-
The coronavirus disease 2019 (COVID-19) pandemic from 2020 to 2023 posed unprecedented challenges to global health, with more than 771 million confirmed cases worldwide, resulting in medical and economic burdens[1]. COVID-19 has widespread and complex effects on the body, with approximately 10 % of individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) developing post-acute sequelae, manifesting as a variety of symptoms across multiple organ systems, including cardiovascular disease, cerebrovascular disease, chronic fatigue syndrome, and dysautonomia[2]. The long-term consequences and mechanisms of these sequelae remain incompletely understood, highlighting the need for a deeper understanding to improve health management and monitoring strategies related to the long-term effects of COVID-19.
Several studies have reported that acute SARS-CoV-2 infection may cause lower urinary tract symptoms (LUTS)[3-5]. LUTS consist of storage, voiding, and post-micturition symptoms, primarily including urinary frequency, urgency, and urinary incontinence (UI), as defined by the International Continence Society[6]. Large population-based epidemiological studies have indicated that the prevalence of LUTS among women ranges from 55.5 % to 66.6 %[7-9], with nocturia, urgency, and UI being the most common symptoms. It is well established that pregnancy and childbirth are independent risk factors for LUTS. The prevalence of UI in women increases over time after delivery, exceeding 30 % after 15 years[10,11], significantly impairing both quality of life and mental well-being[12,13]. However, whether SARS-CoV-2 infection exacerbates LUTS remains controversial. While some studies have reported a higher prevalence of LUTS associated with SARS-CoV-2 infection[3,5,14-16], others have observed a lower prevalence and severity of UI among women during the COVID-19 pandemic[17]. The inconsistency and limitations of study populations leave gaps in our understanding of the impact of prenatal SARS-CoV-2 infection on postpartum LUTS and the potential underlying mechanisms. LUTS may result from various anatomical and functional changes in the pelvic floor muscle (PFM). Case reports have indicated that women experienced UI after recovering from COVID-19, with UI related to PFM weakness benefiting from physical therapy[18]. Additionally, previous studies have demonstrated that COVID-19 can affect the musculoskeletal system, resulting in post-exertional malaise[2]. Since PFM-related disorders are often associated with electrophysiological abnormalities observed through PFM electromyography (EMG)[19,20], and given that our previous study showed consistency between UI outcomes based on questionnaire and EMG values[21], it might be meaningful to explore postpartum LUTS and PFM activity using subjective self-report symptoms and questionnaires, as well as objective EMG assessment.
This study aimed to reveal the correlation between prenatal SARS-CoV-2 infection and postpartum LUTS, as assessed through self-reported symptoms and PFM EMG. We hope to gain valuable evidence and new insights into the underlying mechanisms leading to postpartum LUTS after prenatal SARS-CoV-2 infection, as well as the clinical implications and future research directions.
-
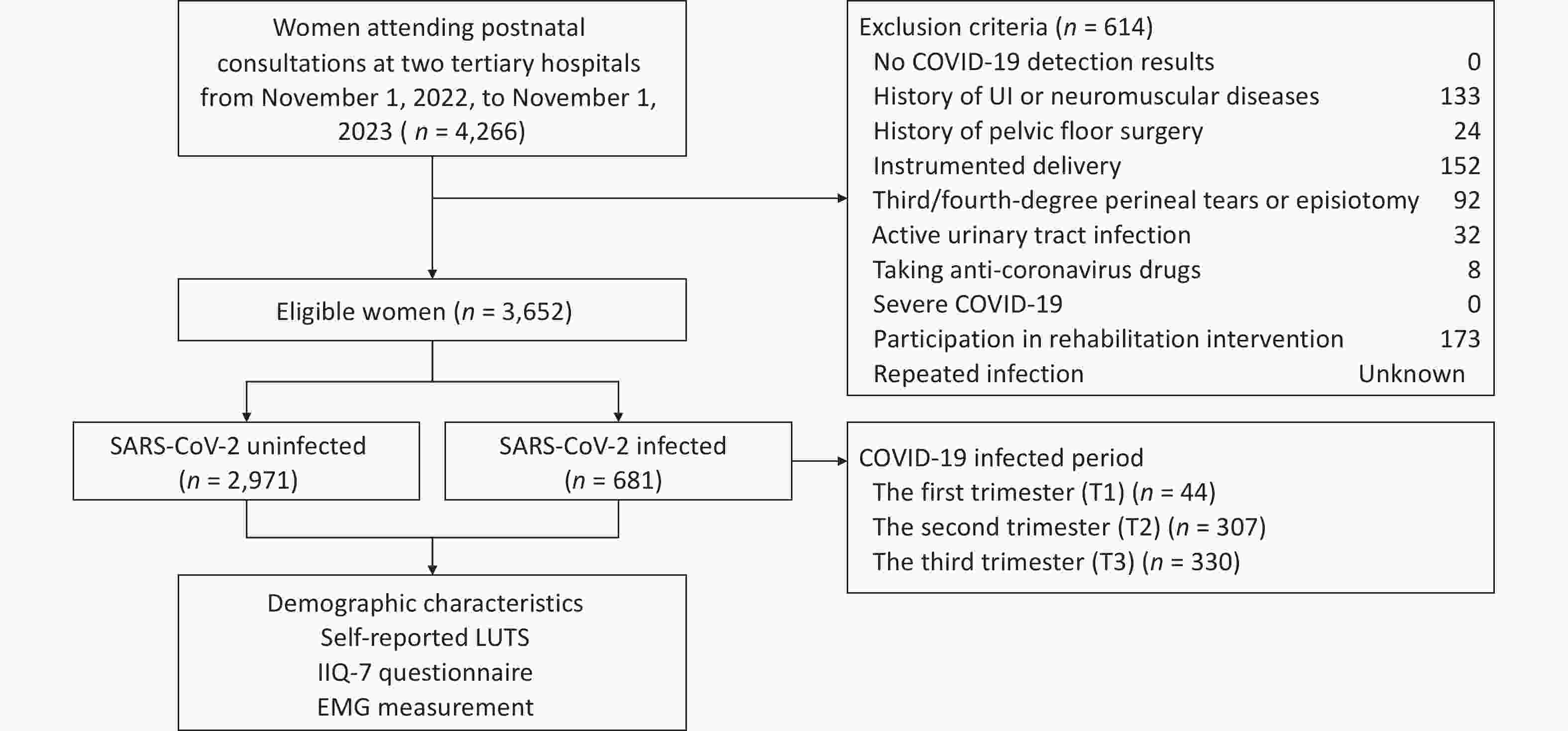
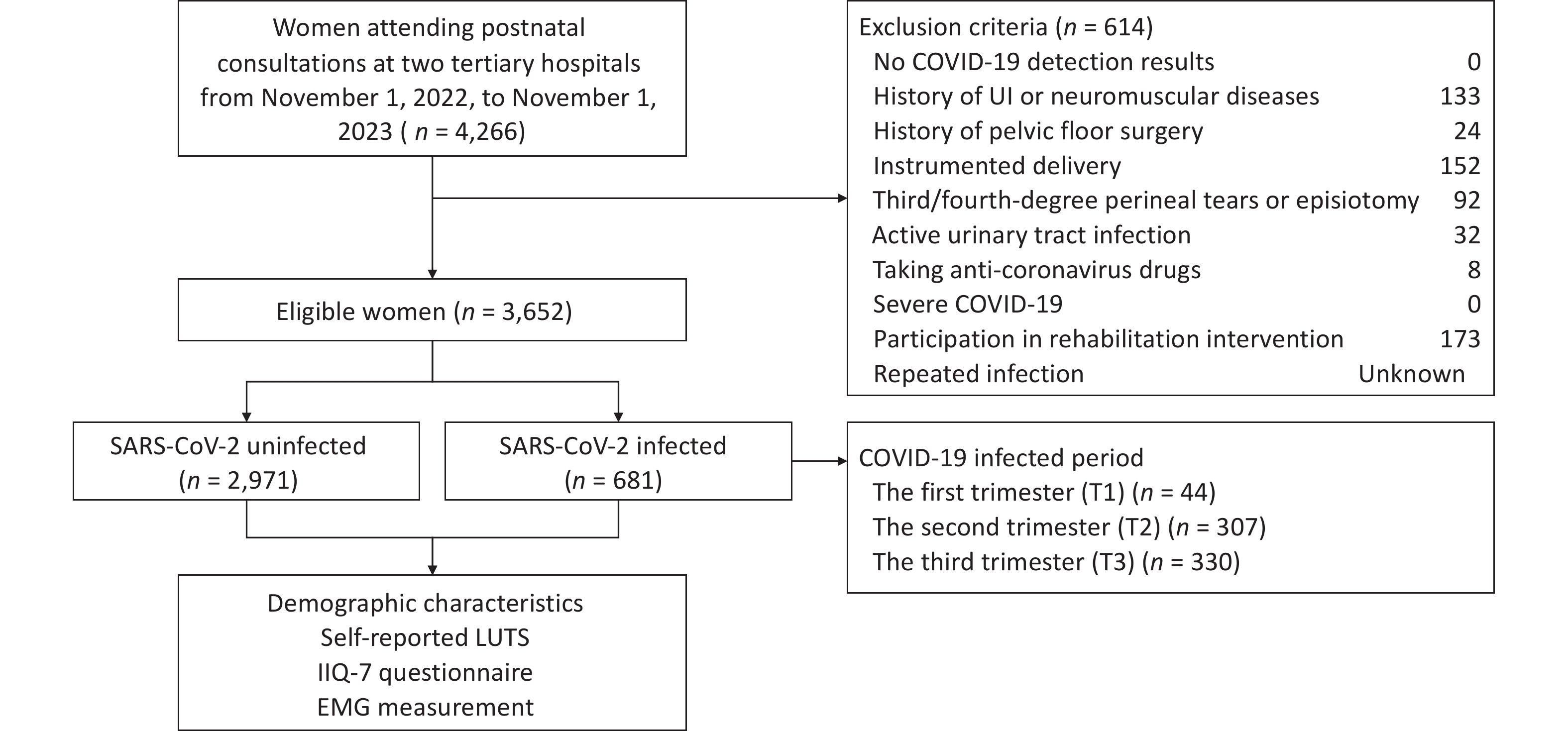
This hospital-based, multicenter, retrospective cohort study was conducted at two tertiary hospitals across northern and southwest China, sequentially involving all women attending postnatal consultations from November 1, 2022, to November 1, 2023. Electronic medical records were used for eligibility screening, group allocation, and baseline analysis. SARS-CoV-2 detection results and demographic characteristics were obtained from medical records. Given the variability in the onset and duration of COVID-19 post-acute sequelae across individuals and symptom types[22], along with findings from previous studies on postpartum UI[21], eligible postnatal consultations were set between 6 and 12 weeks after childbirth. The inclusion criteria were: (1) confirmed prenatal SARS-CoV-2 detection results and (2) age > 18 years. The exclusion criteria were as follows: (1) no SARS-CoV-2 detection results during pregnancy, (2) history of UI or neuromuscular diseases before pregnancy, (3) history of pelvic floor surgery, (4) instrumented delivery, (5) third/fourth-degree perineal tears or episiotomy, (6) active urinary tract infection, (7) taking anti-coronavirus drugs, (8) severe COVID-19, and (9) participation in rehabilitation intervention. Ultimately, based on SARS-CoV-2 antigen positivity or negativity, there were 681 and 2,971 women in the SARS-CoV-2 infected and uninfected groups, respectively. The study was performed in accordance with the Declaration of Helsinki and approved by the Beijing Hospital Medical Ethics Committee (2019BJYYEC-014-02) on February 15, 2019.
During postnatal consultations, eligible women self-reported postpartum LUTS, completed questionnaires, and underwent PFM EMG assessments. LUTS were categorized into storage (stress and urge UI, frequency) and voiding (terminal dribbling) symptoms. The definitions followed those of the International Continence Society[6]. Stress UI was defined as involuntary leakage during exertion, such as sneezing or coughing, whereas urge UI was defined as involuntary leakage immediately preceded by urgency. Participants were considered to have voiding frequency if they reported voiding too often per day. Terminal dribbling was defined as a prolonged final part of micturition. To account for COVID-19 post-acute sequelae, symptoms were defined as those occurring ≥ 3 months after infection and persisting for at least two months based on the World Health Organization guidelines[23]. Potential confounders, including age, body mass index (BMI), postpartum time, mode of delivery, and SARS-CoV-2 infection period, were defined and compared between groups. BMI (kg/m2) was calculated using weight and height, with categories of underweight (< 18.5), normal weight (18.5-24), overweight (24-28), and obesity (≥ 28). Postpartum time was defined as the duration from childbirth to the first postnatal consultation. The period of SARS-CoV-2 infection was categorized into first (T1: < 14 weeks of gestation), second (T2: 14-28 weeks), and third (T3: ≥ 28 weeks) trimester. A physiotherapist examined the participants, collected self-reported LUTS, assisted with completing questionnaires, and conducted EMG measurements (Figure 1). The primary outcomes were the prevalence of self-reported LUTS and the questionnaire scores. The secondary outcome was the EMG value.
-
We collected the Incontinence Impact Questionnaire-Short Form (IIQ-7)[24], a patient-reported outcome measure designed to assess the frequency, severity, and impact on quality of life. IIQ-7 (range: 0-100) reflects higher scores, denoting more severe symptoms and lower quality of life. IIQ-7 has been translated into Chinese and validated in China[25].
-
EMG signals were detected using “MLD V2 Vaginal Electrode” intravaginal electrodes (Medlander Medical Technology Inc.) and processed using Biostim software (Medlander Medical Technology Inc.). The Glazer protocol was used to standardize the PFM activity[20,26]. It consisted of a standardized sequence of muscle contractions and relaxations in the supine position. Before the protocol, the participants were instructed on how to isolate the abdominal, gluteal, or adductor muscles to prevent interference with EMG values. They were trained to differentiate between contractions by practicing maximal fast and endurance contractions. After a 10-minute rest in the supine position, the EMG test was initiated using voice prompts. The protocol included four steps: 60-second pre-baseline rest (PREBR), five maximal phasic contractions with 10-second rests in between (PPC), five 10-second tonic contractions alternated with 10-second rests (MTC), and 60-second post-baseline rest (POSTBR). The mean amplitude (μV) was used for each of the four EMG values. Minimal activation of the abdominal, gluteal, or adductor muscles was monitored during the contractions.
-
The prevalence of self-reported LUTS, severity of UI (indicated by questionnaires), and EMG values (PREBR, PPC, MTC, and POSTBR) were compared between the SARS-CoV-2 infected and uninfected groups. We performed the Kolmogorov-Smirnov test for continuous variables to confirm normality. Categorical variables are described as numbers and percentages. Continuous variables following a normal distribution are reported as mean ± standard deviation. Categorical variables were analyzed using the chi-square test, while continuous normal variables were analyzed using t-tests. ANOVA was used for comparisons across multiple groups. Confounder-adjusted estimations were conducted using stratified analyses, logistic regression was used for dichotomous variables, such as the prevalence of self-reported LUTS, and general linear models were used for continuous variables, such as questionnaire scores and EMG values. General linear models were applied, with SARS-CoV-2 infection (infected vs. uninfected) and mode of delivery (cesarean section vs. vaginal delivery) as factors, and postpartum time as a covariate. Bonferroni correction was applied to adjust the α level to control for the risk of a type I error in multivariate comparisons. A P-value of < 0.05 was considered statistically significant in bivariate comparisons, while a P < 0.01 was considered statistically significant in multivariate comparisons. All statistical analyses were conducted using SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA).
-
All continuous variables followed a normal distribution. The mean age of the participants was 33 years, with a mean BMI of 23.31 kg/m2 and a mean postpartum period of 64 days. There were 681 women in the SARS-CoV-2 infected group and 2,971 in the SARS-CoV-2 uninfected group. In the SARS-CoV-2 infected group, 44 (6.46 %) women were infected during T1, 307 (45.08 %) during T2, and 330 (48.46 %) during T3. The two groups were similar in terms of age, BMI, and postpartum time. However, a higher cesarean section rate was observed in the SARS-CoV-2 infected group (26.1 % vs. 22.4 %, P = 0.038) (Table 1).
Table 1. The Demographic Characteristics of Participants in the SARS-CoV-2 Infected and Uninfected Groups
Characteristics SARS-CoV-2 infected group (n = 681) SARS-CoV-2 uninfected group (n = 2971) P-value Age, years 32.78 ± 3.91 32.69 ± 4.12 0.684 18-30 203 (29.8 %) 880 (29.6 %) 0.732 30-40 457 (67.1 %) 2015 (67.8 %) 40+ 21 (3.1 %) 76 (2.6 %) BMI, kg/m2 23.43 ± 3.27 23.28 ± 3.08 0.321 < 18.5 (underweight) 23 (3.4 %) 103 (3.5 %) 0.283 18.5-24 (normal weight) 391 (57.4 %) 1803 (60.7 %) 24-28 (overweight) 215 (31.6 %) 827 (27.8 %) ≥ 28 (obesity) 52 (7.6 %) 238 (8.0 %) Postpartum time, day 66 ± 48 63 ± 53 0.262 Mode of delivery 0.038 Vaginal delivery 503 (73.9 %) 2305 (77.6 %) Cesarean section 178 (26.1 %) 666 (22.4 %) Note. Data are presented as mean with standard deviation or number with percentage; P < 0.05 denotes statistical significance. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; BMI, body mass index. -
No significant differences were observed in the prevalence of self-reported LUTS between the SARS-CoV-2 infected and uninfected groups (Table 2). Similarly, there were no significant differences in IIQ-7 scores between the two groups (Table 3). Stratified analysis showed no significant differences in IIQ-7 scores between the SARS-CoV-2 infected and uninfected groups, regardless of vaginal delivery or cesarean section. In both groups, women who delivered vaginally had higher IIQ-7 scores. Based on demographic characteristics and our previous studies, logistic regression was performed to assess the risk of developing LUTS, revealing no significant differences in stress UI (OR = 0.970, P = 0.776), urge UI (OR = 0.965, P = 0.762), frequency (OR = 0.855, P = 0.159), or terminal dribble (OR = 1.031, P = 0.776) after SARS-CoV-2 infection. The general linear model found no interaction between SARS-CoV-2 infection and mode of delivery on IIQ-7 (P = 0.440) scores, and no main effect of SARS-CoV-2 infection on IIQ-7 (P = 0.068). However, the main effect of mode of delivery indicated that IIQ-7 scores (P < 0.001) were lower in women who underwent cesarean section (Table 3).
Table 2. Comparison of the prevalence of self-reported LUTS between the SARS-CoV-2 infected and uninfected groups
LUTS SARS-CoV-2 infected group
(n = 681)SARS-CoV-2 uninfected group
(n = 2971)P-value
COVID-19 infectionStorage symptoms Stress UI 32 (4.7 %) 166 (5.6 %) 0.356 Vaginal delivery 26 (5.2 %) 140 (6.1 %) 0.422 Cesarean section 6 (3.4 %) 26 (3.9 %) 0.769 Urge UI 148 (21.7 %) 663 (22.3 %) 0.741 Vaginal delivery 107 (21.2 %) 501 (21.8 %) 0.779 Cesarean section 41 (23.2 %) 162 (24.1 %) 0.801 Frequency 78 (11.4 %) 374 (12.6 %) 0.641 Vaginal delivery 61 (12.1 %) 303 (13.2 %) 0.513 Cesarean section 17 (9.6 %) 71 (10.5 %) 0.713 Voiding symptoms Terminal dribble 65 (9.5 %) 259 (8.7 %) 0.494 Vaginal delivery 51 (10.1 %) 225 (9.8 %) 0.812 Cesarean section 14 (7.9 %) 34 (5.1 %) 0.143 Note. Data are presented as number with percentage; P < 0.05 denotes statistical significance. LUTS, lower urinary tract symptoms; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UI, urinary incontinence. Table 3. Comparison of questionnaire scores between the SARS-CoV-2 infected and uninfected groups
Questionnaire scores SARS-CoV-2 infected group
(n = 681)SARS-CoV-2 uninfected group
(n = 2971)P-value
COVID-19 infectionIIQ-7 6.23 ± 16.73 7.40 ± 16.91 0.391 Vaginal delivery 6.47 ± 16.32 7.98 ± 18.82 0.293 Cesarean section 3.08 ± 9.42 3.55 ± 14.13 0.795 Note. Data are presented as mean with standard deviation; P < 0.05 denotes statistical significance. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IIQ-7, Impact Questionnaire Short Form questionnaire. -
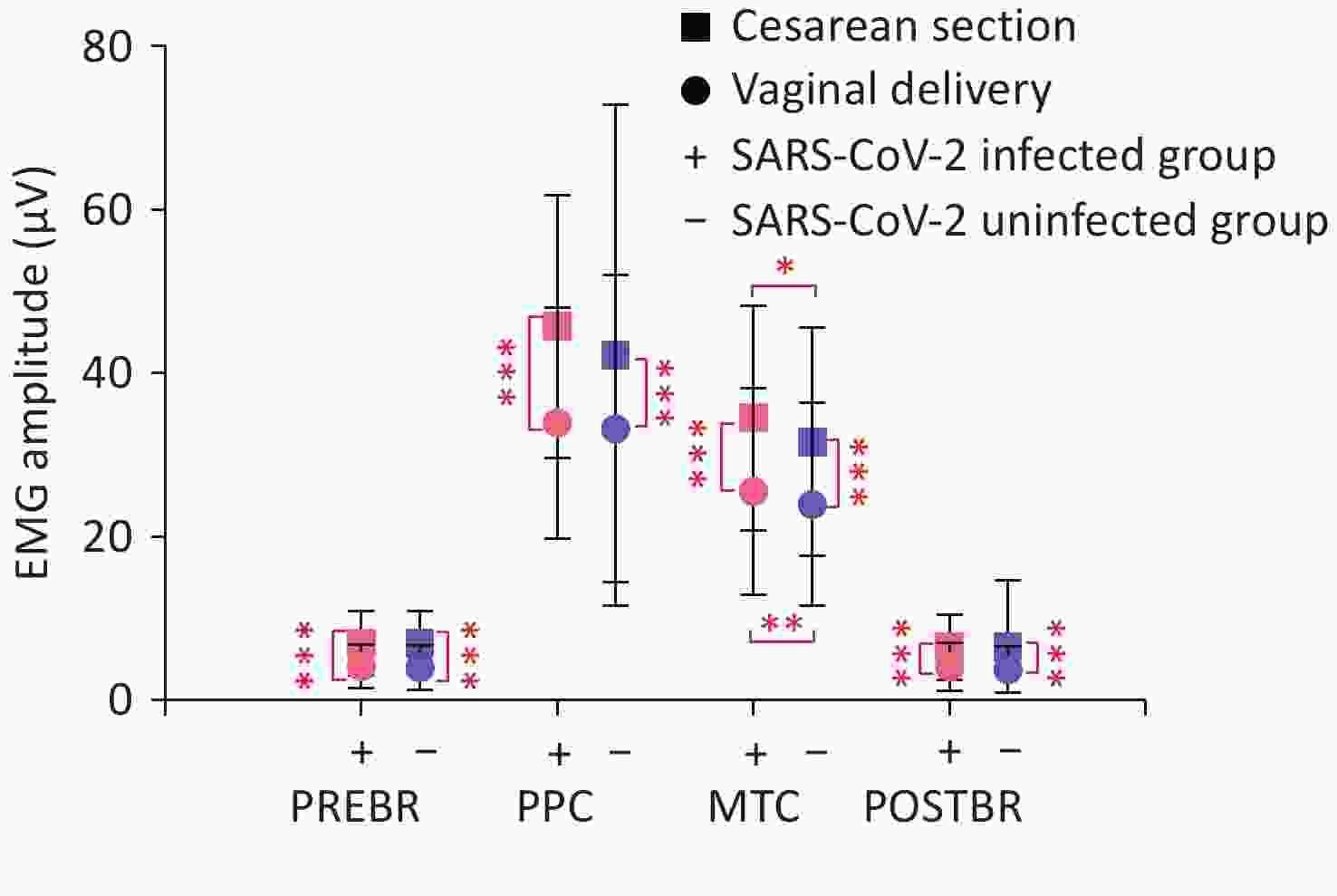
EMG values for PPC (P = 0.045), MTC (P < 0.001), and POSTBR (P = 0.045) were significantly higher in the SARS-CoV-2 infected group than in the uninfected group. Stratified analyses showed that MTC was significantly higher in the SARS-CoV-2 infected group among women who underwent either vaginal delivery (P = 0.008) or cesarean section (P = 0.013) (Figure 2). The general linear model identified SARS-CoV-2 infection as an independent factor influencing MTC (P = 0.001), with no significant main effects on other EMG values (Table 4). Additionally, an interaction between SARS-CoV-2 infection and the mode of delivery was observed for PPC (P = 0.046). Women who underwent cesarean section showed higher EMG values than those who underwent vaginal delivery (Figure 2). The main effects of mode of delivery indicated that PREBR (P < 0.001), PPC (P < 0.001), MTC (P < 0.001), and POSTBR (P < 0.001) were all higher in women who underwent cesarean section (Table 4).
-
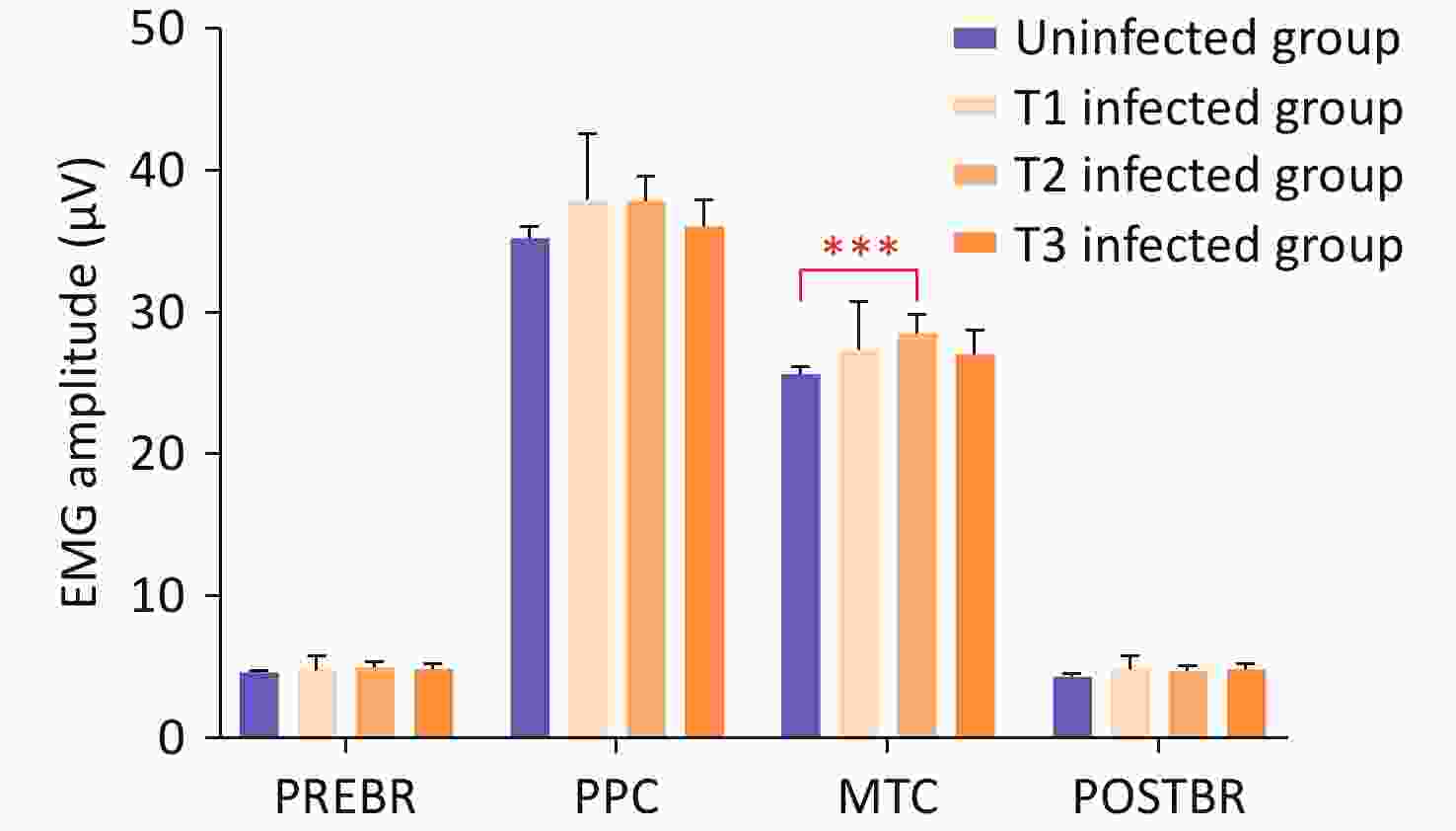
No significant differences were observed in the prevalence of self-reported LUTS, questionnaire scores, or EMG values between T1, T2, and T3. A significant difference was found in MTC when comparing the groups at different SARS-CoV-2 infection periods with the uninfected group (P = 0.001). Pairwise comparisons revealed that only women infected with SARS-CoV-2 during the second trimester had significantly higher MTC (P = 0.001) than those without infection (Figure 3). The general linear model showed no interaction between the SARS-CoV-2 infection period and other factors. However, after post-hoc analysis, the SARS-CoV-2 infection period had a main effect, with MTC being significantly higher in women infected during the second trimester (P = 0.007).
-
In this study, we investigated the correlation between prenatal SARS-CoV-2 infection and postpartum LUTS, as assessed using self-reported symptoms and PFM EMG. Our findings showed that prenatal SARS-CoV-2 infection did not exacerbate postpartum LUTS; however, a higher average mean amplitude of tonic contractions in EMG assessment was observed among infected women, particularly those infected during the second trimester. These results provide valuable evidence and new insights into the underlying mechanisms leading to postpartum LUTS after prenatal SARS-CoV-2 infection.
Vaginal delivery is a well-known risk factor for LUTS because of increased PFM loading and injuries[27]. Our analysis revealed a significantly higher cesarean section rate in the SARS-CoV-2 infected group, which is consistent with previous studies[28]. Additionally, in both the SARS-CoV-2 infected and uninfected groups, women who underwent vaginal delivery exhibited higher IIQ-7 scores. Vaginal delivery, compared with cesarean section, is associated with an almost twofold increase in the risk of long-term stress UI, with the greatest risk observed in younger women[27].
We employed various methods to perform confounder-adjusted estimations of the LUTS outcomes. The conclusion was controversial, as several studies have reported a higher prevalence of LUTS associated with SARS-CoV-2 infection[5], particularly urinary frequency and nocturia[3,15,16]. Another study indicated that the severity of COVID-19 significantly affects LUTS[14]. However, these studies focused on hospitalized patients in the acute infection phase, providing limited insight into the potential molecular mechanisms[15]. This negative effect of SARS-CoV-2 infection on LUTS may subside after the acute phase. In contrast, Ferrari et al. found a lower prevalence and severity of UI among women during the COVID-19 pandemic, focusing on the impact of the pandemic rather than the SARS-CoV-2 infection itself[17].
We also assessed PFM activity to specifically investigate the impact of prenatal SARS-CoV-2 infection on the associated potential mechanisms of postpartum LUTS. Given the subjectivity of questionnaires, we used EMG as a sensitive method to detect PFM activity. Our previous study confirmed the consistency between EMG values and IIQ-7 scores, which increased drastically with time following childbirth[21]. Several studies have proven that EMG is a reliable tool for assessing PFM activity by directly measuring the muscle motor unit action and reflecting the integrated activity of physiological processes[20]. Yang et al. identified pre-baseline rest and endurance contraction as the most correlated values for postpartum stress UI[29], while Ptaszkowski et al. found phasic and endurance contractions to be the most significant in postmenopausal stress UI[30]. Interestingly, we observed a higher average mean amplitude of tonic contractions in the EMG assessment of postpartum women with prenatal SARS-CoV-2 infection. Type I muscle fibers, recruited by tonic contractions, are associated with supporting the pelvic organs, whereas type II muscle fibers engaged during phasic contractions improve continence by preventing leakage during abdominal pressure-increased activities[31]. Our results suggested that, after SARS-CoV-2 infection, the activity of type I muscle fibers of PFM was improved. This finding allows for better tonic contractions during PFM training. Perinatal women with COVID-19 post-acute sequelae exhibit symptoms similar to those of the general population, such as fatigue[32], and the underlying pathophysiological mechanism of musculoskeletal symptoms remains poorly understood[33,34]. One pathophysiological mechanism indicated that reduced skeletal muscle strength after SARS-CoV-2 infection is characterized by local and systemic metabolic disturbances[35,36]. A pilot study showed that fatigue in COVID-19 post-acute sequelae coincided with oxidative stress[37]. Similarly, another study found that exercise intolerance associated with COVID-19 post-acute sequelae is defined by impaired aerobic and anaerobic energy production. Peak venous succinate levels may serve as a potential biomarker of COVID-19 post-acute sequelae[38]. Functional and morphological differences in muscle mitochondria have been discovered in COVID-19 post-acute sequelae, which are probably direct virus-induced alterations[39]. A recent review reported that the mitochondrial dysfunction and ionic disturbance caused by sodium-induced calcium overload are the most likely causes of exertional intolerance and post-exertional malaise[40]. Another pathophysiological mechanism is related to the muscle fiber phenotype shift. Sousa et al. suggested that physiotherapy could yield positive outcomes for women with stress UI caused by muscle weakness following SARS-CoV-2 infection[18]. Since muscle strength is typically significantly affected by fatigue[41], which depends on the recruitment of motor units, a shift toward less oxidative and more glycolytic fiber phenotypes, as demonstrated by Appelman et al., might reduce exercise capacity after SARS-CoV-2 infection[42]. However, given that EMG assessments involved brief contractions that might not induce significant fatigue, and considering the fatigue resistance of type I muscle fibers, the unimpaired PFM activity in our study could be partially explained.
Another perspective is that improved pelvic organ support after SARS-CoV-2 infection might be associated with reduced activity levels. One study reported a lower prevalence and severity of UI among women during the COVID-19 pandemic, ascribing this “protective” effect to decreased physical exertion[17]. Physical activity levels decreased following the COVID-19 outbreak, particularly among perinatal women. Shaw et al.[43] found that moderate to vigorous activity was associated with worse PFM support in postpartum women compared with sedentarism. A trial comparing people with and without COVID-19 post-acute sequelae showed that patients had lower aerobic capacity and less muscle strength, so it recommended cautious exercise adoption to prevent further muscle deconditioning[44].
The reduction in outdoor activities and prolonged bed rest likely alleviated the compression effects on PFM, potentially lessening their impact on PFM activity. Considering that the difference in EMG values between the two groups was minimal (a few microvolts) and was not reflected in LUTS outcomes, this statistical difference in these findings might lack clinical relevance.
The strength of our multicenter study lies in its large sample size, which enabled a comprehensive understanding of COVID-19 post-acute sequelae in perinatal women by analyzing postpartum LUTS. This study utilized both subjective symptom-related questionnaires and objective EMG assessment, with the latter involving disposable vaginal electrodes to prevent cross-infection while providing direct electrophysiological data on PFM. However, this study had several limitations. First, selection bias could not be avoided. This hospital-based study included only postpartum women attending postnatal consultation, limiting its generalizability, but can serve as supplementary evidence for other population-based studies. Second, this study mainly investigated the severity of UI among LUTS, as its high prevalence in postpartum women potentially reflects trends in other LUTS. However, the remaining LUTS have also been reported in individuals with COVID-19 post-acute sequelae[5]. Third, the short follow-up period of 6–12 weeks postpartum may not have captured long-term effects. Previous studies found that two-thirds of individuals with COVID-19 post-acute sequelae had persisting disease for more than a year[45]. Continued follow-up is necessary to understand the lasting impact of COVID-19 post-acute sequelae on postpartum LUTS.
-
Prenatal SARS-CoV-2 infection was not significantly associated with an increased risk of postpartum LUTS. However, it independently altered PFM electromyographic activity, suggesting potential neuromuscular effects of the virus. The discrepancy between PFM electrophysiology and clinical symptoms highlights the need for long-term follow-up to better understand COVID-19 post-acute sequelae.
doi: 10.3967/bes2025.065
Does Prenatal SARS-CoV-2 Infection Exacerbate Postpartum Lower Urinary Tract Symptoms? A Multicenter Retrospective Cohort Study
-
Abstract:
Objective Coronavirus disease 2019 (COVID-19) can result in fatigue and post-exertional malaise; however, whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection exacerbates lower urinary tract symptoms (LUTS) is unclear. This study investigated the association between prenatal SARS-CoV-2 infection and postpartum LUTS. Methods A multicenter, retrospective cohort study was conducted at two tertiary hospitals in China from November 1, 2022, to November 1, 2023. Participants were classified into infected and uninfected groups based on SARS-CoV-2 antigen results. LUTS prevalence and severity were assessed using self-reported symptoms and the Incontinence Impact Questionnaire-Short Form (IIQ-7). Pelvic floor muscle activity was measured using electromyography following the Glazer protocol. Group comparisons were performed to evaluate the association of SARS-CoV-2 infection with LUTS and electromyography parameters, with stratified analyses conducted using SPSS version 26.0. Results Among 3,652 participants (681 infected, 2,971 uninfected), no significant differences in LUTS prevalence or IIQ-7 scores were observed. However, SARS-CoV-2 infection was an independent factor influencing the electromyographic activity of the pelvic floor muscles (mean tonic contraction amplitudes), regardless of delivery mode (P = 0.001). Conclusion Prenatal SARS-CoV-2 infection was not significantly associated with an increased risk of postpartum LUTS but independently altered pelvic floor muscle electromyographic activity, suggesting potential neuromuscular effects. -
Key words:
- COVID-19 post-acute sequelae /
- Lower urinary tract symptoms /
- SARS-CoV-2 /
- Electromyography
The authors declare no conflict of interest.
The study was performed in accordance with the Declaration of Helsinki and approved by the Beijing Hospital Medical Ethics Committee (2019BJYYEC-014-02) on February 15, 2019.
注释:1) Authors’ Contributions: 2) Competing Interests: 3) Ethics: -
Figure 1. Flowchart of the Study Enrollment
Note. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; LUTS, lower urinary tract symptoms; IIQ-7, Impact Questionnaire Short Form questionnaire; EMG, electromyography; COVID-19, coronavirus disease 2019; UI, urinary incontinence; T1, first trimester; T2, second trimester; T3, third trimester.
Figure 2. Stratified analyses of EMG values in the SARS-CoV-2 infected and uninfected groups
Note. P < 0.05 denotes statistical significance. EMG, electromyography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PREBR, average mean amplitude of pre-baseline rest; PPC, average mean amplitude of maximal phasic contractions; MTC, average mean amplitude of tonic contractions; POSTBR, average mean amplitude of post-baseline rest.
Figure 3. Pairwise comparisons of EMG values among women in T1, T2, T3, and uninfected groups.
Note. P < 0.01 denotes statistical significance. EMG, electromyography; T1, first trimester; T2, second trimester; T3, third trimester; PREBR, average mean amplitude of pre-baseline rest; PPC, average mean amplitude of maximal phasic contractions; MTC, average mean amplitude of tonic contractions; POSTBR, average mean amplitude of post-baseline rest.
Table 1. The Demographic Characteristics of Participants in the SARS-CoV-2 Infected and Uninfected Groups
Characteristics SARS-CoV-2 infected group (n = 681) SARS-CoV-2 uninfected group (n = 2971) P-value Age, years 32.78 ± 3.91 32.69 ± 4.12 0.684 18-30 203 (29.8 %) 880 (29.6 %) 0.732 30-40 457 (67.1 %) 2015 (67.8 %) 40+ 21 (3.1 %) 76 (2.6 %) BMI, kg/m2 23.43 ± 3.27 23.28 ± 3.08 0.321 < 18.5 (underweight) 23 (3.4 %) 103 (3.5 %) 0.283 18.5-24 (normal weight) 391 (57.4 %) 1803 (60.7 %) 24-28 (overweight) 215 (31.6 %) 827 (27.8 %) ≥ 28 (obesity) 52 (7.6 %) 238 (8.0 %) Postpartum time, day 66 ± 48 63 ± 53 0.262 Mode of delivery 0.038 Vaginal delivery 503 (73.9 %) 2305 (77.6 %) Cesarean section 178 (26.1 %) 666 (22.4 %) Note. Data are presented as mean with standard deviation or number with percentage; P < 0.05 denotes statistical significance. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; BMI, body mass index. Table 2. Comparison of the prevalence of self-reported LUTS between the SARS-CoV-2 infected and uninfected groups
LUTS SARS-CoV-2 infected group
(n = 681)SARS-CoV-2 uninfected group
(n = 2971)P-value
COVID-19 infectionStorage symptoms Stress UI 32 (4.7 %) 166 (5.6 %) 0.356 Vaginal delivery 26 (5.2 %) 140 (6.1 %) 0.422 Cesarean section 6 (3.4 %) 26 (3.9 %) 0.769 Urge UI 148 (21.7 %) 663 (22.3 %) 0.741 Vaginal delivery 107 (21.2 %) 501 (21.8 %) 0.779 Cesarean section 41 (23.2 %) 162 (24.1 %) 0.801 Frequency 78 (11.4 %) 374 (12.6 %) 0.641 Vaginal delivery 61 (12.1 %) 303 (13.2 %) 0.513 Cesarean section 17 (9.6 %) 71 (10.5 %) 0.713 Voiding symptoms Terminal dribble 65 (9.5 %) 259 (8.7 %) 0.494 Vaginal delivery 51 (10.1 %) 225 (9.8 %) 0.812 Cesarean section 14 (7.9 %) 34 (5.1 %) 0.143 Note. Data are presented as number with percentage; P < 0.05 denotes statistical significance. LUTS, lower urinary tract symptoms; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UI, urinary incontinence. Table 3. Comparison of questionnaire scores between the SARS-CoV-2 infected and uninfected groups
Questionnaire scores SARS-CoV-2 infected group
(n = 681)SARS-CoV-2 uninfected group
(n = 2971)P-value
COVID-19 infectionIIQ-7 6.23 ± 16.73 7.40 ± 16.91 0.391 Vaginal delivery 6.47 ± 16.32 7.98 ± 18.82 0.293 Cesarean section 3.08 ± 9.42 3.55 ± 14.13 0.795 Note. Data are presented as mean with standard deviation; P < 0.05 denotes statistical significance. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IIQ-7, Impact Questionnaire Short Form questionnaire. -
[1] Zhang H, Huang CL, Gu XY, et al. 3-year outcomes of discharged survivors of COVID-19 following the SARS-CoV-2 omicron (B. 1.1. 529) wave in 2022 in China: a longitudinal cohort study. Lancet Respir Med, 2024; 12, 55−66. doi: 10.1016/S2213-2600(23)00387-9 [2] Ballering AV, van Zon SKR, Olde Hartman TC, et al. Persistence of somatic symptoms after COVID-19 in the netherlands: an observational cohort study. Lancet, 2022; 400, 452−61. doi: 10.1016/S0140-6736(22)01214-4 [3] Kaya Y, Kaya C, Kartal T, et al. Could LUTS be early symptoms of COVID-19. Int J Clin Pract, 2021; 75, e13850. [4] Tiryaki S, Egil O, Birbilen AZ, et al. COVID-19 associated lower urinary tract symptoms in children. J Pediatr Urol, 2022; 18, 680.e1−7. doi: 10.1016/j.jpurol.2022.08.018 [5] Khullar V, Lemmon B, Acar O, et al. Does COVID-19 cause or worsen LUT dysfunction, what are the mechanisms and possible treatments? ICI-RS 2023. Neurourol Urodyn, 2024; 43, 1458−63. doi: 10.1002/nau.25441 [6] Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Am J Obstet Gynecol, 2002; 187, 116−26. doi: 10.1067/mob.2002.125704 [7] Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol, 2006; 50, 1306−15. doi: 10.1016/j.eururo.2006.09.019 [8] Zhang L, Zhu L, Xu T, et al. A population-based survey of the prevalence, potential risk factors, and symptom-specific bother of lower urinary tract symptoms in adult Chinese women. Eur Urol, 2015; 68, 97−112. doi: 10.1016/j.eururo.2014.12.012 [9] Wang XJ, Wang HY, Xu P, et al. Epidemiological trends and risk factors related to lower urinary tract symptoms around childbirth: A one-year prospective study. BMC Public Health, 2023; 23, 2134. doi: 10.1186/s12889-023-17065-w [10] Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol, 2014; 123, 1201−6. doi: 10.1097/AOG.0000000000000286 [11] Blomquist JL, Muñoz A, Carroll M, et al. Association of delivery mode with pelvic floor disorders after childbirth. JAMA, 2018; 320, 2438−47. doi: 10.1001/jama.2018.18315 [12] Pang H, Zhang L, Han S, et al. A nationwide population-based survey on the prevalence and risk factors of symptomatic pelvic organ prolapse in adult women in China - a pelvic organ prolapse quantification system-based study. BJOG, 2021; 128, 1313−23. doi: 10.1111/1471-0528.16675 [13] Milsom I, Gyhagen M. Breaking news in the prediction of pelvic floor disorders. Best Pract Res Clin Obstet Gynaecol, 2019; 54, 41−8. doi: 10.1016/j.bpobgyn.2018.05.004 [14] Daryanto B, Janardhana A, Purnomo AF. The effect of covid-19 severity on lower urinary tract symptoms manifestations. Med Arch, 2022; 76, 127−30. doi: 10.5455/medarh.2022.76.127-130 [15] Mumm JN, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does sars-cov-2 cause viral cystitis? Eur Urol, 2020; 78, 624-8. [16] Dhar N, Dhar S, Timar R, et al. De novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J Clin Med Res, 2020; 12, 681−2. doi: 10.14740/jocmr4294 [17] Ferrari A, Corazza I, Mannella P, et al. Influence of the COVID-19 pandemic on self-reported urinary incontinence during pregnancy and postpartum: a prospective study. Int J Gynaecol Obstet, 2023; 160, 187−94. doi: 10.1002/ijgo.14522 [18] de Castro Sousa F, Estevam LF, Silva EP, et al. Possible association of urinary incontinence with post-COVID-19: a report of three cases. J Infect Dev Ctries, 2023; 17, 1544−8. doi: 10.3855/jidc.17431 [19] Prudencio CB, Nunes SK, Pinheiro FA, et al. Gestational diabetes is associated with alteration on pelvic floor muscle activation pattern during pregnancy and postpartum: Prospective cohort using electromyography assessment. Front Endocrinol (Lausanne), 2022; 13, 958909. doi: 10.3389/fendo.2022.958909 [20] Glazer HI, Hacad CR. The glazer protocol: evidence-based medicine pelvic floor muscle (PFM) surface electromyography (SEMG). Biofeedback, 2012; 40, 75−9. doi: 10.5298/1081-5937-40.2.4 [21] Min L, Xudong D, Qiubo L, et al. Two year follow-up and comparison of pelvic floor muscle electromyography after first vaginal delivery with and without episiotomy and its correlation with urinary incontinence: a prospective cohort study. Acta Obstet Gynecol Scand, 2023; 102, 200−8. doi: 10.1111/aogs.14487 [22] Davis HE, McCorkell L, Vogel JM, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol, 2023; 21, 133−46. doi: 10.1038/s41579-022-00846-2 [23] Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis, 2022; 22, e102−7. doi: 10.1016/S1473-3099(21)00703-9 [24] Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the incontinence impact questionnaire and the urogenital distress inventory. Neurourol Urodyn, 1995; 14, 131−9. doi: 10.1002/nau.1930140206 [25] Chan SSC, Choy KW, Lee BPY, et al. Chinese validation of urogenital distress inventory and incontinence impact questionnaire short form. Int Urogynecol J, 2010; 21, 807−12. doi: 10.1007/s00192-010-1102-8 [26] Glazer HI, Romanzi L, Polaneczky M. Pelvic floor muscle surface electromyography. Reliability and clinical predictive validity. J Reprod Med, 1999; 44, 779−82. [27] Tähtinen RM, Cartwright R, Tsui JF, et al. Long-term impact of mode of delivery on stress urinary incontinence and urgency urinary incontinence: a systematic review and meta-analysis. Eur Urol, 2016; 70, 148−58. doi: 10.1016/j.eururo.2016.01.037 [28] Hage-Fransen MAH, Wiezer M, Otto A, et al. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: a systematic review and meta-analysis. Acta Obstet Gynecol Scand, 2021; 100, 373−82. doi: 10.1111/aogs.14027 [29] Yang XY, Zhu LL, Li WJ, et al. Comparisons of electromyography and digital palpation measurement of pelvic floor muscle strength in postpartum women with stress urinary incontinence and asymptomatic parturients: a cross-sectional study. Gynecol Obstet Invest, 2019; 84, 599−605. doi: 10.1159/000501825 [30] Ptaszkowski K, Malkiewicz B, Zdrojowy R, et al. The prognostic value of the surface electromyographic assessment of pelvic floor muscles in women with stress urinary incontinence. J Clin Med, 2020; 9, 1947. doi: 10.3390/jcm9061947 [31] Koelbl H, Strassegger H, Riss PA, et al. Morphologic and functional aspects of pelvic floor muscles in patients with pelvic relaxation and genuine stress incontinence. Obstet Gynecol, 1989; 74, 789−95. [32] Vásconez-González J, Fernandez-Naranjo R, Izquierdo-Condoy JS, et al. Comparative analysis of long-term self-reported COVID-19 symptoms among pregnant women. J Infect Public Health, 2023; 16, 430−40. doi: 10.1016/j.jiph.2023.01.012 [33] Salamanna F, Veronesi F, Martini L, et al. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne), 2021; 8, 653516. [34] dos Santos PK, Sigoli E, Bragança LJG, et al. The musculoskeletal involvement after mild to moderate COVID-19 infection. Front Physiol, 2022; 13, 813924. doi: 10.3389/fphys.2022.813924 [35] Peghin M, Palese A, Venturini M, et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect, 2021; 27, 1507−13. doi: 10.1016/j.cmi.2021.05.033 [36] Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev, 2020; 64, 101185. doi: 10.1016/j.arr.2020.101185 [37] Hofmann H, Önder A, Becker J, et al. Markers of oxidative stress during post-COVID-19 fatigue: a hypothesis-generating, exploratory pilot study on hospital employees. Front Med, 2023; 10, 1305009. doi: 10.3389/fmed.2023.1305009 [38] Leitner BP, Joseph P, Quast AF, et al. The metabolic and physiologic impairments underlying long COVID associated exercise intolerance. Pulm Circ, 2024; 14, e70009. doi: 10.1002/pul2.70009 [39] Bizjak DA, Ohmayer B, Buhl JL, et al. Functional and morphological differences of muscle mitochondria in chronic fatigue syndrome and post-COVID syndrome. Int J Mol Sci, 2024; 25, 1675. doi: 10.3390/ijms25031675 [40] Scheibenbogen C, Wirth KJ. Key pathophysiological role of skeletal muscle disturbance in post COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): accumulated evidence. J Cachexia Sarcopenia Muscle, 2025; 16, e13669. doi: 10.1002/jcsm.13669 [41] Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care, 2014; 31, 562−75. doi: 10.1177/1049909113494748 [42] Appelman B, Charlton BT, Goulding RP, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun, 2024; 15, 17. doi: 10.1038/s41467-023-44432-3 [43] Shaw JM, Wolpern A, Wu JQ, et al. Postpartum sedentary behaviour and pelvic floor support: a prospective cohort study. J Sports Sci, 2023; 41, 141−50. doi: 10.1080/02640414.2023.2202063 [44] Tryfonos A, Pourhamidi K, Jörnåker G, et al. Functional limitations and exercise intolerance in patients with post-COVID condition: a randomized crossover clinical trial. JAMA Netw Open, 2024; 7, e244386. doi: 10.1001/jamanetworkopen.2024.4386 [45] Peter RS, Nieters A, Göpel S, et al. Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: a population-based, nested case-control study. PLoS Med, 2025; 22, e1004511. doi: 10.1371/journal.pmed.1004511 -




 下载:
下载:



 Quick Links
Quick Links