-
The World Health Organization declared Coronavirus disease 2019 (COVID-19) a public health emergency of international concern (PHEIC)[1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of COVID-19, is an enveloped, single-stranded, positive-sense RNA coronavirus. Like severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome (MERS), the causative agent of COVID-19 belongs to the β-genus of the coronavirus family and has a genome sequence with an 82% similarity to that of SARS[1,2]. Therefore, research on human coronaviruses, especially SARS-CoV and MERS-CoV, could be useful for predicting likely environmental fate and subsequent risks of SARS-CoV-2[1]. Transmission from person to person, primarily via respiratory droplets containing viable virus particles, plays an important role in the dissemination of SARS-CoV-1 and MERS-CoV infections[3,4]. However, evidence shows that the viruses are also transmitted through contact with contaminated surfaces and fomites[4].
Human coronaviruses (HCoV), such as SARS-CoV and MERS-CoV, can survive on various surfaces, such as metal, glass, and plastic surfaces, for up to a couple of days[4]. Therefore, contact with an HCoV-contaminated surface is a potential HCoV transmission route[4]. Some investigations have reported that, for some respiratory viruses, such as influenza, indirect transmission routes may be predominant under certain conditions[4,5]. The rate of self-inoculation through repeated hand-to-face contact is an important parameter that may result in respiratory virus transmission through an uncommon mode. During the 2013 SARS outbreak, SARS-CoV RNA was found on hospital surfaces[6]. However, the role of contaminated surfaces and fomites in the transmission of coronavirus infections, especially COVID-19, is still unclear[4,7]. The viability of SARS-CoV and MERS-CoV under various conditions and their prolonged presence in the environment suggest the possibility of coronavirus transmission via contact[2]. MERS-CoV has been recovered from environmental objects, such as bedsheets, bedrails, intravenous fluid hangers, and radiograph devices, and is reported to be more stable in the environment than influenza A H1N1[8]. The higher stability of SARS-CoV than those of other coronaviruses and respiratory viruses has also posed a challenge for SARS-CoV infection control in hospitals[3].
Given the importance of different transmission routes in the epidemiology of emerging viruses[1,4], it is crucial to identify the routes by which these viruses are transmitted. Furthermore, the outbreak of COVID-19 highlights the need for a better understanding of virus transmission control and prevention. Environmental contamination of an emergency room in a hospital in Taiwan, China with the SARS-CoV was suspected to have resulted in transmission among healthcare workers who had no contact with hospitalized SARS patients[6]. To our knowledge, however, the extent of coronavirus contamination of high-touch surfaces in field settings and the role of contact with such surfaces in the transmission of coronaviruses, especially SARS-CoV-2, are unknown. Therefore, this study was designed to investigate the extent to which high-touch surfaces are contaminated with SARS-CoV-2 and identify surfaces that may play a role in transmission of the viral infection during the outbreak of COVID-19.
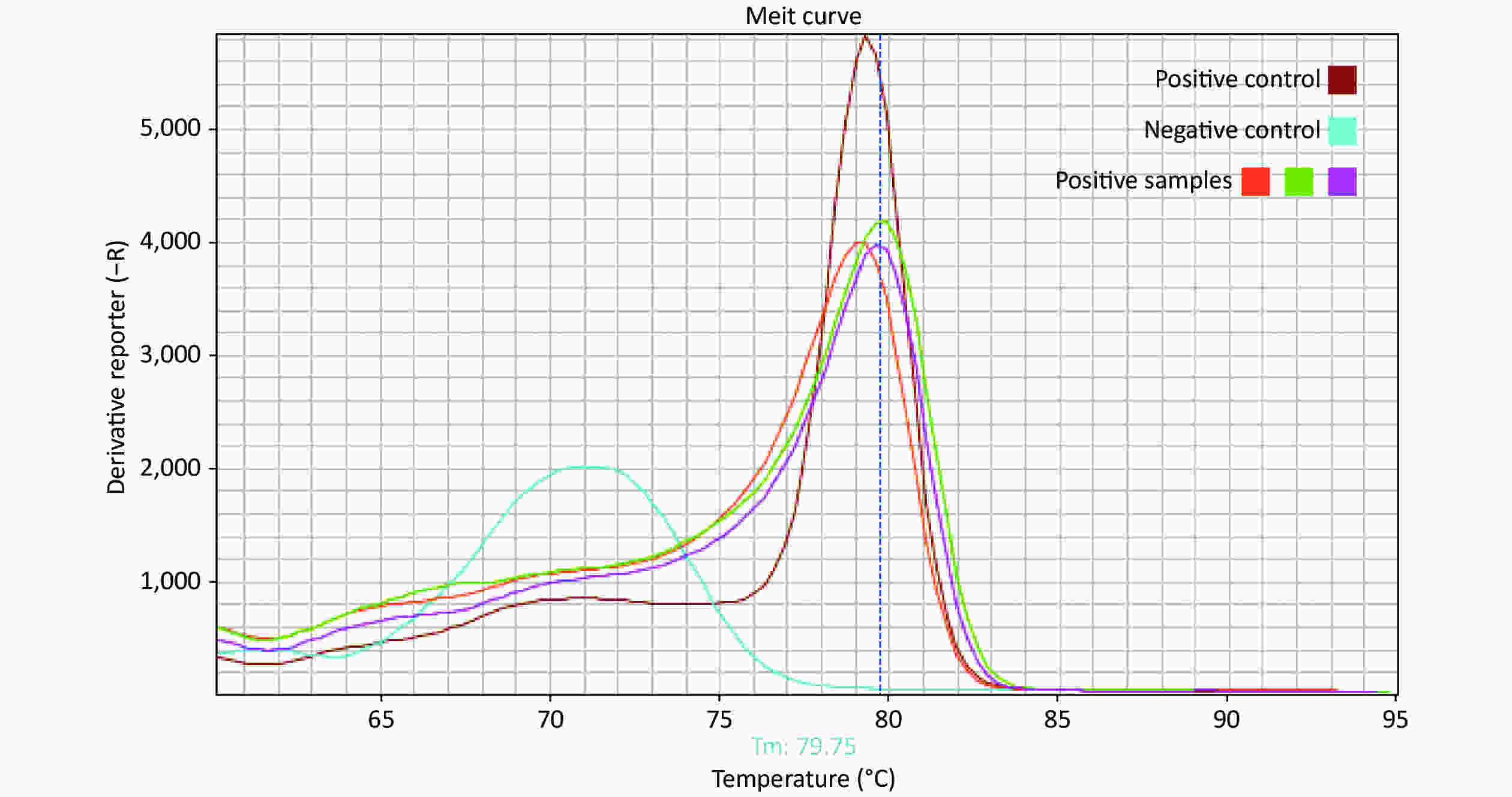
The presence of SARS-CoV-2 on high-touch surfaces was investigated during the early stages of the COVID-19 outbreak in Isfahan, Iran, from March 14 to April 8, 2020. Isfahan is the third most populous city in Iran, with a population of 1.9 million. Crowded areas in the city were selected as sampling points. Premoistened Dacron swabs in viral transport medium[6] were used to sample high-touch surfaces (Table 1). Each swab was moved in horizontal and vertical directions across a surface area of up to 700 cm2. Viral RNA was extracted from swabs using the RNeasy Mini Kit (QIAGEN, Germany) supplemented with β-mercaptoethanol and carrier RNA, following the manufacturer’s instructions. Isolated RNA was used as a template for one-step reverse transcription polymerase chain reaction (RT-PCR). A Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (Sansure Biotech, China) employing specific primers and probes was used for detecting the ORF1ab and N genes of SARS-CoV-2. RT-PCR was also performed using a specific primer set (5’-TGTTAAACCAGGTGGAAC-3’ as the forward primer and 5’-CTGTGTTGTAGATTGCG-3’ as the reverse primer) targeting the RNA-dependent RNA polymerase (RdRP) gene of SARS coronaviruses to detect the presence of SARS-CoV-2. Each RT-PCR run included a positive control (RNA from hospitalized patients with confirmed COVID-19) and negative controls for both RNA extraction and amplification steps. Each 25 μL reaction mix contained 12.5 μL 2 X reaction mix (AMPLIQON, Denmark), 0.25 μL RT enzyme mix (QuantiTect RT-PCR, QIAGEN, Germany), 0.5 μmol/L of each primer (Tag Copenhagen, Denmark), 5 mmol/L MgCl2, 0.25 mg/mL BSA (Sigma, Aldrich) and 8 μL template RNA. The cycling parameters were RT at 50 °C for 30 min, followed by 95 °C for 15 min, 45 cycles at 95 °C for 15 s, and, finally, 57 °C for, 30 s in a Step One real-time PCR system (Applied Biosystems™, CA, USA). A melting curve analysis was performed after the PCR run to differentiate between actual products and primer dimers and eliminate the possibility of false-positive results (Supplementary Figure S1, available in www.besjournal.com). To confirm the presence of SARS-CoV-2 in surface samples, the SARS-CoV-2 gene detected in one positive sample was cloned and sequenced.
Table 1. Detection of SARS-CoV-2 on various objects at sampling locations
Sampling location object Number of
samples
(%)Number of
positive samples
(%)Gas station 29 (28%) 6 (26%) Gas pump handle 19 4 POS system 6 1 Touch screen on the gas pump 4 1 Supermarket 27 (26%) 6 (26%) Freezer or refrigerator doorknob 16 4 POS system 3 1 Trolley handle 4 1 Conveyor belt 2 0 Escalator handrail 2 0 ATM 22 (21%) 4 (18%) Bus station 6 (6%) 1 (4%) Public office 18 (17%) 6 (26%) POS system 1 1 Chair handle 2 2 Public phone 1 0 Elevator button 6 2 Door handle 3 1 Public toilet door handle 1 0 Counter 4 0 Passenger terminal 2 (2%) 0 (0%) Counter 1 0 POS system 1 0 Total 104 (100%) 23 (100%) Note. POS: point of sale; ATM: automated teller machine. Of the 104 surface samples, 23 samples (22%) tested positive for SARS-CoV-2 by RT-PCR (Table 1). High-touch surfaces, such as automated teller machines (ATMs), point of sale (POS) systems, and gas pump handles, showed a relatively high frequency of viral RNA detection (Table 1). Table 2 shows the target genes detected in the positive samples. The RdRP gene was the most frequently detected gene in positive samples (74%), whereas the N and ORF1ab genes were identified in 12 (52%) and 5 (22%) positive samples, respectively. Only two samples were positive for all three target genes (Table 2). In all except one sample, the internal control was amplified, indicating that false-negative results were unlikely.
Table 2. Detection of different target genes in the SARS-CoV-2-positive samples and cycle threshold (Ct) values
Objects Target gene ORF1ab N RdRP ATM ND 37.13 +* ATM ND 34 28.84 ATM ND ND + ATM 37 35.62 ND POS system 36 30 + POS system ND ND + Gas pump handle ND 36.7 27.88 Gas pump handle + POS system 38.86 35.58 + Gas pump handle ND 36.5 ND Gas pump handle ND ND 28.22 Gas pump handle ND 38.26 ND Gas station pump keyboard ND ND + Elevator button ND ND + Elevator button ND 37 ND Freezer doorknob ND ND + Refrigerator doorknob ND ND + Refrigerator doorknob ND 38.23 + Refrigerator doorknob ND ND + Door handle ND ND 27.1 Bus station handrail 37.73 31.19 ND Trolley 37 32.23 ND Counter ND ND 28.56 Counter ND ND + Note. POS: point of sale; ATM: automated teller machine; ND, not detected. *Accurate Ct values were not determined because of primer dimer. In Table 3, the characteristics of positive samples, including the type of material and area of the surface sampled as well as climatic conditions at the time of sampling are presented. Interestingly, more positive swabs were obtained from stainless steel surfaces.
Table 3. Characteristics of sampling surface conditions for positive samples
Object Surface material Surface area
per swab (cm2)
(number of swabs)Air temperature
(˚C)Relative
humidity (%)ATM Stainless steel 20 (1) 23.8 18 ATM Glass 120 (1) 23.8 18 Gas pump handle Stainless steel 50 (1) 23.8 18 Bus station handrail Stainless steel 80 (2) 22.3 43 Gas pump handle + POS system Stainless steel and plastic 30 (3) 22.3 43 Trolley Stainless steel 100 (2) 22.3 43 ATM Stainless steel 20 (2) 18.5 56 Freezer doorknob Stainless steel 150 (1) 18.5 56 Refrigerator doorknob Plastic 200 (1) 18.6 59 POS system Plastic 20 (2) 18.6 59 Refrigerator doorknob Stainless steel 55 (2) 18.6 59 Elevator button Stainless steel 30 (1) 22.1 60 POS system Plastic 20 (3) 22.1 60 Counter Stainless steel 230 (3) 22.1 60 Door handle Stainless steel 50 (3) 22.1 60 Elevator button Stainless steel 10 (2) 22.1 60 Counter Stainless steel 150 (3) 22.1 60 Refrigerator doorknob Glass 100 (1) 22.1 60 Gas pump handle Stainless steel and plastic 30 (3) 22.1 60 Gas station POS system Plastic 20 (3) 22.1 60 Gas station pump keyboard Plastic 20 (3) 22.1 60 Gas pump handle Stainless steel and plastic 30 (3) 22.1 60 ATM Plastic 20 (1) 22.1 60 Note. ATM: automated teller machine; POS: point of sale. Surfaces contaminated with viral and bacterial pathogens can act as a medium for the transfer of microorganisms to hands and, subsequently, the gastrointestinal or respiratory tract[4]. In this study, we detected SARS-CoV-2 RNA in 22% (23 of 104) of surface samples (Table 1). In Ong (2020), 13 of 15 (87%) room sites (including air outlet fans) and 3 of 5 (60%) toilet sites from a patient room were found to be positive for SARS-CoV-2 through RT-PCR[9]. In a study at Huoshenshan hospital in Wuhan, China, surface and air samples from an intensive care unit (ICU) and a general COVID-19 ward (GW) were tested for the presence of SARS-CoV-2. Several samples from the floor and potentially contaminated objects were positive for SARS-CoV-2 RNA, and the rate of SARS-CoV-2 positivity was much higher for the ICU (54/124) than for the GW (9/114)[10]. In study by Dowell et al. (2004), 26 of 94 swab samples from surfaces in two hospitals in Bangkok, Thailand, and Taipei, Taiwan, China were positive for SARS-CoV RNA. However, more positive surface samples (24 from 26) were identified in areas with SARS patients in the most infectious phase of the illness However, all swabs from the surfaces were culture negative[6]. Kim et al. (2016) detected MERS-CoV in 42 and 15 of 68 surface swabs collected from two hospitals treating MERS-CoV patients in South Korea by RT-PCR and viral culture, respectively[7]. Very few studies have reported the detection of coronaviruses in field settings[4,5]. SARS-CoV RNA was recovered from 7.5% (3 of 40) of samples from public surfaces in Jeddah Airport, Saudi Arabia[4]. The nucleic acid of at least one respiratory virus was detected in 9 out of 90 (10%) frequently touched surface samples at airports during the peak period of seasonal influenza in 2015–2016 in Finland[5].
Although SARS-CoV-2 RNA was detected in some surface samples (Table 2), the transmission of the virus through contaminated surfaces is dependent on its ability to survive on the surface[4]. PCR only detects the DNA or RNA of a virus and cannot reflect the viability or infectivity of the virus. Some investigations showed no detection of coronavirus by the culture method, while coronavirus RNA was detected on surface samples. RT-PCR results showed cycle threshold (Ct) values from 27 to 38 for positive samples (Table 2). Ikonen et al. (2018) reported RT-PCR Ct values for respiratory viruses detection ranging from 36.15 to 41.59; because of the relatively high Ct values, they suggested a low viral load, likely lower than the minimum infective dose, on the surfaces that tested positive at airports[5]. Although our results showed a lower range of Ct values (Table 2) than reported by Ikonen et al. (2018), the probability of virus transmission from contaminated surfaces depends on the viability of SARS-CoV-2 particles as well as the minimum infective dose of SARS-CoV-2. Some studies reported higher survival times for SARS and MERS than influenza virus and human coronaviruses[4]. In the study conducted by van Doremalen et al. (2013), viable MERS-CoV was recovered after 48 h, while no viable H1N1 was recovered after 1 h under any of the conditions tested[8]. SARS-CoV can survive for a long time (six days when dried on to Petri dishes) in the environment[4], and it appears that SARS-CoV and MERS-CoV have an unusual capacity to survive on dry surfaces in comparison to other human coronaviruses[8]. Van Doremalen et al. (2020) found that the viability of SARS-CoV-2 in the environment is comparable to that of SARS-CoV-1[3].
Our results showed a high frequency of detection of viral RNA on stainless steel (65%) and plastic surfaces (26%) (Table 3). Although coronaviruses can survive on a wide range of materials, their survival time and therefore recovery from surfaces may be affected by the surface material. Van Doremalen et al. (2020) found that viable SARS-CoV-2 could be detected up to four hours on copper, up to 24 h on cardboard, and up to 2−3 d on plastic and stainless steel; they concluded that SARS-CoV-2 is more stable on plastic and stainless steel than on copper and cardboard[3].
The survival of viruses on surfaces may also be affected by the virus concentration, with higher concentrations being associated with longer survival times. Droplet-contaminated surfaces seem to be more likely involved in influenza transmission than hand-contaminated surfaces, and large surfaces have a higher potential of transmission than small surfaces[4]. Therefore, detection of the SARS-CoV-2 on ATM and gas pump handles (Tables 1 & 3) may be related to the higher frequency of use, and thus higher viral contamination, of these objects.
Generally, the survival of viruses, such as coronaviruses, decreases with increasing temperature due to the denaturation of proteins and increased activity of extracellular enzymes at higher temperatures. We found a higher frequency of detection of viral RNA at a temperature of about 18 ˚C and relative humidity (RH) of 50%−60% than at a higher temperature (23 ˚C) and lower humidity (18%) (Table 3). However, due to the low numbers of positive samples and the lack of any significant change in temperature during the sampling period, the effect of temperature and RH on the presence of SARS-CoV-2 on surfaces was not meaningful. In comparison to those containing the influenza virus, aerosols containing MERS-CoV were more stable under low temperature (20 °C) and low humidity conditions (40% RH)[4].
In conclusion, contact with high-touch surfaces may be a potential route for the community transmission of COVID-19. However, the contribution of this route to COVID-19 transmission depends on factors, such as the virus load on surfaces, viral survival time under different environmental conditions, viral infectious dose, and host factors (frequency of hand contact with the nose). Although further studies are necessary to fully understand the role of surface contamination in field settings in the epidemiology of SARS-CoV-2, hand hygiene and disinfection of frequently hand-touched surfaces may reduce the prevalence of viral infection in the community.
doi: 10.3967/bes2020.126
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Contamination of High-touch Surfaces in Field Settings
-
-
Table 1. Detection of SARS-CoV-2 on various objects at sampling locations
Sampling location object Number of
samples
(%)Number of
positive samples
(%)Gas station 29 (28%) 6 (26%) Gas pump handle 19 4 POS system 6 1 Touch screen on the gas pump 4 1 Supermarket 27 (26%) 6 (26%) Freezer or refrigerator doorknob 16 4 POS system 3 1 Trolley handle 4 1 Conveyor belt 2 0 Escalator handrail 2 0 ATM 22 (21%) 4 (18%) Bus station 6 (6%) 1 (4%) Public office 18 (17%) 6 (26%) POS system 1 1 Chair handle 2 2 Public phone 1 0 Elevator button 6 2 Door handle 3 1 Public toilet door handle 1 0 Counter 4 0 Passenger terminal 2 (2%) 0 (0%) Counter 1 0 POS system 1 0 Total 104 (100%) 23 (100%) Note. POS: point of sale; ATM: automated teller machine. Table 2. Detection of different target genes in the SARS-CoV-2-positive samples and cycle threshold (Ct) values
Objects Target gene ORF1ab N RdRP ATM ND 37.13 +* ATM ND 34 28.84 ATM ND ND + ATM 37 35.62 ND POS system 36 30 + POS system ND ND + Gas pump handle ND 36.7 27.88 Gas pump handle + POS system 38.86 35.58 + Gas pump handle ND 36.5 ND Gas pump handle ND ND 28.22 Gas pump handle ND 38.26 ND Gas station pump keyboard ND ND + Elevator button ND ND + Elevator button ND 37 ND Freezer doorknob ND ND + Refrigerator doorknob ND ND + Refrigerator doorknob ND 38.23 + Refrigerator doorknob ND ND + Door handle ND ND 27.1 Bus station handrail 37.73 31.19 ND Trolley 37 32.23 ND Counter ND ND 28.56 Counter ND ND + Note. POS: point of sale; ATM: automated teller machine; ND, not detected. *Accurate Ct values were not determined because of primer dimer. Table 3. Characteristics of sampling surface conditions for positive samples
Object Surface material Surface area
per swab (cm2)
(number of swabs)Air temperature
(˚C)Relative
humidity (%)ATM Stainless steel 20 (1) 23.8 18 ATM Glass 120 (1) 23.8 18 Gas pump handle Stainless steel 50 (1) 23.8 18 Bus station handrail Stainless steel 80 (2) 22.3 43 Gas pump handle + POS system Stainless steel and plastic 30 (3) 22.3 43 Trolley Stainless steel 100 (2) 22.3 43 ATM Stainless steel 20 (2) 18.5 56 Freezer doorknob Stainless steel 150 (1) 18.5 56 Refrigerator doorknob Plastic 200 (1) 18.6 59 POS system Plastic 20 (2) 18.6 59 Refrigerator doorknob Stainless steel 55 (2) 18.6 59 Elevator button Stainless steel 30 (1) 22.1 60 POS system Plastic 20 (3) 22.1 60 Counter Stainless steel 230 (3) 22.1 60 Door handle Stainless steel 50 (3) 22.1 60 Elevator button Stainless steel 10 (2) 22.1 60 Counter Stainless steel 150 (3) 22.1 60 Refrigerator doorknob Glass 100 (1) 22.1 60 Gas pump handle Stainless steel and plastic 30 (3) 22.1 60 Gas station POS system Plastic 20 (3) 22.1 60 Gas station pump keyboard Plastic 20 (3) 22.1 60 Gas pump handle Stainless steel and plastic 30 (3) 22.1 60 ATM Plastic 20 (1) 22.1 60 Note. ATM: automated teller machine; POS: point of sale. -
[1] Kitajima M, Ahmed W, Bibby K, et al. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci Total Environ, 2020; 739, 139076. doi: 10.1016/j.scitotenv.2020.139076 [2] Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol, 2020; 5, 335−7. doi: 10.1016/S2468-1253(20)30048-0 [3] Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med, 2020; 382, 1564−7. doi: 10.1056/NEJMc2004973 [4] Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect, 2016; 92, 235−50. doi: 10.1016/j.jhin.2015.08.027 [5] Ikonen N, Savolainen-Kopra C, Enstone JE, et al. Deposition of respiratory virus pathogens on frequently touched surfaces at airports. BMC Infect Dis, 2018; 18, 437. doi: 10.1186/s12879-018-3150-5 [6] Dowell SF, Simmerman JM, Erdman DD, et al. Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis, 2004; 39, 652−7. doi: 10.1086/422652 [7] Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East Respiratory Syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis, 2016; 63, 363−9. doi: 10.1093/cid/ciw239 [8] van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eur Surveill, 2013; 18, 20590. [9] Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA, 2020; 323, 1610−2. doi: 10.1001/jama.2020.3227 [10] Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis, 2020; 26, 1583−91. doi: 10.3201/eid2607.200885 -




 下载:
下载:



 Quick Links
Quick Links