-
Cardiovascular diseases (CVD) are major public health conditions that are leading causes of death[1,2] and the identification of predictive cardiovascular event markers is urgently needed. To date, numerous biomarkers have been associated with CVD development and progression, including lipoprotein-associated phospholipase A2 (Lp-PLA2), high-sensitivity C-reactive protein (hs-CRP), interleukin-6, homocysteine, and others[3–6]. Lp-PLA2 is a macrophage derived enzyme and proposed as a pro-inflammatory biomarker with a central role in atherosclerosis progression[7]. Substantial evidence shows that Lp-PLA2 is associated with an increased risk of cardiovascular events[8-10]. Also, ethnic variations in Lp-PLA2 have been identified; levels are higher in Caucasians than other races[11,12].
However, data on Lp-PLA2 to predict cardiovascular events, with long-term follow-up, are limited in Chinese and Asian populations. In addition, the American Association of Clinical Endocrinologists (AACE) proposed Lp-PLA2 standards for predicting the risk of future CVD, however, no studies have yet assessed whether this standard is applicable to a Chinese setting. Therefore, we hypothesized that aberrant increases in Lp-PLA2 levels could be associated with an increased risk of cardiovascular events in a Chinese population. Our remit was to explore the predictive ability of Lp-PLA2 on the occurrence of cardiovascular events, with a long follow-up, in a CVD-free, middle-aged Chinese population. We also explored the feasibility of applying AACE guidelines to the Chinese population.
-
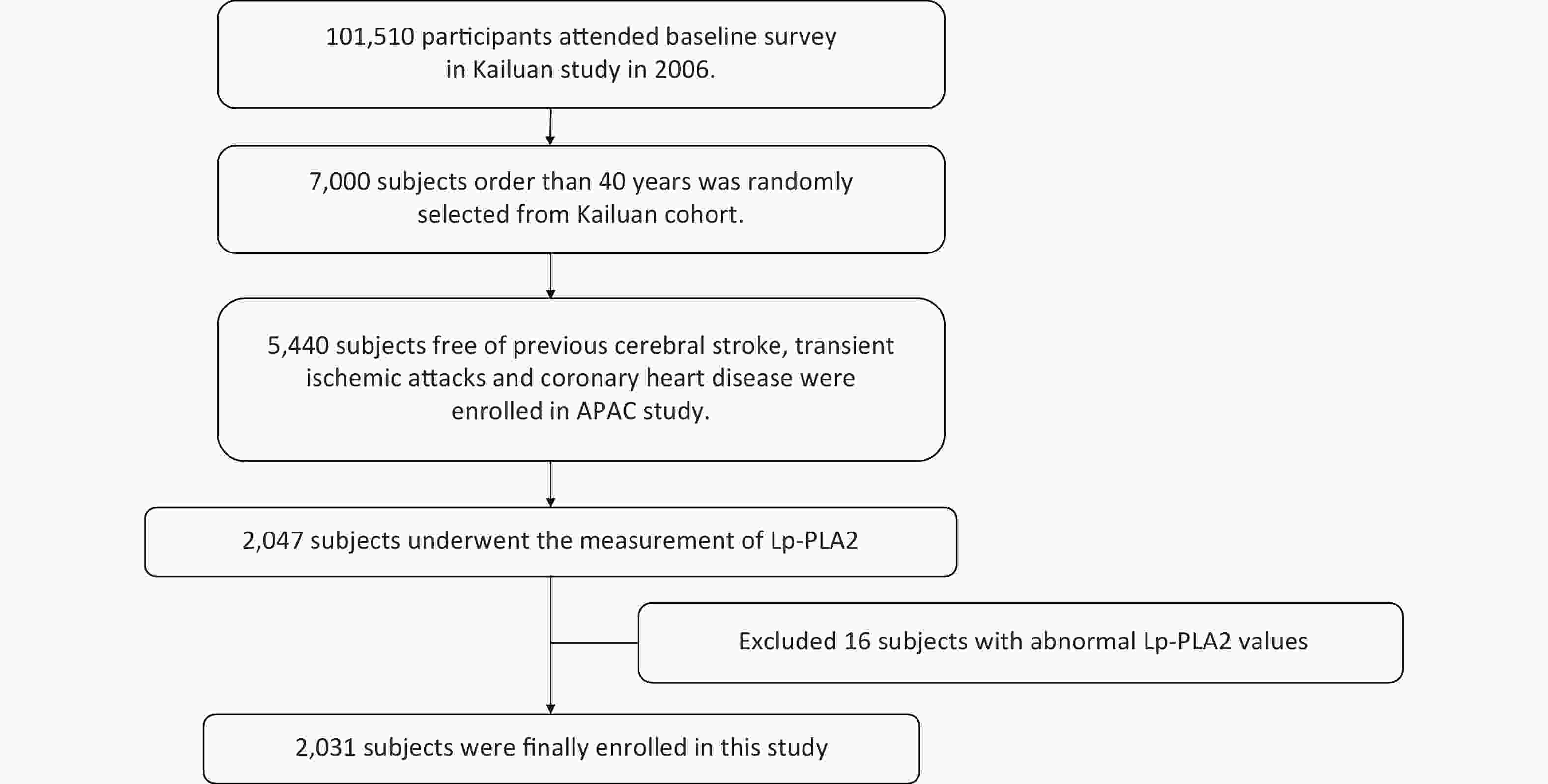
Participants and data came from the Asymptomatic Polyvascular Abnormalities Community study (APAC), a sub-population of the Kailuan study which included 101,510 current employees and retirees at the Kailuan (Group) Co. Ltd, Tangshan city, northern China[13]. From June 2010 to June 2011, 5,440 participants aged > 40 years old and free of stroke, transient ischemic attack, and coronary disease, were eligible and included in the APAC study. The APAC study protocol was previously reported[14]. Among these 5,440 participants, 2,047 participants were undertaken the Lp-PLA2 measurements and were investigated Lp-PLA2 associations with cardiovascular events, from 2010 to 2019. After screening, 2031 participants were included after excluding 16 participants whose Lp-PLA2 values were outside the assay detection range (39–2,500 ng/mL) (Supplementary Figure S1, available in www.besjournal.com). The study was performed according to Helsinki Declaration guidelines and was approved by the Ethics Committee of Kailuan General Hospital and Beijing Tiantan Hospital. Written informed consent was obtained from all participants.
-
Baseline data were collected by research coordinators using standardized questionnaires. These data included, participant demographics, lifestyles, medical conditions, and blood biochemical examinations. Medical conditions included, hypertension, diabetes mellitus, and hyperlipidemia, which were defined according to: 1) documented or self-reported history, 2) taking medication for corresponding diseases, or 3) clinical or laboratory examinations (blood pressure ≥ 140/90 mmHg after repeated measures indicating hypertension[15]; fasting blood glucose levels ≥ 7.0 mmol/L for diabetes mellitus; serum total cholesterol ≥ 5.7 mmol/L, triglycerides ≥ 1.7 mmol/L, and low-density lipoprotein cholesterol levels ≥ 4.1 mmol/L for dyslipidemia[16]). Body mass index (BMI) was calculated by dividing weight (kg) by the height squared (m2) and categorized as < 24.0 kg/m2, 24.0–27.9 kg/m2, and > 28.0 kg/m2 which indicated normal, overweight, and obesity, respectively. Physical activity was categorized as inactive, moderately active, and very active. Family monthly income per capita was also classified as: ≤ ¥1,000, ¥1,001–3,000, ¥3,001–5,000, and > ¥5,000[17]. Participants who currently or previously used alcohol were defined as drinkers. Smoking status was categorized as ex-smoker, current smoker, and never smoked. High-sensitivity C-Reactive Protein (hs-CRP) was measured using a high-sensitivity nephelometry assay (Cias Latex CRP-H; Kanto Chemical Co. Inc., Tokyo, Japan) and results were categorized into two groups (< 1.0 mg/dL and ≥ 1.0 mg/dL) according to guidelines[18]. All blood examinations were performed at the central laboratory at Kailuan Hospital.
-
Venous blood samples were collected from fasting participants into serum separator tubes. Samples were centrifuged for 10 min at 3,000 rpm (serum separator tubes were used to clot blood for 2 h at room temperature before centrifugation). Blood samples were labelled with unique numbers and stored at –80 °C until required. Lp-PLA2 levels were measured using an enzyme linked immunosorbent assay (ELISA) kit (CUSABIO Co., Wuhan, China, Human lipoprotein phospholipase A2 (Lp-PLA2) enzyme immunoassay kit, Catalog number; CSB-E08319h). Intra- and inter-assay coefficients of variation were < 8% and < 10%, respectively. To reduce inter-assay and measurement error, all assays were simultaneously conducted by a technician using the ELISA kit (CUSABIO company, Human lipoprotein phospholipase A2 (Lp-PLA2) enzyme immunoassay kit, Catalog number CSB-E08319h) at Beijing Tiantan Hospital, Capital Medical University, Beijing, China, according to manufacturer’s instructions.
-
Participants were followed-up by face-to-face interviews at routine 2-year medical examinations until 31 December 2019, or when an event of interest or death occurred. Physicians blinded to baseline data performed follow-up interviews. For participants who could not complete a face-to-face follow-up, outcome information was obtained by checking medical records at hospitals and/or the patient's medical insurance. Composite endpoints included, the first-ever stroke, myocardial infarction (MI), or all-cause death. Stroke was diagnosed according to World Health Organization criteria combined with brain computed tomography or magnetic resonance confirmation[19], and included three main types: cerebral infarction (CI), intracerebral hemorrhage (CH), and subarachnoid hemorrhage (SAH). MI was defined according to the 2007 universal definition[20]. Participants experiencing any kind of stroke or MI were deemed as having had a major adverse cardiovascular event (MACE). All-cause deaths were confirmed by checking death certificates from provincial statistics offices. All outcomes were checked by the Data Safety Monitoring Board and the Arbitration Committee for Clinical Outcomes.
-
Participants were classified into four groups according to serum Lp-PLA2 level quartiles. The Kolmogorov-Smirnov test was performed to test the normality of continuous variables which were represented as the mean ± standard deviation (SD) or the median together with the interquartile range (IQR). Categorical variables were expressed as counts (percentages). One-way analysis of variance (ANOVA) was performed to compare continuous variables. The chi-square test or the Fisher test was performed to test intergroup differences in categorical variables. Kaplan-Meier estimates were used to generate cumulative risks, and groups were compared using the Log-rank test. Associations between Lp-PLA2 and outcomes were assessed using time-dependent Cox proportional hazards models by calculating hazard ratios (HR) and 95% confidence intervals (CI). For participants with more than one disease, we used the time from the first event occurred in Cox proportional hazards models. We adjusted possible confounders, including age, gender, BMI, drinking and smoking history, history of hypertension, hyperlipidemia, diabetes, and hs-CRP in regression analysis. Lp-PLA2 associations with outcomes were also tested in participants stratified by age, sex, and hs-CRP. A two-sided P < 0.05 value was considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS 26 (SPSS Italy Inc., Bologna, Italy).
-
Participant characteristics are summarized (Table 1). The mean age of the 2,031 participants was 60.4 years and 73.6% were male. Mean Lp-PLA2 levels were 168.5 ng/mL. Observed Lp-PLA2 level quartiles were: Quartile 1 (< 131.9 ng/mL), Quartile 2 (131.9–141.0 ng/mL), Quartile 3 (141.0–159.1 ng/mL), and Quartile 4 (≥ 159.1 ng/mL). We observed significant differences in age, income status, smoking status, drinking status, BMI, hs-CRP level, and history of hypertension between Lp-PLA2 quartiles. Lp-PLA2 levels showed an increased trend with age. Also, hs-CRP levels tended to be increased in the higher Lp-PLA2 quartile groups (Q4). In addition, we observed a tendency for more participants with hypertension in higher Lp-PLA2 quartile groups.
Table 1. Baseline characteristics of study participants according to serum Lp-PLA2 quartiles
Characteristics Total Lp-PLA2 (ng/mL) P value Quartile 1
(< 131.9)Quartile 2
(131.9–141.0)Quartile 3
(141.0–159.1)Quartile 4
(≥ 159.1)n, % 2,031 509 (25.0) 507 (25.0) 508 (25.0) 507 (25.0) Age (years) 60.4 ± 11.7 55.7 ± 8.7 58.0 ± 10.2 61.7 ± 11.6 66.3 ± 13.1 < 0.001 Male (n, %) 1,495 (73.6) 384 (75.4) 379 (74.8) 363 (71.5) 369 (72.8) 0.460 Income, ¥/month (n, %) < 0.001 ≤ 3,000 1,697 (83.6) 459 (90.2) 428 (84.4) 420 (82.7) 390 (76.9) 3,001–5,000 252 (12.4) 44 (8.6) 65 (12.8) 66 (13.0) 77 (15.2) > 5,000 82 (4.0) 6 (1.2) 14 (2.8) 22 (4.3) 40 (7.9) Physical activity (n, %) 0.900 Inactive 749 (36.9) 192 (37.7) 185 (36.5) 181 (35.6) 191 (37.7) Moderately active 463 (22.8) 123 (24.2) 116 (22.9) 112 (22.1) 112 (22.1) Very active 819 (40.3) 194 (38.1) 206 (40.6) 215 (42.3) 204 (40.2) Smoking status (n, %) < 0.001 Never 1,099 (54.1) 246 (48.3) 260 (51.3) 303 (59.7) 290 (57.2) Ex-smoker 157 (7.7) 34 (6.7) 37 (7.3) 32 (6.3) 54 (10.7) Current smoker 775 (38.2) 229 (45.0) 210 (41.4) 173 (34.1) 163 (32.2) Drinker (n, %) 792 (39.0) 210 (41.3) 219 (43.2) 179 (35.2) 184 (36.3) 0.020 BMI (kg/m2) 0.020 < 24.0 796 (39.2) 179 (35.2) 183 (36.1) 206 (40.6) 228 (45.0) 24.0–27.9 923 (45.5) 244 (47.9) 235 (45.4) 230 (45.3) 214 (42.2) > 28.0 312 (15.4) 86 (16.9) 89 (17.6) 72 (14.2) 65 (12.8) Hypertension (n, %) 675 (33.2) 129 (25.3) 150 (29.6) 189 (37.2) 207 (40.8) < 0.001 Diabetes (n, %) 229 (11.3) 47 (9.2) 55 (10.9) 69 (13.6) 58 (11.4) 0.180 Hyperlipidemia (n, %) 245 (12.1) 62 (12.2) 68 (13.4) 63 (12.4) 52 (10.3) 0.480 hs-CRP (mg/dL) 2.5 ± 4.5 2.1 ± 3.3 2.3 ± 4.0 2.3 ± 4.0 3.1 ± 6.3 0.002 hs-CRP, mg/dL (n, %) 0.510 < 1.0 908 (44.7) 231 (45.4) 232 (45.8) 233 (45.9) 212 (41.8) ≥ 1.0 1,123 (55.3) 278 (54.6) 275 (54.2) 275 (54.2) 295 (58.2) Note. Values for categorical variables are represented as number (percentage); values for continuous variables are represented as the mean ± standard deviation. Lp-PLA2 = lipoprotein-associated phospholipase A2; BMI = body mass index; hs-CRP = high-sensitivity C-reactive protein. -
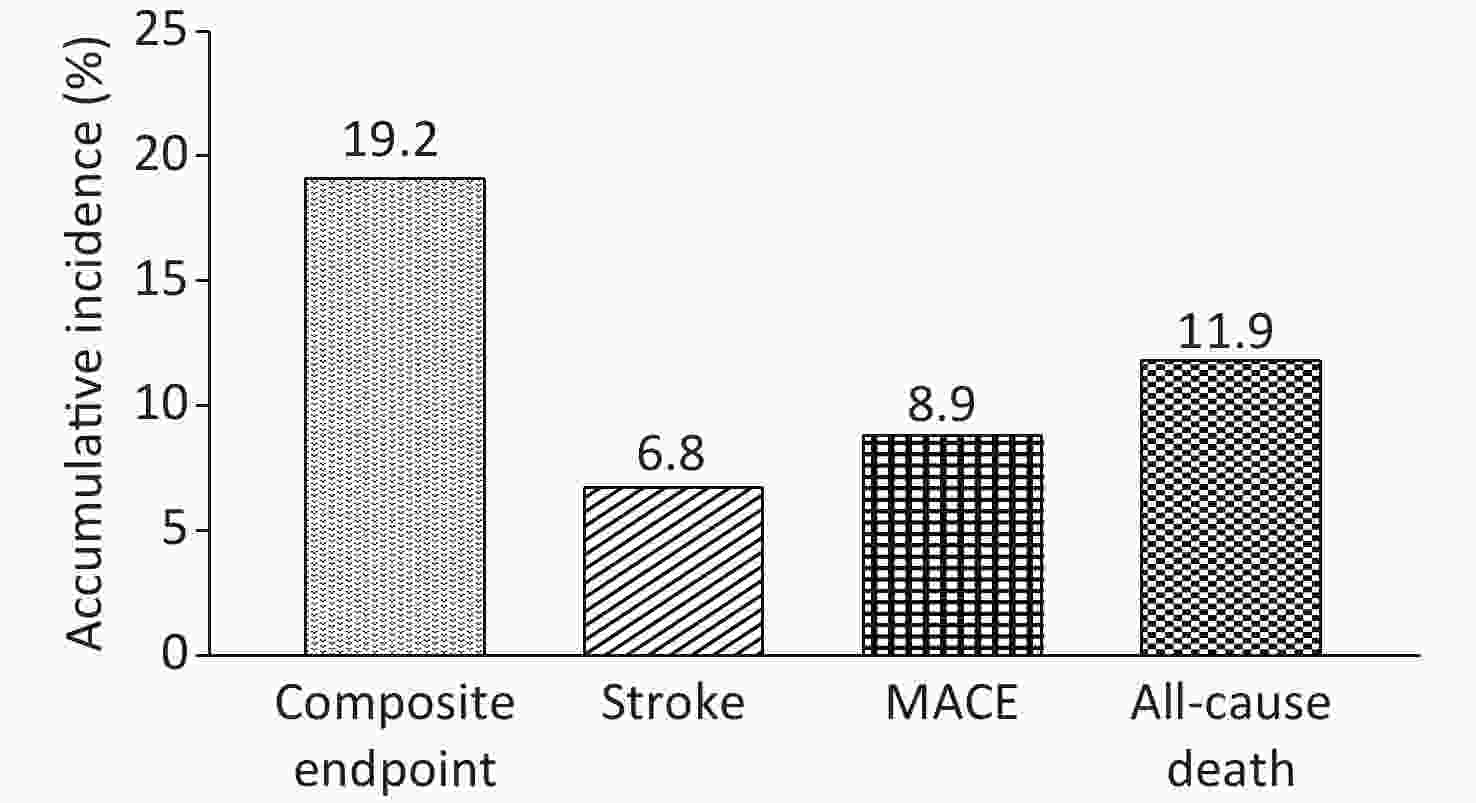
The median follow-up period was 9.1 years (IQR: 8.7–9.3 years). In total, 389 events were identified during the follow-up period, including 137 strokes (6.8%), 43 MIs (2.1%), and 244 all-cause deaths (11.9%). Strokes (137; 6.8%) included, 125 CIs (6.2%), 11 CHs (0.5%), and 3 SAHs (0.1%). With increasing Lp-PLA2 quartiles, the event rate of composite endpoints showed an upward trend. Q4 had the highest composited event rate (28.6%) when compared with Q1 (10.8%), Q2 (14.8%), and Q3 (22.4%). The accumulative incidence of predefined events during the follow-up period is shown (Figure 1).
-
Kaplan-Meier cumulative risk curves for outcomes according to Lp-PLA2 quartiles are shown (Figure 2). Patients in higher Lp-PLA2 groups (Q4) had a significantly higher risk of all four outcome types (all Log-Rank P < 0.05). The HRs with 95% CIs for outcomes according to Lp-PLA2 level quartiles are shown (Table 2). When compared with the lowest Lp-PLA2 quartile, the HR with a 95% CI for developing composite endpoints was 1.77 (1.24–2.54) in the highest quartile. When compared with the lowest quartile, the HR with a 95% CI of the highest quartile (Q4) for developing incident stroke, MACE, and all-cause death was 1.92 (1.03–3.60), 1.69 (1.003–2.84), and 1.94 (1.18–3.18), respectively.
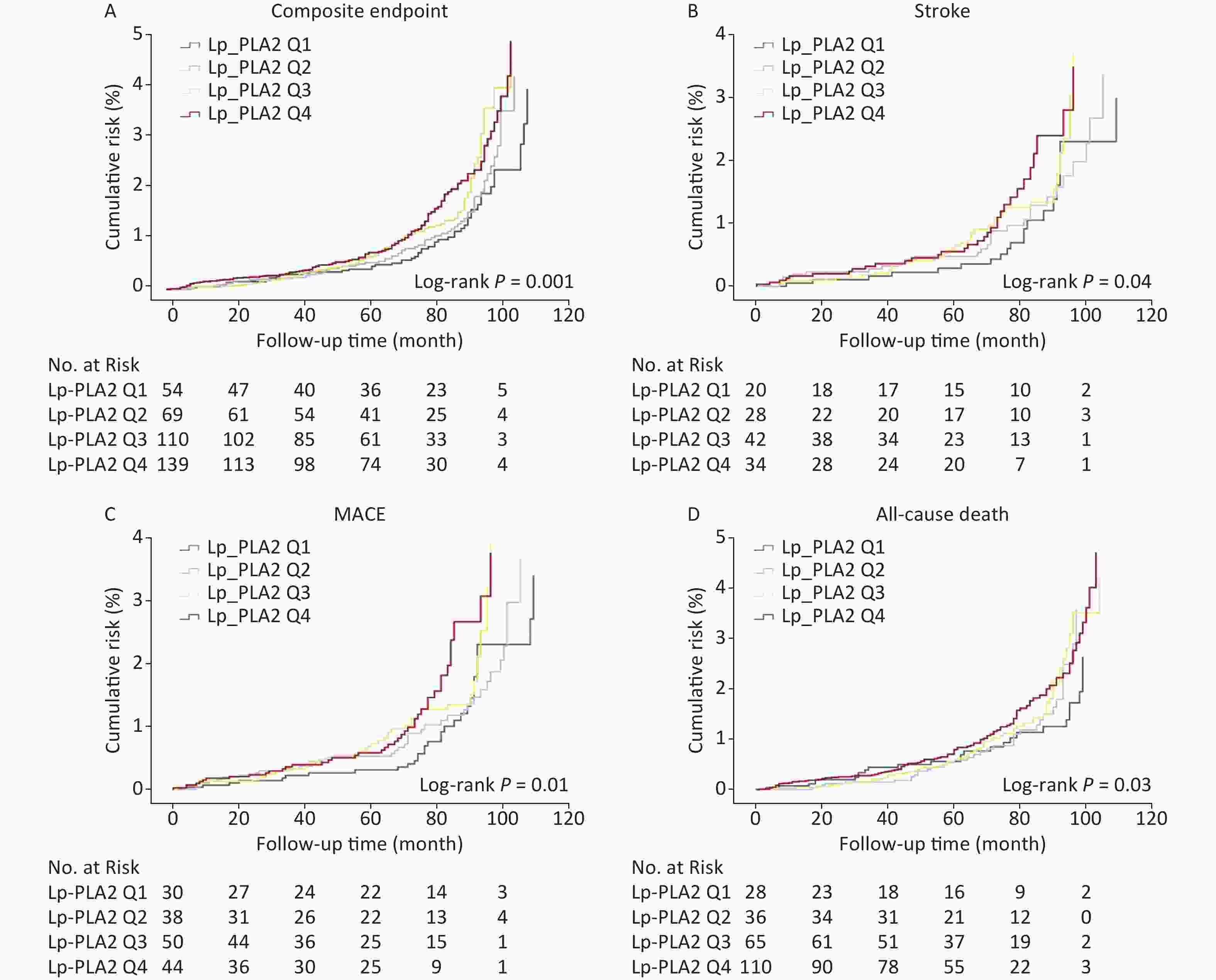
Figure 2. Cumulative risk of outcomes during a median follow-up of 9.1 years in the study population. Kaplan-Meier cumulative risk curves for composite endpoints (A), stroke (B), MACE (C), and all-cause death (D) in participants with different serum Lp-PLA2 levels. Lp-PLA2 Q1(-Q4) = lipoprotein-associated phospholipase A2 Quartile 1 (- Quartile 4); MACE = Major adverse cardiovascular events
Table 2. Association of serum Lp-PLA2 levels with outcomes in participants
Characteristics Events (n, %) Crude model
HR (95% CI)Model 1
HR (95% CI)Model 2
HR (95% CI)Composite endpoint 
Quartile 1 55 (10.8) Reference Reference Reference Quartile 2 75 (14.8) 1.19 (0.83−1.71) 1.22 (0.85−1.75) 1.20 (0.83−1.75) Quartile 3 114 (22.4) 1.36 (0.98−1.89) 1.42 (1.02−1.99) 1.37 (0.96−1.94) Quartile 4 145 (28.6) 1.60 (1.16−2.20) 1.74 (1.23−2.46) 1.77 (1.24−2.54) Stroke Quartile 1 22 (4.3) Reference Reference Reference Quartile 2 30 (5.9) 1.39 (0.77−2.53) 1.38 (0.76−2.52) 1.40 (0.74−2.63) Quartile 3 45 (8.9) 1.51 (0.87−2.64) 1.53 (0.88−2.69) 1.50 (0.82−2.76) Quartile 4 40 (7.9) 1.79 (1.002−3.19) 1.83 (1.01−3.33) 1.92 (1.03−3.60) MACE Quartile 1 31 (6.1) Reference Reference Reference Quartile 2 43 (8.5) 1.16 (0.71−1.91) 1.19 (0.72−1.95) 1.18 (0.71−1.97) Quartile 3 54 (10.6) 1.36 (0.85−2.17) 1.41 (0.88−2.26) 1.36 (0.82−2.26) Quartile 4 52 (10.3) 1.62 (1.004−2.61) 1.73 (1.04−2.87) 1.69 (1.003−2.84) All-cause death Quartile 1 28 (5.5) Reference Reference Reference Quartile 2 37 (7.2) 1.24 (0.75−2.06) 1.32 (0.79−2.20) 1.42 (0.82−2.44) Quartile 3 68 (13.4) 1.30 (0.83−2.04) 1.42 (0.90−2.26) 1.47 (0.89−2.42) Quartile 4 111 (21.8) 1.54 (1.01−2.36) 1.78 (1.13−2.82) 1.94 (1.18−3.18) Note. Model 1: Adjustments made for age and gender. Model 2: Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) indicates statistical significance. Lp-PLA2 = lipoprotein-associated phospholipase A2; MACE = Major adverse cardiovascular events; HR = hazard ratio; CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein. -
In subgroup analyses (Table 3), when compared with participants in the lowest Lp-PLA2 quartile, an increased risk of composite outcome in the highest quartile was statistically significant in males [Q4 vs. Q1: HR 1.91 (1.27–2.88)] but not significant in females (Pinteraction = 0.01). In addition, a significant association was also observed in participants aged > 65 years [Q2 vs. Q1: HR 2.05 (1.01–4.13); Q3 vs. Q1: HR 2.68 (1.37–5.22); and Q4 vs. Q1: HR 2.78 (1.43–5.40)], but not significant in participants aged < 65 years (Pinteraction = 0.04). In subgroup analyses based on hs-CRP, a significant association was observed in participants with higher hs-CRP levels [Q3 vs. Q1: HR 1.68 (1.06–2.65); Q4 vs. Q1: HR 1.80 (1.14–2.83)] (Pinteraction = 0.02). Moreover, similar results were observed for all-cause death and MACE, but no significance for stroke outcomes (Supplementary Table S1, available in www.besjournal.com).
Table 3. Subgroup analyses of serum Lp-PLA2 associations with composite endpoints based on gender, age, and hs-CRP
Characteristics Quartile 1 Quartile 2 Quartile 3 Quartile 4 P for
interactionn, % HR (95% CI) n, % HR (95% CI) n, % HR (95% CI) n, % HR (95% CI) Gender Males 46 (3.1) Reference 69 (4.6) 1.19 (0.79–1.80) 96 (6.4) 1.46 (0.98–2.17) 116 (7.8) 1.91 (1.27–2.88) 0.01 Females 9 (1.7) Reference 6 (1.1) 2.16 (0.62–7.52) 18 (3.4) 0.98 (0.35–2.74) 29 (5.4) 1.21 (0.46–3.16) Age (years) < 65 37 (2.7) Reference 42 (3.0) 0.99 (0.61–1.62) 46 (3.3) 0.94 (0.58–1.51) 34 (2.5) 1.58 (0.95–2.63) 0.04 ≥ 65 18 (2.8) Reference 33 (5.1) 2.05 (1.01–4.13) 68 (10.5) 2.68 (1.37–5.22) 111 (17.1) 2.78 (1.43–5.40) hs-CRP (mg/dL) < 1.0 20 (2.2) Reference 29 (3.2) 1.29 (0.68–2.45) 42 (4.6) 1.16 (0.63–2.11) 45 (5.0) 1.84 (0.98–1.01) 0.02 ≥ 1.0 35 (3.1) Reference 46 (4.1) 1.34 (0.82–2.17) 72 (6.4) 1.68 (1.06–2.65) 100 (8.9) 1.80 (1.14–2.83) Note. Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) and P value indicate statistical significance. Lp-PLA2 = lipoprotein-associated phospholipase A2; HR = hazard ratio; CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein. Table S1. Subgroup analyses of serum Lp-PLA2 associations with stroke, MACE, and all-cause death
Characteristics Quartile 1 Quartile 2 Quartile 3 Quartile 4 P for
interactionn, % HR
(95% CI)n, % HR
(95% CI)n, % HR
(95% CI)n, % HR
(95% CI)Stroke Gender Males 20 (1.3) Reference 26 (1.7) 1.41 (0.70–2.84) 37 (2.5) 1.57 (0.80–3.09) 28 (1.9) 1.98 (0.96–4.08) 0.36 Females 2 (0.4) Reference 4 (0.8) 5.65 (0.59–54.1) 8 (1.5) 2.00 (0.30–13.1) 12 (2.2) 5.68 (0.62–52.5) Age (years) < 65 16 (1.2) Reference 22 (1.6) 1.05 (0.50–2.19) 26 (1.9) 1.05 (0.52–2.13) 16 (1.2) 1.37 (0.58–3.25) 0.06 ≥ 65 6 (0.9) Reference 8 (1.2) 2.02 (0.39–12.6) 19 (2.9) 1.35 (0.20–9.04) 24 (3.7) 2.26 (0.42–12.1) hs-CRP (mg/dL) < 1.0 10 (1.1) Reference 11 (1.2) 2.95 (1.09–8.00) 20 (2.2) 1.42 (0.56–3.59) 15 (1.7) 2.86 (1.03–7.94) 0.07 ≥ 1.0 12 (1.1) Reference 19 (1.7) 2.19 (0.84–5.73) 25 (2.2) 2.19 (0.85–5.68) 25 (2.2) 2.64 (1.03–6.78) MACE Gender Males 27 (1.8) Reference 39 (2.6) 1.21 (0.70–2.09) 46 (3.1) 1.47 (0.85–2.57) 37 (2.5) 1.90 (1.05–3.44) 0.10 Females 4 (0.8) Reference 4 (0.8) 1.32 (0.25–7.01) 8 (1.5) 0.50 (0.11–2.33) 15 (2.8) 0.50 (0.10–2.66) Age (years) < 65 25 (1.8) Reference 33 (2.3) 1.05 (0.58–1.92) 29 (2.1) 0.98 (0.54–1.78) 20 (1.5) 1.16 (0.57–2.36) 0.05 ≥ 65 6 (0.9) Reference 11 (1.7) 2.32 (0.54–9.92) 25 (3.9) 3.19 (0.68–15.0) 32 (4.9) 4.31 (1.01–18.4) hs-CRP (mg/dL) < 1.0 13 (1.4) Reference 16 (1.8) 1.88 (0.79–4.46) 23 (2.5) 1.57 (0.70–3.53) 20 (2.2) 1.54 (0.67–3.54) 0.06 ≥ 1.0 18 (1.6) Reference 27 (2.4) 1.26 (0.62–2.58) 31 (2.8) 1.44 (0.68–3.08) 32 (2.9) 2.08 (0.98–4.40) All-cause death Gender Males 21 (1.4) Reference 35 (2.3) 1.46 (0.80–2.71) 56 (3.8) 1.67 (0.93–2.98) 93 (6.2) 2.26 (1.27–4.03) 0.04 Females 7 (1.3) Reference 2 (0.4) 3.99 (0.45–35.3) 10 (1.9) 1.84 (0.45–7.49) 17 (3.2) 1.78 (0.53–5.98) Age (years) < 65 15 (1.1) Reference 12 (0.9) 1.28 (0.49–3.30) 18 (1.3) 0.92 (0.40–2.15) 17 (1.2) 2.18 (0.88–5.37) 0.30 ≥ 65 13 (2.0) Reference 25 (3.9) 2.09 (0.95–4.60) 48 (7.4) 2.44 (1.15–5.16) 93 (14.4) 2.27 (1.10–4.70) hs-CRP (mg/dL) < 1.0 7 (0.8) Reference 14 (1.5) 1.46 (0.39–5.46) 22 (2.4) 1.09 (0.31–3.86) 30 (3.3) 2.05 (0.57–7.35) 0.08 ≥ 1.0 21 (1.9) Reference 23 (2.1) 1.64 (0.85–3.15) 44 (3.9) 2.02 (1.11–3.65) 80 (7.1) 2.13 (1.20–3.79) Note. Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) and P value indicate statistical significance. -
To the best of our knowledge, ours is the first study to investigate serum Lp-PLA2 associations with a risk of cardiovascular events in a Chinese population, with long-term follow-up. Over a median follow-up period of 9.1 years, composite events including, stroke, MI, and all-cause death occurred in 19.0% of participants. However, approximate 29.0% of participants in the Q4 Lp-PLA2 group experienced composite events. We also determined that higher Lp-PLA2 levels were statistically associated with an increased risk of composite endpoints, and also stroke, MACE, and all-cause death. In addition, Lp-PLA2 associations with composite endpoints were significantly modified by sex, age, and hs-CRP.
Participants with higher Lp-PLA2 levels (e.g., 4th quartile) had a higher cardiovascular event risk. With increasing Lp-PLA2 quartiles, the accumulated risk of cardiovascular events, including composite endpoint, stroke, MACE, and all-cause death, increased significantly during the median follow-up. Participants in the highest Lp-PLA2 quartile had an almost doubled risk of a future composite endpoint, stroke, MACE, and all-cause death when compared with those in the lowest quartile. Our findings agreed with previous studies suggesting that higher Lp-PLA2 levels were associated with an increased risk of cardiovascular events in other ethnicities[21-23]. However, the Multi-Ethnic Study of Atherosclerosis (MESA) study indicated an increased risk across different ethnicities, but not Chinese individuals, which contrasted with our data[24]. The limited number of incident CVD events among Chinese participants in the MESA study may have accounted for this inconsistency. In our study, our cohort was followed-up for a long period and had a relatively high incidence of outcomes. Also, the association of Lp-PLA2 with cardiovascular events was statistically significant. Hence, we suggest a strong association between higher Lp-PLA2 levels and a higher risk of cardiovascular event also exists in our middle-aged Chinese population, similar to other ethnicities. Furthermore, our findings agreed with other studies showing that elevated Lp-PLA2 levels were associated with a high risk of CVD in participants with coronary artery disease, or the high-stroke risk population[10,25].
From subgroup analyses, Lp-PLA2 associated with the risk of cardiovascular events was significantly modified by age and gender in our population. An increased risk of cardiovascular event with elevated serum Lp-PLA2 levels only existed in participants > 65 years. Our findings agreed with our previous study and other studies, including the Cardiovascular Health study and the Rancho Bernardo study[26-28]. In addition, the Lp-PLA2 association was only observed in males and not females. Several studies demonstrated a gender difference in both Lp-PLA2 levels and activity[12,21,22]. The Dallas Heart study reported an association between Lp-PLA2 levels and coronary artery calcification was only observed in men, not women[29]. Thus, combined, these findings suggested a gender difference in Lp-PLA2 associations with CVD and subclinical CVD. Estrogen may be associated with lower serum Lp-PLA2 expression and activity levels[30]. Hence, sex hormone effects may partly explain this gender difference. Nevertheless, limited numbers of incident CVD events in females may also be a potential reason for the lower statistical power in females. Furthermore, we also observed interaction term between hs-CRP and Lp-PLA2 level was statistically significant for composite endpoints. We previously reported a complementary effect of Lp-PLA2 and hs-CRP in carotid atherosclerosis[31]. In addition, previous studies proposed that Lp-PLA2 and hs-CRP may be complementary markers in identifying individuals with high coronary heart disease risk[21]. Hence, combining Lp-PLA2 and hs-CRP levels may provide a more accurate prediction of future cardiovascular events. For patients aged > 65 years with higher Lp-PLA2 levels, especially those combined with higher CRP levels, clinicians should recommend regular check-ups to reduce the risk of cardiovascular events.
AACE recommends 200 ng/mL Lp-PLA2 as a cut-off point to indicate patients with a higher risk of cardiovascular event[32]. In our study, participants with a median Lp-pLA2 level (141.0 ng/mL) or higher, had a higher event risk than the whole population. Ethnicity differences with respect to inflammatory biomarkers, including Lp-PLA2, were previously documented[12,33,34]. In addition, our findings were consistent with a previous Chinese multicenter study which investigated normal Lp-PLA2 reference intervals in a Chinese population aged > 50 years[35]. Similar Lp-PLA2 levels were also observed in a Japanese population[36]. Thus, risk underestimations for future cardiovascular events may occur in Chinese and/or Asian population when using Lp-pLA2 guidelines from European and American populations. However, while we recruited our population from Northern China, it remains to be seen if this group represents baseline levels for the whole Chinese population. Therefore, Lp-PLA2 cut-off values must be identified for cardiovascular event risk for Chinese and/or Asian populations.
This study had several limitations. Firstly, we only accessed all-cause death events rather than classified deaths into cardiovascular or non-cardiovascular, thus we were unable to estimate Lp-PLA2 associations with cardiac mortality. Secondly, we only measured Lp-PLA2 levels at baseline, thus we may have overlooked dynamic changes in Lp-PLA2 levels over time. Finally, most participants were restricted to one community, therefore, our ability to generalize our results to other populations is limited.
Higher Lp-PLA2 levels were significantly associated with an increased risk of cardiovascular events/death in a middle-aged Chinese population. The Lp-PLA2 cut-off point may be lower in Chinese and/or Asian populations for predicting cardiovascular events.
-
The authors thank the survey teams of the study groups for their contribution and also the participants who contributed their time and information.
doi: 10.3967/bes2022.029
The Use of Lipoprotein-Associated Phospholipase A2 in a Chinese Population to Predict Cardiovascular Events
-
Abstract:
Objective To explore associations between lipoprotein-associated phospholipase A2 (Lp-PLA2) and the risk of cardiovascular events in a Chinese population, with a long-term follow-up. Methods A random sample of 2,031 participants (73.6% males, mean age = 60.4 years) was derived from the Asymptomatic Polyvascular Abnormalities Community study (APAC) from 2010 to 2011. Serum Lp-PLA2 levels were determined by enzyme-linked immunosorbent assay (ELISA). The composite endpoint was a combination of first-ever stroke, myocardial infarction (MI) or all-cause death. Lp-PLA2 associations with outcomes were assessed using Cox models. Results The median Lp-PLA2 level was 141.0 ng/mL. Over a median follow-up of 9.1 years, we identified 389 events (19.2%), including 137 stroke incidents, 43 MIs, and 244 all-cause deaths. Using multivariate Cox regression, when compared with the lowest Lp-PLA2 quartile, the hazard ratios with 95% confidence intervals for developing composite endpoints, stroke, major adverse cardiovascular events, and all-cause death were 1.77 (1.24–2.54), 1.92 (1.03–3.60), 1.69 (1.003–2.84), and 1.94 (1.18–3.18) in the highest quartile, respectively. Composite endpoints in 145 (28.6%) patients occurred in the highest quartile where Lp-PLA2 (159.0 ng/mL) was much lower than the American Association of Clinical Endocrinologists recommended cut-off point, 200 ng/mL. Conclusion Higher Lp-PLA2 levels were associated with an increased risk of cardiovascular event/death in a middle-aged Chinese population. The Lp-PLA2 cut-off point may be lower in the Chinese population when predicting cardiovascular events. -
Figure 2. Cumulative risk of outcomes during a median follow-up of 9.1 years in the study population. Kaplan-Meier cumulative risk curves for composite endpoints (A), stroke (B), MACE (C), and all-cause death (D) in participants with different serum Lp-PLA2 levels. Lp-PLA2 Q1(-Q4) = lipoprotein-associated phospholipase A2 Quartile 1 (- Quartile 4); MACE = Major adverse cardiovascular events
Table 1. Baseline characteristics of study participants according to serum Lp-PLA2 quartiles
Characteristics Total Lp-PLA2 (ng/mL) P value Quartile 1
(< 131.9)Quartile 2
(131.9–141.0)Quartile 3
(141.0–159.1)Quartile 4
(≥ 159.1)n, % 2,031 509 (25.0) 507 (25.0) 508 (25.0) 507 (25.0) Age (years) 60.4 ± 11.7 55.7 ± 8.7 58.0 ± 10.2 61.7 ± 11.6 66.3 ± 13.1 < 0.001 Male (n, %) 1,495 (73.6) 384 (75.4) 379 (74.8) 363 (71.5) 369 (72.8) 0.460 Income, ¥/month (n, %) < 0.001 ≤ 3,000 1,697 (83.6) 459 (90.2) 428 (84.4) 420 (82.7) 390 (76.9) 3,001–5,000 252 (12.4) 44 (8.6) 65 (12.8) 66 (13.0) 77 (15.2) > 5,000 82 (4.0) 6 (1.2) 14 (2.8) 22 (4.3) 40 (7.9) Physical activity (n, %) 0.900 Inactive 749 (36.9) 192 (37.7) 185 (36.5) 181 (35.6) 191 (37.7) Moderately active 463 (22.8) 123 (24.2) 116 (22.9) 112 (22.1) 112 (22.1) Very active 819 (40.3) 194 (38.1) 206 (40.6) 215 (42.3) 204 (40.2) Smoking status (n, %) < 0.001 Never 1,099 (54.1) 246 (48.3) 260 (51.3) 303 (59.7) 290 (57.2) Ex-smoker 157 (7.7) 34 (6.7) 37 (7.3) 32 (6.3) 54 (10.7) Current smoker 775 (38.2) 229 (45.0) 210 (41.4) 173 (34.1) 163 (32.2) Drinker (n, %) 792 (39.0) 210 (41.3) 219 (43.2) 179 (35.2) 184 (36.3) 0.020 BMI (kg/m2) 0.020 < 24.0 796 (39.2) 179 (35.2) 183 (36.1) 206 (40.6) 228 (45.0) 24.0–27.9 923 (45.5) 244 (47.9) 235 (45.4) 230 (45.3) 214 (42.2) > 28.0 312 (15.4) 86 (16.9) 89 (17.6) 72 (14.2) 65 (12.8) Hypertension (n, %) 675 (33.2) 129 (25.3) 150 (29.6) 189 (37.2) 207 (40.8) < 0.001 Diabetes (n, %) 229 (11.3) 47 (9.2) 55 (10.9) 69 (13.6) 58 (11.4) 0.180 Hyperlipidemia (n, %) 245 (12.1) 62 (12.2) 68 (13.4) 63 (12.4) 52 (10.3) 0.480 hs-CRP (mg/dL) 2.5 ± 4.5 2.1 ± 3.3 2.3 ± 4.0 2.3 ± 4.0 3.1 ± 6.3 0.002 hs-CRP, mg/dL (n, %) 0.510 < 1.0 908 (44.7) 231 (45.4) 232 (45.8) 233 (45.9) 212 (41.8) ≥ 1.0 1,123 (55.3) 278 (54.6) 275 (54.2) 275 (54.2) 295 (58.2) Note. Values for categorical variables are represented as number (percentage); values for continuous variables are represented as the mean ± standard deviation. Lp-PLA2 = lipoprotein-associated phospholipase A2; BMI = body mass index; hs-CRP = high-sensitivity C-reactive protein. Table 2. Association of serum Lp-PLA2 levels with outcomes in participants
Characteristics Events (n, %) Crude model
HR (95% CI)Model 1
HR (95% CI)Model 2
HR (95% CI)Composite endpoint Quartile 1 55 (10.8) Reference Reference Reference Quartile 2 75 (14.8) 1.19 (0.83−1.71) 1.22 (0.85−1.75) 1.20 (0.83−1.75) Quartile 3 114 (22.4) 1.36 (0.98−1.89) 1.42 (1.02−1.99) 1.37 (0.96−1.94) Quartile 4 145 (28.6) 1.60 (1.16−2.20) 1.74 (1.23−2.46) 1.77 (1.24−2.54) Stroke Quartile 1 22 (4.3) Reference Reference Reference Quartile 2 30 (5.9) 1.39 (0.77−2.53) 1.38 (0.76−2.52) 1.40 (0.74−2.63) Quartile 3 45 (8.9) 1.51 (0.87−2.64) 1.53 (0.88−2.69) 1.50 (0.82−2.76) Quartile 4 40 (7.9) 1.79 (1.002−3.19) 1.83 (1.01−3.33) 1.92 (1.03−3.60) MACE Quartile 1 31 (6.1) Reference Reference Reference Quartile 2 43 (8.5) 1.16 (0.71−1.91) 1.19 (0.72−1.95) 1.18 (0.71−1.97) Quartile 3 54 (10.6) 1.36 (0.85−2.17) 1.41 (0.88−2.26) 1.36 (0.82−2.26) Quartile 4 52 (10.3) 1.62 (1.004−2.61) 1.73 (1.04−2.87) 1.69 (1.003−2.84) All-cause death Quartile 1 28 (5.5) Reference Reference Reference Quartile 2 37 (7.2) 1.24 (0.75−2.06) 1.32 (0.79−2.20) 1.42 (0.82−2.44) Quartile 3 68 (13.4) 1.30 (0.83−2.04) 1.42 (0.90−2.26) 1.47 (0.89−2.42) Quartile 4 111 (21.8) 1.54 (1.01−2.36) 1.78 (1.13−2.82) 1.94 (1.18−3.18) Note. Model 1: Adjustments made for age and gender. Model 2: Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) indicates statistical significance. Lp-PLA2 = lipoprotein-associated phospholipase A2; MACE = Major adverse cardiovascular events; HR = hazard ratio; CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein. Table 3. Subgroup analyses of serum Lp-PLA2 associations with composite endpoints based on gender, age, and hs-CRP
Characteristics Quartile 1 Quartile 2 Quartile 3 Quartile 4 P for
interactionn, % HR (95% CI) n, % HR (95% CI) n, % HR (95% CI) n, % HR (95% CI) Gender Males 46 (3.1) Reference 69 (4.6) 1.19 (0.79–1.80) 96 (6.4) 1.46 (0.98–2.17) 116 (7.8) 1.91 (1.27–2.88) 0.01 Females 9 (1.7) Reference 6 (1.1) 2.16 (0.62–7.52) 18 (3.4) 0.98 (0.35–2.74) 29 (5.4) 1.21 (0.46–3.16) Age (years) < 65 37 (2.7) Reference 42 (3.0) 0.99 (0.61–1.62) 46 (3.3) 0.94 (0.58–1.51) 34 (2.5) 1.58 (0.95–2.63) 0.04 ≥ 65 18 (2.8) Reference 33 (5.1) 2.05 (1.01–4.13) 68 (10.5) 2.68 (1.37–5.22) 111 (17.1) 2.78 (1.43–5.40) hs-CRP (mg/dL) < 1.0 20 (2.2) Reference 29 (3.2) 1.29 (0.68–2.45) 42 (4.6) 1.16 (0.63–2.11) 45 (5.0) 1.84 (0.98–1.01) 0.02 ≥ 1.0 35 (3.1) Reference 46 (4.1) 1.34 (0.82–2.17) 72 (6.4) 1.68 (1.06–2.65) 100 (8.9) 1.80 (1.14–2.83) Note. Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) and P value indicate statistical significance. Lp-PLA2 = lipoprotein-associated phospholipase A2; HR = hazard ratio; CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein. S1. Subgroup analyses of serum Lp-PLA2 associations with stroke, MACE, and all-cause death
Characteristics Quartile 1 Quartile 2 Quartile 3 Quartile 4 P for
interactionn, % HR
(95% CI)n, % HR
(95% CI)n, % HR
(95% CI)n, % HR
(95% CI)Stroke Gender Males 20 (1.3) Reference 26 (1.7) 1.41 (0.70–2.84) 37 (2.5) 1.57 (0.80–3.09) 28 (1.9) 1.98 (0.96–4.08) 0.36 Females 2 (0.4) Reference 4 (0.8) 5.65 (0.59–54.1) 8 (1.5) 2.00 (0.30–13.1) 12 (2.2) 5.68 (0.62–52.5) Age (years) < 65 16 (1.2) Reference 22 (1.6) 1.05 (0.50–2.19) 26 (1.9) 1.05 (0.52–2.13) 16 (1.2) 1.37 (0.58–3.25) 0.06 ≥ 65 6 (0.9) Reference 8 (1.2) 2.02 (0.39–12.6) 19 (2.9) 1.35 (0.20–9.04) 24 (3.7) 2.26 (0.42–12.1) hs-CRP (mg/dL) < 1.0 10 (1.1) Reference 11 (1.2) 2.95 (1.09–8.00) 20 (2.2) 1.42 (0.56–3.59) 15 (1.7) 2.86 (1.03–7.94) 0.07 ≥ 1.0 12 (1.1) Reference 19 (1.7) 2.19 (0.84–5.73) 25 (2.2) 2.19 (0.85–5.68) 25 (2.2) 2.64 (1.03–6.78) MACE Gender Males 27 (1.8) Reference 39 (2.6) 1.21 (0.70–2.09) 46 (3.1) 1.47 (0.85–2.57) 37 (2.5) 1.90 (1.05–3.44) 0.10 Females 4 (0.8) Reference 4 (0.8) 1.32 (0.25–7.01) 8 (1.5) 0.50 (0.11–2.33) 15 (2.8) 0.50 (0.10–2.66) Age (years) < 65 25 (1.8) Reference 33 (2.3) 1.05 (0.58–1.92) 29 (2.1) 0.98 (0.54–1.78) 20 (1.5) 1.16 (0.57–2.36) 0.05 ≥ 65 6 (0.9) Reference 11 (1.7) 2.32 (0.54–9.92) 25 (3.9) 3.19 (0.68–15.0) 32 (4.9) 4.31 (1.01–18.4) hs-CRP (mg/dL) < 1.0 13 (1.4) Reference 16 (1.8) 1.88 (0.79–4.46) 23 (2.5) 1.57 (0.70–3.53) 20 (2.2) 1.54 (0.67–3.54) 0.06 ≥ 1.0 18 (1.6) Reference 27 (2.4) 1.26 (0.62–2.58) 31 (2.8) 1.44 (0.68–3.08) 32 (2.9) 2.08 (0.98–4.40) All-cause death Gender Males 21 (1.4) Reference 35 (2.3) 1.46 (0.80–2.71) 56 (3.8) 1.67 (0.93–2.98) 93 (6.2) 2.26 (1.27–4.03) 0.04 Females 7 (1.3) Reference 2 (0.4) 3.99 (0.45–35.3) 10 (1.9) 1.84 (0.45–7.49) 17 (3.2) 1.78 (0.53–5.98) Age (years) < 65 15 (1.1) Reference 12 (0.9) 1.28 (0.49–3.30) 18 (1.3) 0.92 (0.40–2.15) 17 (1.2) 2.18 (0.88–5.37) 0.30 ≥ 65 13 (2.0) Reference 25 (3.9) 2.09 (0.95–4.60) 48 (7.4) 2.44 (1.15–5.16) 93 (14.4) 2.27 (1.10–4.70) hs-CRP (mg/dL) < 1.0 7 (0.8) Reference 14 (1.5) 1.46 (0.39–5.46) 22 (2.4) 1.09 (0.31–3.86) 30 (3.3) 2.05 (0.57–7.35) 0.08 ≥ 1.0 21 (1.9) Reference 23 (2.1) 1.64 (0.85–3.15) 44 (3.9) 2.02 (1.11–3.65) 80 (7.1) 2.13 (1.20–3.79) Note. Adjustments made for age, gender, body mass index (BMI), drinker status, smoking status, history of hypertension, hyperlipidemia, diabetes, and hs-CRP. Bold HR (95% CI) and P value indicate statistical significance. -
[1] Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol, 2020; 76, 2982−3021. doi: 10.1016/j.jacc.2020.11.010 [2] GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet, 2020; 396, 1223−49. doi: 10.1016/S0140-6736(20)30752-2 [3] Aulin J, Siegbahn A, Hijazi Z, et al. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J, 2015; 170, 1151−60. doi: 10.1016/j.ahj.2015.09.018 [4] Davidson MH, Corson MA, Alberts MJ, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol, 2008; 101, 51F−7F. doi: 10.1016/j.amjcard.2008.04.019 [5] Mani P, Puri R, Schwartz GG, et al. Association of initial and serial C-reactive protein levels with adverse cardiovascular events and death after acute coronary syndrome: a secondary analysis of the VISTA-16 trial. JAMA Cardiol, 2019; 4, 314−20. doi: 10.1001/jamacardio.2019.0179 [6] Veeranna V, Zalawadiya SK, Niraj A, et al. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol, 2011; 58, 1025−33. doi: 10.1016/j.jacc.2011.05.028 [7] Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol, 2006; 26, 2523−9. doi: 10.1161/01.ATV.0000244681.72738.bc [8] Vittos O, Toana B, Vittos A, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2): A review of its role and significance as a cardiovascular biomarker. Biomarkers, 2012; 17, 289−302. doi: 10.3109/1354750X.2012.664170 [9] Zhang H, Gao Y, Wu D, et al. The relationship of lipoprotein-associated phospholipase A2 activity with the seriousness of coronary artery disease. BMC Cardiovasc Disord, 2020; 20, 295. doi: 10.1186/s12872-020-01580-4 [10] Sheng GH, Zhou J, Chang C, et al. Relationship between Lp-PLA2 and in-stent restenosis after coronary stenting: a 3-year follow-up study. Scott Med J, 2021; 66, 178−85. doi: 10.1177/00369330211034809 [11] Lee KK, Fortmann SP, Varady A, et al. Racial variation in lipoprotein-associated phospholipase A2 in older adults. BMC Cardiovasc Disord, 2021; 11, 38. [12] Brilakis ES, Khera A, Mcguire DK, et al. Influence of race and sex on lipoprotein- associated phospholipase A2 levels: observations from the Dallas heart study. Atherosclerosis, 2008; 199, 110−5. doi: 10.1016/j.atherosclerosis.2007.10.010 [13] Wu SL, Huang ZR, Yang XC, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a Northern Chinese industrial city. Circ Cardiovasc Qual Outcomes, 2012; 5, 487−93. doi: 10.1161/CIRCOUTCOMES.111.963694 [14] Zhou Y, Li Y, Xu L, et al. Asymptomatic Polyvascular Abnormalities in Community (APAC) study in China: objectives, design and baseline characteristics. PLoS One, 2013; 8, e84685. doi: 10.1371/journal.pone.0084685 [15] Song DY, Wang XW, Wang S, et al. Jidong cognitive impairment cohort study: objectives, design, and baseline screening. Neural Regen Res, 2020; 15, 1111−9. doi: 10.4103/1673-5374.266070 [16] Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA, 2001; 285, 2486−97. doi: 10.1001/jama.285.19.2486 [17] Qiu MS, Wang XW, Yao Y, et al. Protocol of Jidong women health cohort study: rationale, design, and baseline characteristics. Biomed Environ Sci, 2019; 32, 144−52. [18] Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation, 2003; 107, 499−511. doi: 10.1161/01.CIR.0000052939.59093.45 [19] World Health Organization. Stroke—1989 recommendations on stroke prevention, diagnosis, and therapy report of the WHO task force on stroke and other cerebrovascular disorders. Stroke, 1989; 20, 1407−31. doi: 10.1161/01.STR.20.10.1407 [20] Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol, 2007; 50, 2173−95. doi: 10.1016/j.jacc.2007.09.011 [21] Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in Communities (ARIC) study. Circulation, 2004; 109, 837−42. doi: 10.1161/01.CIR.0000116763.91992.F1 [22] Oei HHS, Van Der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation, 2005; 111, 570−5. doi: 10.1161/01.CIR.0000154553.12214.CD [23] Sudhir K. Lipoprotein-associated phospholipase A2, a novel inflammatory biomarker and independent risk predictor for cardiovascular disease. J Clin Endocrinol Metab, 2005; 90, 3100−5. doi: 10.1210/jc.2004-2027 [24] Garg PK, McClelland RL, Jenny NS, et al. Lipoprotein-associated phospholipase A2 and risk of incident cardiovascular disease in a multi-ethnic cohort: the multi ethnic study of atherosclerosis. Atherosclerosis, 2015; 241, 176−82. doi: 10.1016/j.atherosclerosis.2015.05.006 [25] Zhang FJ, Guo JW, Yang FF, et al. Lp-PLA2 evaluates the severity of carotid artery stenosis and predicts the occurrence of cerebrovascular events in high stroke-risk populations. J Clin Lab Anal, 2011; 35, e23691. [26] Jenny NS, Solomon C, Cushman M, et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and risk of cardiovascular disease in older adults: results from the cardiovascular health study. Atherosclerosis, 2010; 209, 528−32. doi: 10.1016/j.atherosclerosis.2009.09.021 [27] Wang YX, Zhou B, Zhou PG, et al. Association of lipoprotein-associated phospholipase A2 mass with asymptomatic cerebral artery stenosis. J Cell Mol Med, 2018; 22, 2329−36. doi: 10.1111/jcmm.13521 [28] Daniels LB, Laughlin GA, Sarno MJ, et al. Lipoprotein-associated phospholipase A2 is an independent predictor of incident coronary heart disease in an apparently healthy older population: the rancho Bernardo study. J Am Coll Cardiol, 2008; 51, 913−9. doi: 10.1016/j.jacc.2007.10.048 [29] Brilakis ES, Khera A, Saeed B, et al. Association of lipoprotein-associated phospholipase A2 mass and activity with coronary and aortic atherosclerosis: findings from the Dallas heart study. Clin Chem, 2008; 54, 1975−81. doi: 10.1373/clinchem.2008.107359 [30] Miyaura S, Maki N, Byrd W, et al. The hormonal regulation of platelet-activating factor acetylhydrolase activity in plasma. Lipids, 1991; 26, 1015−20. doi: 10.1007/BF02536494 [31] Liu HM, Yao Y, Wang YX, et al. Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study. J Cell Mol Med, 2018; 22, 5145−50. doi: 10.1111/jcmm.13803 [32] Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologists and American college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract, 2017; 23, 479−97. doi: 10.4158/EP171764.GL [33] Anuurad E, Ozturk Z, Enkhmaa B, et al. Association of lipoprotein-associated phospholipase A2 with coronary artery disease in African-Americans and Caucasians. J Clin Endocrinol Metab, 2010; 95, 2376−83. doi: 10.1210/jc.2009-2498 [34] The Lp-PLA2 Studies Collaboration. Collaborative meta-analysis of individual participant data from observational studies of Lp-PLA2 and cardiovascular diseases. Eur J Cardiovasc Prev Rehabil, 2007; 14, 3−11. doi: 10.1097/01.hjr.0000239464.18509.f1 [35] Lin YH, Gong ML, Zhang P, et al. Establishment of normal reference interval of lipoprotein-associated phospholipase A2 quality concentration in apparent healthy people aged over 50 years. Chin J Lab Med, 2021; 32−8. (In Chinese [36] Ueshima H, Kadowaki T, Hisamatsu T, et al. Lipoprotein-associated phospholipase A2 is related to risk of subclinical atherosclerosis but is not supported by Mendelian randomization analysis in a general Japanese population. Atherosclerosis, 2016; 246, 141−7. doi: 10.1016/j.atherosclerosis.2015.12.027 -
 21315Supplementary Materials.pdf
21315Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links