-
The mammalian brain has high energy requirements. Approximately 20% of the oxygen and 25% of the glucose consumed by the human body are consumed by cerebral metabolism, yet the brain constitutes only -2% of the total body weight [1]. In addition to this extremely high energy consumption, the brain appears to have very limited energy storage. Energy supply and expenditure are tightly coupled with neurovascular and neurometabolic mechanisms. Astrocytes, which are the most abundant cells in the mammalian brain and vastly outnumber neurons, are important in the neurovascular coupling of neuronal activity and cerebral blood flow (CBF), which provides an uninterrupted supply of oxygen and glucose to meet the high metabolic demand of the neurons [2-3].
Glutamate, an important excitatory neurotransmitter in the central nervous system, has several functions in the nervous system. It acts both pre- and postsynaptically by activating glutamate receptors, which are responsible for excitatory neurotransmission and are pivotal elements of complex systems underlying synaptic plasticity, learning, memory and other fundamental events/functions in neurophysiology [4]. In addition to being an excitatory transmitter, much of the glutamate taken up by glutamate transporters in astrocytes is destined for oxidative phosphorylation to generate energy, which requires conversion to the tricarboxylic acid (TCA) cycle intermediate a-ketoglutarate [5].
Astrocytes are important in glutamate uptake, metabolism, and release in the central nervous system. Recent studies indicated that disturbance of astrocyte metabolism is a significant cause of neuronal dysfunction and neurodegenerative processes [6-7]. Glutamate is also involved in pathological conditions such as cerebral ischemia and other neurodegenerative diseases. In these instances, extracellular glutamate levels are increased significantly, resulting in neurotoxic effects. Numerous studies have shown that nervous system diseases could be regulated by the dysfunctions of glutamate receptors and/or glutamate uptake [8-9]. Although previous studies have shown that glutamate administration could increase glycolysis and acidification in astrocytes [10-11], the effect of glutamate on cellular metabolism and changes in glycolysis in rat astrocytes remains to be fully characterized. In the current study, astrocytes were cultured, identified and exposed to glutamate. The respiration and glycolysis changes in cultured astrocytes were analyzed using a Seahorse XF-24 Metabolic Flux Analyzer. Lactate release was also detected to confirm the shift to glycolysis in astrocytes after glutamate exposure.
-
All of the rats were provided by the Institute of Laboratory Animal Science affiliated with the Chinese Academy of Medical Sciences [Certificate No, SCSK (Beijing) 2005-0013]. The animal protocol was approved by the Animal Care and Use Committee of Beijing Neurosurgical Institute and was consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Dimethyl Sulphoxide (DMSO) and 3-(4, 5-dimethyl-thiazol- 2-yl)-2, 5-diphenyltetrazoliumbromide (MTT) were obtained from Sigma (St.Louis, MO, USA). Trypsin, Minimum Essential Medium (MEM) and fetal bovine serum (FBS) were obtained from GIBCO-BRL (Grand Island, NY, USA). Rabbit anti-glial fibrillary acidic protein (GFAP) was purchased from Dako (Glostrup, Denmark). Mounting medium containing 4', 6-Diamidino-2-phenylindole dihydrochloride DAPI (ZLI-9557) and goat anti-rabbit secondary antibodies conjugated to fluorescein isothiocyanate (FITC) (ZF-0311) were obtained from ZSGB-BIO (Beijing, China). Cell Lysis Buffer and Enhanced BCA Protein Assay Kit were obtained from Beyotime (Haimen, China). The XF Glycolysis Stress Test Kit, XF Cell Mito Stress Test Kit, and XF Base Medium were acquired from Seahorse Bioscience (North Billerica, MA, USA). Sodium pyruvate, glucose and L-glutamine were purchased from Sigma-Aldrich (Saint Louis, MO, USA). The Lactate Assay Kit was obtained from Biovision (California, USA).
-
Astrocyte cultures were prepared according to a previously described method [12] with slight modifications [13]. One-day-old Sprague Dawley rats were anesthetized with ether, and then dipped into 75% alcohol for sterilization. The cerebral cortex was removed from the skulls, and the meninges were carefully stripped away. The cerebral tissues were cut into small pieces and dissociated into single cells by gentle pipetting. After filtering, the isolated cells were maintained in MEM supplemented with 10% FBS. All of the cultures were incubated with at 37 ℃ in air containing 5% CO2 , and the culture medium was replaced every 3-4 d. To obtain more astrocytes, subculturing was performed when the cultured astrocytes were confluent on the tenth day of primary culture, and the secondary cultured astrocytes were used in all of the experiments.
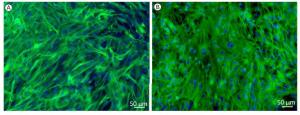
The purities of the cultured astrocytes were confirmed by immunofluorescence staining for GFAP, a marker of astrocytes. Astrocytes were fixed with 95% ethanol for 15 min at room temperature. Next, the astrocytes were incubated with polyclonal rabbit antibody against GFAP (1:50) diluted in PBS overnight at 4 ℃. After three 3 min washes in PBS, the astrocytes were incubated with FITC-conjugated goat anti-rabbit IgG (1:200) for 1 h at room temperature. Nuclei were counterstained with DAPI. Images were acquired using an inverted Zeiss Axio Observer fluorescence microscope.
-
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured using a Seahorse XF-24 Metabolic Flux Analyzer (Seahorse Bioscience, North Billerica, MA). Astrocytes were plated at 5000 cells/well and cultured on Seahorse XF-24 plates for 24 h. On the day of analysis, cells were pretreated with 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 25 mmol/L glucose, 2 mmol/L sodium pyruvate, and 2 mmol/L glutamine (pH 7.4) followed by incubation at 37 ℃ in a non-CO2 incubator for 1 h. All of the medium and injection reagents were adjusted to pH 7.4 on the day of assay. The initial 35 min reading established the baseline. Three subsequent injections followed, comprising 1 μmol/L oligomycin (complex V inhibitor), 1 μmol/Lcarbonyl cyanide phospho- (p)-trifluoromethoxy phenylhydrazone (FCCP, a proton gradient uncoupler that collapses proton electrochemical gradients and allows the respiratory chain to operate maximally), and 0.5 μmol/L rotenone/ antimycin (complex I inhibitor). After each injection, 4 time points were recorded with approximately 35 min between each injection. The OCR and ECAR were automatically recorded and calculated by the Seahorse XF-24 software.
For the ECAR assay, astrocytes were plated at 5000 cells/well and cultured on Seahorse XF-24 plates for 24 h. Cells were pretreated with 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 2 mmol/Lglutamine (pH 7.4) and incubated at 37 ℃ in a non-CO2 incubator for 1 h. Glucose, oligomycin and 2-deoxyglucose (2-DG) (a glucose analog, which inhibits glycolysis through competitive binding to glucose hexokinase, the first enzyme in the glycolytic pathway) were subsequently injected into the medium to final concentrations of 10 mmol/L, 1 μmol/L, 50 mmol/L, respectively.
-
Astrocyte viability was determined using the MTT assay. Cells were plated on 96-well plates at 2.5 × 104 cells/well. Approximately 20 μL of MTT at 5 mg/mL was added to each well immediately after either 1 h or 24 h of glutamate treatment. Plates were incubated for 4 h at 37 ℃. The medium was aspirated from each well, and 100 μL of DMSO was added to dissolve the formazan crystals. The optical density of each well was read at 492 nm using a microplate reader (M200 Pro, Tecan). The results are presented as percentages of the control group, which was defined as 100%.
-
Astrocytes were plated at 5000 cells/well and cultured on 96-well plates for 24 h. Cells were pretreated with glutamate (0.1, 1, and 10 mmol/L) for 1 h or 24 h, and the lactate concentration was measured with a colorimetric kit according to the manufacturer's instructions with a microplate reader (M200 Pro, Tecan). Then cells were lysed with Cell Lysis Buffer and protein concentration was measured using a BCA Protein Assay Kit. The lactate concentration in the samples was calculated from a standard curve and normalized by total protein content.
-
Data are expressed as the mean ± SD of at least 3 independent experiments. Group results were analyzed for variance using ANOVA. Comparisons of two groups were performed using Student's t-test. All analyses were conducted with GraphPad Prism 5.0 software. P < 0.05 was defined as statistically significant.
-
The monolayer cultures of astrocytes were presented as uniform shapes by immunofluorescence staining, which revealed that over 95% of cells were GFAP-positive (Figure 1A). With the treatment of 1 mmol/L glutamate for 1 h, there was no significant change in cell numbers and cell morphology (Figure 1B).
-
The optimal FCCP concentration was first determined using Seahorse Metabolic Analyzer. As shown in Figure 2A, after sequentially injecting oligomycin and FCCP (0-2 μmol/L), cells get the maximal OCR. Compared to each group, 1 μmol/L FCCP could obtain the maximal OCR, which was basically the same with that in 2 μmol/L FCCP group. So 1 μmol/L FCCP was used as the optimal concentration in further research.
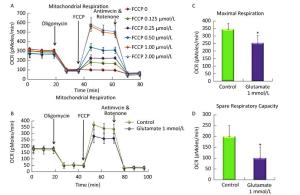
Figure 2. Effect of glutamate on mitochondrial respiration in astrocytes. (A) The optimal FCCP concentration was first determined by sequentially injecting oligomycin (1 mmol/L) and FCCP (0-2 μmol/L). 1 μmol/L FCCP was used as the optimal concentration for the following research. (B) Astrocytes were pretreated with 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 25 mmol/L glucose, 2 mmol/L sodium pyruvate, and 2 mmol/L glutamine (pH 7.4) followed by incubation at 37 ℃ in a non-CO2 incubator for 1 h. Three subsequent injections followed, comprising 1 μmol/L oligomycin, 1 μmol/L FCCP, and 0.5 μmol/L rotenone/ antimycin. The OCR value was automatically recorded and calculated by the Seahorse XF-24 software. The oxygen consumption rate (OCR) (B), maximal respiration (C), and spare respiratory capacity (D) changes in astrocytes treated with or without glutamate were shown. Error bars represent the mean ± SD, (n = 5). P < 0.05 versus the control group.
Then we determined the OCR and ECAR in astrocytes using Seahorse Metabolic Analyzer and analyzed the results using the XF Mito Stress Test / Glycolysis Stress Test Report Generator provided by Seahorse Bioscience. The spare respiratory capacity indicates the capability of the cell to respond to an energetic demand as well as how closely the cell is to respiring to its theoretical maximum, which can be an indicator of cell fitness or flexibility. Spare respiratory capacity was calculated by subtracting the basal OCR from the maximum OCR after FCCP addition. As shown in Figure 2B and C, the basal OCR was not affected by the glutamate, whereas upon addition of FCCP, the maximal respiration decreased from 343.5 ± 41.8 to 253.2 ± 51.4. In addition, the spare respiratory capacity in the glutamate group was only half that of the control, which decreased from 199.2 ± 53.3 to 100.0 ± 52.6 (Figure 2D), indicating that the astrocytes' respiration was impaired by the glutamate treatment.
-
We then determined the ECAR by sequential injection of glucose, oligomycin, and 2-DG in either control astrocytes or astrocytes pretreated with glutamate. Glycolytic capacity is the maximum ECAR rate reached by cells following the addition of oligomycin, effectively shutting down oxidative phosphorylation and driving the cell to use glycolysis to its maximum capacity. Glycolytic reserve indicates the capability of a cell to respond to an energetic demand as well as how close the glycolytic function is to the cell's theoretical maximum, and equal to glycolytic capacity divided by basal ECAR. Figure 3A showed that when combined with oligomycin, a mitochondrial aerobic respiration inhibitor, glutamate treatment significantly increased the ECAR value. Further analysis showed that the glycolytic capacity and glycolytic reserve in astrocytes increased nearly 4-fold and 2-fold, respectively. Our results indicating that the glutamate treatment increased the glycolytic functions in astrocytes.

Figure 3. Effect of glutamate on the glycolytic activity of astrocytes. Astrocytes were pretreated with or without 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 2 mmol/L glutamine (pH 7.4) and incubated at 37 ℃ in a non-CO2 incubator for 1 h. Glucose (10 mmol/L), oligomycin (1 μmol/L) and 2-DG (50 mmol/L) were subsequently injected into the medium. The ECAR value was automatically recorded and calculated by the Seahorse XF-24 software. Changes in the glyolytic function (A), glycolytic capacity (B), and glycolytic reserve (C) in astrocytes induced by glutamate. Error bars represent the mean ± SD, n = 5. P < 0.05 versus the control group.
-
Glycolysis is closely related to the extracellular lactate concentration. Cell viability was first evaluated using the MTT assay (Figure 4A), which showed that astrocyte viability was not affected by glutamate treatment. Lactate release into the astrocyte culture medium was detected at 1 h and 24 h after glutamate treatment. As shown in Figure 4B, 1 h treatment with glutamate (0.1-10 mmol/L) significantly increased the lactate concentration, which was further increased after 24 h of glutamate treatment.

Figure 4. Effect of glutamate on cell viability and lactate production. (A) Astrocyte viability was determined immediately after 1 h or 24 h of glutamate (0.1 mmol/L, 1 mmol/L, and 10 mmol/L) treatment. Cell viability was determined by MTT assay. The results are presented as percentages of the control group, which was defined as 100%. (B) Astrocytes were pretreated with glutamate (0.1, 1, and 10 mmol/L) for 1 h or 24 h, and the lactate concentration in culture medium was calculated from a standard curve and normalized by total protein content. Error bars represent the mean ± SD, n = 3. **P < 0.01 vs. the 1-h control group; ##P < 0.01 vs. the 24-h control group; ###P < 0.001 vs. the 24-h control group.
-
Although many studies have focused on the dysfunction of glutamate receptors and/or glutamate uptake during brain ischemia, less is known about the effect of glutamate on astrocyte metabolism. In the current study, we directly demonstrate that exogenous glutamate treatment could impair astrocyte respiration and enhance aerobic glycolysis (as measured by a metabolic analyzer) as well as further increase in lactate release.
Glutamate is the principal excitatory neurotransmitter in the brain. One of the most essential roles of astrocytes is to maintain a low resting glutamate concentration of 1-10 mmol/L by removing glutamate from the synaptic cleft after neuron depolarization and convert it to glutamine within astrocytes [14-15]. When the extracellular concentration of glutamate becomes pathologically elevated, it acts as a neurotoxin and can induce severe neurological damage [16]. Previous studies have shown that in rat cortical neurons, glutamate exerted its excitotoxicity effect at a concentration of 20 mmol/L [17-18]. In contrast to neurons, the current study showed that the viability of astrocytes after exposure to 1 mmol/L glutamate was not decreased, which is consistent with a previous study [19]. In addition, the shift in astrocyte metabolism after glutamate treatment was monitored and calculated by measuring the extracellular oxygen consumption rate with a Seahorse Metabolic Analyzer, and we found that the maximum respiration capacity and reserve capacity were significantly reduced in astrocytes upon treatment with 1 mmol/L glutamate. These results showed that glutamate could impair astrocytic respiration, which was consistent with previous studies on glutamate-induced deficiencies in neuronal respiration [20].
A former study has also shown that acute addition of glutamate lowered mitochondrial pH and mitochondrial respiration, suggesting that the inhibition of astrocytic respiration by glutamate was related to the mitochondrial matrix pH as well as the intracellular acidification [10]. As glucose consumption by mitochondrial oxidative phosphorylation not only fuels astrocytes and neuronal activity but also provides substrates for the biosynthesis of proteins, lipids, nucleic acids and amino acids. We speculated that inhibition of astrocytic respiration could impair the synthesis of neurotransmitter precursors and further reduced energy generation derived by oxidation, which could reduce glutamate uptake, and may further enhance glutamate-induced damage to the brain [21].
The rate of glycolysis increases significantly during neurological disorders and is also a recognized feature of cerebral metabolism immediately after injury [22-23]. Glutamate content is upregulated during brain injuries, including cerebral ischemia, tumors and traumatic brain injury [24-25], which suggests that increased glutamate may lead to the change in the glycolysis rate and be involved in the pathological changes in the brain. In the present study, we directly proved that glutamate enhanced aerobic glycolysis by improving the cellular glycolytic reserves. The increased glycolytic capacity was further confirmed by the detected changes in lactate release, which was consistent with previous studies [11, 26-27].
However, under physiological conditions, the glycolysis and lactate release was an important regulator to maintain metabolic homeostasis in the brain. Exogenous glutamate uptaken by astrocytes via glutamate transporters requires Na/K-ATPase pump and ATP, and further increases glucose transport, glycolysis, glycogen degradation, and the production of lactate [28]. A recent study has indicated that mitochondrial respiration inhibition induced ATP reduction in astrocytes could be compensated by the enhanced glycolysis, and this process could be regulated by the NAD(P)H/NAD(P)+ redox state and the mitochondrial electrochemical gradient [29]. It is also known that the lactate taken up by neurons can be used as an energy source [30-32]. The uptake of glutamate induced glycolysis and lactate release in astrocytes could be used as metabolism substrate for the neighboring neurons to be metabolized [6]. As the product of glycolysis, lactate could be shuttled into mitochondria via the mitochondrial monocarboxylate transporter (MCT) where it is oxidized to pyruvate and entered into the TCA cycle to be converted into a series of organic acids with the liberation of carbon dioxide [33]. In addition, the lactate was also related to learning and long-term memory formation [31, 34]. So our research may provide new insight into the bioenergetics of astrocytes and their contribution to the neuron astrocyte lactate shuttle. Moreover, the lactate could also be a cytoprotective agent for astrocytes. A recent study demonstrated that lactate could also be a neuroprotective agent during glutamate-induced excitotoxicity, which required the functional malate-aspartate NADH shuttle [35]. We speculate that the cytoprotective effect of lactate might explain why the astrocytes' viability was not affected by the glutamate treatment.
Glutamate in the brain acts as a ‘double-edged sword', both as an essential neurotransmitter and as a contributor to a variety of neurological diseases by acting as a neurotoxin. Glutamate metabolism in astrocytes is complex; therefore, further research on other metabolic pathways and the regulatory mechanisms of glutamate function in astrocytes is still required.
In conclusion, we found that glutamate treatment could inhibit cell respiration and enhance aerobic glycolysis in astrocytes. The changes in cellular metabolism and lactate release after glutamate treatment might uncover the mechanisms of glutamate injury or protection in neurological disorders. However, further studies are still necessary to investigate the pathogenesis and mechanisms related to astrocytic glutamate metabolism in central nervous system disorders.
-
YAN Xu performed OCR and ECAR assay and wrote the manuscript. SHI Zhong Fang performed immunofluorescence staining. XU Li Xin performed the astrocyte culture. LI Jia Xin and WU Min performed the MTT assay. WANG Xiao Xuan and JIA Mei performed the determination of lactate release. DONG Li Ping performed the statistical analysis. YANG Shao Hua revised the manuscript. YUAN Fang designed research and got a grant from the National and Beijing Natural Science Foundation of China.
doi: 10.3967/bes2017.005
Glutamate Impairs Mitochondria Aerobic Respiration Capacity and Enhances Glycolysis in Cultured Rat Astrocytes
-
Abstract:
Objective To study the effect of glutamate on metabolism, shifts in glycolysis and lactate release in rat astrocytes. Methods After 10 days, secondary cultured astrocytes were treated with 1 mmol/L glutamate for 1 h, and the oxygen consumption rates (OCR) and extra cellular acidification rate (ECAR) was analyzed using a Seahorse XF 24 Extracellular Flux Analyzer. Cell viability was then evaluated by MTT assay. Moreover, changes in extracellular lactate concentration induced by glutamate were tested with a lactate detection kit. Results Compared with the control group, treatment with 1 mmol/L glutamate decreased the astrocytes' maximal respiration and spare respiratory capacity but increased their glycolytic capacity and glycolytic reserve. Further analysis found that 1-h treatment with different concentrations of glutamate (0.1-1 mmol/L) increased lactate release from astrocytes, however the cell viability was not affected by the glutamate treatment. Conclusion The current study provided direct evidence that exogenous glutamate treatment impaired the mitochondrial respiration capacity of astrocytes and enhanced aerobic glycolysis, which could be involved in glutamate injury or protection mechanisms in response to neurological disorders. -
Key words:
- Astrocytes /
- Glutamate /
- Mitochondrial metabolism /
- Glycolysis /
- Lactate
-
Figure 2. Effect of glutamate on mitochondrial respiration in astrocytes. (A) The optimal FCCP concentration was first determined by sequentially injecting oligomycin (1 mmol/L) and FCCP (0-2 μmol/L). 1 μmol/L FCCP was used as the optimal concentration for the following research. (B) Astrocytes were pretreated with 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 25 mmol/L glucose, 2 mmol/L sodium pyruvate, and 2 mmol/L glutamine (pH 7.4) followed by incubation at 37 ℃ in a non-CO2 incubator for 1 h. Three subsequent injections followed, comprising 1 μmol/L oligomycin, 1 μmol/L FCCP, and 0.5 μmol/L rotenone/ antimycin. The OCR value was automatically recorded and calculated by the Seahorse XF-24 software. The oxygen consumption rate (OCR) (B), maximal respiration (C), and spare respiratory capacity (D) changes in astrocytes treated with or without glutamate were shown. Error bars represent the mean ± SD, (n = 5). P < 0.05 versus the control group.
Figure 3. Effect of glutamate on the glycolytic activity of astrocytes. Astrocytes were pretreated with or without 1 mmol/L glutamate for 1 h and then changed to base medium supplemented with 2 mmol/L glutamine (pH 7.4) and incubated at 37 ℃ in a non-CO2 incubator for 1 h. Glucose (10 mmol/L), oligomycin (1 μmol/L) and 2-DG (50 mmol/L) were subsequently injected into the medium. The ECAR value was automatically recorded and calculated by the Seahorse XF-24 software. Changes in the glyolytic function (A), glycolytic capacity (B), and glycolytic reserve (C) in astrocytes induced by glutamate. Error bars represent the mean ± SD, n = 5. P < 0.05 versus the control group.
Figure 4. Effect of glutamate on cell viability and lactate production. (A) Astrocyte viability was determined immediately after 1 h or 24 h of glutamate (0.1 mmol/L, 1 mmol/L, and 10 mmol/L) treatment. Cell viability was determined by MTT assay. The results are presented as percentages of the control group, which was defined as 100%. (B) Astrocytes were pretreated with glutamate (0.1, 1, and 10 mmol/L) for 1 h or 24 h, and the lactate concentration in culture medium was calculated from a standard curve and normalized by total protein content. Error bars represent the mean ± SD, n = 3. **P < 0.01 vs. the 1-h control group; ##P < 0.01 vs. the 24-h control group; ###P < 0.001 vs. the 24-h control group.
-
[1] Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism:focus on astrocyte-neuron metabolic cooperation. Cell Metab, 2011; 14, 724-38. doi: 10.1016/j.cmet.2011.08.016 [2] Choudhury GR, Winters A, Rich RM, et al. Methylene blue protects astrocytes against glucose oxygen deprivation by improving cellular respiration. PloS One, 2015; 10, e0123096. doi: 10.1371/journal.pone.0123096 [3] Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron, 2011; 71, 782-97. doi: 10.1016/j.neuron.2011.08.009 [4] Köles L, Kató E, Hanuska A, et al. Modulation of excitatory neurotransmission by neuronal/glial signalling molecules:interplay between purinergic and glutamatergic systems. Purinergic Signal, 2015; 12, 1-24. https://www.researchgate.net/publication/283543859_Modulation_of_excitatory_neurotransmission_by_neuronalglial_signalling_molecules_interplay_between_purinergic_and_glutamatergic_systems [5] Kreft M, Bak LK, Waagepetersen HS, et al. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro, 2012; 4, AN20120007. doi: 10.1042/AN20120007 [6] Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci, 2014; 7, 38. http://www.sigmaaldrich.com/catalog/papers/23596393 [7] Di Malta C, Fryer JD, Settembre C, et al. Astrocyte dysfunction triggers neurodegeneration in a lysosomal storage disorder. Proc Natl Acad Sci USA, 2012; 109, E2334-E42. doi: 10.1073/pnas.1209577109 [8] Golubeva AV, Moloney RD, O'connor RM, et al. Metabotropic Glutamate Receptors in Central Nervous System Diseases. Curr Drug Targets, 2016; 17, 538-616. doi: 10.2174/1389450116666150316224011 [9] Kou T, Foster JB, Lin CLG. Glutamate transporter EAAT2:regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci, 2015; 72, 3489-506. doi: 10.1007/s00018-015-1937-8 [10] Azarias G, Perreten H, Lengacher S, et al. Glutamate transport decreases mitochondrial pH and modulates oxidative metabolism in astrocytes. J Neurosci, 2011; 31, 3550-9. doi: 10.1523/JNEUROSCI.4378-10.2011 [11] Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis:a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA, 1994; 91, 10625-29. doi: 10.1073/pnas.91.22.10625 [12] Mccarthy KD, De Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol, 1980; 85, 890-902. doi: 10.1083/jcb.85.3.890 [13] Shi ZF, Zhao WJ, Xu LX, et al. Downregulation of Aquaporin 4 Expression through Extracellular Signal-regulated Kinases1/3 Activation in Cultured Astrocytes Following Scratch-injury. Biomed Environ Sci, 2015; 28, 199-205. http://www.cnki.com.cn/Article/CJFDTotal-SWYX201503004.htm [14] Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci, 2005; 25, 7538-47. doi: 10.1523/JNEUROSCI.1927-05.2005 [15] Meldrum BS. Glutamate as a neurotransmitter in the brain:Review of physiology and pathology. J Nutr, 2000; 130, 1007S-15S. https://www.researchgate.net/publication/12580625_Glutamate_as_a_neurotransmitter_in_the_brain_Review_of_physiology_and_pathology?ev=auth_pub [16] Kritis AA, Stamoula EG, Paniskaki KA, et al. Researching glutamate-induced cytotoxicity in different cell lines:a comparative/collective analysis/study. Front Cell Neurosci, 2015; 9, 91. https://www.researchgate.net/publication/273956066_Researching_glutamate_-_induced_cytotoxicity_in_different_cell_lines_A_comparativecollective_analysisstudy [17] Cong L, Cao C, Cheng Y, et al. Green tea polyphenols attenuated glutamate excitotoxicity via antioxidative and antiapoptotic pathway in the primary cultured cortical neurons. Oxid Med Cell Longev, 2016; 2016, 2050435. [18] Yin WY, Ye Q, Huang HJ, et al. Salidroside protects cortical neurons against glutamate-induced cytotoxicity by inhibiting autophagy. Mol Cell Biochem, 2016; 419, 53-64. doi: 10.1007/s11010-016-2749-3 [19] Shen Y, Tian Y, Shi X, et al. Exposure to high glutamate concentration activates aerobic glycolysis but inhibits ATP-linked respiration in cultured cortical astrocytes. Cell Biochem Funct, 2014; 32, 530-37. doi: 10.1002/cbf.v32.6 [20] Laird MD, Clerc P, Polster BM, et al. Augmentation of Normal and Glutamate-Impaired Neuronal Respiratory Capacity by Exogenous Alternative Biofuels. Transl Stroke Res, 2013; 4, 643-51. doi: 10.1007/s12975-013-0275-0 [21] Hertz L, Zielke HR. Astrocytic control of glutamatergic activity:astrocytes as stars of the show. Trends Neurosci, 2004; 27, 735-43. doi: 10.1016/j.tins.2004.10.008 [22] Yang SH, Li W, Sumien N, et al. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers:methylene blue connects the dots. Prog Neurobiol, 2015. [23] Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury:new discoveries with implications for treatment. Front Neurosci, 2015; 8, 408. https://www.researchgate.net/profile/George_Brooks/publication/272365466_Cerebral_Metabolism_Following_Traumatic_Brain_Injury_New_Discoveries_with_Implications_for_Treatment/links/550085110cf2d61f820e20e3.pdf [24] Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem, 2008; 105, 287-95. doi: 10.1111/j.1471-4159.2008.05301.x [25] Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med, 1994; 330, 613-22. doi: 10.1056/NEJM199403033300907 [26] Dienel GA, Mckenna MC. A dogma-breaking concept:glutamate oxidation in astrocytes is the source of lactate during aerobic glycolysis in resting subjects. J Neurochem, 2014; 131, 395-98. doi: 10.1111/jnc.2014.131.issue-4 [27] Bittner CX, Valdebenito R, Ruminot I, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci, 2011; 31, 4709-13. doi: 10.1523/JNEUROSCI.5311-10.2011 [28] Chatton JY, Magistretti PJ, Barros LF. Sodium signaling and astrocyte energy metabolism. Glia, 2016; 64, 1667-76. doi: 10.1002/glia.22971 [29] Thevenet J, De MU, Domingo JS, et al. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J, 2016; 30, 1913-26. doi: 10.1096/fj.201500182 [30] Wyss MT, Jolivet R, Buck A, et al. In vivo evidence for lactate as a neuronal energy source. J Neurosci, 2011; 31, 7477-85. doi: 10.1523/JNEUROSCI.0415-11.2011 [31] Steinman MQ, Gao V, Alberini CM. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci, 2016; 10, 10. [32] Van Hall G, Stømstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab, 2009; 29, 1121-9. doi: 10.1038/jcbfm.2009.35 [33] Schurr A. Cerebral glycolysis:a century of persistent misunderstanding and misconception. Front Neurosci, 2015; 8, 360. https://www.researchgate.net/publication/269186683_Cerebral_Glycolysis_A_Century_of_Persistent_Misunderstanding_and_Misconception [34] Dinuzzo M. Astrocyte-Neuron Interactions during Learning May Occur by Lactate Signaling Rather than Metabolism. Front Integr Neurosci, 2016; 10, 2. https://www.researchgate.net/publication/292213402_Astrocyte-Neuron_Interactions_during_Learning_May_Occur_by_Lactate_Signaling_Rather_than_Metabolism [35] Llorente-Folch I, Rueda CB, Pérez-Liébana I, et al. l-Lactate-Mediated Neuroprotection against Glutamate-Induced Excitotoxicity Requires ARALAR/AGC1. J Neurosci, 2016; 36, 4443-56. -




 下载:
下载:



 Quick Links
Quick Links