Pirillo A, et al. Novel strategies to target proprotein convertase subtilisin kexin 9: beyond monoclonal antibodies. Cardiovasc Res, 2019; 115, 510−8. doi: 10.1093/cvr/cvz003 [11] Hao Y, Yang YL, Wang YC, et al. Effect of the early application of evolocumab on blood lipid profile and cardiovascular prognosis in patients with extremely high-risk acute coronary syndrome. Int Heart J, 2022; 63, 669−77. doi: 10.1536/ihj.22-052 [12] Chan JCY, Piper DE, Cao Q, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA, 2009; 106, 9820−5. doi: 10.1073/pnas.0903849106 [13] Chikowore T, Cockeran M, Conradie KR, et al. C679X loss-of-function PCSK9 variant lowers fasting glucose levels in a black South African population: a longitudinal study. Diabetes Res Clin Pract, 2018; 144, 279−85. doi: 10.1016/j.diabres.2018.09.012 [14] Banach M, Penson PE, Vrablik M, et al. Optimal use of lipid-lowering therapy after acute coronary syndromes: a Position Paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res, 2021; 166, 105499. doi: 10.1016/j.phrs.2021.105499 [15] Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376, 1713−22. doi: 10.1056/NEJMoa1615664 [16] Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med, 2018; 379, 2097−107. doi: 10.1056/NEJMoa1801174 [17] Shapiro MD, Tavori H, Fazio S. PCSK9: from basic science discoveries to clinical trials. Circ Res, 2018; 122, 1420−38. doi: 10.1161/CIRCRESAHA.118.311227 [18] Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet, 2010; 375, 735−42. doi: 10.1016/S0140-6736(09)61965-6 [19] Päth G, Perakakis N, Mantzoros CS, et al. PCSK9 inhibition and cholesterol homeostasis in insulin producing β-cells. Lipids Health Dis, 2022; 21, 138. doi: 10.1186/s12944-022-01751-6 [20] Schmidt AF, Swerdlow DI, Holmes MV, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol, 2017; 5, 97−105. doi: 10.1016/S2213-8587(16)30396-5 [21] Fryirs M, Barter PJ, Rye KA. Cholesterol metabolism and pancreatic β-cell function. Curr Opin Lipidol, 2009; 20, 159−64. doi: 10.1097/MOL.0b013e32832ac180 [22] Khan SU, Rahman H, Okunrintemi V, et al. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc, 2019; 8, e011581. doi: 10.1161/JAHA.118.011581 [23] Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med, 2016; 375, 2144−53. doi: 10.1056/NEJMoa1604304 [24] Lotta LA, Sharp SJ, Burgess S, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA, 2016; 316, 1383−91. doi: 10.1001/jama.2016.14568 [25] Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet, 2015; 385, 351−61. doi: 10.1016/S0140-6736(14)61183-1 [26] Bonnefond A, Yengo L, Le May C, et al. The loss-of-function PCSK9 p. R46L genetic variant does not alter glucose homeostasis. Diabetologia, 2015; 58, 2051−5. doi: 10.1007/s00125-015-3659-8 [27] Basiak M, Hachula M, Kosowski M, et al. Effect of PCSK9 inhibitors on hemostasis in patients with isolated hypercholesterolemia. J Clin Med, 2022; 11, 2542. doi: 10.3390/jcm11092542 [28] Ramin-Mangata S, Wargny M, Pichelin M, et al. Circulating PCSK9 levels are not associated with the conversion to type 2 diabetes. Atherosclerosis, 2020; 293, 49−56. doi: 10.1016/j.atherosclerosis.2019.11.027 [29] Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol, 2017; 5, 941−50. doi: 10.1016/S2213-8587(17)30313-3 [30] Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med, 2017; 376, 1527−39. doi: 10.1056/NEJMoa1701488 [31] Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med, 2000; 19, 1793−819. doi: 10.1002/1097-0258(20000715)19:13<1793::AID-SIM482>3.0.CO;2-Q [32] Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ, 1995; 310, 170. doi: 10.1136/bmj.310.6973.170 [33] Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol, 2017; 2, 598−607. doi: 10.1001/jamacardio.2017.0747 [34] Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol, 2019; 7, 618−28. doi: 10.1016/S2213-8587(19)30158-5 [35] Leiter LA, Tinahones FJ, Karalis DG, et al. Alirocumab safety in people with and without diabetes mellitus: pooled data from 14 ODYSSEY trials. Diabet Med, 2018; 35, 1742−51. doi: 10.1111/dme.13817 [36] Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J, 2016; 37, 2981−9. doi: 10.1093/eurheartj/ehw292 [37] Leiter LA, Müller-Wieland D, Baccara-Dinet MT, et al. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med, 2018; 35, 121−30. doi: 10.1111/dme.13450 [38] Monami M, Sesti G, Mannucci E. PCSK9 inhibitor therapy: a systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes Metab, 2019; 21, 903−8. doi: 10.1111/dom.13599 [39] Chen T, Wang ZW, Xie J, et al. Efficacy and safety of PCSK9 inhibitors in patients with diabetes: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis, 2023; 33, 1647−61. doi: 10.1016/j.numecd.2023.05.033 [40] Peyot ML, Roubtsova A, Lussier R, et al. Substantial PCSK9 inactivation in β-cells does not modify glucose homeostasis or insulin secretion in mice. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids, 2021; 1866, 158968. [41] Ramin-Mangata S, Thedrez A, Nativel B, et al. Effects of proprotein convertase subtilisin kexin type 9 modulation in human pancreatic beta cells function. Atherosclerosis, 2021; 326, 47−55. doi: 10.1016/j.atherosclerosis.2021.03.044 [42] Sattar N, Preiss D, Robinson JG, et al. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol, 2016; 4, 403−10. doi: 10.1016/S2213-8587(16)00003-6 [43] Lee J, Hegele RA. PCSK9 inhibition and diabetes: turning to Mendel for clues. Lancet Diabetes Endocrinol, 2017; 5, 78−9. doi: 10.1016/S2213-8587(16)30398-9 [44] de Carvalho LSF, Campos AM, Sposito AC. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and incident type 2 diabetes: a systematic review and meta-analysis with over 96, 000 patient-years. Diabetes Care, 2018; 41, 364−7. doi: 10.2337/dc17-1464 [45] Goldman A, Raschi E, Cukierman-Yaffe T, et al. Hyperglycaemic disorders associated with PCSK9 inhibitors: a real-world, pharmacovigilance study. Eur J Prev Cardiol, 2022; 29, 1334−42. doi: 10.1093/eurjpc/zwab209 [46] Ference BA, Holmes MV, Smith GD. Using mendelian randomization to improve the design of randomized trials. Cold Spring Harb Perspect Med, 2021; 11, a040980. doi: 10.1101/cshperspect.a040980 -




 下载:
下载:



 Quick Links
Quick Links