-
Stroke is the second leading cause of death and the leading cause of acquired disability worldwide[1-2]. Over two-thirds of stroke deaths occur in developing countries[3]. In China, there are 2.5 million new stroke cases each year and 7.5 million stroke survivors. Stroke now accounts for 21.6% and 20.8% of total mortality in men and women, respectively[4]. As such, stroke poses a major public health burden in China.
Epidemiological studies have demonstrated that stroke is a complex, multifactorial disease caused by a combination of vascular risk factors and environmental and hereditary factors[5-7]. Family history of cardiovascular diseases (CVD) is considered a non-modifiable, hereditary risk factor for stroke. Accumulating evidence suggested that individuals with a family history of CVD have a genetic susceptibility to stroke when compared to individuals in a shared environment without a family history of CVD[8-10]. Some studies also suggested that serum C-reactive protein (CRP), a classic inflammatory biomarker, is a modifiable risk factor for the morbidity and mortality associated with stroke[11-13]. In a previous study, higher levels of CRP were observed in children with a family history of CVD[14]. As there is a potential association between high CRP levels and a family history of CVD, and both are risk factors for stroke, the effect of high CRP level on the risk of stroke in people with a family history of CVD needs to be further clarified. However, to date, there are no published studies analyzing the cumulative effect of a family history of CVD and CRP level on stroke incidence. In the present study, we analyzed the association between a family history of CVD, CRP level, and future stroke risk in a 9.2-year follow-up study among Mongolians, in Inner Mongolia, China. We also investigated the potential role of CRP levels in enhancing risk prediction of stroke events in people in this population with a family history of CVD.
-
This prospective cohort study was conducted from June 2002 to July 2012 in Inner Mongolia, an autonomous region in north China. According to the objective of the study, we needed to select Mongolian farmers and herdsmen with a traditional Mongolian lifestyle and a stable place of residence. Based on these criteria, we selected as study fields the two townships in the counties of Kezuohou Banner and Naiman Banner with the highest proportion of Mongolians in their populations. There were 32 nature villages in the two townships and most of the residents (over 95%) were Mongolian farmers and herdsmen. A total of 3, 475 Mongolian people aged 20 years and over lived in these villages. Of these, 886 people were excluded because they refused to participate or had existing cardiovascular or endocrine diseases, including hyper/hypothyroi-dism. A total of 2, 589 participants were included in the study. This study was approved by the Soochow University Ethics Committee. Written informed consent was obtained from all study participants.
-
Data on the demographic characteristics, lifestyle risk factors, family history of CVD, and personal medical history of the participants were gathered from standard questionnaires written in Chinese and administered by four trained staff. In the questionnaires, we asked participants how many of their first-degree and second-degree relatives suffered from CVD; whether they smoked, how many years they had smoked and how many cigarettes they smoked a day; and whether they drank alcohol, how many years they had drunk alcohol, and how much alcohol they consumed in a day. Family history of CVD was defined as having at least one person with diagnosed CVD among first-degree relatives, or at least two people with diagnosed CVD among second-degree relatives. Cigarette smoking was defined as having smoked at least one cigarette per day for one year or more. Alcohol consumption was defined as consuming any alcoholic beverage containing at least 25 g alcohol once a day on average for one year or more during the past three years.
Three consecutive sitting blood pressure (BP) measurements for each participant were taken by trained staff using a mercury sphygmomanometer according to a standard protocol[15], after the subjects had rested for at least five minutes. The first and fifth Korotkoff sounds were recorded as systolic BP (SBP) and diastolic BP (DBP), respectively. The mean of these three BP measurements was used in the data analysis. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or use of antihypertensive medication in the last two weeks[16]. Body weight and height were measured by trained staff with participants wearing light clothing and without shoes. The body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). The waist circumference (WC) was measured at 1 cm above the umbilicus.
Fasting blood samples were collected from all participants during the morning, after at least eight hours of fasting. All plasma and serum samples were frozen at -80 ℃ until laboratory testing. A modified hexokinase enzymatic method was applied to test fasting plasma glucose (FPG) levels[17]. Levels of triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were assessed enzymatically on a Beckman Synchrony CX5 Delta Clinical System (Beckman Coulter, Fullerton, CA, USA) using commercial reagents[18]. The low-density lipoprotein cholesterol (LDL-C) level was calculated using the Friedewald equation for participants with TG less than 400 mg/dL[19]. Serum CRP level was assessed using an immunoturbidimetric assay on a Beckman Synchron CX5 Delta Clinical System with commercial reagents.
-
The methods of follow-up and outcome assessment have been reported previously[20]. Briefly, all participants were followed from June 2002 to July 2012. The study outcome was the incidence of stroke, including ischemic stroke and hemorrhagic stroke, during the follow-up period. Stroke was defined as evidence of an acute disturbance in focal areas of the brain lasting for ≥ 24 h and thought to be due to intracranial hemorrhage or ischemia[21]. Participants who did not have a stroke, who died from other causes, or who were lost to follow-up were defined as censored. If the participant was contacted and found to have had a stroke, the stroke incidence date was considered the end-point date. Data were censored at the time of the contact if the participant was reached and was found not to have had a stroke, and on the day we last contacted the participant if he or she was lost to follow-up. For those who died from other causes, data were censored at the death date in the medical records. Four county hospitals with modern diagnostic facilities, including computed tomography (CT) and magnetic resonance imaging (MRI), provide residents of the 32 villages with medical services. Since 2004, household surveys of all participants were conducted on an average of every two years to identify new stroke cases. Trained staff interviewed either the participants or their relatives (if participants were dead or unable to communicate) and completed a medical status questionnaire. If a participant reported a stroke during the period since the last survey, the staff reviewed hospital records, including outpatient or admission records, the discharge summary, and especially CT or MRI scan results, to confirm.
-
CRP level was categorized into two groups based on the median CRP value: < 5.92 mg/L (low CRP level) and ≥ 5.92 mg/L (high CRP level). We categorized all participants into four groups: no family history of CVD/low CRP level, no family history of CVD/high CRP level, family history of CVD/low CRP level, and family history of CVD/high CRP level. Conventional cardiovascular risk factors were compared across the four groups using analysis of variance for continuous variables and chi-squared tests for categorical variables. The cumulative stroke risk among the four groups was estimated using the Kaplan-Meier curves and compared by log-rank test. We used Cox proportional hazards models to compute the hazard ratios (HRs) of stroke among the four groups by adjusting for important confounding factors including age, sex, smoking status, drinking status, hypertension, BMI, WC, and levels of blood glucose, TG, LDL-C, and HDL-C. We set a multiplicative interaction term of family history of CVD and CRP in the Cox proportional hazards model and tested its effect on the stroke incidence, independent of family history of CVD, CRP, and other confounding factors. We also compared cumulative risk and computed HRs of ischemic stroke and hemorrhagic stroke among the four groups. Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS statistical software (version 9.4, Cary, NC, USA).
-
As of July 31, 2012, we had followed-up the participants for an average of 9.2 years. Among the 2, 589 participants, six were lost to follow-up, giving a follow-up rate of 99.8%. Forty-five participants were excluded for missing key variables, and a total of 2, 544 people were included in the final analysis (Figure 1). During the follow-up period, a total of 120 patients with stroke were identified, with 76 ischemic and 44 hemorrhagic stroke events. The cumulative incidence rates of stroke, ischemic stroke, and hemorrhagic stroke were 4.7%, 3.0%, and 1.7%, respectively; the incidence densities were 510, 323, and 187 per 100, 000 person-years, respectively.
-
Table 1 presents a comparison of baseline characteristics among the four groups. Conventional stroke risk factors such as age, sex, smoking status, drinking status, BMI, WC, SBP, DBP, hypertension, TG, HDL-C, LDL-C, and FPG, were significantly different among the four groups. Participants with a family history of CVD in either the low CRP level or the high CRP level group tended to be older, men, drinkers, and hypertensive with higher SBP and DBP measures. Participants with high CRP levels in either the family history of CVD or the no family history of CVD groups were likely to be smokers, and to have higher levels of BMI, WC, TC, TG, LDL-C, FPG, and lower levels of HDL-C.
Table 1. Demographic and Clinical Characteristics of 2, 544 Participants according to Four Groups
Variables No family history of CVD/low CRP No family history of CVD/high CRP Family history of CVD/low CRP Family history of CVD/high CRP P No. of participants 1, 160 1, 054 109 221 Age, mean ± SD, y 44.98 ± 12.13 47.01 ± 11.81 48.84 ± 15.28* 50.71 ± 13.49* < 0.001 Male, n (%) 361 (31.12) 461 (43.74) 71 (65.14)* 149 (67.42)* < 0.001 Smoking, n (%) 455 (39.22) 497 (47.15)* 50 (45.87) 126 (57.01)* < 0.001 Drinking, n (%) 306 (26.38) 388 (36.81) 50 (45.87)* 105 (47.51)* < 0.001 BMI, mean ± SD, kg/m2 21.57 ± 3.03 22.92 ± 3.81* 22.15 ± 2.95 22.78 ± 3.61* < 0.001 WC, mean ± SD, cm 78.25 ± 8.43 82.82 ± 10.20* 81.07 ± 7.72 84.18 ± 9.87* < 0.001 SBP, mean ± SD, mmHg 124.77 ± 22.81 129.99 ± 23.30 141.15 ± 29.23* 148.71 ± 26.96* < 0.001 DBP, mean ± SD, mmHg 81.49 ± 12.13 85.30 ± 12.30 91.07 ± 13.86* 93.72 ± 13.09* < 0.001 HBP, n (%) 313 (26.98) 404 (38.33) 67 (61.47)* 168 (76.02)* < 0.001 TC (mmol/L) 3.44 (2.84-4.06) 3.75 (3.11-4.65)* 3.53 (2.76-4.33) 3.93 (3.20-4.74)* < 0.001 TG (mmol/L) 0.75 (0.54-1.04) 1.21 (0.86-1.84)* 0.80 (0.58-1.05) 1.22 (0.84-1.82)* < 0.001 HDL-C (mmol/L) 1.20 (1.02-1.42) 1.07 (0.91-1.30)* 1.14 (0.96-1.38) 1.09 (0.94-1.35)* < 0.001 LDL-C (mmol/L) 1.99 (1.51-2.58) 2.34 (1.69-3.15)* 2.13 (1.43-3.00) 2.41 (1.92-3.18)* < 0.001 FPG (mmol/L) 4.50 (4.10-5.10) 5.10 (4.50-5.60)* 5.00 (4.40-5.60) 5.30 (4.90-5.90)* < 0.001 Note. All values are expressed with median (inter-quartile range) unless otherwise noted. Low CRP level was defined as serum C-reactive protein concentration < 5.92 mg/L; and High CRP level was defined as serum C-reactive protein concentration ≥ 5.92 mg/L. CRP, C-reactive protein; CVD, cardiovascular disease; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HBP, diagnosis of hypertension; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fast plasma glucose; *P < 0.05 compared to group with no family history of CVD/low CRP level. -
Family history of CVD and CRP level served as independent variables in analyzing their effects on stroke. The association between a family history of CVD or CRP level and stroke is shown in Table 2. The age-sex adjusted HRs [95% confidence interval (CI)] of stroke for a family history of CVD and high CRP level were 1.76 (1.18-2.63) and 1.24 (0.81-1.90), respectively. After adjusting for age; sex; smoking and drinking status; hypertension; BMI; WC; levels of FPG, TG, LDL-C, HDL-C; and CRP level (or family history of CVD), HRs (95% CI) of stroke for family history of CVD and high CRP level were 1.24 (0.81-1.90) and 1.40 (0.94-2.09), respectively.
Table 2. Adjusted HRs (95% CI) for the Stroke incidence according to Family History of CVD Status and Serum CRP Concentration
Variables Cases Person-years Age-sex Adjusted Multivariate Adjusted HR 95% CI P HR 95% CI P Family history of CVD* No 85 20, 527 1.00 1.00 Yes 35 2, 983 1.76 1.18-2.63 0.006 1.24 0.81-1.90 0.321 High CRP** No 46 11, 726 1.00 1.00 Yes 74 1, 784 1.44 0.99-2.07 0.055 1.40 0.94-2.09 0.103 Total*** No family history of CVD/low CRP 38 10, 732 1.00 1.00 No family history of CVD/high CRP 47 9, 795 1.23 0.80-1.89 0.344 1.25 0.79-1.98 0.335 Family history of CVD/low CRP 8 994 1.26 0.58-2.71 0.560 0.92 0.42-2.00 0.823 Family history of CVD/high CRP 27 1, 989 2.34 1.42-3.86 < 0.001 1.78 1.03-3.07 0.039 Note. HR, hazard ratio; CI, confidential interval. Low CRP was defined as serum C-reactive protein concentration < 5.92 mg/L; and High CRP level was defined as serum C-reactive protein concentration ≥ 5.92 mg/L. *Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C, HDL-C and C-reactive protein. **Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, family history of cardiovascular disease, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. ***Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. -
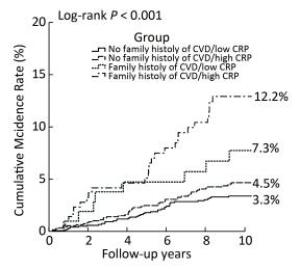
Figure 2 shows the Kaplan-Meier curves for cumulative incidence rates of stroke for the four groups. From June 2002 to July 2012, the cumulative incidence rates of stroke in the no family history of CVD/low CRP level, no family history of CVD/high CRP level, family history of CVD/low CRP level, and family history of CVD/high CRP level groups were 3.3%, 4.5%, 7.3%, and 12.2%, respectively (log-rank P < 0.001). Participants with both a family history of CVD and high CRP levels had the highest cumulative stroke incidence rate.
Table 2 presents the age-sex adjusted and multivariate adjusted HRs (95% CI) of stroke according to family history of CVD status and CRP level. Compared with the no family history of CVD/low CRP level group, the age-sex adjusted HRs (95% CI) of stroke in the no family history of CVD/high CRP level, family history of CVD/low CRP level, and family history of CVD/high CRP level groups were 1.23 (0.80-1.89), 1.26 (0.58-2.71), and 2.34 (1.42-3.86), respectively. After further adjusting for other cardiovascular risk factors, the HR (95% CI) of stroke for the family history of CVD/high CRP level group was 1.78 (1.03-3.07), and remained significant although the HR value decreased slightly. Therefore, we set a multiplicative interaction term of family history of CVD and serum CRP in a multivariable model, but no significant interaction was detected between family history of CVD and CRP level on stroke risk (P = 0.343). In subgroup analyses stratified by age and sex, the highest stroke risk was observed in participants with both a family history of CVD and high CRP level in all subgroups, and it reached significant levels in several subgroups (Table 3). Statistical tests for interactions between the group and age or sex on stroke risk were not significant (all P > 0.05).
Table 3. Subgroup Analysis of Adjusted HRs (95% CI) for the stroke incidence according to Family History of CVD Status and serum CRP Concentration
Subgroup No. of cases/ participants Group Pinteraction No family history of CVD/low CRP No family history of CVD/high CRP Family history of CVD/low CRP Family history of CVD/high CRP Age, years* 0.528 < 45 7/1, 208 1.00 1.40 (0.19-10.07) NA 5.99 (0.61-59.06) ≥ 45 113/1, 336 1.00 1.30 (0.81-2.09) 1.12 (0.51-2.47) 1.86 (1.05-3.29) Sex** 0.793 Men 77/1, 042 1.00 1.12 (0.63-2.00) 0.91 (0.36-2.29) 1.95 (1.01-3.78) Women 43/1, 502 1.00 1.52 (0.72-3.23) 1.43 (0.31-6.55) 2.15 (0.79-5.84) Note.NA: not available for limited sample size. The age cut-off point is 45 years (the median of age at baseline). *Multivariate model adjusted for sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. **Multivariate model adjusted for age, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. -
Figure 3A shows the cumulative incidence rates and multivariate adjusted HRs (95% CI) of ischemic stroke in the four groups. The cumulative incidence rates of ischemic stroke in the no family history of CVD/low CRP level, no family history of CVD/high CRP level, family history of CVD/low CRP level, and family history of CVD/high CRP level groups were 2.1%, 2.7%, 3.7%, and 9.1%, respectively (log-rank P < 0.001). Participants with a family history of CVD and high CRP levels had the highest cumulative ischemic stroke incidence rate. Compared with the no family history of CVD/low CRP level group, the multivariable adjusted HR of ischemic stroke for the family history of CVD/high CRP levels group was 2.14 (95% CI, 1.09-4.20; P = 0.027). No significant interaction was detected between a family history of CVD and CRP level on ischemic stroke risk (P = 0.091).
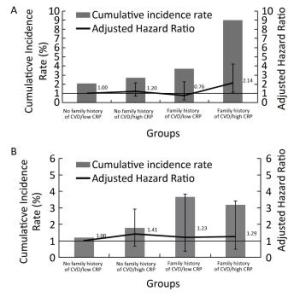
Figure 3. Cumulative incidence rate and multivariate adjusted HR (95% CI) of ischemic stroke (A) and hemorrhagic stroke (B) in four groups according to family history of CVD status and serum CRP concentration.
Figure 3B shows the cumulative incidence rates and multivariate adjusted HRs (95% CI) of hemorrhagic stroke in the four groups. The cumulative incidence rates of hemorrhagic stroke in the no family history of CVD/low CRP levels, no family history of CVD/high CRP levels, family history of CVD/low CRP levels, and family history of CVD/high CRP levels groups were 1.2%, 1.8%, 3.7%, and 3.2%, respectively (log-rank P = 0.052). Compared with the no family history of CVD/low CRP level group, the multivariable adjusted HRs of hemorrhagic stroke in other three groups were not statistically significant (all P > 0.05). Nor did we detect a significant interaction between a family history of CVD and CRP level on hemorrhagic stroke risk (P = 0.662).
-
In this population-based prospective cohort study of an Inner Mongolian population, we did not find that either family history of CVD or CRP level was significantly and independently associated with stroke risk after adjusting for important confounding factors. However, in further analyses, which combined the effects of family history of CVD and CRP level, we observed that participants with a family history of CVD and high CRP level had a significantly higher stroke risk, especially for ischemic stroke, compared to those with no family history of CVD and low CRP levels. These findings indicate that there is a combined effect of a family history of CVD and CRP level on the stroke incidence. To our knowledge, this is the first study to evaluate the combined effects of a family history of CVD and CRP level on stroke risk in the Mongolian population.
Several epidemiological studies have demonstrated that a family history of CVD can lead to an increased stroke risk as an important genetic risk factor, and this history can serve as a tool for identifying individuals at high risk of suffering a stroke[8-9]. The Framingham Study[10] found that parental stroke was associated with a three-fold increase in stroke risk in their offspring, which persisted after adjustment for conventional stroke risk factors. An association has also been reported between low-grade chronic inflammation and an elevated stroke risk[22-23]. CRP is a classic inflammatory biomarker, and high levels can indicate the presence of chronic inflammation. The value of CRP to predict stroke risk has been assessed in several large epidemiological studies[11-13]. However, studies by Brass et al.[24] and Siegerink et al.[25] found that a family history of CVD could not predict future stroke risk, and the Northern Manhattan[26] and Rotterdam Scan[27] studies found no association between CRP levels and stroke risk.
The conflicting results from these studies may be due to differences in study design, study population, sample size, or the methods used to identify CVD events in family members. This means that instability may exist when either family history of CVD or CRP level is used to predict stroke risk. In our study, we did not find a significant association between family history of CVD or CRP level and stroke. However, we found high CRP level may increase stroke risk in people with a family history of CVD, suggesting that family history of CVD and CRP levels together may be more efficient in predicting the stroke incidence. In the subgroup analysis stratified by age and sex, we found the highest stroke risk in participants with both a family history of CVD and high CRP level in all subgroups, and it reached significant levels in the subgroups of the elderly and men. The decreased statistical power of subgroup analysis and the lower prevalence of cardiovascular risk factors in young people and women may have led to this study being underpowered to detect any significant association in these subgroups. Therefore, studies with large sample sizes are needed to clarify the association in young people or women.
Several lines of evidence have led to the hypothesis that the role of systemic inflammation in stroke is probably small when compared with the effects of traditional risk factors. The Honolulu Heart Program study[28] found that the association between high CRP levels and increased stroke risk was strongest in individuals aged under 55 years who had no hypertension or diabetes. In consequence, the value of CRP level combined with a family history of CVD to predict stroke is more practical and stronger in younger people, among whom traditional risk factors such as hypertension are less common. As mentioned above, family history of CVD is a non-modifiable risk factor. However, diet modification[29], exercise[30], and drugs such as statins[31] are reported to reduce CRP levels and modulate other risk factors for stroke. Thus, it is important for people with a family history of CVD to decrease their serum CRP levels through diet, exercise, and even statins to reduce stroke risk, especially before traditional risk factors occur.
The exact mechanism underlying the combined effect of family history of CVD and CRP level on stroke risk is not completely understood. Considering the stroke process, it is plausible for a family history of CVD to increase the stroke incidence through (1) familial sharing of environmental, cultural and lifestyle factors; (2) genetic heritability of stroke risk factors, such as elevated BP; (3) inherited susceptibility to the effects of such risk factors; and (4) the interaction between environmental and genetic factors[8]. High CRP level is likely to increase stroke risk through its important role in the pathogenesis and progression of atherosclerosis, plaque rupture, platelet aggregation, and intravascular thrombosis[32]. Therefore, there may be a biological interaction between a family history of CVD and CRP levels, and high CRP level can improve risk prediction for ischemic stroke for individuals with a family history of CVD.
This study has several strengths that deserve mention. First, it is a cohort study based on a minority population of China, where the study participants were homogeneous in terms of genetic background and environmental exposures. Second, the study data were collected with rigid quality control and important covariables were measured and controlled for in the analysis. Third, this cohort had low loss to follow-up due to stability of the in-field population, and a relatively long follow-up time with an average of 9.2 years. However, there are also some limitations of the study that should be mentioned. First, the sample size was comparatively small, and the number of hemorrhagic stroke events was relatively low, so the relatively low statistical power might have underestimated the combined effect of a family history of CVD and high CRP level on hemorrhagic stroke. Second, the socioeconomic status, educational status, dietary patterns, and physical activity status of participants were not recorded, so we were unable to adjust for them in our analyses. However, all the participants were Mongolians whose families had have lived in the area for multiple generations and had maintained traditional manners and customs of Mongolian ethnicity, their professions were farmers and herdsmen, and their diets were high in fat and salt. Therefore, the socioeconomic status, educational status, dietary patterns and physical activity status of the participants were similar, and their effect on the results would be relatively low. Third, we did not evaluate the reproducibility of questionnaire measures (i.e. intraclass correlation coefficient). However, during the on-site investigation period, the four trained staff administered the questionnaire to a unified standard, and each day's questionnaires were checked that night, and any abnormal or missing values were reinvestigated the next day, according to the research proposal. Finally, 889 people from the two adjacent townships did not participate in this study cohort, which might have introduced some selection bias. We believe this bias would be minimal because it is unlikely that these individuals chose not to participate due to their family history of CVD or CRP levels.
-
In conclusion, our study showed that participants with both, a family history of CVD and high CRP levels had the highest stroke risk among the four groups of Mongolian participants. This finding suggests that high CRP levels may increase stroke risk, especially for ischemic stroke, among people with a family history of CVD.
-
We would like to thank all participants in this study and the Kezuohouqi Banner Center for Disease Prevention and Control and the Naiman Banner Center for Disease Prevention and Control for their support and assistance.
-
ZHU Zheng Bao collected and researched data, and wrote the manuscript. HUANGFU Xin Feng, ZHOU Yi Peng, TIAN Yun Fan, ZHONG Chong Ke, BUREN Batu, and XU Tian collected and researched data. LI Hong Mei, ZHANG Ming Zhi, and WANG Ai Li researched data. ZHANG Yong Hong designed the study, collected and researched data, and revised the manuscript. ZHANG Yong Hong is the guarantor of this work and, as such, had full access to the data and takes responsibility for the accuracy of data analysis.
-
No conflict of interest to declare.
doi: 10.3967/bes2017.084
Combined Effects of Family History of Cardiovascular Disease and Serum C-reactive Protein Level on the Risk of Stroke:A 9.2-year Prospective Study among Mongolians in China
-
Abstract:
Objective We aimed to evaluate the combined effect of a family history of cardiovascular disease (CVD) and high serum C-reactive protein (CRP) on the stroke incidence in an Inner Mongolian population in China. Methods A prospective cohort study was conducted from June 2002 to July 2012, with 2, 544 participants aged 20 years and over from Inner Mongolia, China. We categorized participants into four groups based on the family history of CVD and CRP levels. Results We adjusted for age; sex; smoking; drinking; hypertension; body mass index; waist circumference; and blood glucose, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels. Compared with the group with no family history of CVD/low CRP levels, the group with family history of CVD/high CRP levels had a hazard ratio (HR) of 1.78[95% confidence interval (CI), 1.03-3.07; P=0.039] of stroke, and an HR of 2.14 (95% CI, 1.09-4.20; P=0.027) of ischemic stroke. The HRs of hemorrhagic stroke for the other three groups were not statistically significant (all P > 0.05). Conclusion Participants with both a family history of CVD and high CRP levels had the highest stroke incidence, suggesting that high CRP levels may increase stroke risk, especially of ischemic stroke, among individuals with a family history of CVD. -
Table 1. Demographic and Clinical Characteristics of 2, 544 Participants according to Four Groups
Variables No family history of CVD/low CRP No family history of CVD/high CRP Family history of CVD/low CRP Family history of CVD/high CRP P No. of participants 1, 160 1, 054 109 221 Age, mean ± SD, y 44.98 ± 12.13 47.01 ± 11.81 48.84 ± 15.28* 50.71 ± 13.49* < 0.001 Male, n (%) 361 (31.12) 461 (43.74) 71 (65.14)* 149 (67.42)* < 0.001 Smoking, n (%) 455 (39.22) 497 (47.15)* 50 (45.87) 126 (57.01)* < 0.001 Drinking, n (%) 306 (26.38) 388 (36.81) 50 (45.87)* 105 (47.51)* < 0.001 BMI, mean ± SD, kg/m2 21.57 ± 3.03 22.92 ± 3.81* 22.15 ± 2.95 22.78 ± 3.61* < 0.001 WC, mean ± SD, cm 78.25 ± 8.43 82.82 ± 10.20* 81.07 ± 7.72 84.18 ± 9.87* < 0.001 SBP, mean ± SD, mmHg 124.77 ± 22.81 129.99 ± 23.30 141.15 ± 29.23* 148.71 ± 26.96* < 0.001 DBP, mean ± SD, mmHg 81.49 ± 12.13 85.30 ± 12.30 91.07 ± 13.86* 93.72 ± 13.09* < 0.001 HBP, n (%) 313 (26.98) 404 (38.33) 67 (61.47)* 168 (76.02)* < 0.001 TC (mmol/L) 3.44 (2.84-4.06) 3.75 (3.11-4.65)* 3.53 (2.76-4.33) 3.93 (3.20-4.74)* < 0.001 TG (mmol/L) 0.75 (0.54-1.04) 1.21 (0.86-1.84)* 0.80 (0.58-1.05) 1.22 (0.84-1.82)* < 0.001 HDL-C (mmol/L) 1.20 (1.02-1.42) 1.07 (0.91-1.30)* 1.14 (0.96-1.38) 1.09 (0.94-1.35)* < 0.001 LDL-C (mmol/L) 1.99 (1.51-2.58) 2.34 (1.69-3.15)* 2.13 (1.43-3.00) 2.41 (1.92-3.18)* < 0.001 FPG (mmol/L) 4.50 (4.10-5.10) 5.10 (4.50-5.60)* 5.00 (4.40-5.60) 5.30 (4.90-5.90)* < 0.001 Note. All values are expressed with median (inter-quartile range) unless otherwise noted. Low CRP level was defined as serum C-reactive protein concentration < 5.92 mg/L; and High CRP level was defined as serum C-reactive protein concentration ≥ 5.92 mg/L. CRP, C-reactive protein; CVD, cardiovascular disease; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HBP, diagnosis of hypertension; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fast plasma glucose; *P < 0.05 compared to group with no family history of CVD/low CRP level. Table 2. Adjusted HRs (95% CI) for the Stroke incidence according to Family History of CVD Status and Serum CRP Concentration
Variables Cases Person-years Age-sex Adjusted Multivariate Adjusted HR 95% CI P HR 95% CI P Family history of CVD* No 85 20, 527 1.00 1.00 Yes 35 2, 983 1.76 1.18-2.63 0.006 1.24 0.81-1.90 0.321 High CRP** No 46 11, 726 1.00 1.00 Yes 74 1, 784 1.44 0.99-2.07 0.055 1.40 0.94-2.09 0.103 Total*** No family history of CVD/low CRP 38 10, 732 1.00 1.00 No family history of CVD/high CRP 47 9, 795 1.23 0.80-1.89 0.344 1.25 0.79-1.98 0.335 Family history of CVD/low CRP 8 994 1.26 0.58-2.71 0.560 0.92 0.42-2.00 0.823 Family history of CVD/high CRP 27 1, 989 2.34 1.42-3.86 < 0.001 1.78 1.03-3.07 0.039 Note. HR, hazard ratio; CI, confidential interval. Low CRP was defined as serum C-reactive protein concentration < 5.92 mg/L; and High CRP level was defined as serum C-reactive protein concentration ≥ 5.92 mg/L. *Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C, HDL-C and C-reactive protein. **Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, family history of cardiovascular disease, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. ***Multivariate model adjusted for age, sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. Table 3. Subgroup Analysis of Adjusted HRs (95% CI) for the stroke incidence according to Family History of CVD Status and serum CRP Concentration
Subgroup No. of cases/ participants Group Pinteraction No family history of CVD/low CRP No family history of CVD/high CRP Family history of CVD/low CRP Family history of CVD/high CRP Age, years* 0.528 < 45 7/1, 208 1.00 1.40 (0.19-10.07) NA 5.99 (0.61-59.06) ≥ 45 113/1, 336 1.00 1.30 (0.81-2.09) 1.12 (0.51-2.47) 1.86 (1.05-3.29) Sex** 0.793 Men 77/1, 042 1.00 1.12 (0.63-2.00) 0.91 (0.36-2.29) 1.95 (1.01-3.78) Women 43/1, 502 1.00 1.52 (0.72-3.23) 1.43 (0.31-6.55) 2.15 (0.79-5.84) Note.NA: not available for limited sample size. The age cut-off point is 45 years (the median of age at baseline). *Multivariate model adjusted for sex, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. **Multivariate model adjusted for age, smoking and drinking status, hypertension, BMI, WC and levels of blood glucose, TG, LDL-C and HDL-C. -
[1] Bonita R, Mendis S, Truelsen T, et al. The global stroke initiative. Lancet Neurol, 2004; 3, 391-3. doi: 10.1016/S1474-4422(04)00800-2 [2] Strong K, Mathers C, Bonita R. Preventing stroke:saving lives around the world. Lancet Neurol, 2007; 6, 182-7. doi: 10.1016/S1474-4422(07)70031-5 [3] Feigin VL. Stroke epidemiology in the developing world. Lancet, 2005; 365, 2160-1. doi: 10.1016/S0140-6736(05)66755-4 [4] He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med, 2005; 353, 1124-34. doi: 10.1056/NEJMsa050467 [5] Della-Morte D, F Guadagni, R Palmirotta, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics, 2012; 13, 595-613. doi: 10.2217/pgs.12.14 [6] O'Donnell MJ, D Xavier, L Liu, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study):a case-control study. Lancet, 2010; 376, 112-23. doi: 10.1016/S0140-6736(10)60834-3 [7] Sacco RL. Newer risk factors for stroke. Neurology, 2001; 57, S31-4. doi: 10.1212/WNL.57.suppl_2.S31 [8] Liao D, R Myers, S Hunt, et al. Familial history of stroke and stroke risk. The Family Heart Study. Stroke, 1997; 28, 1908-12. http://www.biomedcentral.com/content/xml/1471-2377-5-20.xml [9] Flossmann E, UG Schulz, PM Rothwell. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke, 2004; 35, 212-27. http://circgenetics.ahajournals.org/content/5/2/226 [10] Seshadri S, A Beiser, A Pikula, et al. Parental occurrence of stroke and risk of stroke in their children:the Framingham study. Circulation, 2010; 121, 1304-12. doi: 10.1161/CIRCULATIONAHA.109.854240 [11] Kaptoge S, E Di Angelantonio, G Lowe, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality:an individual participant meta-analysis. Lancet, 2010; 375, 132-40. doi: 10.1016/S0140-6736(09)61717-7 [12] Kuo HK, CJ Yen, CH Chang, et al. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population:systematic review and meta-analysis. Lancet Neurol, 2005; 4, 371-80. doi: 10.1016/S1474-4422(05)70099-5 [13] Ridker PM, JE Buring, J Shih, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation, 1998; 98, 731-3. doi: 10.1161/01.CIR.98.8.731 [14] E L, N Fragakis, E Ioannidou, et al. Increased levels of proinflammatory cytokines in children with family history of coronary artery disease. Clin Cardiol, 2010; 33, E6-10. https://www.ncbi.nlm.nih.gov/pubmed/20229495 [15] Perloff D, C Grim, J Flack, et al. Human blood pressure determination by sphygmomanometry. Circulation, 1993; 88, 2460-70. doi: 10.1161/01.CIR.88.5.2460 [16] 1993 guidelines for the management of mild hypertension. Memorandum from a World Health Organization/International Society of Hypertension meeting. Guidelines Subcommittee of the WHO/ISH Mild Hypertension Liaison Committee. Hypertension, 1993; 22, 392-403. [17] Sitzmann FC, P Eschler. Enzymatic determination of blood glucose with a modified hexokinase method. Med Klin, 1970; 65, 1178-83. https://www.ncbi.nlm.nih.gov/pubmed/5517345 [18] Allain CC, LS Poon, CSG Chan, et al. Enzymatic determination of total serum cholesterol. Clin Chem, 1974; 20, 470-5. doi: 10.1021/ed055p63?src=recsys [19] Friedewald WT, RI Levy, DS Fredrickson. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 1972; 18, 499-502. https://www.ncbi.nlm.nih.gov/pubmed/4337382 [20] Tang L, T Xu, H Li, et al. Hypertension, alcohol drinking and stroke incidence:a population-based prospective cohort study among inner Mongolians in China. J Hypertens, 2014; 32, 1091-6. doi: 10.1097/HJH.0000000000000142 [21] Stroke——1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke, 1989; 20, 1407-31. [22] Pearson TA, GA Mensah, RW Alexander, et al. Markers of inflammation and cardiovascular disease:application to clinical and public health practice:A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 2003; 107, 499-511. doi: 10.1161/01.CIR.0000052939.59093.45 [23] Ridker PM, M Cushman, MJ Stampfer, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med, 1997; 336, 973-9. doi: 10.1056/NEJM199704033361401 [24] Brass LM, LA Shaker. Family history in patients with transient ischemic attacks. Stroke, 1991; 22, 837-41. doi: 10.1161/01.STR.22.7.837 [25] Siegerink B, FR Rosendaal, A Algra. Family history differs between young women with myocardial infarction and ischemic stroke:results from the RATIO case-control study. Atherosclerosis, 2012; 223, 235-8. doi: 10.1016/j.atherosclerosis.2012.04.024 [26] Elkind MS, JM Luna, YP Moon, et al. High-sensitivity C-reactive protein predicts mortality but not stroke:the Northern Manhattan Study. Neurology, 2009; 73, 1300-7. doi: 10.1212/WNL.0b013e3181bd10bc [27] van Dijk EJ, ND Prins, SE Vermeer, et al. C-reactive protein and cerebral small-vessel disease:the Rotterdam Scan Study. Circulation, 2005; 112, 900-5. doi: 10.1161/CIRCULATIONAHA.104.506337 [28] Curb JD, RD Abbott, BL Rodriguez, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation, 2003; 107, 2016-20. doi: 10.1161/01.CIR.0000065228.20100.F7 [29] Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U. S. adults. Epidemiology, 2002; 13, 561-8. http://circgenetics.ahajournals.org/content/7/2/178 [30] Esposito K, R Marfella, M Ciotola, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome:a randomized trial. Jama, 2004; 292, 1440-6. doi: 10.1001/jama.292.12.1440 [31] Albert MA, E Danielson, N Rifai, et al. Effect of statin therapy on C-reactive protein levels:the pravastatin inflammation/CRP evaluation (PRINCE):a randomized trial and cohort study. Jama, 2001; 286, 64-70. doi: 10.1001/jama.286.1.64 [32] Esenwa CC, MS Elkind. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol, 2016; 12, 594-604. doi: 10.1038/nrneurol.2016.125 -




 下载:
下载:



 Quick Links
Quick Links