-
Arsenic is a metal-like carcinogen widely found in water, soil, and air environments[1]. At present, more than 200 million people, in 70 countries, are exposed to arsenic contaminated drinking water[2]. In chronic arsenic exposure, trivalent arsenic is the most carcinogenic and can induce diseases including skin, liver, lung, and bladder cancers[3]. However, the mechanism of carcinogenesis induced by arsenic exposure is not yet fully elucidated.
microRNAs are a class of endogenous non-coding RNAs with lengths of approximately 19-24 nucleotides. Their main function is to combine with the 3' non-coding region of target gene mRNA to regulate gene expression and participate in the development, proliferation, and apoptosis of cells[4]. Expression of microRNA-21 is involved in the occurrence and development of many cancers including those of the lung, liver, and pancreas[5].
Arsenic can activate signal transducer and activator of transcription 3 (STAT3) through phosphorylation. Phosphorylated STAT3 can subsequently participate in the malignant transformation of cells and the proliferation, migration, invasion, and apoptosis of cancer cells[6]. Moreover, phosphorylated (p)STAT3 can up-regulate the expression of miRNA-21 in cancer cells[7, 8], which can inhibit the expression of tumor suppressor genes such as programmed cell death protein 4 (PDCD4), phosphatase and tensin homolog (PTEN), and protein sprouty homolog 1 (Spry1) and ultimately lead to the malignant proliferation of cancer cells. However, this is not the case in all studies. Gu Jingyi et al. found that low doses of arsenic inhibited the expression of miRNA-21 in leukemic cells[9]. Furthermore, Cárdenas-González M found that chronic arsenic exposure did not significantly change miRNA-21 expression[10].
Expression of miRNA-21 as a mechanism of action in the process of arsenic-induced carcinogenesis remains controversial. Therefore, we undertook a meta-analysis of arsenic and miRNA-21-related experimental studies to delineate the role of miRNA-21 in arsenic-induced carcinogenesis.
-
Inclusion criteria were determined according to PICO principles[11].
Study design: Experimental studies published in Chinese and English.
Participants (P): All cells, animals, or human.
Intervention (I): The experimental groups were treated with arsenic or arsenic compounds. The arsenic model group had a dose or time relationship with cell proliferation, STAT3, and miRNA-21, and the highest dose or the longest time was used in the analysis.
Comparison (C): The control group was a blank control group without any intervention.
Outcome (O): Indexes related to cell proliferation and STAT3, pSTAT3, miRNA-21, anti-miRNA-21, miRNA-21 mimic, PDCD4, PTEN, Spry1, Vimentin, E-cadherin, and N-cadherin.
-
1) Literature in languages other than Chinese and English, irrelevant titles, or does not contain arsenic and miRNA-21 in the manuscript. 2) Repeated publications (the same study published in both Chinese and English journals). 3) A document in which data are incomplete or unavailable, or in which valid data are unavailable. 4) Summary literature, conference literature, academic dissertation. 5) No control group.
-
The databases form which studies were retrieved include: Cochrane library, PubMed, Web of Science, Excerpta Medica database (EMBASE), China National Knowledge Infrastructure (CNKI), Wan Fang Data databases, Viper database, China Biology Medicine disc (CBMdisc). The retrieval period is from the beginning of the publication to July 1, 2018.
The key words used included: arsenic, arsenite, arsenic trioxide (ATO), As2O3, microRNA-21, miRNA-21, miR-21, STAT3, pSTAT3, PDCD4, PTEN, Spry1, E-cadherin, N-cadherin, and Vimentin. The specific search strategy was: [(arsenic) OR (arsenite) OR (ATO)] AND [(microRNA-21) OR (miRNA-21) OR (miR-21)] AND [(STAT3) OR (pSTAT3) OR (PDCD4) OR (PTEN) OR (Spry1) OR (E-cadherin) OR (N-cadherin) OR (Vimentin)].
-
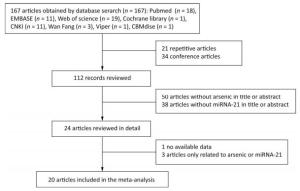
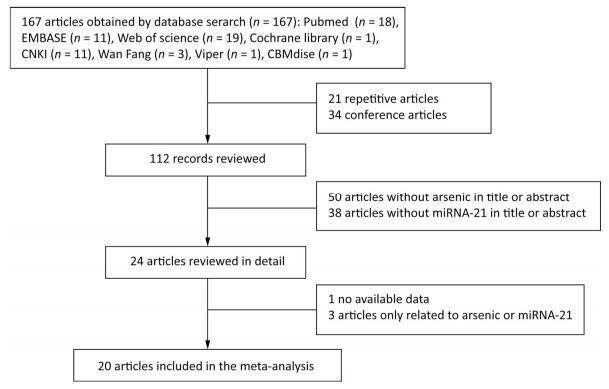
A total of 167 articles were retrieved using the described search strategy, and 20 articles were retained based on the inclusion and exclusion criteria[32]. Our search strategy is that two researchers independently used the same key words to search. First, according to PICO principle, 167 articles were included based on the title and abstract information of the articles. Then, 21 duplicates and 34 conference papers with only abstracts were deleted according to the inclusion exclusion criteria, and 112 articles remained. After read the titles and abstracts, 50 and 38 articles without arsenic and miRNA-21 were excluded, respectively, and 24 remained. Then, after reading the full text of the articles, one article that could not extract valid data and three articles containing only arsenic or miRNA-21 were excluded. Finally, we included 20 articles. The search deadline is July 1, 2018, and the search results are shown in Figure 1.
-
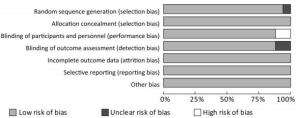
The Cochrane risk offset quality assessment tool was used to evaluate the quality of the 17 articles included. The assessment included seven items: 1) Random sequence generation (selection); 2) Allocation concealment (selection); 3) Blinding of participants and personnel (performance bias); 4) Blinding of outcome assessment (detection bias); 5) Incomplete outcome data (attrition bias); 6) Selective reporting (reporting bias); and 7) Other bias.
-
In this study, because of arsenic could cause many kinds of cancers, and miRNA-21 was involved in the occurrence and development of many kinds of cancers, however, there were few reports about the relationship between arsenic and miRNA-21, so our study was aimed to observe the effect of arsenic on the expression of PDCD4, PTEN and Spry1 by miRNA-21 and explore the influence on the malignant proliferation of cells. Our main intervention was to treat cells with arsenic alone and with arsenic in combination with miRNA-21 agonists or inhibitors.
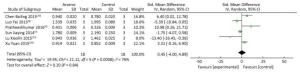
Data were analyzed using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration 2012, Portland, OR, USA) and Stata 12.0 (Stata Corp LP, College Station, TX, USA) software packages. In the quality assessment of included studies, using Review Manager 5.3 software scoring system to evaluate the quality of studies. Standard mean difference (SMD) was used to compare and analyze numerical data with large differences in means. In this study we tested many different indicators, including protein levels and mRNA levels. The units of these indicators were different. We used the standard mean difference in order to facilitate comparison among the various articles. This method was also used in the article of LI Dong Jie[11] and others. This avoided the problem that different indicators were inconvenient to compare due to different units. SMD was also increasingly used in a wide range of applications. The formula for calculating the standard mean difference is ${d_i} = \frac{{\overline {{x_{1i}}} - \overline {{x_{2i}}} }}{{{s_d}}}$, i = 1, 2, 3 … k. The heterogeneity[33] was assessed by testing I2, according to the Cochrane Handbook[32], heterogeneity was divided into four categories by I2: 0% to 40%, which may not be important; 30% to 60%, there may be moderate heterogeneity; 50% to 90%, there may be substantial heterogeneity; 75% to 100%, there was greater heterogeneity. The random effects model was chosen when P < 0.05 and I2 > 50%. The fixed effect model was chosen when P > 0.05 and I2 ≤ 50%. Heterogeneity sources within the 17 articles were detected by subgroup analysis. According to the study on the dose-response relationship by Crippa et al.[37], we used spline models and subgroup analyses to analyze the bidirectional effects of arsenic on miRNA-21. The subgroups were determined based on exposure duration (≤ 24 h or > 24 h) and exposure concentration (≤ 5 μmol/L and > 5 μmol/L). The combined results of the experimental and control groups were expressed by SMD and 95% confidence intervals (95% CI). We used the funnel plot to assess whether there was a publication bias. The hypothesis test used chi-square test. When P < 0.05, we think there was a publication bias in the results. We used Stata 12.0 software for sensitivity analysis; I2 > 50%, using a random effects model; sensitivity analysis plots were provided in the results. All statistical analyses were performed with two-sided, and the differences were deemed statistically significant when P ≤ 0.05.
-
The basic features of the 17 articles included in this analysis are shown in Table 1. The experimental groups were treated with different forms of arsenic, including sodium arsenite (NaAsO2) and arsenic trioxide (As2O3). The control groups were blank controls without arsenic exposure. In the subgroup analysis, the arsenic exposure duration differed, so the exposure duration was divided into two groups: ≤ 24 h (n = 13) and > 24 h (n = 4). The arsenic exposure dose also differed and was divided into two groups: ≤ 5 µmol/L (n = 13) and > 5 μmol/L (n = 4). Outcome variables were apoptosis-related (colony number, E-cadherin, N-cadherin, and Vimentin expression) and miRNA-21 related (miRNA-21, STAT3, pSTAT3, PDCD4, PTEN, and Spry1 expression). Three articles, Lu Xiaolin et al.[12], Pratheeshkumar et al.[14], and Luo Fei et al.[8], provided the most information. All three articles analyzed at least five indicators.
Table 1. Characteristics of the Studies Included in the Meta-analysis
Authors Year Language n Arsenic Type Arsenic Dose (μmol/L) Exposure Duration (h) Outcome Indicators Country Lu Xiaolin et al.[12] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 1, 2, 3, 7, 9 China Ling Min et al.[13] 2012 English 3 NaAsO2 ≤ 5 ≤ 24 1, 4, 5, 6 China Pratheeshkumar et al.[6] 2016 English 3 As2O3 > 5 > 24 1, 2, 3, 4, 7 America Gu Jingyi et al.[9] 2011 English 3 As2O3 ≤ 5 ≤ 24 1 China Luo Fei et al.[8] 2013 English 3 NaAsO2 ≤ 5 ≤ 24 1, 2, 3, 7, 8, 9 China Liu Xinlu et al.[4] 2016 English 3 NaAsO2 > 5 ≤ 24 1, 4, 5, 6 China Banerjee Nilanjana et al.[15] 2017 English 45 As2O3 ≤ 5 > 24 4, 5 India Xu Yuan et al.[16] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 2, 3, 5 China Luo Fei et al.[17] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 1, 4, 7, 8, 9 China Li Yumin et al.[18] 2010 English 3 As2O3 ≤ 5 ≤ 24 4 China Zhao Yue et al.[19] 2013 English 3 NaAsO2 ≤ 5 > 24 1 China Zhao Xin et al.[20] 2015 English 6 As2O3 ≤ 5 ≤ 24 1, 6 China Lu Shen et al.[21] 2013 English 3 NaAsO2 ≤ 5 ≤ 24 1, 6 China Cárdenas-González M et al.[10] 2016 English 27 As2O3 ≤ 5 > 24 1 Mexico Sun Jiaying et al.[7] 2014 English 3 As2O3 > 5 ≤ 24 1, 2, 3 China Chen Bailing et al.[22] 2013 English 3 As2O3 > 5 ≤ 24 1, 2, 3 China Li Yumin et al.[23] 2010 Chinese 3 As2O3 ≤ 5 ≤ 24 4 China He Qian et al.[34] 2013 English 3 As2O3 ≤ 5 > 24 1, 4, 5 China Lu Lu et al.[35] 2018 English 3 As2O3 > 5 > 24 1, 5 China Liu Haiwei et al.[36] 2018 English 3 As2O3 > 5 ≤ 24 1, 5 China Note. n, number within the experimental group; miRNA-21, an endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; E-cadherin, a type of cell adhesion molecule; N-cadherin, a type of cell adhesion molecule; Vimentin, a type Ⅲ intermediate filament protein. 1, miRNA-21; 2, STAT3; 3, pSTAT3; 4, PDCD4; 5, PTEN; 6, Spry1; 7, E-cadherin; 8, N-cadherin; 9, Vimentin. -
The 17 articles included in this study were evaluated for quality, the low risk rate of bias was found to be more than 75%, the high risk rate of bias was found to be less than 10% (Figure 2).
-
The colony numbers produced by cells of the arsenic exposed groups were 7.35-fold greater than those of the normal control groups (95% CI: 1.11, 13.59). The E-cadherin levels produced by cells exposed to arsenic were 25.74-fold lower than those produced by the normal control groups (95% CI: -50.44, -1.04). The level of Vimentin was 10.27-fold higher in cells exposed to arsenic than in the cells of the normal control groups (95% CI: -13.4, 15.95). The level of N-cadherin in the arsenic exposed groups was 20.27-fold lower than that in the normal control groups (95% CI: -51.16, 10.62), however, the effect of arsenic on N-cadherin was non-significant. (Figure 3). These results suggest that arsenic can down-regulate the expression of E-cadherin, and increase the number of cell colonies, potentially leading to the malignant proliferation of cells.
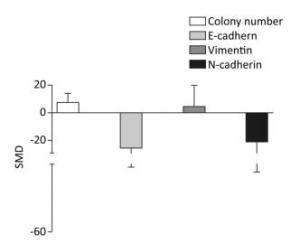
Figure 3. Effect of arsenic on malignant cell proliferation. SMD, standardized mean difference; E-cadherin, a type of cell adhesion molecule; N-cadherin, a type of cell adhesion molecule; Vimentin, a type Ⅲ intermediate filament (IF) protein. Arsenic can inhibit the expression of E-cadherin and N-cadherin, promote the expression of Vimentin, and cause malignant proliferation of cells.
-
There was no significant difference in STAT3 expression in the arsenic exposure groups and normal control groups (P > 0.05) (Figure 4). However, in the meta-analysis of the association between arsenic and pSTAT3, pSTAT3 expression was higher in the arsenic treated groups that in the normal control groups (SMD = 4.80, 95% CI: -0.52, 10.12) (Figure 5), but the results also showed there was no significant difference in pSTAT3 expression in the arsenic exposure groups and normal control groups. However, arsenic had the tendency to activate pSTAT3 during the process of arsenic-induced malignant cell proliferation.
-
The expression of miRNA-21 in the arsenic exposure groups was higher than that in normal control groups (SMD = 4.65, 95% CI: 2.50, 6.80) (Figure 6). The results show that arsenic can induce increased miRNA-21 expression in the cells. These results indicate that arsenic could cause the overexpression of miRNA-21 in the process of arsenic-induced malignant cell proliferation.
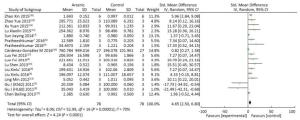
Figure 6. The Effect of ARSENIC on miRNA-21. Forest plot shows the effect of arsenic treatment on miRNA-21 expression in treatment and control groups. SMD, standardized mean difference; Ⅳ, independent variable; 95% CI, 95% confidence interval; SD, standard deviation. Arsenic can promote the expression of miRNA-21.
-
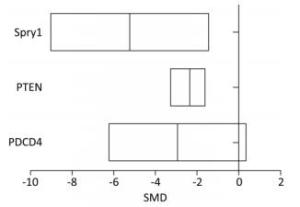
The level of PDCD4 expression in the arsenic exposure group was 2.86-fold lower than that in the normal control group (95% CI: -6.23, 0.35). The level of PTEN expression in the arsenic exposure group was 2.20-fold lower than that of the normal control group (95% CI: -3.27, -1.56). The level of Spry1 expression in the arsenic exposure group was 5.26 fold-lower than that of the normal control group (95% CI: -9.03, -1.42) (Figure 7). These results show that arsenic can inhibit the expression of PDCD4, PTEN and Spry1, but there was a marginally significant in the expression of PDCD4 by arsenic.
-
PDCD4, PTEN, and Spry1 may be miRNA-21 target genes[21, 24]. To determine whether arsenic can further alter the expression of PDCD4, PTEN, and Spry1 by regulating miRNA-21 expression, we used the arsenic treatment group as the control group, and arsenic combined with miRNA-21 inhibitor and arsenic combined with miRNA-21 mimic groups as experimental groups. This comparison was designed to observe the effect of arsenic on the expression of PDCD4, PTEN, and Spry1 following the addition of miRNA-21 inhibitor and miRNA-21 mimic. The results showed that treatment with arsenic alone could inhibit PDCD4 (SMD = -2.65, 95% CI: -5.95, 0.66), PTEN (SMD = -1.8, 95% CI: -2.57, -1.03), and Spry1 (SMD = -4.85, 95% CI: -8.46, -1.24) expression. After arsenic and miRNA-21 inhibitor treatment, the expression of PDCD4 (SMD = 7.63, 95% CI: 3.95, 11.31) was resumed, the expression of PTEN (SMD = 5.30, 95% CI: 0.06, 10.66) was marginally resumed, and Spry1 (SMD = 3.83, 95% CI: 5.46, 13.12) was not resumed. Arsenic and miRNA-21 mimic had no significant inhibitory effect on PDCD4 (SMD = 5.90, 95% CI: 12.16, 0.36) and PTEN (SMD = 3.58, 95% CI: 14.50, 7.33), but had significant inhibitory effect on Spry1 (SMD = 9.55, 95% CI: 17.86, 1.24) (Figure 8).
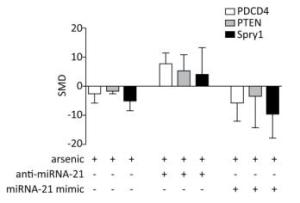
Figure 8. The effects of arsenic on PDCD4, PTEN, and Spry1. SMD, standardized mean difference; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; anti-miRNA-21, miRNA-21 inhibitor; miRNA-21 mimic, miRNA-21 agonist. Compared with arsenic group, anti-miRNA-21 could promote the expression of PDCD4, PTEN, and Spry1; miRNA-21-mimic could inhibit the expression of PDCD4, PTEN, and Spry1.
-
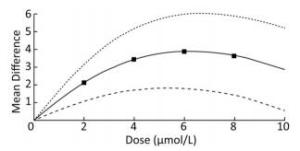
To demonstrate the dose-response relationship between arsenic and miRNA-21, the spline model was used to quantitatively analyze the standardized mean difference in multiple studies. We analyzed some of the articles included in our study, and the basic information was shown in Table 2. The results showed that the mean difference between the different doses were 2.19 (95% CI: 1.27, 3.09) for 2 μmol/L, 3.42 (95% CI: 1.70, 5.06) for 4 μmol/L; 3.92 (95% CI: 1.82, 6.04) for 6 μmol/L; 3.67 (95% CI: 1.42, 5.92) for 8 μmol/L. The results showed that when the dose of arsenic was less than 6 μmol/L, the dose-response relationship between arsenic and miRNA-21 was positive. When the dose was more than 6 μmol/L, the mean difference did not increase with the dose (Figure 9). This result showed more clearly that the regulation of miRNA-21 expression by arsenic dose was bidirectional.
Table 2. Comprehensive Dose-response Data from Five Articles on miRNA-21 at Different Doses of Arsenic
ID Author Year Dose [Mean ± SD) μmol/L] 0 2 5 10 15 20 1 Pratheeshkumar et al.[6] 2016 10.30 ± 0.01 13.56 ± 0.14 15.43 ± 0.04 13.54 ± 0.02 - - 2 Liu Xinlu et al.[4] 2016 10.02 ± 0.12 15.32 ± 0.14 11.73 ± 0.11 10.42 ± 0.13 9.23 ± 0.16 - 3 Gu J (K562) et al.[9] 2011 10.34 ± 1.23 13.56 ± 2.43 16.34 ± 2.43 15.87 ± 2.13 14.32 ± 1.90 13.42 ± 1.63 4 Gu J (K562) et al.[9] 2011 11.00 ± 7.13 11.50 ± 7.01 15.80 ± 6.93 13.31 ± 7.32 11.67 ± 7.32 - 5 Sun J et al.[7] 2014 10.60 ± 5.32 11.30 ± 5.14 12.43 ± 5.43 11.43 ± 6.92 - - Note. Comprehensive dose-response data from five articles on miRNA-21 at different doses of arsenic. Dose, the dose of arsenic. Mean, the average expression of miRNA-21 under arsenic. SD, standard deviation of miRNA-21 expression under arsenic. -
Low-dose arsenic exposure (≤ 5 μmol/L) could increase the expression of miRNA-21 (SMD = 7.58, 95% CI: 2.80, 12.36; I2 = 72.0%), pSTAT3 (SMD = 9.06, 95% CI: 2.06, 16.06; I2 = 74.2%) and decrease the expression of Spry1 (SMD = -10.34, 95% CI: -16.08, -4.60; I2 = 34.3%), however, arsenic exposure in the low-dose group had no significant effect on the expression of PDCD4 (SMD = -6.94, 95% CI: -15.38, 1.49). High-dose arsenic exposure (> 5 μmol/L) increased the expression of miRNA-21 (SMD = 2.94, 95% CI: 0.38, 5.49; I2 = 16.0%) and decreased the expression of Spry1 (SMD = -1.93, 95% CI: -3.35, -0.52; I2 = 0.0%) and E-cadherin (SMD = -95.87, 95% CI: -162.32, -29.42; I2 = 0.0%) (Figure 10). These results show that pSTAT3 activation was more obvious at a low arsenic dose than at a high arsenic dose, and that the inhibitory effect of low-dose arsenic on Spry1 expression was more obvious than that of high-dose arsenic. However, high-dose arsenic had a stronger inhibitory effect on E-cadherin than did low-dose arsenic. Because the I2 values of miRNA-21 and pSTAT3 in the low dose group were larger, the heterogeneity probably came from the miRNA-21 and pSTAT3 in the low dose group.
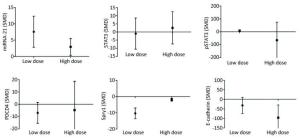
Figure 10. Subgroup analysis of arsenic exposure dose. SMD, standardized mean difference. miRNA-21, An endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; E-cadherin, a type of cell adhesion molecule. Arsenic can promote the expression of miRNA-21 under low or high dose exposure. The inhibitory effect of low dose of arsenic on PDCD4 and Spry1 was more obvious. The inhibitory effect of high dose arsenic on pSTAT3 and E-cadherin is more obvious.
-
The results showed that when the arsenic exposure time was less than 24 h, the expression of miRNA-21 (SMD = 5.36, 95% CI: 1.88, 8.84; I2 = 64.0%), pSTAT3 (SMD = 13.93, 95% CI: 3.37, 24.50; I2 = 89.0%) increased, and the expression of PDCD4 (SMD = -4.43, 95% CI: -6.79, -2.06; I2 = 18.5%) decreased. When the arsenic exposure duration was more than 24 h, the expression of miRNA-21 (SMD = 13.93, 95% CI: 3.37, 24.50; I2 = 0.0%), STAT3 (SMD = 13.73, 95% CI: 4.08, 23.37; I2 = 0.0%), and pSTAT3 (SMD = 3.12, 95% CI: 0.43, 5.81; I2 = 0.0%) increased, but the expression of PDCD4 (SMD = -19.31, 95% CI: -85.88, 47.25; I2 = 91.1%) was non-significant. (Figure 11). The effect of arsenic on the activation of miRNA-21 and the inhibition of pSTAT3 was more obvious after the longer exposure duration than after the shorter exposure duration. Because the I2 values of miRNA-21 and pSTAT3 in the shorter exposure duration group were larger, the heterogeneity probably came from the miRNA-21 and pSTAT3 in the shorter exposure duration group.
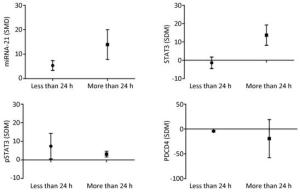
Figure 11. Subgroup analysis of arsenic exposure time. SMD, standardized mean difference. miRNA-21, An endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4. Prolonged arsenic exposure could promote the expression of miRNA-21 and STAT3 and inhibit the expression of pSTAT3 and PDCD4.
-
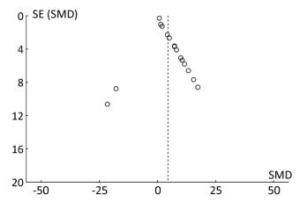
The funnel plot shows that all the results of the study are symmetrically distributed, which indicates there is no publication bias (Figure 12).
-
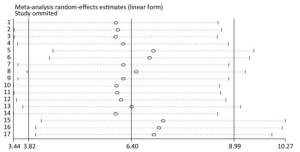
Taking the sensitivity analysis of arsenic and miRNA-21 as an example, we found that all results were located on both sides of the midline with no obvious deviation (Figure 13). These results show that the results of the research included in the literature are relatively stable, and that no individual results affect the overall results.
-
Arsenic can induce malignant proliferation of cells and cause a variety of cancerous diseases, including skin, lung, liver, and bladder cancers[3]. At present, increasing numbers of studies have found that miRNAs play an important role in the process of arsenic induced cancer[14, 25]. miRNA-21 has been identified as a key oncogene in this process and is involved in the occurrence and development of many cancers[24, 26, 27]. The results of this study show that exposure to arsenic increases miRNA-21 levels, reduces PTEN, and Spry1 levels. Subsequently, E-cadherin and N-cadherin protein expression decreases and expression of the Vimentin protein increases, ultimately leading to the malignant transformation of cells. The results provide a theoretical basis for the mechanism of arsenic induced cancer.
In this study, we focused on the role of miRNA-21 in arsenic-induced carcinogenesis. The main function of miRNA-21 is to bind to the 3'-UTR of target genes such as PDCD4, PTEN, and Spry1 and to inhibit the post-transcriptional translation of target genes. Reduced expression of miRNA-21, or increased expression of PDCD4, PTEN, or Spry1 can inhibit tumor formation. Therefore, the relationship between miRNA-21 and PDCD4, PTEN, and Spry1 is very important for the treatment of cancer. First, we found that arsenic had the tendency to activate pSTAT3. Studies conducted by Chen Beiling et al.[22] and Lu Xiaoling et al.[12] revealed that when arsenic enters cells it increases the level of IL-6, and subsequently increase the level of pSTAT3. IL-6, as an inflammatory stimulator, which transmits the inflammatory signal to STAT3 to induce STAT3 phosphorylation. pSTAT3 then transmits the inflammatory signal to the nucleus.
We also observed that arsenic increased the levels of miRNA-21, and we speculated that this process may be related to the observed changes in pSTAT3 levels. Sun Jiaying et al.[7] and Luo Fei et al.[8] found that expression of miRNA-21 was activated when pSTAT3 levels increased, indicating that arsenic likely activated pSTAT3 which then increased miRNA-21 levels. We found that arsenic induced malignant transformation of cells and inhibited the expression of the tumor suppressor genes PDCD4, PTEN, and Spry1. These results indicated that the expression of PDCD4, PTEN, and Spry1 was affected by miRNA-21 during arsenic induced carcinogenesis. Therefore, we observed the relationship between arsenic, miRNA-21 inhibitor, and miRNA-21 mimic. The results showed that arsenic alone could inhibit the expression of PDCD4, PTEN, and Spry1, while the combination of arsenic and miRNA-21 inhibitor could increase the expression of PDCD4 and PTEN. Arsenic combined with miRNA-21 mimic could inhibit the expression of Spry1 more strongly than could arsenic alone. These results suggest that expression of PDCD4, PTEN, and Spry1 may be inhibited by increasing the expression of miRNA-21 in arsenic induced carcinogenesis. When the expression of tumor suppressor genes PDCD4, PTEN, and Spry1 is reduced, malignant cell proliferation can occur. However, studies by Radha Madhyastha et al.[28] and Amit Bera et al.[29] found that inhibition of PDCD4, PTEN, and Spry1 did not directly result in malignant cell transformation, but further inhibited the phosphorylation of AKT. AKT phosphorylation results in the binding of IκB to the monomeric dimer IKKβ-NF-κB in the cytoplasm, after which the signal is transduced to the nucleus resulting in the malignant proliferation of cancer cells. Therefore, NF-κB signaling pathway is closely involved in the process of arsenic carcinogenesis involving miRNA-21.
The activation of miRNA-21 by arsenic exposure at different doses and for different durations varied. A study conducted by Luo Fei et al.[17] found that low-dose and short-term arsenic exposure could activate miRNA-21, a result supported by other studies[12, 16]. However, it has also been shown that low-dose and long-term arsenic exposure can activate miRNA-21[14]. Therefore, we preformed subgroup analysis based on the duration and dose of arsenic exposure. The results showed that low-dose and long-term arsenic exposure had the strongest effect on miRNA-21 activation, while high-dose and short-term arsenic exposure had a weaker miRNA-21 activation effect. According to the result of the dose-response relationship between arsenic and miRNA-21, our results found the bidirectional regulation effect of arsenic on miRNA-21 expression, in which the dosage of arsenic played an essential role according to the spline model and the dose subgroup analysis. Thus, some new ways could be adopted to change the dose of arsenic in order to regulate the expression of miRNA-21.
Therefore, we suggest that low-dose and long-term arsenic exposure can activate pSTAT3 to further promote the expression of miRNA-21 and inhibit the expression of PDCD4, PTEN, and Spry1 target genes, ultimately resulting in the malignant cell proliferation. We also found that inhibiting the expression of miRNA-21 could increase the expression of PDCD4, PTEN, and Spry1, and inhibit the malignant transformation of cells. These results reveal that miRNA-21 inhibitors can be used in the treatment of cancer, and provide a feasible method for the treatment of cancers caused by arsenic exposure.
In the studies we included, since the results obtained show a high I2 value on the forest plot, indicating that our results are heterogeneous, we used a subgroup analysis method to analyze the source of heterogeneity. It was found that the dose and time of arsenic produced heterogeneity in the expression of PDCD4 and pSTAT3, which indicates that the dose and time of arsenic are important sources of heterogeneity. We have clearly compared the differences of different subgroups. We identified high levels of heterogeneity related to many factors other than those included in the subgroup analysis. For example, the subjects of the studies differed and included animals, humans, and cells. The methods employed to expose the subjects to arsenic also differed and included exposure via drinking water and gavage. The effects of arsenic on the development of cancer may also involve other factors such as the ERK/NF-κB signaling pathway and IL-6. Indeed, miRNA-21 can regulate the ERK/NF-κB signaling pathway and further influence the occurrence and development of tumors[30, 31]. Lu Xiaolin et al.[12] showed that the activation of STAT3 is closely related to IL-6 levels. Therefore, the interaction between miRNA-21, ERK/NF-κB signaling pathway and IL-6 will become the focus of our future research.
doi: 10.3967/bes2018.090
The Bidirectional Effects of Arsenic on miRNA-21: A Systematic Review and Meta-analysis
-
Abstract:
Objective Arsenic is a metalloid environmental carcinogen involved in the occurrence and development of many cancers. miRNA-21 plays a crucial role in arsenic-induced carcinogenesis. We aimed to elucidate the mechanism by which miRNA-21 influences arsenic-induced cancer. Methods We used meta-analysis of published studies to determine how arsenic induces cancerous cells through miRNA-21. Results Low-dose arsenic exposure (≤ 5 μmol/L) can increase miRNA-21 and phosphorylated signal transducter and activator of transcription 3 (pSTAT3) expression, and decrease programmed cell death protein 4 (PDCD4) and protein sprouty homolog 1 (Spry1) expression. High-dose arsenic exposure (> 5 μmol/L), can increase miRNA-21 expression, and decrease Spry1 and E-cadherin expression. Short-term arsenic exposure (≤ 24 h) can increase miRNA-21 and pSTAT3 expression, and decrease PDCD4 expression. Moreover, long-term arsenic exposure (> 24 h) can increase the miRNA-21, STAT3, and pSTAT3 expression, and decrease PDCD4 expression. We found that activation of miRNA-21 and pSTAT3 were most pronounced following long-term arsenic exposure at low doses, and the effects on PDCD4 expression were most pronounced following short-term arsenic exposure at low doses. miRNA-21 inhibitors increased the expression of tumor suppressor genes PDCD4, PTEN, and Spry1 and miRNA-21-mimics suppressed the expression of these tumor suppressor genes. Conclusion Arsenic can cause cancer by activating miRNA-21 and inhibiting the expression of PDCD4, PTEN, and Spry1. -
Figure 3. Effect of arsenic on malignant cell proliferation. SMD, standardized mean difference; E-cadherin, a type of cell adhesion molecule; N-cadherin, a type of cell adhesion molecule; Vimentin, a type Ⅲ intermediate filament (IF) protein. Arsenic can inhibit the expression of E-cadherin and N-cadherin, promote the expression of Vimentin, and cause malignant proliferation of cells.
Figure 6. The Effect of ARSENIC on miRNA-21. Forest plot shows the effect of arsenic treatment on miRNA-21 expression in treatment and control groups. SMD, standardized mean difference; Ⅳ, independent variable; 95% CI, 95% confidence interval; SD, standard deviation. Arsenic can promote the expression of miRNA-21.
Figure 8. The effects of arsenic on PDCD4, PTEN, and Spry1. SMD, standardized mean difference; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; anti-miRNA-21, miRNA-21 inhibitor; miRNA-21 mimic, miRNA-21 agonist. Compared with arsenic group, anti-miRNA-21 could promote the expression of PDCD4, PTEN, and Spry1; miRNA-21-mimic could inhibit the expression of PDCD4, PTEN, and Spry1.
Figure 10. Subgroup analysis of arsenic exposure dose. SMD, standardized mean difference. miRNA-21, An endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; E-cadherin, a type of cell adhesion molecule. Arsenic can promote the expression of miRNA-21 under low or high dose exposure. The inhibitory effect of low dose of arsenic on PDCD4 and Spry1 was more obvious. The inhibitory effect of high dose arsenic on pSTAT3 and E-cadherin is more obvious.
Figure 11. Subgroup analysis of arsenic exposure time. SMD, standardized mean difference. miRNA-21, An endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4. Prolonged arsenic exposure could promote the expression of miRNA-21 and STAT3 and inhibit the expression of pSTAT3 and PDCD4.
Table 1. Characteristics of the Studies Included in the Meta-analysis
Authors Year Language n Arsenic Type Arsenic Dose (μmol/L) Exposure Duration (h) Outcome Indicators Country Lu Xiaolin et al.[12] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 1, 2, 3, 7, 9 China Ling Min et al.[13] 2012 English 3 NaAsO2 ≤ 5 ≤ 24 1, 4, 5, 6 China Pratheeshkumar et al.[6] 2016 English 3 As2O3 > 5 > 24 1, 2, 3, 4, 7 America Gu Jingyi et al.[9] 2011 English 3 As2O3 ≤ 5 ≤ 24 1 China Luo Fei et al.[8] 2013 English 3 NaAsO2 ≤ 5 ≤ 24 1, 2, 3, 7, 8, 9 China Liu Xinlu et al.[4] 2016 English 3 NaAsO2 > 5 ≤ 24 1, 4, 5, 6 China Banerjee Nilanjana et al.[15] 2017 English 45 As2O3 ≤ 5 > 24 4, 5 India Xu Yuan et al.[16] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 2, 3, 5 China Luo Fei et al.[17] 2015 English 3 NaAsO2 ≤ 5 ≤ 24 1, 4, 7, 8, 9 China Li Yumin et al.[18] 2010 English 3 As2O3 ≤ 5 ≤ 24 4 China Zhao Yue et al.[19] 2013 English 3 NaAsO2 ≤ 5 > 24 1 China Zhao Xin et al.[20] 2015 English 6 As2O3 ≤ 5 ≤ 24 1, 6 China Lu Shen et al.[21] 2013 English 3 NaAsO2 ≤ 5 ≤ 24 1, 6 China Cárdenas-González M et al.[10] 2016 English 27 As2O3 ≤ 5 > 24 1 Mexico Sun Jiaying et al.[7] 2014 English 3 As2O3 > 5 ≤ 24 1, 2, 3 China Chen Bailing et al.[22] 2013 English 3 As2O3 > 5 ≤ 24 1, 2, 3 China Li Yumin et al.[23] 2010 Chinese 3 As2O3 ≤ 5 ≤ 24 4 China He Qian et al.[34] 2013 English 3 As2O3 ≤ 5 > 24 1, 4, 5 China Lu Lu et al.[35] 2018 English 3 As2O3 > 5 > 24 1, 5 China Liu Haiwei et al.[36] 2018 English 3 As2O3 > 5 ≤ 24 1, 5 China Note. n, number within the experimental group; miRNA-21, an endogenous non-coding RNA; STAT3, signal transducer and activator of transcription 3; pSTAT3, phosphorylated signal transduction and activator of transcription 3; PDCD4, programmed cell death protein 4; PTEN, phosphatase and tensin homolog; Spry1, protein sprouty homolog 1; E-cadherin, a type of cell adhesion molecule; N-cadherin, a type of cell adhesion molecule; Vimentin, a type Ⅲ intermediate filament protein. 1, miRNA-21; 2, STAT3; 3, pSTAT3; 4, PDCD4; 5, PTEN; 6, Spry1; 7, E-cadherin; 8, N-cadherin; 9, Vimentin. Table 2. Comprehensive Dose-response Data from Five Articles on miRNA-21 at Different Doses of Arsenic
ID Author Year Dose [Mean ± SD) μmol/L] 0 2 5 10 15 20 1 Pratheeshkumar et al.[6] 2016 10.30 ± 0.01 13.56 ± 0.14 15.43 ± 0.04 13.54 ± 0.02 - - 2 Liu Xinlu et al.[4] 2016 10.02 ± 0.12 15.32 ± 0.14 11.73 ± 0.11 10.42 ± 0.13 9.23 ± 0.16 - 3 Gu J (K562) et al.[9] 2011 10.34 ± 1.23 13.56 ± 2.43 16.34 ± 2.43 15.87 ± 2.13 14.32 ± 1.90 13.42 ± 1.63 4 Gu J (K562) et al.[9] 2011 11.00 ± 7.13 11.50 ± 7.01 15.80 ± 6.93 13.31 ± 7.32 11.67 ± 7.32 - 5 Sun J et al.[7] 2014 10.60 ± 5.32 11.30 ± 5.14 12.43 ± 5.43 11.43 ± 6.92 - - Note. Comprehensive dose-response data from five articles on miRNA-21 at different doses of arsenic. Dose, the dose of arsenic. Mean, the average expression of miRNA-21 under arsenic. SD, standard deviation of miRNA-21 expression under arsenic. -
[1] Zhou Youyou, Wang Yanfu, Su Juan, et al. Integration of microRNAome, proteomics and metabolomics to analyze arsenic induced malignant cell transformation. Oncotarget, 2017; 53, 90879-96. http://cn.bing.com/academic/profile?id=0f2adf26b9fc0c0eb6fb06e435e62788&encoded=0&v=paper_preview&mkt=zh-cn [2] Brenda C Minatel, Adam P Sage, Christine Anderson, et al. Environmental arsenic exposure:From genetic susceptibility to pathogenesis. Environ Int, 2018; 112, 183-97. doi: 10.1016/j.envint.2017.12.017 [3] Katherine M Hunt, Ritesh K Srivastava, Craig A Elmets, et al. The mechanistic basis of arsenicosis:Pathogenesis of skin cancer. Cancer Lett, 2014; 354, 211-9. doi: 10.1016/j.canlet.2014.08.016 [4] Liu Xinlu, Luo Fei, Ling Min, et al. MicroRNA-21 activation of ERK signaling via PTEN is involved in arsenite-induced autophagy in human hepatic L-02 cells. Toxicol Lett, 2016; 252, 1-10. doi: 10.1016/j.toxlet.2016.04.015 [5] Tang Yiting, Zhou Xifa, Ji Jianfeng, et al. High expression levels of miR-21 and miR-210 predict unfavorable survival in breast cancer:a systemic review and meta-analysis. Int J Biol Marker, 2015; 30, e347-58. doi: 10.5301/jbm.5000160 [6] Poyil Pratheeshkumar, Young-Ok Son, Sasidharan Padmaja Divya, et al. Oncogenic transformation of human lung bronchial epithelial cells induced by arsenic involves ROS-dependent activation of STAT3-miR-21-PDCD4 mechanism. Sci Rep-uk, 2016; 6, 37227. doi: 10.1038/srep37227 [7] Sun Jiaying, Yu Miaomiao, Lu Yongju, et al. Carcinogenic metalloid arsenic induces expression of mdig oncogene through JNK and STAT3 activation. Cancer Lett, 2014; 346, 257-63. doi: 10.1016/j.canlet.2014.01.002 [8] Luo Fei, Xu Yuan, Ling Min, et al. Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicol Appl Pharm, 2013; 273, 27-34. doi: 10.1016/j.taap.2013.08.025 [9] Gu Jingyi, Zhu Xuejiao, Li Yumin, et al. miRNA-21 regulates arsenic-induced anti-leukemia activity in myelogenous cell lines. Med Oncol, 2011; 28, 211-8. doi: 10.1007/s12032-009-9413-7 [10] Cárdenas-González M, Osorio-Yáñez C, Gaspar-Ramírez O, et al. Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ Res, 2016; 150, 653-62. doi: 10.1016/j.envres.2016.06.032 [11] Li Dongjie, Wei Yutao, Xu Shangzhi, et al. A systematic review and meta‑analysis of bidirectional effect of arsenic on ERK signaling pathway. Mol Med Rep, 2017; 17, 4422-32. http://cn.bing.com/academic/profile?id=45fe68234a44a0e11ad8a159747e4956&encoded=0&v=paper_preview&mkt=zh-cn [12] Lu Xiaolin, Luo Fei, Liu Yi, et al. The IL-6/STAT3 pathway via miR-21 is involved in the neoplastic and metastatic properties of arsenite-transformed human keratinocytes. Toxicol Lett, 2015; 237, 191-9. doi: 10.1016/j.toxlet.2015.06.011 [13] Ling Min, Li Yuan, Xu Yuan, et al. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radical Bio Med, 2012; 52, 1508-18. doi: 10.1016/j.freeradbiomed.2012.02.020 [14] Wang Zhao-Xia, Lu Bin-Bin, Wang He, et al. MicroRNA-21 Modulates Chemosensitivity of Breast Cancer Cells to Doxorubicin by Targeting PTEN. Arch Med Res, 2011; 42, 281-90. doi: 10.1016/j.arcmed.2011.06.008 [15] Nilanjana Banerjee, Apurba K Bandyopadhyay, Suman Dutta, et al. Increased microRNA 21 expression contributes to arsenic induced skin lesions, skin cancers and respiratory distress in chronically exposed individuals. Toxicology, 2017; 378, 10-6. doi: 10.1016/j.tox.2017.01.006 [16] Xu Yuan, Luo Fei, Liu Yi, et al. Exosomal miR-21 derived from arsenite-transformed human bronchial epithelial cells promotes cell proliferation associated with arsenite carcinogenesis. Arch Toxicol, 2015; 89, 1071-82. doi: 10.1007/s00204-014-1291-x [17] Luo Fei, Ji Jie, Liu Yi, et al. MicroRNA-21, up-regulated by arsenite, directs the epithelial mesenchymal transition and enhances the invasive potential of transformed human bronchial epithelial cells by targeting PDCD4. Toxicol Lett, 2015; 232, 301-9. doi: 10.1016/j.toxlet.2014.11.001 [18] Li Yumin, Zhu Xuejiao, Gu Jingyi, et al. Anti-miR-21 oligonucleotide sensitizes leukemic K562 cells to arsenic trioxide by inducing apoptosis. Cancer Res, 2010; 101, 948-54. http://cn.bing.com/academic/profile?id=0bdc4af2bd9894c983e46104f33315b9&encoded=0&v=paper_preview&mkt=zh-cn [19] Zhao Yue, Xu Yuan, Luo Fei, et al. Angiogenesis, mediated by miR-21, is involved arsenite-induced carcinogenesis. Toxicol Lett, 2013; 223, 35-41. doi: 10.1016/j.toxlet.2013.08.020 [20] Zhao Xin, Shi Yuan-Qi, Yan Cai-Chuan, et al. Up-regulation of miR-21 and miR-23a Contributes to As2O3-induced hERG Channel Deficiency. Basic Clin Pharmacol, 2015; 116, 516-23. doi: 10.1111/bcpt.2015.116.issue-6 [21] Shen Lu, Ling Min, Li Yuan, et al. Feedback regulations of miR-21 and MAPKs via Pdcd4 and Spry1 Are Involved in Arsenite-Induced Cell Malignant Transformation. Plos One, 2013; 8, e57652. doi: 10.1371/journal.pone.0057652 [22] Chen Bailing, Liu Jia, Chang Qingshan, et al. JNK and STAT3 signaling pathways converge on Akt-mediated phosphorylation of EZH2 in bronchial epithelial cells induced by arsenic. Cell Cycle, 2013; 12, 112-21. doi: 10.4161/cc.23030 [23] Li Yuming, Gu Jingyi, Zhu Xuejiao, et al. Study on the sensitivity of leukemic cells to arsenic trioxide enhanced by targeted suppression of mIRNA21. Chin J Integr Trad West Med, 2010; 30, 170-3. (In Chinese) http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zxyjh201002016 [24] Wang Zhao-Xia, Lu Bin-Bin, Wang He, et al. MicroRNA-21 Modulates Chemosensitivity of Breast Cancer Cells to Doxorubicin by Targeting PTEN. Arch Med Res, 2011; 42, 281-90. doi: 10.1016/j.arcmed.2011.06.008 [25] Rebecca T Marquez, Erik Wendlandt, Courtney Searcey Galle, et al. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-κB signaling. AM J Physiol-gastr L, 2010; 298, G535-41. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_2853303 [26] Sha Min, Ye Jun, Zhang Li-xin, et al. Celastrol Induces Apoptosis of Gastric Cancer Cells by miR-21 Inhibiting PI3K/Akt-NF-κB Signaling Pathway. Pharmacology, 2014; 93, 39-46. doi: 10.1159/000357683 [27] Mao Xu-Hua, Chen Min, Wang Yan, et al. MicroRNA-21 regulates the ERK/NF-κB signaling pathway to affect the proliferation, migration and apoptosis of human melanoma A375 cells by targeting SPRY1, PDCD4 and PTEN. Mol Carcinogen, 2016; 56, 886-910. http://www.ncbi.nlm.nih.gov/pubmed/27533779 [28] Radha Madhyastha, Harish Kumar Madhyastha, Yutthana Pengjam, et al. NFkappaB activation is essential for miR-21 induction by TGFb1 in high glucose conditions. Biochem bioph Res Co, 2014; 451, 615-21. doi: 10.1016/j.bbrc.2014.08.035 [29] Amit Bera, Nandini Ghosh-Choudhury, Nirmalya Dey, et al. NFκB-mediated cyclin D1 expression by microRNA-21 influences renal cancer cell proliferation. Cell Signal, 2013; 25, 2575-86. doi: 10.1016/j.cellsig.2013.08.005 [30] Ling Min, Li Yuan, Xu Yuan, et al. Regulation of miRNA-21 by reactive oxygen species-activated ERK/NF-κB in arsenite-induced cell transformation. Free Radical Bio med, 2012; 52, 1508-18. doi: 10.1016/j.freeradbiomed.2012.02.020 [31] Wei C, Li L, Kim IK, et al. NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radical Res, 2014; 48, 282-91. doi: 10.3109/10715762.2013.865839 [32] Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.2[updated June 2017]. In: The Cochrane Library, version 5.2. Chichester, UK: John Wiley & Sons, Ltd, 2017. [33] Xu Mengchuan, Rui Dongsheng, Yan Yizhong, et al. Oxidative Damage Induced by Arsenic in Mice or Rats:A Systematic Review and Meta-Analysis. Biol Trace Elem Res, 2017; 176, 154-75. doi: 10.1007/s12011-016-0810-4 [34] He Qian, Cai Lei, Shuai Ling, et al. Ars2 Is Overexpressed in Human Cholangiocarcinomas and Its Depletion Increases PTEN and PDCD4 by Decreasing MicroRNA-21. Molecular Carcinogenesis, 2013; 52, 286-96. doi: 10.1002/mc.v52.4 [35] Lu Lu, Xu Hui, Yang Ping, et al. Involvement of HIF-1α-regulated miR-21, acting via the Akt/NF-κB pathway, in malignant transformation of HBE cells induced by cigarette smoke extract. Toxicol Lett, 2018; 289. http://www.ncbi.nlm.nih.gov/pubmed/29501572 [36] Liu Haiwei, Cheng Le, Cao Dengke, et al. Suppression of miR-21 Expression Inhibits Cell Proliferation and Migration of Liver Cancer Cells by Targeting Phosphatase and Tension Homolog (PTEN). Med Sci Monitor, 2018; 24, 3571-7. doi: 10.12659/MSM.907038 [37] Alessio Crippa, Nicola Orsini. Dose-response meta-analysis of differences in means. BMC Med Res Methodol, 2016; 16, 91. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4971698/ -




 下载:
下载:




 Quick Links
Quick Links