-
Atmospheric particulate matter (PM) 2.5 is a major carcinogen and is associated with an increased risk of developing various cancers and pulmonary fibrous diseases[1–2]. Non-small cell lung cancer (NSCLC) is the major type of lung cancer with high morbidity and mortality rates. Epidemiological studies report that the incidence of NSCLC, particularly adenocarcinoma, significantly differs with the gender[3]. Female patients with NSCLC have unique epidemiologic characteristics, imaging profiles, clinical manifestations, and pathological characteristics[4]. Non-smoking women have a 2.5-times higher risk of developing NSCLC than non-smoking men, suggesting the effect of estrogen and its receptors[5–6]. Activated estrogen receptors (ERs) can regulate the expression of many genes and interact with several signaling pathways to regulate the malignancy of cancers; however, the ER-related mechanism underlying the regulation of PM2.5-modulated malignancy of NSCLC is unclear.
ERα and ERβ are members of the nuclear receptor family, and the interaction of ERs and their ligands can activate the ERs and promote their nuclear translocation, thereby regulating gene expression. ERβ is the main functional receptor of estrogen in the lungs[7], and NSCLC has been a concern to the prognostic significance of ERβ. The expression of the ERα gene in NSCLC varies and has been detected in the nucleus and cytoplasm of cancer cells[8]. Furthermore, high levels of ERα mRNA transcripts were detectable in NSCLC tissues from female patients with NSCLC[9], suggesting that ERα may contribute to the development of NSCLC in women; however, the importance of ERα expression in PM2.5-modulated malignancy of NSCLC cells, and whether exposure to PM2.5 can modulate ERα expression are unclear. This study aimed to explore whether PM2.5 can modulate the malignancy of human NSCLC and to identify the potential role of ERα-related mechanisms.
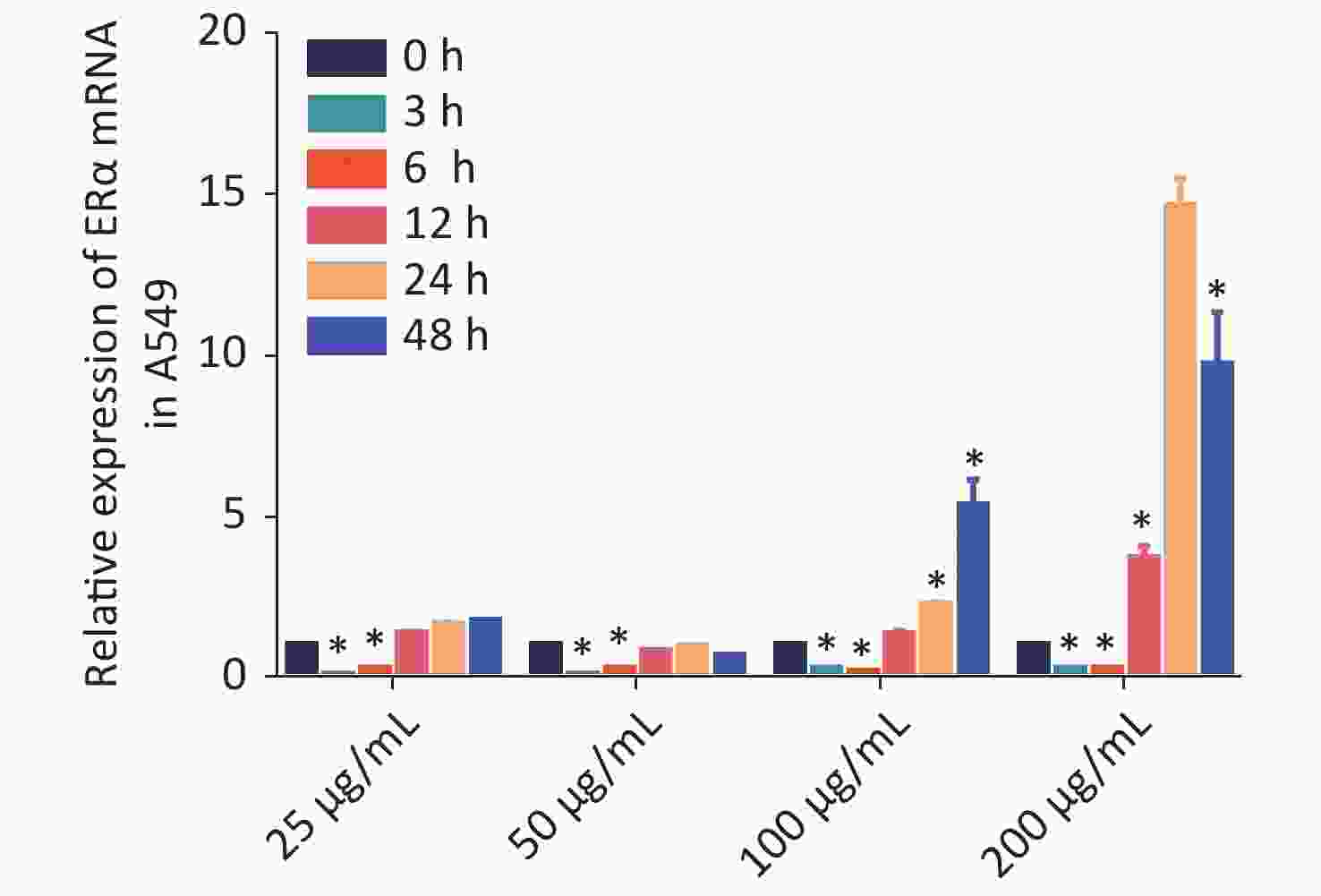
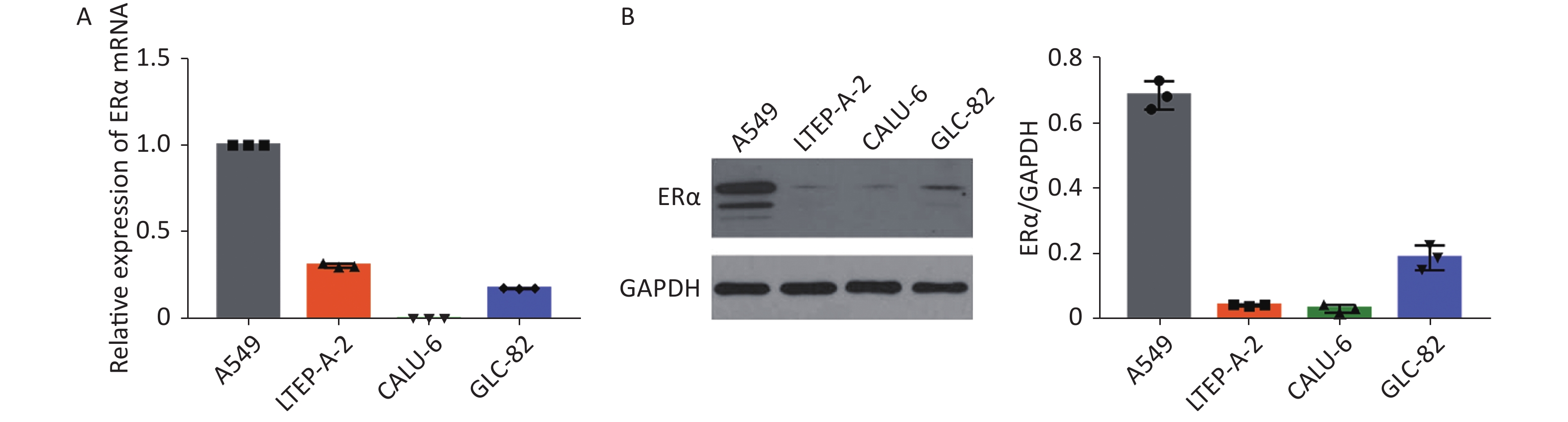
Using RT-qPCR and Western blot, we examined the relative expression levels of the mRNA transcript and protein expression of ERα in human NSCLC A549, LTEP-A-2, CALU-6, and lung adenocarcinoma GLC-82 (Supplementary Figure S1 available in www.besjournal.com). The results indicated different expression levels of ERα in different types of cancer cells and that A549 cells highly expressed ERα. Subsequently, we examined the effect of PM2.5 in modulating ERα expression in A549 cells using RT-qPCR. A549 cells were incubated with different concentrations of PM2.5 (0, 25, 50, 100, and 200 µg/mL) for 0, 3, 6, 12, 24, and 48 h, and the relative expression levels of ERα mRNA transcripts were quantified by using RT-qPCR (Supplementary Figure S2 available in www.besjournal.com). Treatment with 25 or 50 µg/mL PM2.5 significantly decreased ERα mRNA transcript levels in A549 cells, whereas treatment with higher doses of PM2.5 (> 50 µg/mL) for 24 h significantly increased the relative levels of ERα mRNA transcripts in A549 cells. The highest levels of ERα mRNA transcripts in A549 cells were observed after treatment with 200 µg/mL PM2.5 for 24 h. We used these experimental conditions for subsequent experiments. We also tested the effect of PM2.5 on ERα expression in CALU-6 cells.
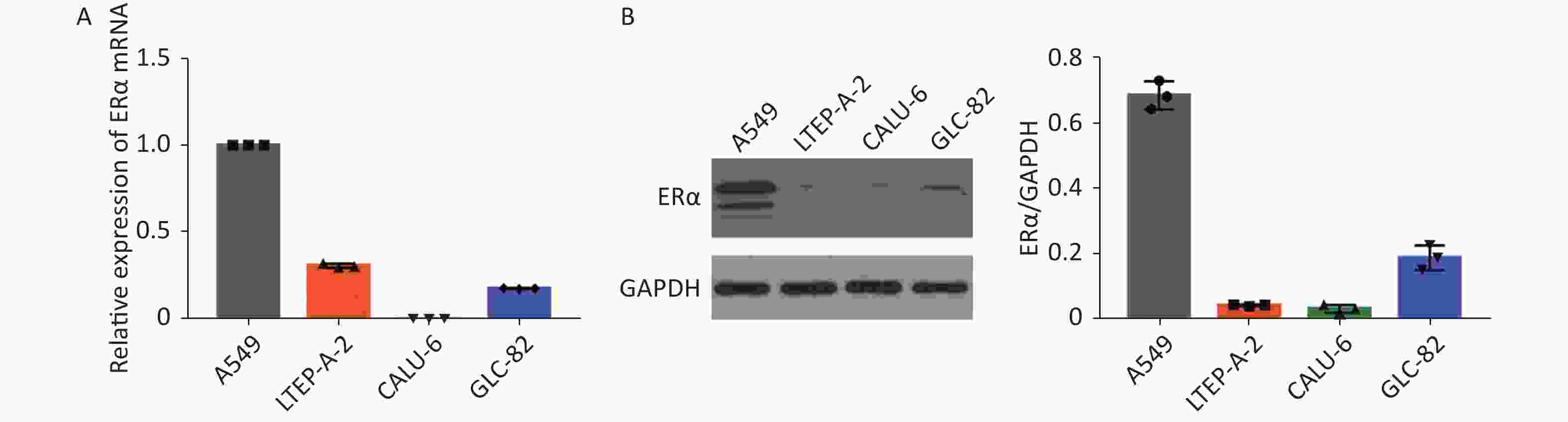
Figure S1. ERα expression in NSCLC cell lines A549, LTEP-A-2, CALU-6 and lung adenocarcinoma cell line GLC-82.
We incubated CALU-6 and A549 cells with 200 µg/mL PM2.5 for 0, 24, 48, and 72 h to determine the effect of PM2.5 on the proliferation of NSCLC. MTT assay was performed to evaluate cell proliferation (Figure 1A); the results indicated that cell proliferation in the PM2.5 treatment group significantly increased compared to that in the group without PM2.5 in A549 cells. In CALU-6 cells, the change of cell proliferation was not obvious compared to that in A549 cells. To identify the role of ERα, A549 cells were transfected with controls or ERα-specific siRNA. The cells were treated with or without 200 µg/mL PM2.5 for 0, 24, 48, and 72 h, and their proliferation was quantified by using an MTT assay. As shown in Figure 1B, we observed no significant difference between the proliferation of the control and ERα-silenced A549 cells, indicating that ERα silencing did not affect the proliferation of A549 cells. Furthermore, while PM2.5 treatment stimulated the proliferation of control A549 cells in a time-dependent manner, the same treatment only moderately increased the proliferation of ERα-silenced A549 cells, although their proliferation remained significantly higher than that of the PM2.5-untreated cells (P < 0.05). Thus, PM2.5 stimulates the proliferation of A549 cells, and A549 cells are partially dependent on high levels of ERα expression.
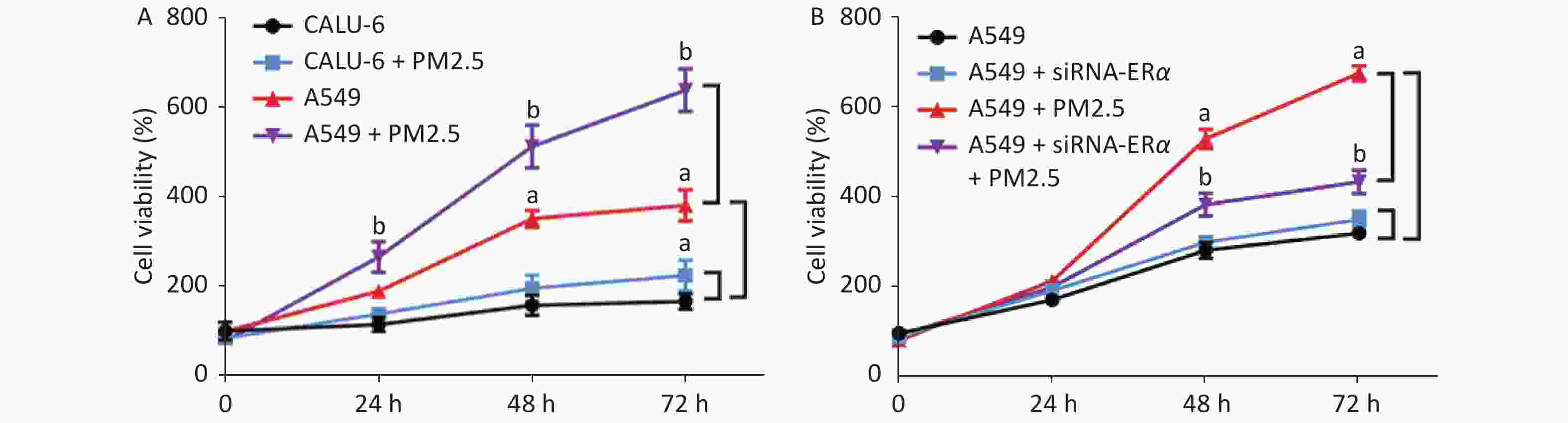
Figure 1. PM2.5 exposure stimulated the proliferations of CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
Next, we tested whether PM2.5 could modulate PI3K/AKT signaling in CALU-6 and A549 cells. Using western blot, the expression levels of ERα, PI3K, AKT, and AKT phosphorylation were quantified. The treatment mode and grouping of the two cell lines were consistent with that in the MTT experiments, except that the treatment mode of PM2.5 was changed to a concentration of 200 µg/mL for 24 h. In the PM2.5 treatment group of CALU-6 cells, the expression levels of ERα, PI3K, AKT, and p-AKT were higher than those in the control group; the trend of expression levels in A549 cells was similar to that in CALU-6 cells (Figure 2A). Moreover, transfection with siRNA resulted in a nearly 50% decrease in ERα protein expression, whereas treatment with PM2.5 slightly enhanced ERα expression in A549 cells, which was significantly mitigated in ERα-silenced A549 cells. We identified a similar expression pattern of PI3K protein across different groups of cells. Furthermore, ERα silencing did not alter the relative levels of AKT expression and phosphorylation in A549 cells, whereas treatment with PM2.5 significantly enhanced AKT expression and phosphorylation, which were abrogated in ERα-silenced A549 cells (Figure 2B). Accordingly, PM2.5 enhanced PI3K/AKT signaling in A549 cells, partially dependent on high levels of ERα expression.
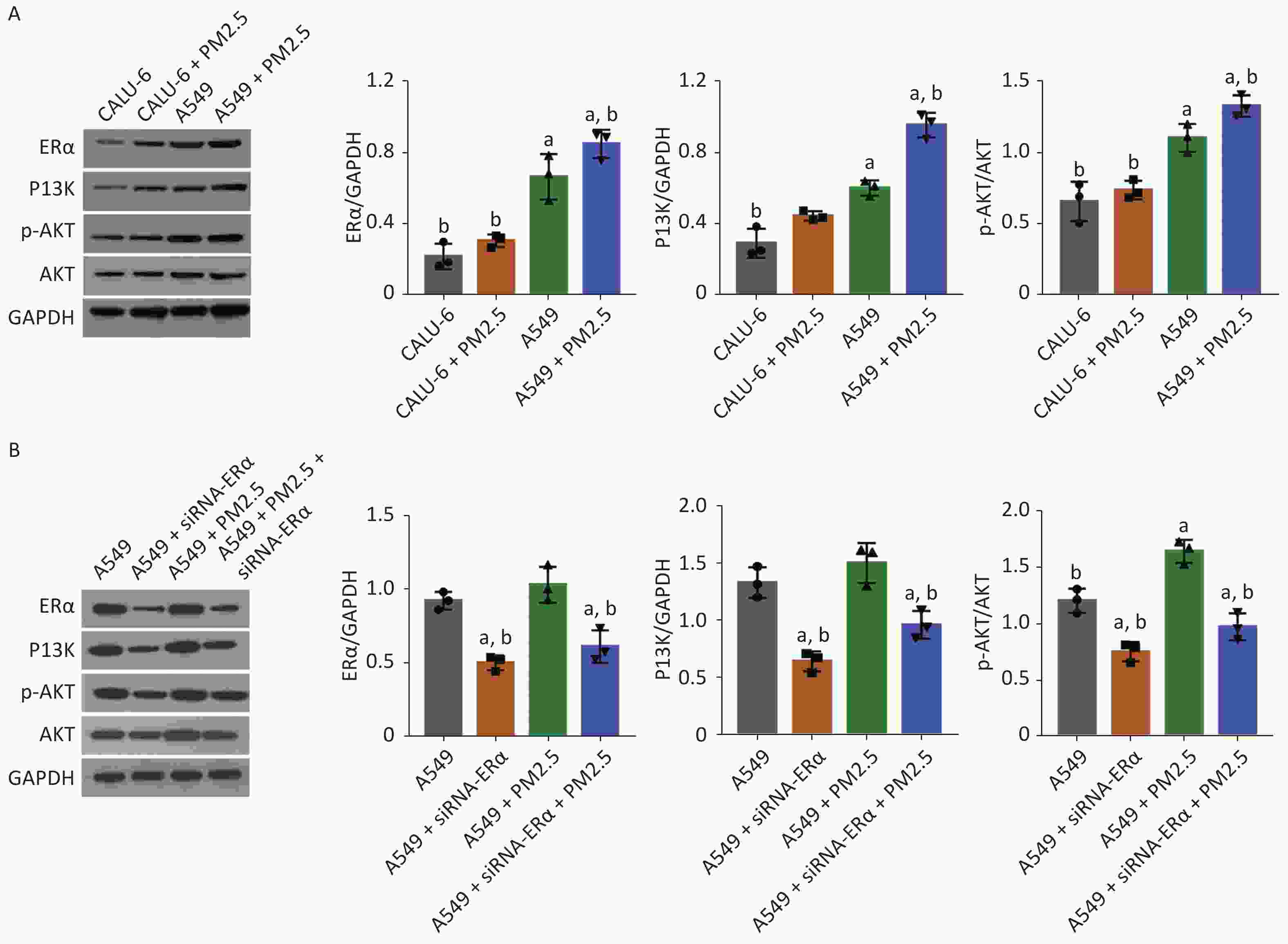
Figure 2. PM2.5 exposure enhanced the PI3K/AKT activation in CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
We further examined the role of PM2.5 using wound-healing experiments in A549 cells and transwell invasion assay in A549 and CALU-6 cells. We found that ERα silencing slightly inhibited wound healing, whereas treatment with 200 µg/mL PM2.5 significantly enhanced wound healing in A549 cells in a time-dependent manner (P < 0.05, Figure 3A). Compared to that in PM2.5-treated control cells, the proportion of wound healing areas in ERα-silenced A549 cells were significantly reduced (P < 0.05), but remained significantly higher than that in the control (P < 0.05). In the transwell invasion assay of CALU-6 cells, the number of invaded cells was significant increased in the PM2.5 group compared to the control group after incubation for 48 h (P < 0.05) (Figure 3B). In A549 cells, there was no significant change in the number of invaded cells after incubation for 24 h, whereas PM2.5 significantly enhanced the invasion and ERα silencing inhibited the invasion after incubation for 48 h (Figure 3C). Altogether, these findings reveal that PM2.5 promotes wound healing and invasion of A549 cells.
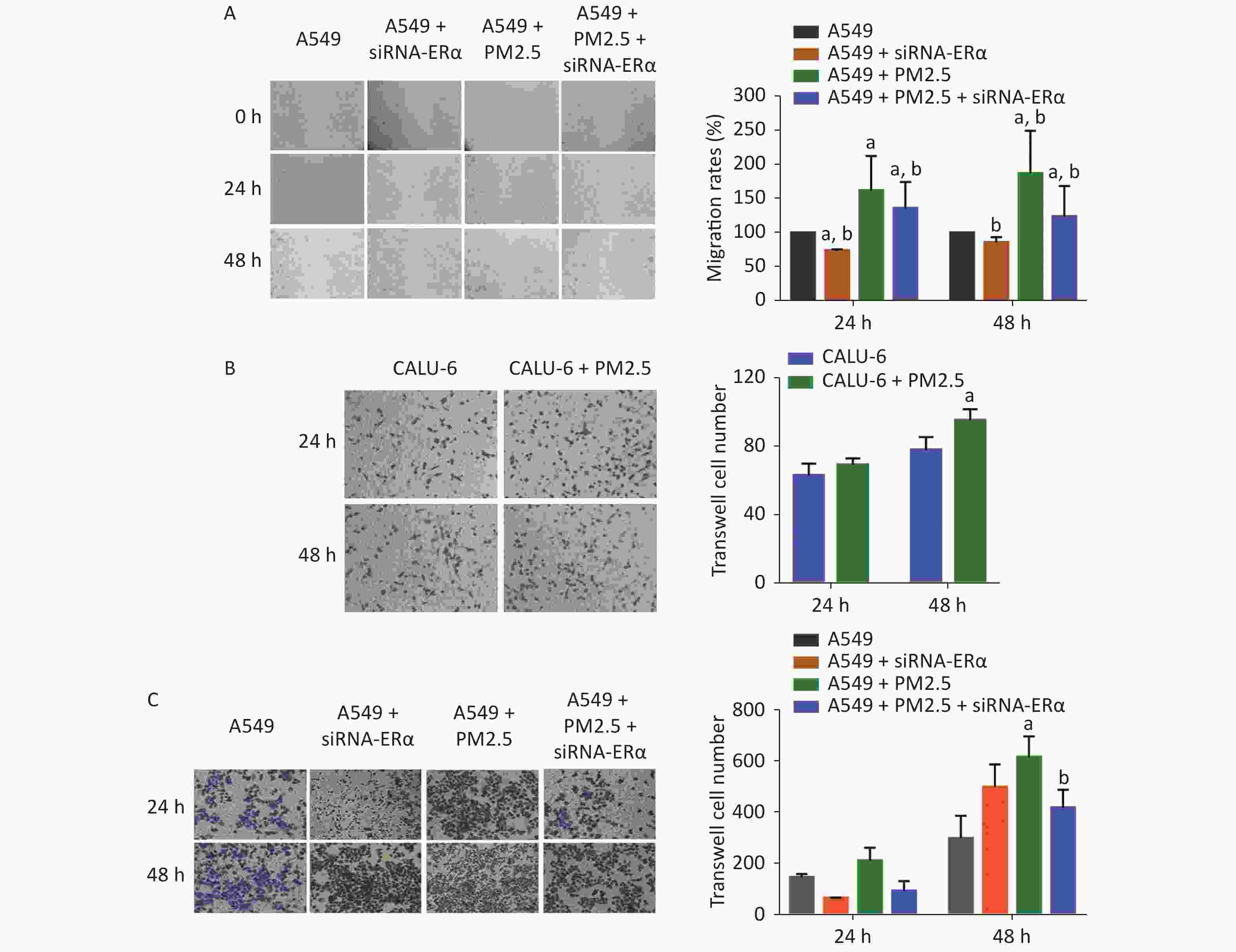
Figure 3. PM2.5 exposure increased the wound healing of A549 cells and invasions of CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
In summary, our data indicated that PM2.5 exposure significantly increased the proliferation, wound healing, and invasion of A549 and CALU-6 cells. The proliferation, wound healing, and invasion of A549 cells were significantly higher than those in CALU-6 cells; this finding was consistent with previous observations[10]; however, the effects of PM2.5 on malignant behaviors were significantly mitigated or abrogated in ERα-silenced A549 cells. These findings clearly indicate that PM2.5 enhances the malignancy of A549 cells. More importantly, exposure to PM2.5 enhanced PI3K expression and AKT activation, which were both significantly mitigated or demolished in ERα-silenced A549 cells. These findings indicate that PM2.5 enhanced PI3K/AKT activation in A549 cells. Accordingly, ERα may be essential in the PM2.5 exposure-enhanced progression of NSCLC and could be a new therapeutic target in women with NSCLC. Given that PI3K/AKT signaling inhibitors could benefit patients with NSCLC, the combination of tyrosine kinase inhibitors (TKIs) for ERs may be valuable in controlling NSCLC progression and preventing TKI-related drug resistance.
doi: 10.3967/bes2021.041
Exposure to PM2.5 Enhances the PI3K/AKT Signaling and Malignancy of ERα Expression-dependent Non-small Cell Lung Carcinoma
-
&These authors contributed equally to this work.
注释: -
S1. ERα expression in NSCLC cell lines A549, LTEP-A-2, CALU-6 and lung adenocarcinoma cell line GLC-82.
The relative levels of ERα protein expression and mRNA transcripts were quantified by Western blot and RT-qPCR. All data were representative images or expressed as the mean ± SD of each group from three separate experiments. (A) RT-qPCR analysis of ERα mRNA transcripts in different cell lines. (B) Western Blot analysis of ERα protein expression in different cell lines.
S2. PM2.5 exposure stimulated ERα mRNA transcription in A549 cells.
A549 cells were treated in triplicate with the indicated concentrations of PM2.5 for the indicated varying time periods. The cells were harvested and the relative levels of ERα mRNA transcripts were quantified by RT-qPCR. All data were expressed as the mean ± SD of each group from three separate experiments. *P < 0.05 vs. the untreated control.
Figure 1. PM2.5 exposure stimulated the proliferations of CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
(A) Changes of CALU-6 and A549 cell proliferation after 200 µg/mL PM2.5 treatment for 0, 24 , 48 , 72 h. The cell proliferation was measured by MTT. All data were expressed as the mean ± SD of each group from six separate experiments. aP < 0.05 vs. the CALU-6 group. bP < 0.05 vs. the A549 group. (B) A549 cells were transfected with control or ERα-specific siRNA and the cells were treated with or without 200 µg/mL PM2.5 for the indicated time periods. The cell proliferation was measured by MTT. All data were expressed as the mean ± SD of each group from six separate experiments. aP < 0.05 vs. the control group. bP < 0.05 vs. the PM2.5 group.
Figure 2. PM2.5 exposure enhanced the PI3K/AKT activation in CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
(A) Western blot showing the expression changes of ERα, PI3K, AKT and p-AKT in CALU-6 and A549 cells after 200 µg/mL PM2.5 treatment for 24 h. All data were representative images or expressed as the mean ± SD of each group from three separate experiments. aP < 0.05 vs. the CALU-6 group. bP < 0.05 vs. the A549 group. (B) A549 cells were transfected with control or ERα-specific siRNA and the cells were treated with or without 200 µg/mL PM2.5 for 24 h. Western blot showing the expression changes of ERα, PI3K, AKT and p-AKT in each group of A549 cells. All data were representative images or expressed as the mean ± SD of each group from three separate experiments. aP < 0.05 vs. the control group. bP < 0.05 vs. the PM2.5 group.
Figure 3. PM2.5 exposure increased the wound healing of A549 cells and invasions of CALU-6 and A549 cells, partially dependent on high levels of ERα expression.
(A) Following transfection with siRNA for 24 h, the cells were cultured up to 65% of confluence. Subsequently, the cells were wounded and treated in triplicate with or without 200 µg/mL PM2.5 to culture for the indicated time periods, followed by photoimaged. All data were representative images or expressed as the mean ± SD of each group from three separate experiments. aP < 0.05 vs. the control group. bP < 0.05 vs. the PM2.5 group. (B)–(C) These two types of cells (5 × 104 cells/well) were cultured with serum-free medium in the top chamber that had been coated with Matrigel. The bottom chambers were filled with 20% FBS medium. The cells were cultured for 24 or 48 h and invaded cells were photoimaged and counted. Data were representative images or expressed as the mean ± SD of each group from three separate experiments. (B) The invasion results of CALU-6 cell with or without 200 µg/mL PM2.5 for 24 h. aP < 0.05 vs. the CALU-6 group. (C) The invasion results of A549 cells that were transfected with control or ERα-specific siRNA and treated with or without 200 µg/mL PM2.5 for 24 h. aP < 0.05 vs. the control group. bP < 0.05 vs. the PM2.5 group.
-
[1] Pope III CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA, 2002; 287, 1132−41. doi: 10.1001/jama.287.9.1132 [2] Tomczak A, Miller AB, Weichenthal SA, et al. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer, 2016; 139, 1958−66. doi: 10.1002/ijc.30255 [3] de Perrot M, Licker M, Bouchardy C, et al. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg, 2000; 119, 21−6. doi: 10.1016/S0022-5223(00)70213-3 [4] Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroidsm, 2007; 72, 135−43. doi: 10.1016/j.steroids.2006.11.019 [5] Brueckl WM, Al-Batran SE, Ficker JH, et al. Prognostic and predictive value of estrogen receptor 1 expression in completely resected non-small cell lung cancer. Int J Cancer, 2013; 133, 1825−31. doi: 10.1002/ijc.28209 [6] Siegfried JM, Stabile LP. Estrogenic steroid hormones in lung cancer. Semin Oncol, 2014; 41, 5−16. doi: 10.1053/j.seminoncol.2013.12.009 [7] Zhang GF, Yanamala N, Lathrop KL, et al. Ligand-independent antiapoptotic function of estrogen receptor-β in lung cancer cells. Mol Endocrinol, 2010; 24, 1737−47. doi: 10.1210/me.2010-0125 [8] Gomez-Fernandez C, Mejias A, Walker G, et al. Immunohistochemical expression of estrogen receptor in adenocarcinomas of the lung: the antibody factor. Appl Immunohistochem Mol Morphol, 2010; 18, 137−41. doi: 10.1097/PAI.0b013e3181bec23b [9] McHugh MK, Lopez MS, Ho CH, et al. Use of the cytokinesis-blocked micronucleus assay (CBMN) to detect gender differences and genetic instability in a lung cancer case-control study. Cancer Epidemiol Biomarkers Prev, 2013; 22, 135−45. doi: 10.1158/1055-9965.EPI-12-0435 [10] Yang B, Chen DM, Zhao H, et al. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. Springerplus, 2016; 5, 2059. doi: 10.1186/s40064-016-3734-8 -




 下载:
下载:



 Quick Links
Quick Links