-
Preterm birth (PTB), defined as a live birth occurring before 37 weeks of gestation, is associated with numerous adverse outcomes. These include poor growth, respiratory disorders, heightened susceptibility to infections because of low body weights, underdeveloped body functions, and low immunity. Such complications considerably affect infant health, pose life-threatening risks, and impose substantial social and economic burdens. The incidence of PTB in China has increased from 5.36% in 1990–1994 to 7.04% in 2015–2016[1]. The etiology of PTB is complex, with environmental factors such as the air pollutant PM2.5 (aerodynamic diameter ≤ 2.5 μm) playing a crucial role. PM2.5 exposure is associated with increased risk of infant mortality and adverse health outcomes, including endocrine disorders, intrauterine infections, and placental ischemia[2]. Although previous studies have established associations between PM2.5 and adverse pregnancy outcomes, including PTB, birth weight, gestational diabetes mellitus, and preeclampsia[3], the causal relationship remains unclear.
Mendelian randomization (MR) has become a widely used technique in recent years for causal analysis in clinical and epidemiological studies. This study aimed to investigate the causal relationship between PM2.5 exposure and birth outcomes, including PTB, birth weight, and miscarriage, using MR to provide a reference basis for the clinical prevention of PTB and to reduce the social and economic burden.
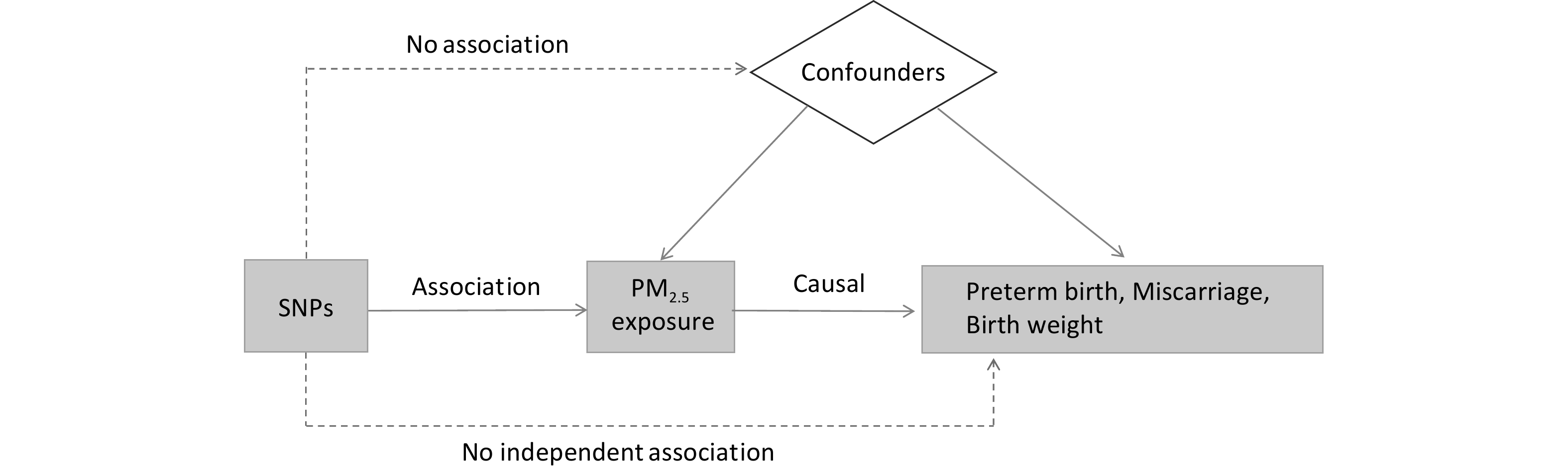
In this study, single-nucleotide polymorphisms (SNPs) from genome-wide association studies were used as the basis for two-sample MR analyses to assess the association and causal relationship between PM2.5 and birth outcomes, including PTB, miscarriage, and birth weight. Sensitivity analysis was performed to verify the validity of the results. The effects of environmental and other confounding factors can be minimized using the MR analysis, requiring variables to satisfy three main core assumptions: (1) The instrumental variables screened must be related to exposure. In this study, SNPs related to PM2.5 exposure were identified through a genome-wide analysis (threshold set at P < 5.0 × 10-8, set parameters: R2 = 0.001, kb > 10,000). (2) The instrumental variables must influence the outcome only through exposure; if they affect the outcome through other pathways, it indicates genetic pleiotropy. To address this, sensitivity analyses and pleiotropy tests were conducted to eliminate potential interference. (3) The screened instrumental variables must be independent of confounding factors. A flowchart illustrating the MR analysis is shown in Figure 1.
SNPs associated with PM2.5 exposure were obtained from the UK Biobank dataset (GWAS ID: ukb-b-11312). Data on the outcome variables (PTB, miscarriage, and birth weight) were derived from the Early Growth Genetics summary data[4]. MR analysis methods, including inverse-variance weighted (IVW), weighted median (WM), MR-Egger, simple mode, and weighted mode were used to determine the relationship between exposure to PM2.5, and birth outcomes. Of these, IVW provides the most precise assessment with an integrated assessment of the causal effects of multiple genetic variants that are less affected by heterogeneity and instrumental variable pleiotropy. Therefore, the results of IVW were used as an evaluation indicator, and the final effect size was assessed using additional analytical methods, which were robust when their effect size (β-value) was consistent with IVW. The MR-Egger regression effects model was used in the sensitivity analyses to assess pleiotropy and exclude instrumental variables from influencing outcomes through pathways other than exposure. Heterogeneity in IVW analyses was examined using Cochran's Q statistic, and P > 0.05 indicated that no heterogeneity existed in the estimates of SNP effect. Simultaneously, the selected SNPs were eliminated individually using the leave-one-out method and analyzed to determine whether they affected overall causality. The F statistic was calculated according to the formula (F = β2 / SE2) to determine the potential bias of instrumental variables[5]. Instrumental variables were considered strongly correlated with exposure when the F statistic exceeded 10. The PM2.5 exposure dataset, comprising 423,796 individuals of European ancestry was analyzed, identifying eight independent SNPs for PM2.5 exposure, as shown in Supplementary Table S1.
Table S1. Single nucleotide polymorphisms of PM2.5 included in the MR analyses
SNP CHR β SE EAF P F rs114708313 6 0.025 0.004 0.066 4.20E-08 30.076 rs12203592 6 0.022 0.003 0.213 6.20E-17 69.918 rs1372504 5 0.012 0.002 0.374 3.10E-08 30.674 rs1537371 9 0.012 0.002 0.5 8.50E-09 33.149 rs6749467 2 -0.012 0.002 0.466 1.40E-08 32.228 rs72642437 18 0.113 0.019 0.004 3.10E-09 35.119 rs77205736 8 0.014 0.002 0.274 2.10E-08 31.399 rs77255816 6 0.031 0.006 0.037 4.20E-08 30.041 Note. EAF is the effect allele frequency and β is the effect value, CHR is the chromosome numbering. The analysis results revealed a causal relationship between PM2.5 exposure and PTB (OR = 1.99, 95% CI: 1.21–3.29, PIVW < 0.01). The effect size across the remaining four analytical methods was consistent with the IVW results (Supplementary Figure S1). The results suggest that PM2.5 exposure leads to an increased risk of PTB. The heterogeneity test using Cochran’s Q test showed P = 0.61 > 0.05, indicating that the results were not heterogeneous. Horizontal pleiotropy was assessed using the MR-Egger regression method and the intercept term (P = 0.43 > 0.05). The selected SNPs were eliminated individually to analyze whether they affected the overall causality. The results showed that no SNP sites significantly affected the overall causality (Supplementary Figure S2). The two methods showed that no horizontal pleiotropy or heterogeneity existed among the selected SNPs and that the screened instrumental variables were reliable. Our results confirm that exposure to PM2.5 during pregnancy is contributes to PTB risk. However, according to the World Health Organization’s Maternal Health Surveys in 24 countries, no notable association exists between PM2.5 exposure during pregnancy and PTB. This relationship has been previously confirmed by several studies. The potential mechanisms by which PM2.5 exposure causes PTB during pregnancy remain unclear, and both the composition and exposure status of PM2.5 may influence the occurrence of PTB. In a cohort study of 336 Chinese cities, He et al. found a positive correlation between prenatal exposure to five anthropogenic PM2.5 constituents (OC, BC, SO42-, NH4+, and NO3-) and the occurrence of PTB, with carbonaceous constituents (OC and BC) likely playing a more critical role[6]. Determining the critical window of PM2.5 exposure is crucial for PTB prevention. Late-pregnancy PM2.5 exposure may be a critical period for triggering PTB. However, Guan et al. found in a time-series study in Beijing, China that chronic exposure to PM2.5 had a greater effect on PTB than acute exposure[7].
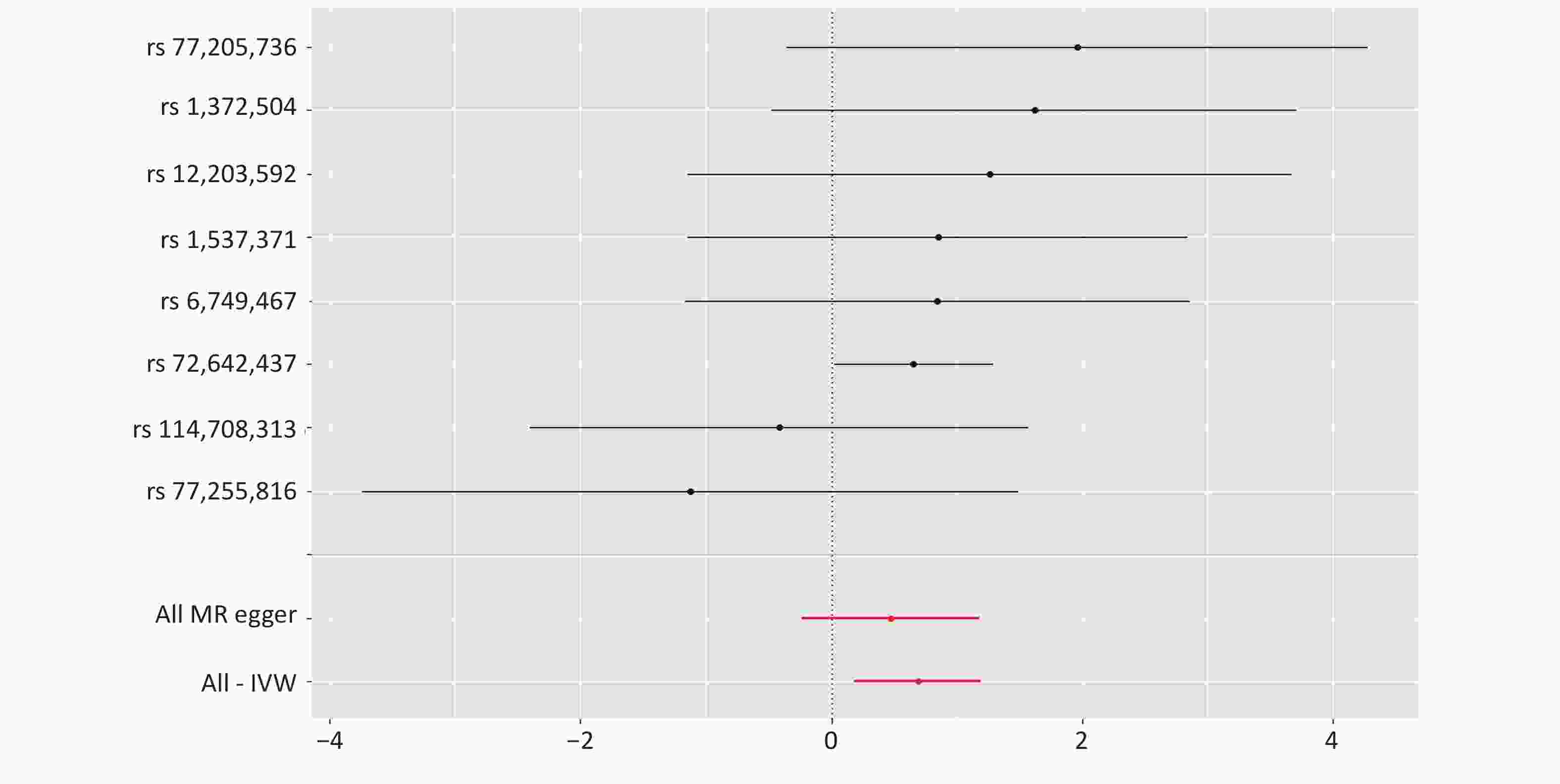
No causal relationship was found between PM2.5 exposure and miscarriage or infant birth weight (P > 0.05) (Figure 2). This may be influenced by the number of SNPs associated with PM2.5, and should be supported and validated using more relevant data in future studies. Air pollution is associated with low birth weight, intrauterine growth retardation, preterm births, and birth defects. Several studies have explored the mechanisms involved in this process. Exposure to airborne particulate matter during pregnancy may cause inflammation in the lungs of the mother, affecting the oxygen and nutrient supply of the body, and the inflammatory response of the placenta may affect the blood and nutrient supply between the mother and the baby, leading to low birth weight[8]. In addition, the components of PM2.5 and the chemicals it carries, such as polycyclic aromatic hydrocarbons and the heavy metals lead, arsenic, and nickel, may lead to impaired function of the umbilical cord, which may affect fetal development. National and international studies on the relationship between PM2.5 exposure and miscarriage, and estimates of stillbirths because of ambient fine particulate matter from 137 countries suggest that at least a quarter of all stillbirths are attributable to PM2.5 exposure during pregnancy[9]. This may be because PM2.5 crosses the placental barrier directly, triggers hypoxia or immune-mediated damage, resulting in irreversible embryonic damage and stillbirth[10].

Figure 2. Forest plot of the Mendelian randomization study on PM2.5 exposure, and birth outcomes. nSNP: number of single nucleotide polymorphisms associated with PM2.5.
This inconsistency in the results may be influenced by factors such as region, population, PM2.5 concentration, exposure time, particle size, and composition. The application of the MR methodology in this study circumvents the inherent limitations of traditional observational studies and enables more accurate causal inferences. However, this study has some limitations. The data used in this study were obtained from individuals of European origin, and the conclusions lack generalizability. Data from populations of different races should be included for additional validation. Although a causal association between PM2.5 exposure and preterm birth was verified in this study, future efforts are still needed to identify critical windows and explore potential mechanisms of action.
In conclusion, this study used MR to find a causal relationship between PM2.5 exposure and the risk of PTB. PM2.5 exposure led to increased occurrence of PTBs. No causal relationship was found in the analysis between PM2.5 exposure and miscarriage or infant birth weight.
doi: 10.3967/bes2025.009
Causal Association between PM2.5 Exposure and Preterm Birth based on Mendelian Randomization Analysis
-
The authors declare that there are no conflict of interest. Data sharing The supplementary materials will be available in www.besjournal.com.
This study is exempt from ethical review.
注释:1) Competing interests: 2) Ethichs: -
S1. Single nucleotide polymorphisms of PM2.5 included in the MR analyses
SNP CHR β SE EAF P F rs114708313 6 0.025 0.004 0.066 4.20E-08 30.076 rs12203592 6 0.022 0.003 0.213 6.20E-17 69.918 rs1372504 5 0.012 0.002 0.374 3.10E-08 30.674 rs1537371 9 0.012 0.002 0.5 8.50E-09 33.149 rs6749467 2 -0.012 0.002 0.466 1.40E-08 32.228 rs72642437 18 0.113 0.019 0.004 3.10E-09 35.119 rs77205736 8 0.014 0.002 0.274 2.10E-08 31.399 rs77255816 6 0.031 0.006 0.037 4.20E-08 30.041 Note. EAF is the effect allele frequency and β is the effect value, CHR is the chromosome numbering. -
[1] Jing SW, Chen C, Gan YX, et al . Incidence and trend of preterm birth in China, 1990-2016: a systematic review and meta-analysis. BMJ Open,2020 ;10 ,e039303 . doi: 10.1136/bmjopen-2020-039303[2] Lavigne É, Burnett RT, Stieb DM, et al . Fine particulate air pollution and adverse birth outcomes: effect modification by regional nonvolatile oxidative potential. Environ Health Perspect,2018 ;126 ,077012 . doi: 10.1289/EHP2535[3] Wang WR, Mu SQ, Yan WZ, et al . Prenatal PM2.5 exposure increases the risk of adverse pregnancy outcomes: evidence from meta-analysis of cohort studies. Environ Sci Pollut Res Int,2023 ;30 ,106145 −97 . doi: 10.1007/s11356-023-29700-5[4] Warrington NM, Beaumont RN, Horikoshi M, et al . Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet,2019 ;51 ,804 −14 . doi: 10.1038/s41588-019-0403-1[5] Kuś A, Kjaergaard AD, Marouli E, et al . Thyroid function and mood disorders: a mendelian randomization study. Thyroid,2021 ;31 ,1171 −81 . doi: 10.1089/thy.2020.0884[6] He Y, Jiang YX, Yang Y, et al . Composition of fine particulate matter and risk of preterm birth: a nationwide birth cohort study in 336 Chinese cities. J Hazard Mater,2022 ;425 ,127645 . doi: 10.1016/j.jhazmat.2021.127645[7] Guan TJ, Xue T, Gao SH, et al . Acute and chronic effects of ambient fine particulate matter on preterm births in Beijing, China: a time-series model. Sci Total Environ,2019 ;650 ,1671 −7 . doi: 10.1016/j.scitotenv.2018.09.279[8] Yuan Z. Progress of research on the association between PM2.5 pollution and the risk of low birth weight development. 2017. (In Chinese) [9] Xue T, Tong MK, Li JJH, et al . Estimation of stillbirths attributable to ambient fine particles in 137 countries. Nat Commun,2022 ;13 ,6950 . doi: 10.1038/s41467-022-34250-4[10] Green R, Sarovar V, Malig B, et al . Association of stillbirth with ambient air pollution in a California cohort study. Am J Epidemiol,2015 ;181 ,874 −82 . doi: 10.1093/aje/kwu460 -
 24313+Supplementary Materials.pdf
24313+Supplementary Materials.pdf

-





 下载:
下载:






 Quick Links
Quick Links