-
Shortwave belongs to the radio frequency band of the electromagnetic spectrum and has a frequency range of 3-30 MHz. Currently, shortwave is widely used in wireless communications, industrial manufacturing, radar observations and medical treatments. High-intensity exposure to shortwave radiation may induce adverse effects on human health[1, 2]; however, very few studies have been reported regarding this issue. The central nervous system (CNS) plays a major role in controlling all behaviours of the body, and many animal experiments have shown that the CNS was vulnerable to electromagnetic radiation, which could induce decreased cognitive function[3, 4], an impaired hippocampal structure[5-7] and abnormal EEG[8] in rats. Nevertheless, these results are mainly based on microwave band (300 MHz-300 GHz). As a part of the electromagnetic spectrum, whether the shortwave with a specific intensity could damage the CNS remains unknown. Furthermore, studies have indicated that electromagnetic radiation could affect the expression of CREB and NMDAR in rat hippocampal tissue or PC12 cells[5, 9, 10]. CREB is considered to be an essential transcription factor, modulating the gene expression necessary for the survival and proliferation of neurons and the formation and consolidation of memory[11, 12]. It was located downstream of Ca2+-dependent signal pathways activated by Ca2+ inflow caused by NMDAR activation[13, 14]. Moreover, NR1, NR2A and NR2B were important subunits for NMDAR function; the transcriptional regulation by CREB relied on its phosphorylation at Serine 133[15]. Accordingly, investigation of the expression of NMDAR subunits, CREB and p-CREB in hippocampal tissue could contribute to exploring the biological mechanisms of shortwave exposure.
Therefore, this study was designed to determine the potential effects of shortwave exposure on cognitive function, EEG activities, hippocampal morphology structure, and neuron Nissl bodies in rats. Furthermore, the NMDAR-related CREB pathway was analysed to explore the mechanisms.
-
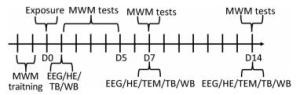
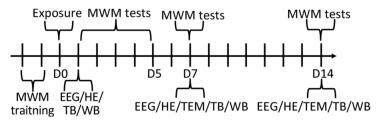
One hundred male Wistar rats (weight 200- 220 g, age 6-8 weeks) were provided by the Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China) and raised in a standard animal laboratory with free access to food and water. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were randomly divided into four groups: the control group (Con), 5 mW/cm2 exposure group, 10 mW/cm2 exposure group, and 30 mW/cm2 exposure group, with 25 rats per group. The experimental design was outlined in Figure 1.
-
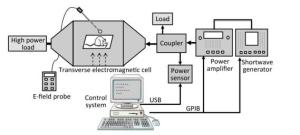
A schematic diagram of the exposure setup for shortwave exposure is shown in Figure 2. The shortwave generator (SMF100A, Rohde & Schwarz, Germany) is connected to a power amplifier (BBA100, Rohde & Schwarz, Germany), followed by a dual-directional coupler with a power sensor (NRP-Z91, Rohde & Schwarz, Germany). The forward coupling port transmits a continuous wave signal with a standard power density (e.g., 10 mW/cm2) at the frequency point of 27 MHz into a transverse electromagnetic cell (117486, ETS Lindgren, USA), which which is terminated with a high power load to make the reflection coefficient valid. The electric field strength in the electromagnetic cell is measured with an E-field probe (HI-6053, ETS Lindgren, USA) and transferred into the power density unit. The control system was designed by China Academy of Information and Communications Technology, Beijing, China.
During the exposure, the rats were restrained in a closed Plexiglas cage with holes of 1.5 cm in diameter to facilitate breathing and were placed in the middle of the electromagnetic cell. The whole bodies of the rats were exposed to shortwave with an average power density of 5, 10, or 30 mW/cm2, respectively, for 6 min. Correspondingly, the average specific absorption rates (SARs) of the rat brains were 1.70 × 10-4, 3.40 × 10-4, and 1.02 × 10-3 W/kg, respectively. The SAR distribution was calculated by the finite domain time difference (FDTD) method, which has been reported in a previous study[16]. The rats in the Con underwent a sham exposure.
-
The MWM is an important method to assess cognitive function (spatial learning and memory) in rats. In this test, the MWM setup (Beijing Sunny Instrument Co. Ltd., China) mainly consists of an open circular pool (150 cm in diameter and 50 cm in height), a circular platform (12 cm in diameter) and a computer-assisted tracking system. Following suggested protocols[17, 18], the pool was filled approximately half-way with water (20-22 ℃) and was divided equally into four quadrants; the platform was submerged 2 cm below the water surface and placed in the middle of a fixed quadrant; the animal (10 rats per group) was released into the water at water-level in the desired start position in one of the four quadrants, facing the tank wall. Prior to the shortwave exposure, the rats were placed in the pool, from one quadrant to another, to train them to find the platform in 60 s. The rats who failed to find the platform within this time limit were guided to it. The animal was then left on the platform for 15 s to allow it to orient to its position in space prior to the next training. The time to find the platform was recorded as the escape latency time for its spatial training score. On days 1-4, 7, and 14 after exposure, the average escape latency (AEL) of the four quadrants was analysed. To assess the retention of spatial memory, a probe test was conducted after the last training trail (5 days) in which the hidden platform was removed. Thereafter, the animal was placed in a novel start position farthest from the platform; the percent time in the target (platform) quadrant and the number of crossings over the original platform position in 60 s were subsequently calculated.
-
The EEG recordings were performed using a Biopac MP150 system (Biopac Systems Inc., USA). On 1, 7, and 14 days after shortwave exposure, the rats (5 rats per group) were weighed and anaesthetized with 1% sodium pentobarbital (5 mL/kg); EEG signals were subsequently conducted by a four-electrode configuration placed on the surface of the rats' scalps and were recorded for 5 min. The gravity frequency of the rats' EEG power spectra and the powers of the slow waves δ and θ were analysed.
-
Morphological changes of the hippocampus were observed by HE staining. On 1, 7, and 14 days after shortwave exposure, animals of the four groups (5 rats per group) were sacrificed immediately after the EEG recording, the brains were immediately removed. The left cerebral hemispheres were fixed in 10% neutral buffered formalin solution, the right hemispheres' hippocampi were separated and stored at -80 ℃ for the following tests. After dehydration, clearing, wax impregnation and embedding, the paraffin-embedded tissues were sectioned at a thickness of 4 μm. Following HE staining procedures, histological images of the brains were observed and captured using a light microscope (Leica, Germany).
Hippocampal specimens of 1 mm3 cubes of the four groups on 7 days after exposure were separated and fixed in 2.5% glutaraldehyde solution, followed by 1% osmium tetroxide solution. After being dehydrated with a graded alcohol solution and embedded in EPON618, the specimens were cut into ultrathin sections, stained in uranyl acetate and lead citrate. The ultrastructure features of the hippocampus were subsequently observed by TEM (Hitachi, Japan).
-
Neuron Nissl bodies in the rats' hippocampi of the four groups (5 rats per group) were assessed on 1, 7, and 14 days after exposure by toluidine blue staining. After being deparaffinized and hydrated with distilled water, the paraffin sections of the brain tissue were stained in toluidine blue working solution for 30 min and then washed in distilled water three times. Following rapid dehydration with 95% and 100% alcohols, the sections were cleared in xylene and mounted with coverslips. The features of the hippocampal dentate gyrus (DG) area were subsequently captured, the mean optical density (MOD) of the Nissl bodies was calculated using Image Pro-Plus software (Media Cybernetics, USA).
-
The expressions of NMDAR subunits (NR1, NR2A, and NR2B), CREB and p-CREB in the rats' hippocampi of the Con and 30 mW/cm2 groups (5 rats per group) on 1, 7, and 14 days after exposure were measured by Western blot. Total protein (for NMDAR detection) and nuclear protein (for CREB and p-CREB detection) were extracted from the hippocampal specimens according to the manufacturer's protocols (BestBio, China). After incubation with loading buffer in boiling water for 10 min, equivalent amounts of protein from each sample were loaded and run on polyacrylamide gels and subsequently transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, USA). Following blocking with 5% skimmed milk at room temperature for 1 h, the membranes were incubated with primary antibodies against NR1/2A/2B (all 1:1, 000, Abcam, USA), CREB and p-CREB (1:800, Abcam, USA), GAPDH (1:5, 000, Bioworld, USA), and Lamin B1 (1:1, 000, Bioworld, USA) at 4 ℃ overnight (GAPDH and Lamin B1 acted as internal references of total protein and nuclear protein respectively). The membranes were subsequently washed in tris-buffered saline with 0.1% Tween-20 (TBST) four times (5 min each time) and incubated with horseradish peroxidase conjugated secondary antibodies (1:10, 000, ZSGB-Bio, China) for 45 min at room temperature. After washing with TBST and incubation with enhanced chemiluminescence (ECL), the protein bands were visualized and recorded; the optical density values were then analysed with ImageJ software (NIH, USA).
-
Data were presented as the mean ± standard deviation (SD), statistical analyses were performed using SPSS 19.0 software (IBM, USA). The AEL data were analysed by repeated measurements of two way ANOVA with Dunnett Post Hoc; the percent time and crossing number data in the MWM test, the EEG data and the MOD of the Nissl bodies were analysed by one way ANOVA with Dunnett Post Hoc; the data related to protein expressions were analysed by independent-samples t test. P < 0.05 was considered statistically significant.
-
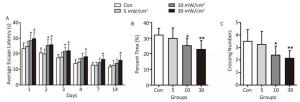
Compared with the Con, the rats in the 5 mW/cm2 group performed normal during the MWM test (Figure 3). However, the AEL in the 10 mW/cm2 group on 1-3 days after exposure was significantly prolonged, and a longer AEL was also identified in the 30 mW/cm2 group on 1-4, 7, and 14 days after exposure (P < 0.05; Figure 3A). Moreover, the percent time in the target quadrant and the crossing number of the rats in the 10 and 30 mW/cm2 groups were significantly decreased after shortwave exposure, and the high dose (30 mW/cm2) group performed worst (P < 0.05 or P < 0.01; Figure 3B and C).
-
The results showed that the gravity frequency of the EEG power spectra in the 10 and 30 mW/cm2 groups on 1 day after exposure was significantly reduced, and this reduction continued on 7 and 14 days in the 30 mW/cm2 group (P < 0.01; Figure 4A). The lower gravity frequency indicated the migration of EEG activities to slow waves. Furthermore, the powers of the slow waves δ and θ were analysed, it indicated that the δ wave power in the 10 mW/cm2 group on 1 day and the 30 mW/cm2 group on 1, 7, and 14 days significantly increased (P < 0.05; Figure 4B). Similarly, the θ wave power in the 10 and 30 mW/cm2 groups on 1 and 7 days were significantly increased (P < 0.05 or P < 0.01; Figure 4C). These results demonstrated the depression of the rats' EEG activities caused by shortwave exposure.
-
The HE staining slices (Figure 5) showed that the shrinkage in shape and dark staining in the cytoplasm of neurons in the hippocampal DG area (shown by the arrows) were visible in the exposure groups, particularly in the 10 mW/cm2 group on 7 days and the 30 mW/cm2 group on 7 and 14 days after exposure. Furthermore, the hippocampal ultrastructure observed by TEM indicated that shortwave exposure damaged the mitochondria and blood capillaries. Compared to the Con, the ultrastructure of the 5 mW/cm2 group slightly changed (Figure 6B and F), whereas clear swelling or cavitation in the mitochondria (shown by the arrows) (Figure 6C and D) and a widened gap around the blood capillaries (shown by the triangles) (Figure 6G and H) were present in the 10 and 30 mW/cm2 groups on 7 days after exposure; moreover, the 30 mW/cm2 group had the most serious lesions. These lesions in the exposure groups indicated that shortwave exposure damaged the morphological structure and ultrastructure of the rats' hippocampi.
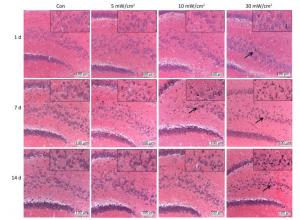
Figure 5. Changes of morphological structure in rat's hippocampal dentate gyrus area after shortwave exposure (HE staining, scale bar = 100 μm). The Con showed normal hippocampal structure, the exposure groups showed varying degrees of shrinkage and dark staining in hippocampal pyramidal cells (shown by the arrows). The upper right corners showed magnified views.
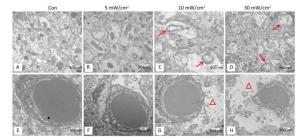
Figure 6. Ultrastructure changes of mitochondria and blood capillaries in the rats' hippocampi on 7 days after shortwave exposure (TEM, scale bar = 500 nm). (A) Normal mitochondria. (B-D) Increasing lesions of swollen mitochondria (shown by the arrows). (E) Normal blood capillary and surroundings. (F-H) Gradually aggravated lesions of widened gap around blood capillary (shown by the triangles).
-
Nissl bodies are the unique structures of nerve cells in the nerve tissue, and they reflect the development and function of neurons. In the Con group, the Nissl bodies of normal neurons in the hippocampal DG area were dyed navy blue and located around the nucleus, whereas there were varying degrees of fading in the exposure groups (Figure 7A). The quantitative analysis results showed that a significant decrease of Nissl body contents occurred in the 10 mW/cm2 group on 7 days and the 30 mW/cm2 group on 1, 7, and 14 days after exposure (P < 0.05 or P < 0.01; Figure 7B). Moreover, the last group had the most substantial reduction.
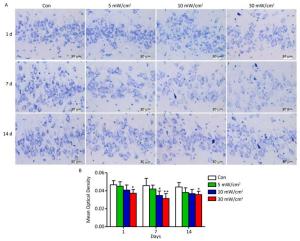
Figure 7. Nissl bodies changes in the rats' hippocampal dentate gyrus area after shortwave exposure. (A) Toluidine blue staining of hippocampal slices after exposure (scale bar = 30 μm). The Con showed normal performance, while the exposure groups were stained slightly. (B) Quantitative analysis of mean optical density on the content of Nissl bodies (n = 5). vs. Con: *P < 0.05 and **P < 0.01.
-
The protein expressions of the NMDAR subunits (NR1, NR2A, and NR2B) and the transcription factors CREB and p-CREB were analysed to explore the potential mechanisms of the behavioral impairments caused by shortwave exposure. There were no significant alterations in the NR1 content in the hippocampal tissue after 30 mW/cm2 shortwave exposure (Figure 8A). The content of NR2A in the 30 mW/cm2 group significantly increased on 1 and 7 days (P < 0.05 or P < 0.01; Figure 8B), more NR2B were expressed in the 30 mW/cm2 group on 1, 7, and 14 days after exposure (P < 0.05 or P < 0.01; Figure 8C). Furthermore, the hippocampal tissues in the 30 mW/cm2 group had lower level expressions of CREB on 1 day and p-CREB on 1 and 7 days after exposure (P < 0.05 or P < 0.01; Figure 8D and E), which indicated a significant suppression of the CREB pathway.
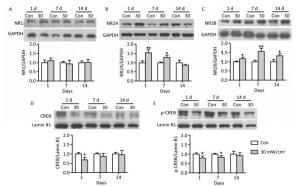
Figure 8. The protein expressions in rats' hippocampal tissue after shortwave exposure and quantitative analysis of ratio to respective internal references. (A) NR1. (B) NR2A. (C) NR2B. (D) CREB. (E) p-CREB. GAPDH and Lamin B1 acted as internal references of total protein and nuclear protein respectively. vs. Con: *P < 0.05 and **P < 0.01.
-
Spatial learning and memory represent important indicators to evaluate brain cognitive function in rats, and the MWM test has comprised an extensive approach in animal experiments[18, 19]. Previous studies have shown that electromagnetic radiation, mainly microwave radiation, could impair the spatial learning and memory abilities of rats[5, 8, 20]. Similar results were also identified in this study, in which the rats in the shortwave exposure groups had poor spatial learning and memory, characterized by a prolonged latency of AEL, as well as reduced platform-crossing numbers and percent time in the target quadrant during the MWM test. Moreover, worse performance occurred in the higher power intensity group. These findings indicated that shortwave exposure with a specific intensity could damage the cognitive function of rats.
EEG, which measures the spontaneous and rhythmic electrical activity of large-scale neuronal networks in the brain, has been considered a feasible approach for reflecting brain function states; most cognitive processes have been linked to the traditional frequency bands in the δ, θ, α, and β ranges[21-23]. Previous studies have indicated significant effects of electromagnetic radiation on EEG activities. One study showed that exposure to radio frequency fields, at 1-10 MHz (15 Hz modulation) at the level of 0.5-1 kV/m for 2 h per day for 6 weeks, enhanced the low frequency components and decreased the high frequency activities of EEG in rabbits[24]. Another study showed an increase in the total power of EEG after exposure to a 2.45 GHz continuous wave (30 mW/cm2) for 10 min in rats[25]. Moreover, an increased power of the δ wave caused by 2.856 GHz microwave exposure (30 mW/cm2) in rats was described in Li's report[8]. Correspondingly, this study showed the decline of the gravity frequency of the EEG power spectra, accompanied by the increase of δ and θ wave powers. Both the migration to low frequency and increased powers of slow waves demonstrated that shortwave exposure could depress EEG activities, considering that the low-frequency activities were involved in the inhibitory state[21]. Moreover, the depression of EEG activities might partially explain the reasons for cognitive impairment caused by shortwave exposure.
It is well-established that different types of memory disorders may be caused by the injury of different brain regions, of which hippocampal lesions are closely related to spatial memory impairment[26, 27]. However, the effects of exposure to a specific intensity of shortwave on the hippocampus in rats have not previously been reported. This study showed that shortwave exposure resulted in lesions of the morphological structure in the hippocampal DG areas, as well as the ultrastructure in mitochondria and blood capillaries. Moreover, shortwave exposure induced the decrease of neuron Nissl bodies, also referred to as chromatolysis. Nissl bodies mainly consist of rough endoplasmic reticulum. As the unique structure of nerve cells, Nissl bodies play major role in synthesizing proteins; the content and morphology of Nissl bodies could reflect the functional activities of neurons. The decreased content also provided evidence of the neural impairment caused by shortwave exposure.
Based on the combination of the changes in hippocampal structure and cognitive function, it could be concluded that shortwave exposure with a specific intensity could induce structural and functional impairments in the rat hippocampus. Furthermore, functional impairment occurred earlier than structural lesions, both injuries tended to ease gradually.
To further investigate the mechanisms of structural and functional impairments in the rat hippocampus, memory related proteins were analysed.
CREB, a nuclear-localized transcription factor, was located downstream of Ca2+ signal transduction pathways and acted as a conduit between upstream signals and downstream target-gene transcription[28]. Transcriptional activation was triggered by the phosphorylation of CREB at S133[15]; brain-derived neurotrophic factor (BDNF), c-fos, and activity-regulated cytoskeleton-associated protein (Arc) have been established to be CREB target genes[11, 29]. Importantly, the gene expressions regulated by CREB are considered to be essential for long-term memory formation and enhancement[11, 30]. The phosphorylation of CREB was controlled by upstream Ca2+-dependent signals, including MAP kinase (MAPK) and Ca2+/calmodulin-dependent kinase (CaMK)[13, 31, 32]. These signals were activated by Ca2+ inflow, which could be triggered by activation of the NMDA glutamate receptor[33]. Among the NMDAR subunits, NR1, NR2A, and NR2B played critical roles in the receptor's function. Consequently, it is commonly thought that the increased expression of NMDAR subunits contributed to CREB activation, as well as the transcription of memory related genes[11, 34]. In this study, surprisingly, we identified an increased expression of NMDAR, which was followed by the suppression of the CREB pathway, which might be explained by the opposing roles of synaptic (syn-) and extrasynaptic (ex-) NMDAR[35, 36]. Studies have indicated that the activation of syn-NMDAR stimulated pro-survival signals, such as CREB activation, in neurons, whereas elevated ex-NMDAR expression contributed to the shutoff of CREB, thus resulting in neuronal degeneration and dysfunction[36-38]. As a result, it was assumed that shortwave exposure mainly induced the increase of ex-NMDAR expression, suppressed the CREB pathway, and subsequently aggravated the structural and functional impairments of hippocampal neurons, which will be investigated by selective separation or intervention to syn- or ex-NMDAR in further studies.
In summary, this study demonstrated that shortwave exposure impaired spatial learning and memory, and this behavioral abnormality was accompanied by a series of dose-dependent pathophysiologic changes, including depressed EEG activities, an injured hippocampal structure, and decreased neuron Nissl bodies. Further data suggested that NMDAR-related CREB pathway suppression might be involved in shortwave-induced structural and functional impairments in rat hippocampus. In view of these detrimental effects in rats, people who work in high-intensity shortwave exposure environment may also suffer from radiation damage on recognition memory, and attentions need be pained on shortwave-related occupations. Therefore, future studies are planned to determine the possible brain injury on the workers exposed to shortwave and provide effective medical prevention.
-
No conflict of interest to declare.
-
YU Chao and BAI Yan Xin conducted experiments and YU Chao wrote the paper; XU Xin Ping and HAO Yan Hui collected the EEG data; GAO Ya Bing, WANG Hui, and TAN Sheng Zhi performed the tissue sections and observation; LI Wen Chao, ZHANG Jing, YAO Bin Wei, and DONG Ji conducted the rats exposure; PENG Rui Yun and ZHAO Li designed this study.
doi: 10.3967/bes2019.026
Behavioral Abnormality along with NMDAR-related CREB Suppression in Rat Hippocampus after Shortwave Exposure
-
Abstract:
Objective To estimate the detrimental effects of shortwave exposure on rat hippocampal structure and function and explore the underlying mechanisms. Methods One hundred Wistar rats were randomly divided into four groups (25 rats per group) and exposed to 27 MHz continuous shortwave at a power density of 5, 10, or 30 mW/cm2 for 6 min once only or underwent sham exposure for the control. The spatial learning and memory, electroencephalogram (EEG), hippocampal structure and Nissl bodies were analysed. Furthermore, the expressions of N-methyl-D-aspartate receptor (NMDAR) subunits (NR1, NR2A, and NR2B), cAMP responsive element-binding protein (CREB) and phosphorylated CREB (p-CREB) in hippocampal tissue were analysed on 1, 7, and 14 days after exposure. Results The rats in the 10 and 30 mW/cm2 groups had poor learning and memory, disrupted EEG oscillations, and injured hippocampal structures, including hippocampal neurons degeneration, mitochondria cavitation and blood capillaries swelling. The Nissl body content was also reduced in the exposure groups. Moreover, the hippocampal tissue in the 30 mW/cm2 group had increased expressions of NR2A and NR2B and decreased levels of CREB and p-CREB. Conclusion Shortwave exposure (27 MHz, with an average power density of 10 and 30 mW/cm2) impaired rats' spatial learning and memory and caused a series of dose-dependent pathophysiological changes. Moreover, NMDAR-related CREB pathway suppression might be involved in shortwave-induced structural and functional impairments in the rat hippocampus. -
Key words:
- Shortwave exposure /
- Learning and memory /
- Hippocampus /
- NMDAR /
- CREB
-
Figure 5. Changes of morphological structure in rat's hippocampal dentate gyrus area after shortwave exposure (HE staining, scale bar = 100 μm). The Con showed normal hippocampal structure, the exposure groups showed varying degrees of shrinkage and dark staining in hippocampal pyramidal cells (shown by the arrows). The upper right corners showed magnified views.
Figure 6. Ultrastructure changes of mitochondria and blood capillaries in the rats' hippocampi on 7 days after shortwave exposure (TEM, scale bar = 500 nm). (A) Normal mitochondria. (B-D) Increasing lesions of swollen mitochondria (shown by the arrows). (E) Normal blood capillary and surroundings. (F-H) Gradually aggravated lesions of widened gap around blood capillary (shown by the triangles).
Figure 7. Nissl bodies changes in the rats' hippocampal dentate gyrus area after shortwave exposure. (A) Toluidine blue staining of hippocampal slices after exposure (scale bar = 30 μm). The Con showed normal performance, while the exposure groups were stained slightly. (B) Quantitative analysis of mean optical density on the content of Nissl bodies (n = 5). vs. Con: *P < 0.05 and **P < 0.01.
Figure 8. The protein expressions in rats' hippocampal tissue after shortwave exposure and quantitative analysis of ratio to respective internal references. (A) NR1. (B) NR2A. (C) NR2B. (D) CREB. (E) p-CREB. GAPDH and Lamin B1 acted as internal references of total protein and nuclear protein respectively. vs. Con: *P < 0.05 and **P < 0.01.
-
[1] Israel M, Vangelova K, Ivanova M. Cardiovascular risk under electromagnetic exposure in physiotherapy. Environmentalist, 2007; 27, 539-43. doi: 10.1007/s10669-007-9065-0 [2] Altpeter ES, Roosli M, Battaglia M, et al. Effect of short-wave (6-22 MHz) magnetic fields on sleep quality and melatonin cycle in humans:the Schwarzenburg shut-down study. Bioelectromagnetics, 2006; 27, 142-50. doi: 10.1002/(ISSN)1521-186X [3] Narayanan SN, Kumar RS, Karun KM, et al. Possible cause for altered spatial cognition of prepubescent rats exposed to chronic radiofrequency electromagnetic radiation. Metab Brain Dis, 2015; 30, 1193-206. doi: 10.1007/s11011-015-9689-6 [4] Deshmukh PS, Megha K, Nasare N, et al. Effect of low level subchronic microwave radiation on rat brain. Biomed Environ Sci, 2016; 29, 858-67. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=bes201612002 [5] Wang H, Peng RY, Zhao L, et al. The relationship between NMDA receptors and microwave-induced learning and memory impairment:A long-term observation on Wistar rats. Int J Radiat Biol, 2015; 91, 262-9. doi: 10.3109/09553002.2014.988893 [6] Hussein S, El-Saba AA, Galal MK. Biochemical and histological studies on adverse effects of mobile phone radiation on rat's brain. J Chem Neuroanat, 2016; 78, 10-9. doi: 10.1016/j.jchemneu.2016.07.009 [7] Chauhan P, Verma H, Sisodia R, et al. Microwave radiation (2.45 GHz)-induced oxidative stress:Whole-body exposure effect on histopathology of Wistar rats. Electromagn Biol Med, 2017; 36, 20-30. http://www.ncbi.nlm.nih.gov/pubmed/27362544 [8] Li HJ, Peng RY, Wang CZ, et al. Alterations of cognitive function and 5-HT system in rats after long term microwave exposure. Physiol Behav, 2015; 140, 236-46. doi: 10.1016/j.physbeh.2014.12.039 [9] Gökçek-Saraç Ç, Er H, Kencebay MC, et al. Effects of acute and chronic exposure to both 900 MHz and 2100 MHz electromagnetic radiation on glutamate receptor signaling pathway. Int J Radiat Biol, 2017; 93, 980-9. doi: 10.1080/09553002.2017.1337279 [10] Zuo HY, Lin T, Wang DW, et al. RKIP regulates neural cell apoptosis induced by exposure to microwave radiation partly through the MEK/ERK/CREB pathway. Mol Neurobiol, 2015; 51, 1520-9. doi: 10.1007/s12035-014-8831-5 [11] Kida S, Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull, 2014; 105, 17-24. doi: 10.1016/j.brainresbull.2014.04.011 [12] Silva AJ, Kogan JH, Frankland PW, et al. CREB and memory. Annu Rev Neurosci, 1998; 21, 127-48. doi: 10.1146/annurev.neuro.21.1.127 [13] Ma H, Groth RD, Cohen SM, et al. γCaMKⅡ shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell, 2014; 159, 281-94. doi: 10.1016/j.cell.2014.09.019 [14] Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci, 2001; 4, 261-7. doi: 10.1038/85109 [15] Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation:a Ca2+-and stimulus duration-dependent switch for hippocampal gene expression. Cell, 1996; 87, 1203-14. doi: 10.1016/S0092-8674(00)81816-4 [16] Li C, Yang L, Li CH, Xie Y, et al. Dosimetric variability of the rats' exposure to electromagnetic pulses. Electromagn Biol Med, 2015; 34, 334-43. doi: 10.3109/15368378.2014.925472 [17] Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth, 1984; 11, 47-60. doi: 10.1016/0165-0270(84)90007-4 [18] Vorhees CV, Williams MT. Morris water maze:procedures for assessing spatial and related forms of learning and memory. Nat Protoc, 2006; 1, 848-58. doi: 10.1038/nprot.2006.116 [19] D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev, 2001; 36, 60-90. doi: 10.1016/S0165-0173(01)00067-4 [20] Sharma A, Sisodia R, Bhatnagar D, et al. Spatial memory and learning performance and its relationship to protein synthesis of Swiss albino mice exposed to 10 GHz microwaves. Int J Radiat Biol, 2014; 90, 29-35. doi: 10.3109/09553002.2013.835883 [21] Herrmann CS, Struber D, Helfrich RF, et al. EEG oscillations:From correlation to causality. Int J Psychophysiol, 2016; 103, 12-21. doi: 10.1016/j.ijpsycho.2015.02.003 [22] Vecchio F, Miraglia F, Quaranta D, et al. Cortical connectivity and memory performance in cognitive decline:A study via graph theory from EEG data. Neuroscience, 2016; 316, 143-50. doi: 10.1016/j.neuroscience.2015.12.036 [23] Purdon PL, Pierce ET, Mukamel EA, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci USA, 2013; 110, E1142-E51. doi: 10.1073/pnas.1221180110 [24] Takashima S, Onaral B, Schwan HP. Effects of modulated RF energy on the EEG of mammalian brains. Effects of acute and chronic irradiations. Radiat Environ Biophys, 1979; 16, 15-27. doi: 10.1007/BF01326893 [25] Thuröczy G, Kubinyi G, Bodo M, et al. Simultaneous response of brain electrical activity (EEG) and cerebral circulation (REG) to microwave exposure in rats. Rev Environ Health, 1994; 10, 135-48. http://europepmc.org/abstract/med/8047672 [26] Wang JX, Rogers LM, Gross EZ, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science, 2014; 345, 1054-57. doi: 10.1126/science.1252900 [27] Bannerman DM, Sprengel R, Sanderson DJ, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci, 2014; 15, 181-92. doi: 10.1038/nrn3677 [28] Mantamadiotis T, Papalexis N, Dworkin S. CREB signalling in neural stem/progenitor cells:recent developments and the implications for brain tumour biology. BioEssays, 2012; 34, 293-300. doi: 10.1002/bies.v34.4 [29] Dalla Massara L, Osuru HP, Oklopcic A, et al. General anesthesia causes epigenetic histone modulation of c-Fos and brain-derived neurotrophic factor, target genes important for neuronal development in the immature rat hippocampus. Anesthesiologists, 2016; 124, 1311-27. doi: 10.1097/ALN.0000000000001111 [30] Yu XW, Curlik DM, Oh MM, et al. CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. Elife, 2017; 6, e19358. doi: 10.7554/eLife.19358 [31] Perkinton MS, Ip J, Wood GL, et al. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J Neurochem, 2002; 80, 239-54. doi: 10.1046/j.0022-3042.2001.00699.x [32] Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci, 2013; 14, 593-608. doi: 10.1038/nrn3531 [33] Lau CG, Takeuchi K, Rodenasruano A, et al. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans, 2009; 37, 1369-74. doi: 10.1042/BST0371369 [34] Cammarota M, Bevilaqua LR, Ardenghi P, et al. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning:abolition by NMDA receptor blockade. Mol Brain Res, 2000; 76, 36-46. doi: 10.1016/S0169-328X(99)00329-0 [35] Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron, 2014; 82, 279-93. doi: 10.1016/j.neuron.2014.03.030 [36] Kaufman AM, Milnerwood AJ, Sepers MD, et al. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci, 2012; 32, 3992-4003. doi: 10.1523/JNEUROSCI.4129-11.2012 [37] Dau A, Gladding CM, Sepers MD, et al. Chronic blockade of extrasynaptic NMDA receptors ameliorates synaptic dysfunction and pro-death signaling in Huntington disease transgenic mice. Neurobiol Dis, 2014; 62, 533-42. doi: 10.1016/j.nbd.2013.11.013 [38] Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci, 2002; 5, 405-14. doi: 10.1038/nn835 -




 下载:
下载:




 Quick Links
Quick Links