-
Human beings are usually exposed to microwaves originated from the equipment in industry, communication, military, and aerospace, which has led to interest in health effects of long-term low level microwave radiation exposure, particularly on the brain, one of the most sensitive organs to microwaves [1]. Morris water maze, oxidative stress related typical markers of corticosteroids (CORT), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD) and total antioxidant capacity (TAOC), ultrastructure, dendritic spine growth and molecular signaling pathway are important indicators in the study of learning and memory effects[2]. Among them, Synbindin (Sbdn), closely associated to dendritic spine growth, learning and memory, was found to be a remarkable microwave sensitive molecule in our previous work [3]. Additionally, it’s well known that both the CA1 and Dentate Gyrus (DG) regions of the hippocampus are involved in learning and memory, however studies on the effect of microwave on DG region were less, so, this paper further focused on DG region in the study of dendrite spine, ultrastructure of neurons and phospho-extracellular signal-regulated kinase (pERK) protein expression. Therefore, Synbindin was focused on and the above indicators were integrated in this paper to study the influence and mechanism of long-term low-intensity 2,100 MHz microwave on learning and memory.
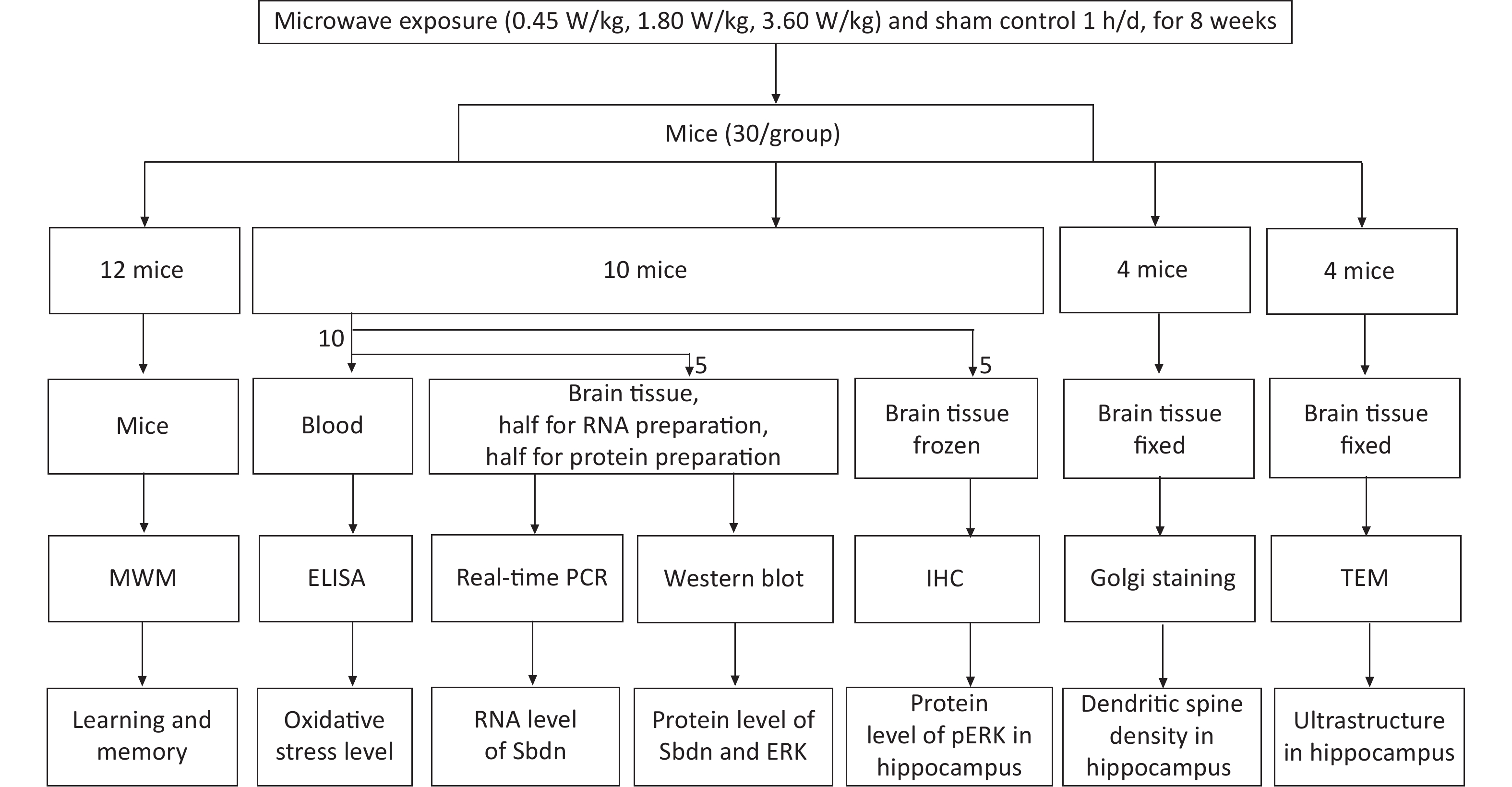
Male Kunming mice (6–8 w) obtained from the Laboratory Animal Center (Beijing, China) were used in the study and the animal experiment was approved by the ethnic committee. Mice were divided randomly into three exposure groups (with whole body average SAR of 0.45 W/kg, 1.80 W/kg and 3.60 W/kg, according to power densities of 1, 4, and 8 mW/cm2) and one sham control group. Exposure group mice were irradiated to 2,100 MHz microwaves for 1 h daily for 8 weeks while sham control group animals were processed in parallel to the exposed groups, without microwave exposure [3]. There were 30 mice per group for following test experiment. Among them 12 mice per group were conducted to perform Morris Water Maze experiment to test learning and memory ability one day after the end of irradiation experiment, and 18 mice per group were used for blood and brain tissues collection immediately after the end of irradiation experiment for evaluation of ultrastructure, dendritic spine, gene expression and oxidative stress respectively. A flow chart of experimental phase is shown in Figure 1. The Morris water maze test was performed to test learning and memory. The test involved navigation and space exploration tasks. For the navigation task, each mouse was trained three times a day from 3 different starting site to find the safety platform for 5 consecutive days. For the space exploration task, the platform was removed on day 6, mice were put into 3 nontarget quadrants. The mice behavior such as escape latency and swimming path length were monitored by a CRE camera suspended over the center of the pool. Tests of Ultrastructure in the DG region from 4 mice per group were performed by a Hitachi-H7650 transmission electron microscope (TEM, Hitachi, Japan). Dendritic spine densities in the DG region from 4 mice per group were determined by the Golgi silver-plating method. Images of Golgi-stained neurons in the DG area were captured using a light microscope (Nikon H550S, Japan) and the Nikon H550S image analysis system. Uniformly distributed dendrites with intact soma were used to count dendritic spines. The number of dendritic spines on each 30 μm long dendrite was counted and averaged, which was expressed as the number of dendritic spines per 10 μm. Measurements of pERK protein expression in DG region from 5 mice per group were performed by the Rabbit Hypersensitivity Two-Step Immunohistochemical Detection Kit and Concentrated DAB Kit were used to detect using an anti-pERK antibody P-ECK (K23) (dilution 1:500). The mRNA level of Sbdn was measured by quantitively Real time PCR. Total RNA was isolated using Trizol. Primers designed for Sbdn and GAPDH, which served as an internal reference gene, were provided by the Beijing Hooseen Biotechnology Co., Ltd. (Beijing, China). The forward primer for Sbdn was ACT TTC AGT TAC CCG CTG GA and the reverse primer was GCT CGC ATC TGA TAG GCA TT. The forward primer of GAPDH was GGT TGT CTC CTG CGA CTT CA and the reverse primer was TGG TCC AGG GTT TCT TAC TCC. Real-time PCR was performed using TaqMan Mixture and 2×Taq PCR MasterMix (Beijing Hooseen Biotechnology Co., Ltd.) in a qPCR instrument (qTOWER 2.2, Germany) according to the manufacturer’s protocols. Amplification was performed for 40 cycles at 94 °C for 20 s, 60 °C for 20 s, and 72 °C for 20 s. Five animals per group were statistically analyzed. The protein level of Sbdn was measured by Western blotting. Total protein was isolated using radioimmunoprecipitation assay (RIPA) lysis solution (P0013B, Beyotime, Shanghai, China) containing a protease inhibitor cocktail (04693159001, Roche, Nutley, NJ, USA). The primary antibody of Synbindin (dilution 1:600), extracellular signal-regulated kinase (ERK) 1/2(dilution 1:1,000), and pERK1/2(dilution 1:1,000) were used to target tested proteins, and Tubulin (dilution 1:1,000) was used as an internal protein control according to the manufacture instructions. Mouse anti-rabbit (dilution 1:2,000) was used as secondary antibody. Quantification of the band density was performed by densitometric analysis using Image Lab Software. 5 animals per group were statistically analyzed. The oxidative stress marker levels of CORT, SOD, MDA, TAOC, and CAT in serum were measured using commercially available ELISA assay kits (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. SPSS 22.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Indexes in acquisition trials, such as escape latency, total path length, and speed in MWM test, dendritic spine density, the biochemical concentration changes, and the expressions of mRNA and proteins were analyzed by one-way ANOVA, followed by LSD’s post hoc test. Spearman double-tail test was used to analyze the correlation significance. A two-sided alpha level of 0.05 was used to assess statistical significance. Data are expressed as means ± SEM.
As suggested by our previous work [3], this study confirmed that microwaves reduced learning and memory from the Morris water Maze experiment. In the navigation task, compared with the sham control group, the total path lengths of the exposure groups increased significantly on days 1, 3, and 5 of training (Figure 2). On day 5 of training, the total path length increased with increasing irradiation intensity, and the total path length in 3.60 W/kg group was significantly higher than that in the sham control group. A representative path map on day 5 is shown in Figure 2. As irradiation intensity increased, the path of mice searching for the safety platform increased. There was a significant correlation between the total path length and microwave intensity on day 5 (P < 0.01, R = 1.0; linear slope 45.41). The above results have shown that microwave significantly inhibits learning and memory, although that of other indicators (results not presented) did not. Our results were strongly supported by Sharma A et al reporting that microwave radiation (2,100 MHz) originating from mobile phone for 4 hours/day (5 days/week) for 3 months produced significant learning ability inhibition [4]. Furthermore, what’s the mechanism? First of all, the dendritic spine density and ultrastructure in DG region involved in learning and memory was studied. Results showed that spine density of the sham control group was (7.08 ± 0.29)/10 μm, and that of the 0.45, 1.80, and 3.60 W/kg groups was (6.36 ± 0.35)/10 μm, (6.21 ± 0.31)/10 μm, (6.04 ± 0.25)/10 μm, respectively (Supplementary Table S1, available in www.besjournal.com), and the higher the SAR value, the more obvious the decrease of dendritic spines density. Supplementary Figure S1 (available in www.besjournal.com) was representative images of dendritic spines. Microwave induced the ultrastructure in DG region abnormal. In the sham control group, the synaptic structure was distinct and intact, and the neuron cell structure was ordinary (Supplementary Figure S2, available in www.besjournal.com). In the 0.45 W/kg group, the synaptic cleft was occasionally blurred, but the neuron structure was normal. In the 1.80 W/kg group, the synaptic cleft was occasionally blurred, the number of synaptic vesicles had increased, the perinuclear space of neurons had widened, the mitochondria were swollen, and the cristae were occasionally broken. In the 3.60 W/kg group, the synaptic space was blurred, some of the synaptic vesicles had increased, the mitochondria of neurons were swollen, there was occasional cavitation, and the endoplasmic reticulum had expanded. There appeared a trend that the ultrastructural anomalies in the DG region became more and more obvious with the increase of microwave intensity, which was verified by Wang H et al. [5] reporting that 2.856 GHz microwaves with the average power density of 5 and 10 mW/cm2 for 6 min/day, 5 days/week and up to 6 weeks produced neuronal degeneration, enlarged perivascular spaces in the hippocampus, swollen mitochondria, and decrease in the quantity of synaptic vesicles. Then the oxidative stress related indicators of CORT, MDA, SOD, TAOC, and CAT was studied. Results showed that as irradiation intensity increased, the level of serum CORT, MDA, and TAOC tended to increase. The concentrations of the above substances increased from (53.18 ± 5.77) µg/L, (1.52 ± 0.11) nmol/L, and (3.57 ± 0.25) U/mL in the sham control group to (82.05 ± 3.69) µg/L, (2.56 ± 0.34) nmol/L, and (4.88 ± 0.33) U/mL in the 3.60 W/kg group. For 1.80 W/kg and 3.60 W/kg group, their differences against sham control group were significant (Supplementary Figure S3, available in www.besjournal.com). However, the changes of SOD and CAT concentration were not obvious. The results were similar to the following studies. Shahin et al. [6] found that long-term (30 and 60 days) low-level 2.45 GHz continuous microwave exposure (overall average whole body SAR value 0.0146 W/Kg) reduced spatial memory and increased serum CORT level in adult male mice by local stress-induced suppression of hippocampal memory formation signaling. Sharma et al. [4] revealed that rats exposed to microwave radiation (900 MHz) for different durations over 90 days showed diminished brain working memory and significantly increased level of MDA with concomitantly depleted levels of SOD, CAT, and redox enzymes. Different from the common understanding that stress leads to the increase of oxidizing substances and the decrease of antioxidant capacity, this paper found that microwave caused the decrease of both oxidation and antioxidant capacity, indicating that microwave could activate these two processes simultaneously, and the enhancement of oxidative capacity exceeded the antioxidant capacity, which was consistent with the results of ultrastructural damage caused by microwave. Next, based on Sbdn clues discovered in previous work, the molecular mechanism of microwave inhibition of learning and memory was explored. It was found that after microwave radiation exposure, both the mRNA and protein level of Sbdn in the 0.45, 1.80, and 3.60 W/kg groups in mouse brain tissue decreased in a dose-dependent manner (Supplementary Table S2, available in www.besjournal.com). There was a significant correlation between microwave intensity and Sbdn mRNA level (P < 0.01, R = 1.0; linear slope –0.088) and Sbdn protein level (P < 0.01, R = 1.0; linear slope –0.267). The protein levels of ERK and pERK in the irradiation group were significantly higher than in the sham control group. Therefore, the ratio of pERK to ERK in the irradiation group was lower than in the sham control group (Figure 3). In addition, by light microscopy, blue-stained particles in the DG region of hippocampus, indicating pERK protein expression levels, decreased obviously after microwave exposure with the lowest in the 3.60 W/kg groups (Supplementary Figure S4, available in www.besjournal.com), which was consistent with our results of Western blotting and Friedman et al.[7] reporting that long-term radiation exposure (875 MHz, 0.11 mW/cm2, 30 min) decreased ERK activity and the pERK level. Cell loss glial cell proliferation was also found, indicating inflammation occurred. As was known, Sbdn was the physiological ligand of adhesion molecule syndecan-2, an important regulator of synaptic function [8] and was the interacting protein of ERK2 [9], related to transcription, synthesis and transport of synaptic proteins and learning and memory function [10]. Therefore, Sbdn and ERK inhibition could be inferred to be the crucial molecules basis mediating the decline of learning and memory induced by long-term low-intensity microwave in the study.
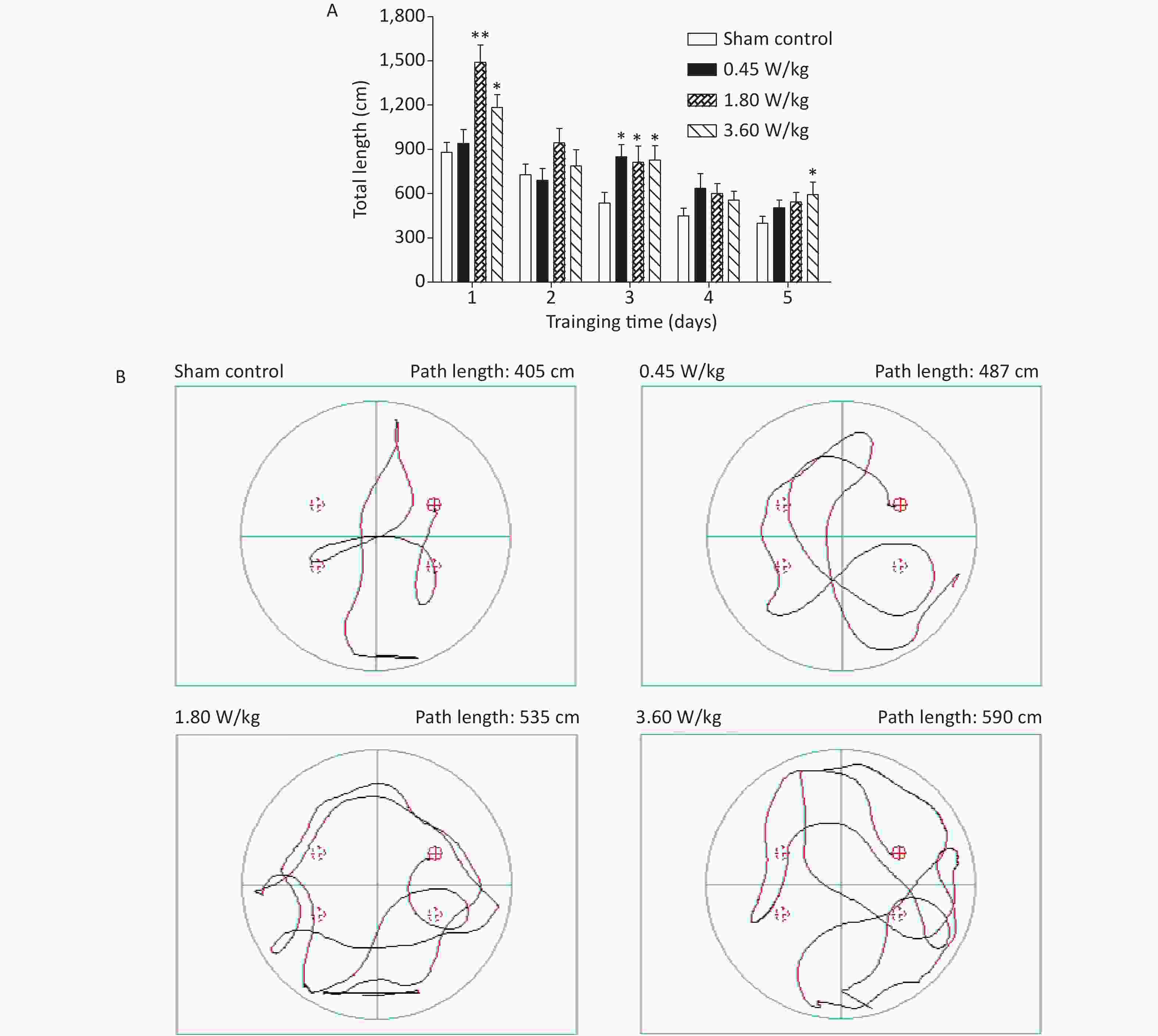
Figure 2. Statistic results and representative photographs of total swimming trails in a Morris water maze after 2,100 MHz microwave exposure. (A) Statistic results of total swimming path length. (B) Representative photographs of total swimming trails. *P < 0.05; **P < 0.01 vs. sham control. n = 12. All data shown are mean ± SEM.
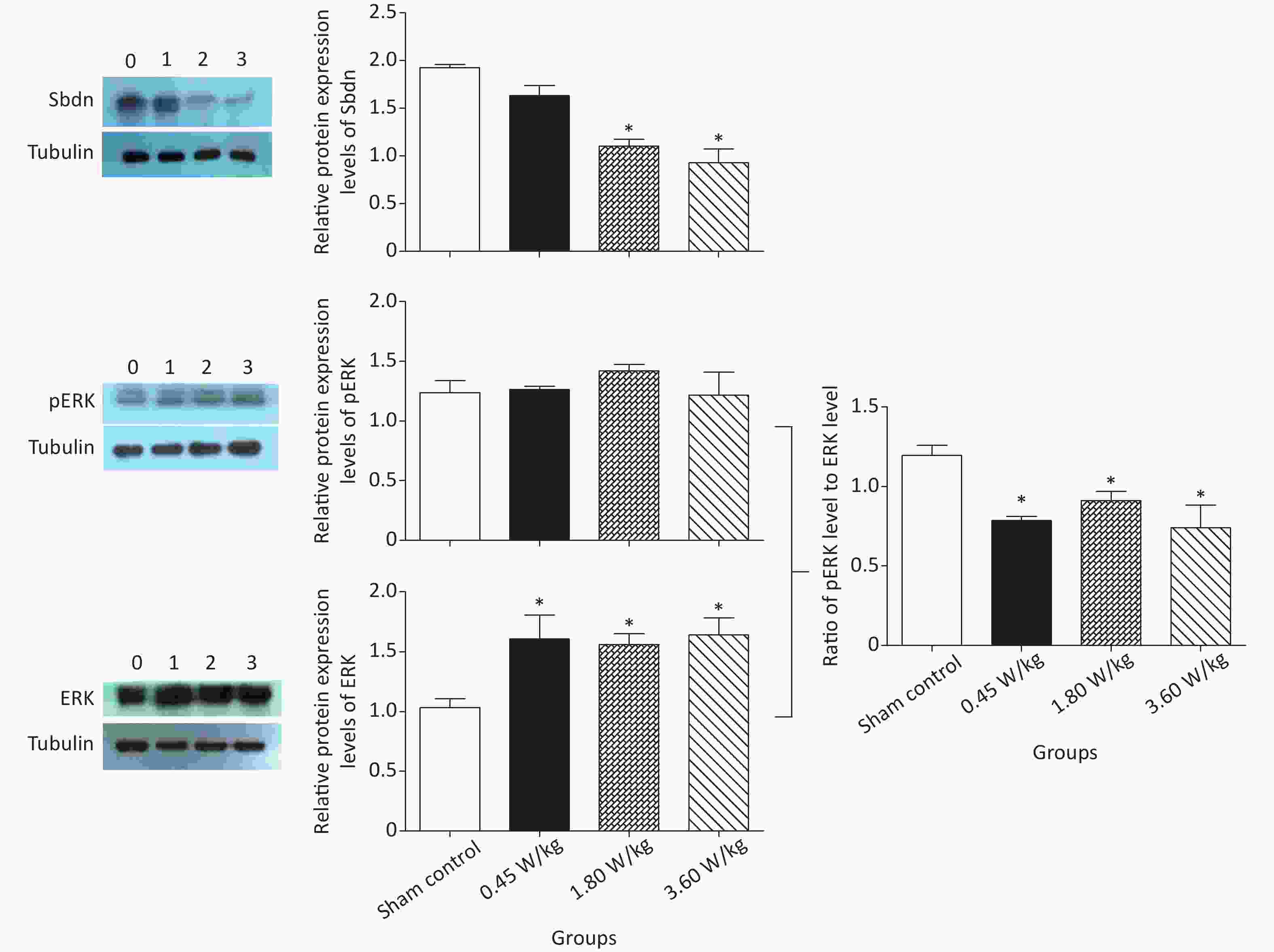
Figure 3. Sbdn and pERK/ERK protein levels in the mouse brain after 2,100 MHz microwave exposure. *P < 0.05 vs. sham control. n = 5. All data shown are mean ± SEM.
Table S1. Dendritic spine density in the hippocampal DG region after 2,100 MHz microwave exposure.
Groups Dendritic spine density (#/10 μm) Sham control 7.08 ± 0.29 0.45 W/kg 6.36 ± 0.35* 1.80 W/kg 6.21 ± 0.31* 3.60 W/kg 6.04 ± 0.25** Note. *P < 0.05, **P < 0.01 vs. sham control. n = 4. All data shown are mean ± SEM. 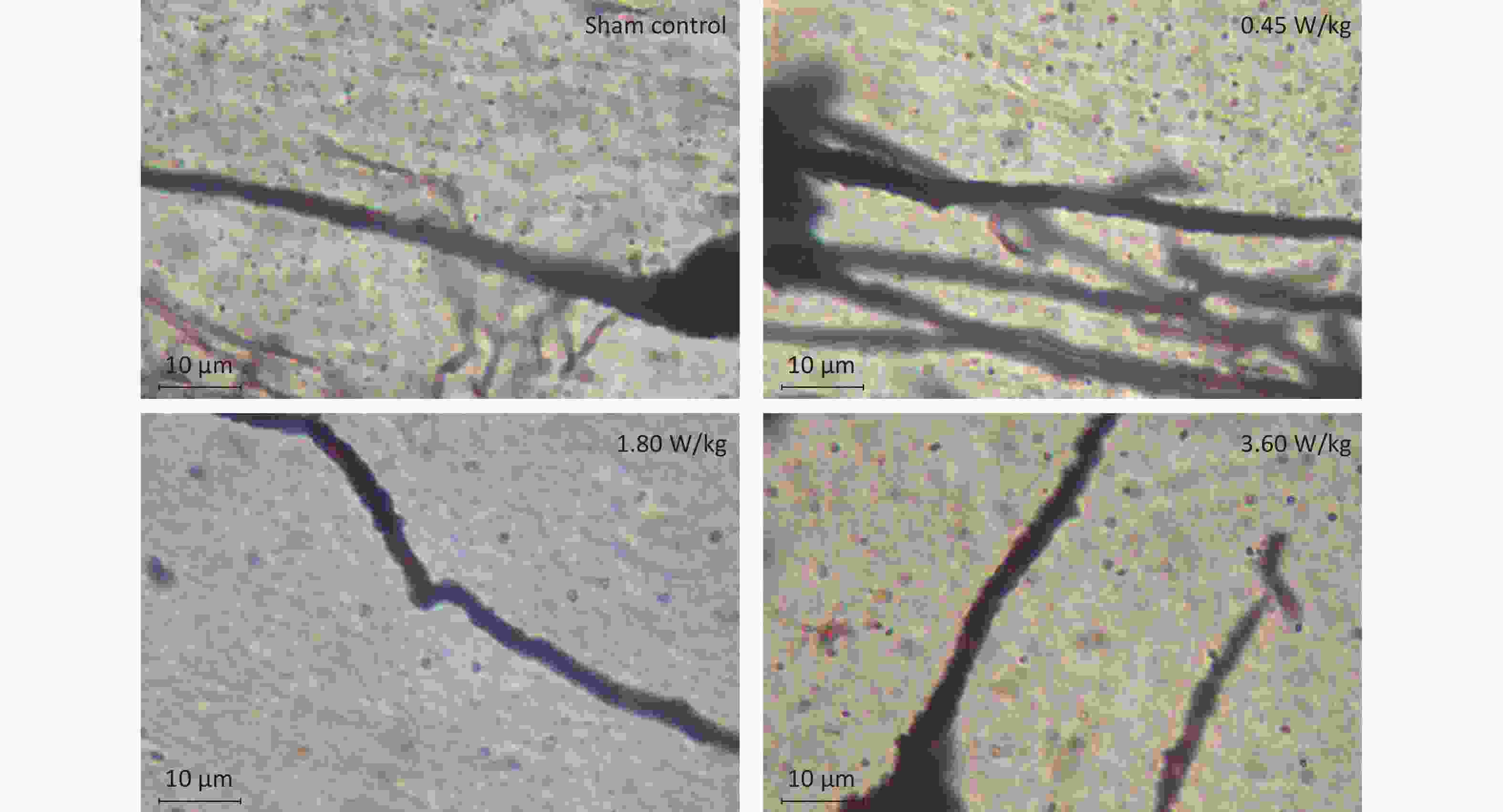
Figure S1. Representative photographs of dendritic spines in the hippocampal DG region after 2,100 MHz microwave exposure (oil microscope ×100).
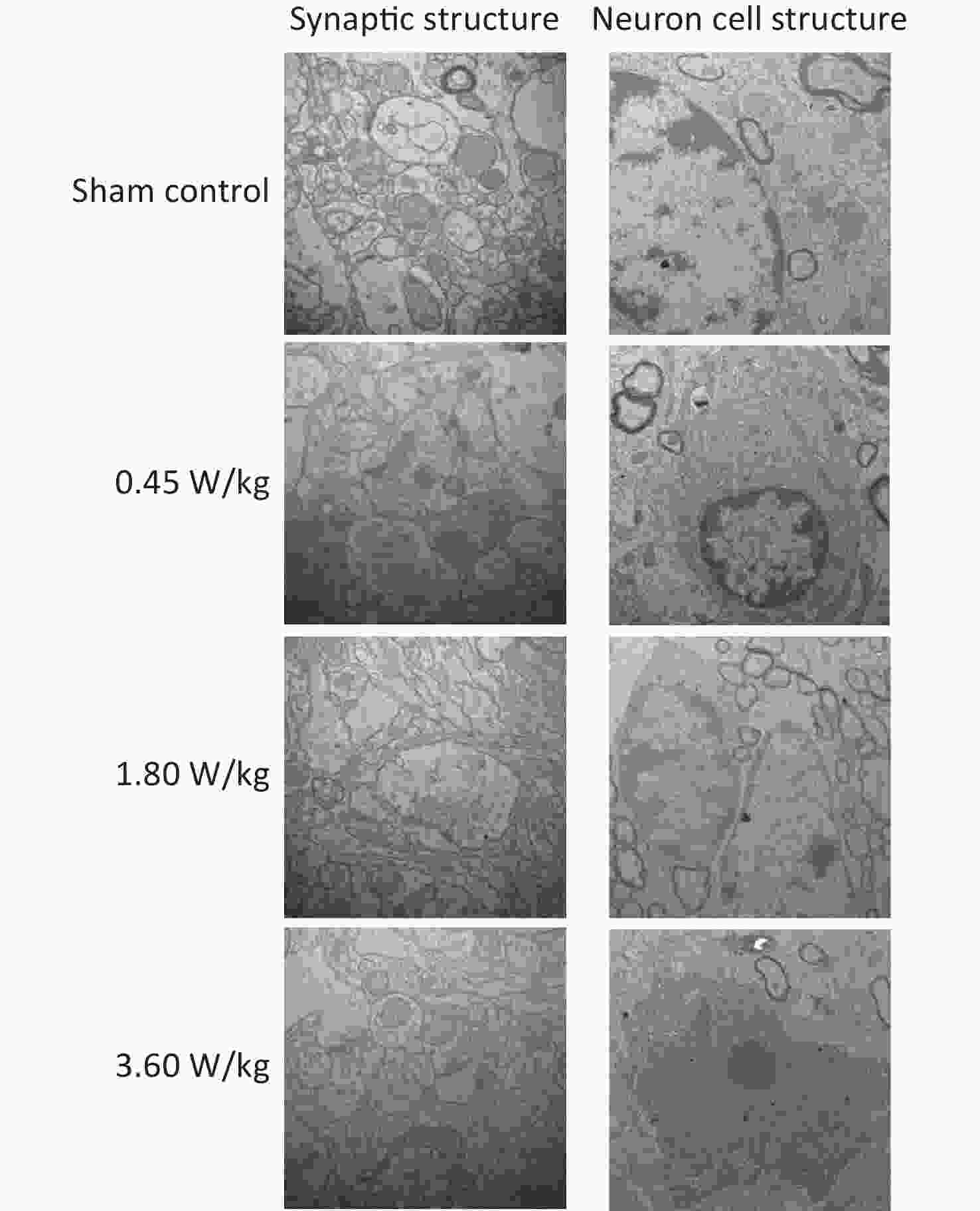
Figure S2. Representative photographs of mouse hippocampal neurons by electron microscopy after 2,100 MHz microwave exposure (Scale bar = 500 nm).
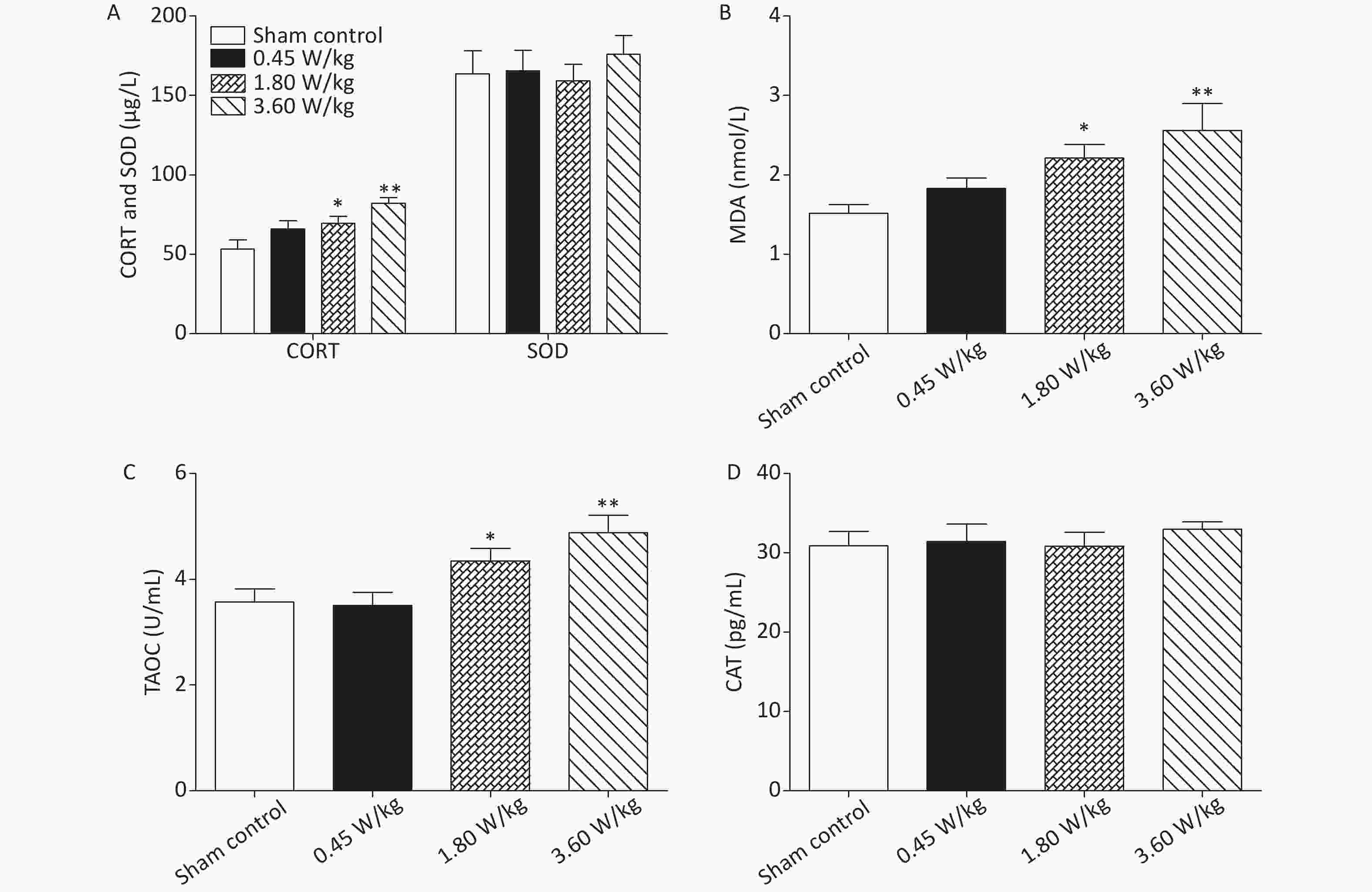
Figure S3. Oxidative stress response in the serum after 2,100 MHz microwave exposure. (A) Serum CORT and SOD levels. (B) Serum MDA level. (C) Serum TAOC level. (D) Serum CAT level. *P < 0.05; **P < 0.01 vs. sham control. n = 8. All data shown are mean ± SEM.
Table S2. mRNA level of Sbdn in the mouse brain after 2,100 MHz microwave exposure.
Groups Relative Sbdn expression level Sham control 1.00 ± 0.06 0.45 W/kg 0.70 ± 0.01** 1.80 W/kg 0.65 ± 0.06** 3.60 W/kg 0.59 ± 0.07** Note. **P < 0.01 vs. sham control. n = 5. All data shown are mean ± SEM. 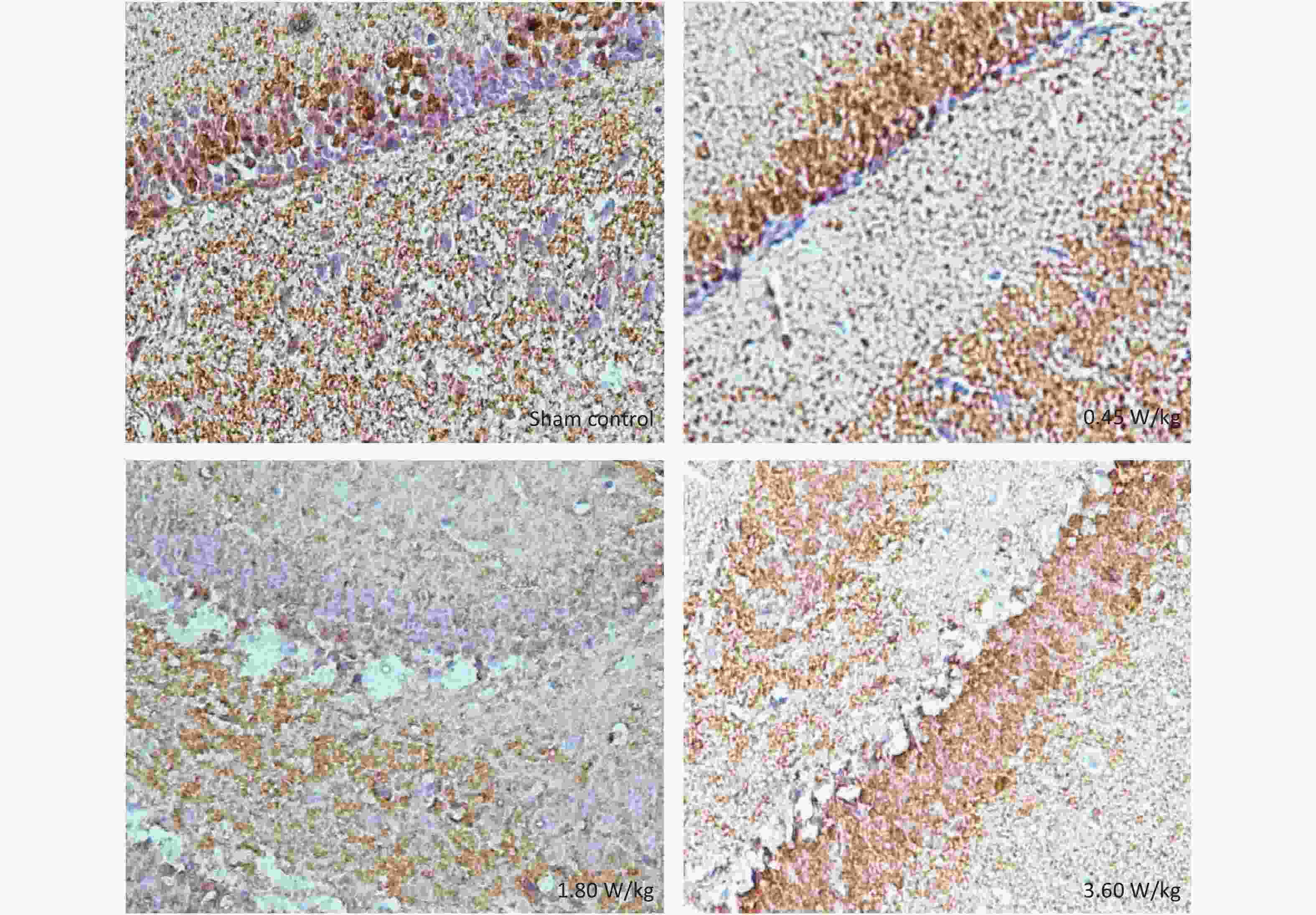
Figure S4. Representative immunohistochemical images of pERK in the DG region after 2,100 MHz microwave exposure.
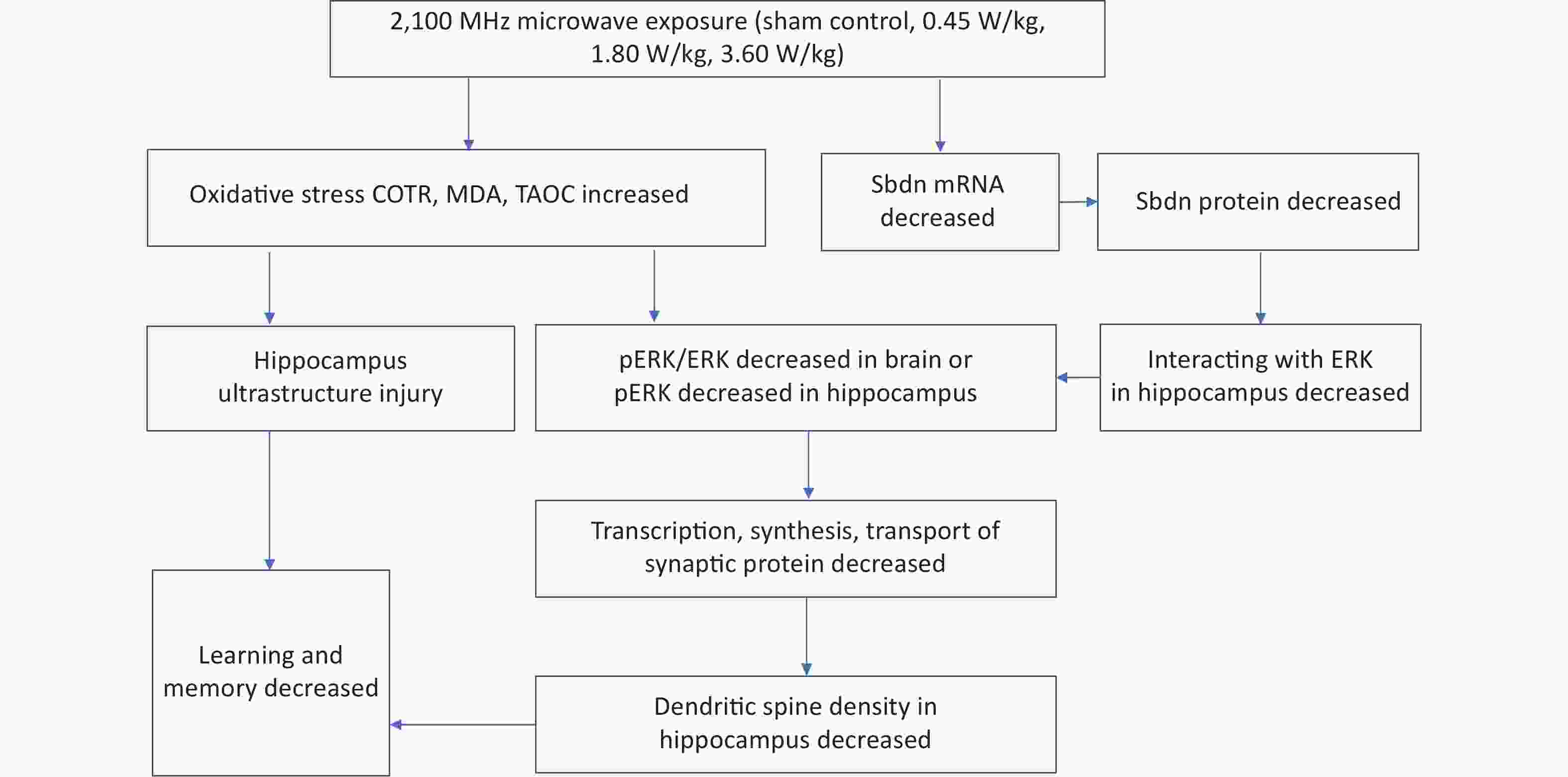
Summarily, the study found that long-term low-intensity 2,100 MHz microwave radiation reduced spatial learning and memory ability of mice by the following possible mechanisms: (1) neuron ultrastructure destruction caused by oxidative stress; (2) dendritic spine growth inhibition caused by decreased mRNA and protein expression level of Sbdn, pERK or pERK/ERK level, and transcription, synthesis and transport of synaptic proteins. This study for the first time revealed a good intensity-response relationship between Sbdn expression and microwave intensity, suggesting an important mediating role of Sbdn in microwave inhibiting learning and memory by the way of microwave decreasing the expression of Sbdn and its downstream signal molecule pERK/ERK and then reducing the density of dendritic spine. See the mechanism of long-term low-intensity microwave inhibiting learning and memory (Supplementary Figure S5, available in www.besjournal.com). This study is of great value for the establishment of limits, risk assessment and protection of microwave occupational exposure population, and also has important value for the health impact of 5G microwave exposure on the public.
Conflicts of Interest The authors declare no conflict of interest.
Acknowledgment We thank senior engineer WU Tong Ning from China academy of information communication for his contribution in dose calculation.
doi: 10.3967/bes2022.074
Long-Term, Low-Level Microwave Radiation Impairs Learning and Memory via Synbindin: Molecular Basis and Underlying Mechanism
-
-
Figure 2. Statistic results and representative photographs of total swimming trails in a Morris water maze after 2,100 MHz microwave exposure. (A) Statistic results of total swimming path length. (B) Representative photographs of total swimming trails. *P < 0.05; **P < 0.01 vs. sham control. n = 12. All data shown are mean ± SEM.
S1. Dendritic spine density in the hippocampal DG region after 2,100 MHz microwave exposure.
Groups Dendritic spine density (#/10 μm) Sham control 7.08 ± 0.29 0.45 W/kg 6.36 ± 0.35* 1.80 W/kg 6.21 ± 0.31* 3.60 W/kg 6.04 ± 0.25** Note. *P < 0.05, **P < 0.01 vs. sham control. n = 4. All data shown are mean ± SEM. S2. mRNA level of Sbdn in the mouse brain after 2,100 MHz microwave exposure.
Groups Relative Sbdn expression level Sham control 1.00 ± 0.06 0.45 W/kg 0.70 ± 0.01** 1.80 W/kg 0.65 ± 0.06** 3.60 W/kg 0.59 ± 0.07** Note. **P < 0.01 vs. sham control. n = 5. All data shown are mean ± SEM. -
[1] Belpomme D, Hardell L, Belyaev I, et al. Thermal and non-thermal health effects of low intensity non-ionizing radiation: an international perspective. Environ Pollut, 2018; 242, 643−58. doi: 10.1016/j.envpol.2018.07.019 [2] Lin YY, Gao P, Guo YC, et al. Effects of long-term exposure to L-Band high-power microwave on the brain function of male mice. Biomed Res Int, 2021; 2021, 2237370. [3] Zhao YL, Li YX, Ma HB, et al. The screening of genes sensitive to long-term, low-level microwave exposure and bioinformatic analysis of potential correlations to learning and memory. Biomed Environ Sci, 2015; 28, 558−70. [4] Sharma S, Shukla S. Effect of electromagnetic radiation on redox status, acetylcholine esterase activity and cellular damage contributing to the diminution of the brain working memory in rats. J Chem Neuroanat, 2020; 106, 101784. doi: 10.1016/j.jchemneu.2020.101784 [5] Wang H, Tan SZ, Xu XP, et al. Long term impairment of cognitive functions and alterations of NMDAR subunits after continuous microwave exposure. Physiol Behav, 2017; 181, 1−9. doi: 10.1016/j.physbeh.2017.08.022 [6] Shahin S, Singh SP, Chaturvedi CM. 2. 45 GHz microwave radiation induced oxidative and nitrosative stress mediated testicular apoptosis:Involvement of a p53 dependent bax-caspase-3 mediated pathway. Environ Toxicol, 2018; 33, 931−45. [7] Friedman J, Kraus S, Hauptman Y, et al. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J, 2007; 405, 559−68. doi: 10.1042/BJ20061653 [8] Ethell IM, Hagihara K, Miura Y, et al. Synbindin, a novel syndecan-2-binding protein in neuronal dendritic spines. J Cell Biol, 2000; 151, 53−68. doi: 10.1083/jcb.151.1.53 [9] Weng YR, Kong X, Yu YN, et al. The role of ERK2 in colorectal carcinogenesis is partly regulated by TRAPPC4. Mol Carcinogen, 2014; 53, E72−84. doi: 10.1002/mc.22031 [10] Hart G, Balleine BW. Consolidation of goal-directed action depends on MAPK/ERK signaling in rodent prelimbic cortex. J Neurosci, 2016; 36, 11974−86. doi: 10.1523/JNEUROSCI.1772-16.2016 -
 21483Supplementary Materials.pdf
21483Supplementary Materials.pdf

-




 下载:
下载:





 Quick Links
Quick Links