-
Endometrial cancer (EC) is a malignant tumour that occurs in the epithelial cells of the endometrium and represents one of the most common malignancies involving the female reproductive system, with endometrioid adenocarcinoma as the most common type. In recent years, with an increasingly aging society and the growing number of obese people, the incidence of EC is constantly rising, posing a serious threat to women’s health. Some studies have reported that the interruption of digestion and absorption caused by imbalance in intestinal microbiota may lead to conditions such as obesity, hypertension, diabetes, and hormone imbalance, which are all risk factors for EC. Meanwhile, intestinal bacteria produce a series of metabolites during colonization and reproduction, which can rapidly respond to changes in the microenvironment of the body. Changes in their types and quantities can serve as sensitive indicators of physiological and pathological changes in the body. Patients with EC often suffer from metabolic diseases, which can lead to metabolic disorders involving carbohydrates, fats, and amino acid in their bodies.
The intestinal microbiota affects the occurrence and development of EC, but to date, there have been no reports regarding combining intestinal DNA sequencing and metabolomics to conduct correlation analysis in EC. This study adopted advanced intestinal DNA sequencing and metabolomic techniques to conduct a detailed analysis of the intestinal microbial composition and metabolites of patients with EC and a control group. This is expected to provide more precise diagnosis and treatment options for patients with endometrial cancer.
Ten patients diagnosed with EC who received treatment at the Gynaecology Inpatient Department of the First Hospital of Shanxi Medical University from January 2023 to July 2023 were selected as the experimental group (EC group). Ten healthy women undergoing outpatient examinations were chosen randomly as a control group (N group). All the participants were residents of Shanxi Province. The average age of the experimental group was 55.20 ± 5.85 years, and the average age of the control group was 55.30 ± 4.81 years. There were no statistically significant differences in age between the two groups (P > 0.05), ensuring comparability. Approximately 3 g of stool sample was collected from the participants using sterile collection tubes and stored at −80 °C for long-term preservation.
16S rRNA sequencing primarily utilises CTAB for DNA extraction. 16S rRNA genes of distinct V4 regions were amplified using specific primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) and barcoded. Sequencing libraries were generated using NEB Next® Ultra DNA Library Prep Kit (Illumina, USA) following the manufacturer’s recommendations and index codes were added. Finally, the library underwent DNA sequencing on an Illumina NovaSeq platform and 250 bp paired-end reads were generated.
Tissues (100 mg) from each sample were individually ground in liquid nitrogen and the homogenate was resuspended in 80% prechilled methanol thorough vortexing. The samples were incubated on ice for 5 min and were then centrifuged at 15,000 ×g for 20 min at 4 °C. The supernatant was diluted to a final concentration containing 53% methanol using Liquid chromatography–mass spectrometry (LC-MS) grade water. The samples were subsequently transferred to fresh Eppendorf tubes and were centrifuged at 15,000 ×g for 20 min at 4 °C. Finally, the supernatant was injected into a LC-MS/MS system for analysis.
The experimental data were organized and analysed using the software SPSS Statistics 26.0. P-values < 0.05 were considered statistically significant, indicating a significant difference. The analysis of sequencing and metabolite data was performed using QIIME 2 and R software packages.
At the OTU level, β-diversity analysis based on unweighted UniFrac distances that were visualized using PCoA analysis revealed a clear separation between the two groups, indicating differences between the groups. Further analysis was performed using PERMANOVA, a permutational multivariate analysis of variance method that is used to compare discrete data between two or more groups. The results indicated that there was a statistically significant difference in terms of diversity between the EC and N groups, with P < 0.05 (Table 1). Previous studies have associated the decrease in microbial diversity with EC, diabetes, obesity, and various other diseases[1,2], which is consistent with the findings of this study. These analyses imply that a diversity in intestinal microbiota composition may be beneficial to human health.
Table 1. Statistical analysis for inter-group difference
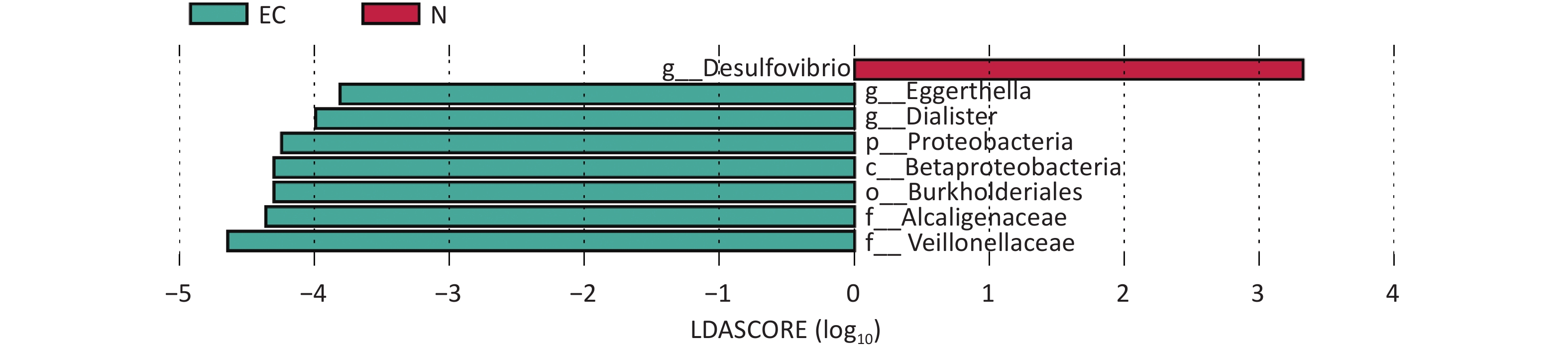
Group 1 Group 2 Sample size Permutations pseudo-F P-value EC N 20 999 1.75 0.03 Note. EC, endometrial cancer (experimental group); N, control group. Linear discriminant analysis Effect Size was performed to compare differences in gut microbiota between the two groups. At genus level, the results revealed that the EC group mainly demonstrated an enrichment of Eggerthella and Dialister, whereas the relative abundance of Desulfovibrio was lower than that of the N group. Other taxonomic level differences were manifested as an enrichment of Proteobacteria, Betaproteobacteria, Burkholderiales, Alcaligenaceae, and Veillonellaceae in the EC group. (LDA score > 2.00, P < 0.05) (Figure 1). A recent Mendelian randomization study reported that γ-Proteobacteria have a causal relationship with EC[3], suggesting that changes in the composition of this bacteria in the gut may play a role in the pathogenesis of EC. Dialister is a pathogenic bacterial genus mainly associated with oral diseases. However, in recent years, increasing evidence has shown that Dialister is also associated with obesity and cancer[4]. The increased abundance of Dialister and microbial genes encoding carbohydrate-active enzymes in the intestine are associated with difficulty in weight loss, as this bacterium may enhance energy utilization from carbohydrate breakdown in individuals struggling with weight reduction[5]. In this study, the presence of Dialister in the EC group was significantly higher than that in the N group, suggesting that it may indirectly affect the pathogenesis of the disease in patients with EC by interfering with the energy absorption process.
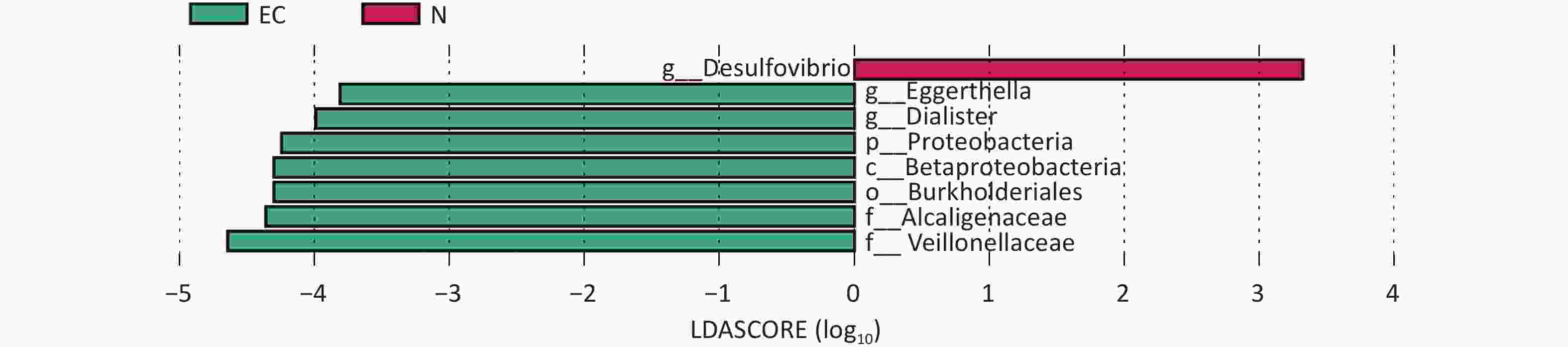
Figure 1. Linear discriminant analysis EFfect Size diagram between two sets of samples, the length of the bar chart represents the impact of different species (i.e. LDA value). EC, experimental group; N, control group.
The total ion flow diagram of the EC and the N group revealed a total of 1,799 metabolites detected from the two sample groups. Supervised Orthogonal Partial Least Squares Discriminant Analysis plots (Figure 2A) along with further validation (Figure 2B) indicated significant differences in the gut metabolome between the two groups (P ≤ 0.05, Q2 = 0.56). We used t-tests to compare specific significantly altered metabolites between the two groups (P ≤ 0.05) (Figure 2C). Equol is a specific end-metabolite produced in the body by the metabolism of soy isoflavones by certain intestinal bacteria. Some studies have shown that germ-free animals do not produce equol[6]. When the level of oestrogen in the body is low, equol can play a role similar to oestrogen. However, when the level of oestrogen in the body is high, equol may bind to oestrogen receptors, thus reducing the chances of oestrogen binding to its cognate receptors. This competitive binding helps reduce the overall proliferative effect of oestrogen, thereby reducing the risk of diseases related to increased levels of oestrogen in the body[7]. Oestrogen is closely associated with an increased risk of EC. In this study, equol levels were significantly reduced in the intestines of patients with EC. When the amount of oestrogen in the body is excessive, the competitive inhibition of oestrogen is limited, which may be an important cause of EC, although the specific mechanisms involved deserve further study.
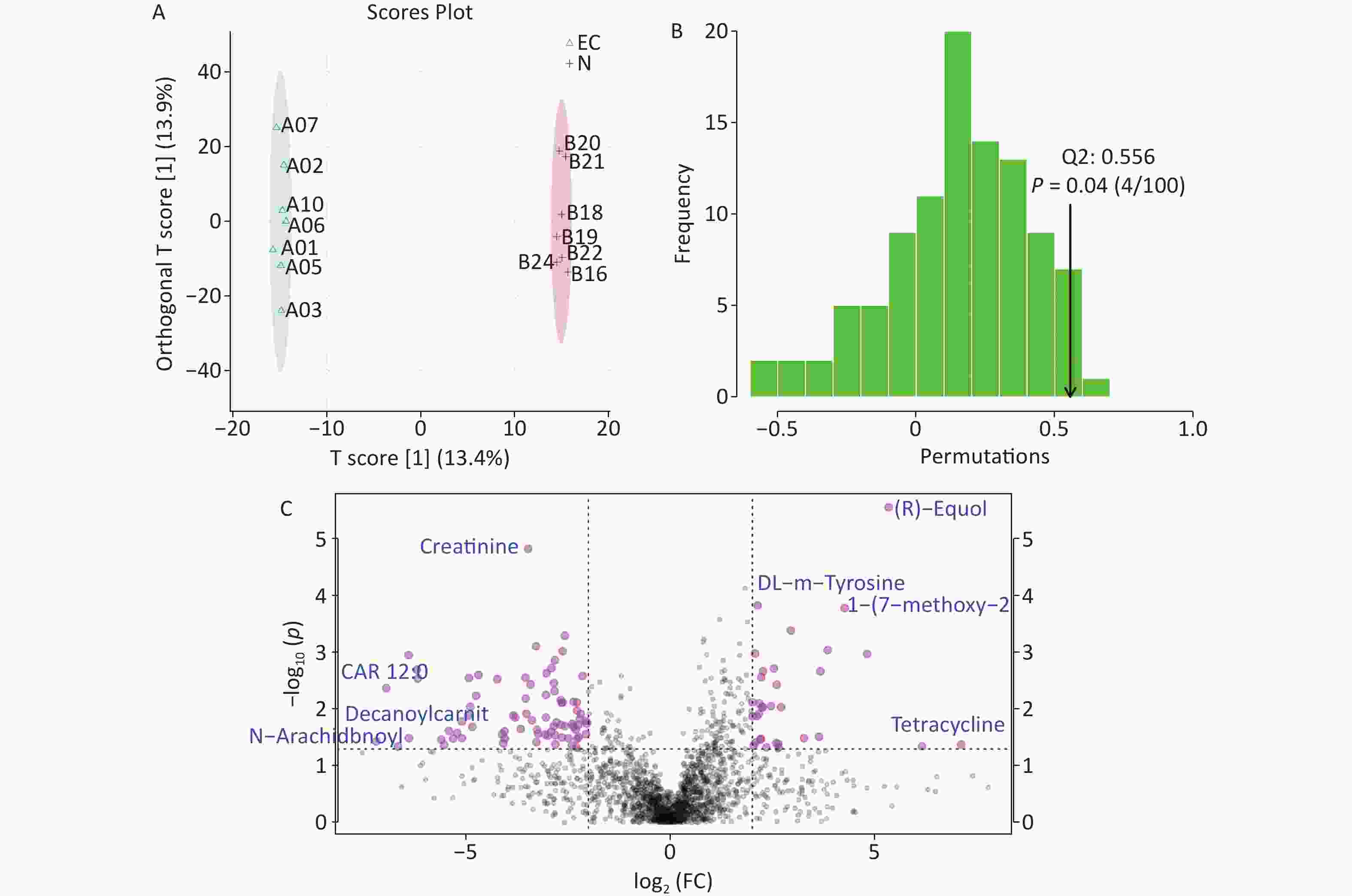
Figure 2. Analysis of different metabolites between two groups. (A) Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) score chart, (B) Distribution of the test statistic (Q2) and P-value of the OPLS-DA permutation test, (C) Multiple variation volcano map. EC, experimental group; N, control group.
Pathway enrichment analysis of different metabolites revealed that the significantly enriched metabolic pathways included the taurine and hypotaurine metabolism, pyrimidine metabolism, starch and sucrose metabolism, and other biosynthetic pathways. These metabolic pathways might play crucial roles in the biological processes investigated. Taurocholic acid is one of the key differential metabolites in the metabolic pathway of taurine and hypotaurine synthesis Bile acids that are synthesized in the liver from cholesterol can promote fat metabolism. They mainly exist in the enterohepatic circulation system and function through recycling. Taurine-conjugated bile acids, such as taurocholic acid, promote the absorption of lipids in the digestive tract thereby affecting the digestion and absorption of lipid substances in the body. Long-term metabolic disorders may lead to weight gain and obesity[8]. Obesity is a known risk factor for EC, and taurocholic acid may indirectly promote the occurrence of EC through the absorption of lipids in the digestive tract. In this study, changes in the pyrimidine metabolism pathway were noted. This pathway mainly synthesizes pyrimidine nucleotides that are key molecules in DNA replication[9]. Uncontrolled growth and abnormal proliferation are the basic characteristics of tumour cells. Pyrimidine metabolic pathway is extremely sensitive to cell proliferation and apoptosis and may play a key role in tumour progression[10]. These metabolites, on further validation can be used as markers for the non-invasive diagnosis of endometrial cancer.
In summary, regulating the composition of gut microbiota and restoring gut microbial balance may present a novel strategy for the prevention, diagnosis, and treatment of EC. Furthermore, faecal metabolites may potentially serve as non-invasive diagnostic markers for EC.
doi: 10.3967/bes2024.145
Endometrial Cancer Research Based on Gut Microbiomics and Metabolomics: An Analysis of Correlation and Differences
-
Ruifang Zhai, Sanyuan Zhang and Zhe Wang supervised the experimental design and contributed to manuscript revision. Dan Xu conducted experimental research, collected and analysed data, and wrote the paper. Peiyue Yu and Fengqin Xue assisted in experimental research and data analysis. All authors contributed to the manuscript and have read and approved the final version.
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
注释:1) Author Contributions: 2) Competing Interests: -
Figure 2. Analysis of different metabolites between two groups. (A) Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) score chart, (B) Distribution of the test statistic (Q2) and P-value of the OPLS-DA permutation test, (C) Multiple variation volcano map. EC, experimental group; N, control group.
Table 1. Statistical analysis for inter-group difference
Group 1 Group 2 Sample size Permutations pseudo-F P-value EC N 20 999 1.75 0.03 Note. EC, endometrial cancer (experimental group); N, control group. -
[1] Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature, 2009; 457, 480−4. doi: 10.1038/nature07540 [2] Lambeth SM, Carson T, Lowe J, et al. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes, 2015; 2, 1−7. [3] Long YW, Tang LH, Zhou YY, et al. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med, 2023; 21, 66. doi: 10.1186/s12916-023-02761-6 [4] Sonnenburg ED, Smits SA, Tikhonov M, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature, 2016; 529, 212−5. doi: 10.1038/nature16504 [5] Muñiz Pedrogo DA, Jensen MD, Van Dyke CT, et al. Gut microbial carbohydrate metabolism hinders weight loss in overweight adults undergoing lifestyle intervention with a volumetric diet. Mayo Clin Proc, 2018; 93, 1104−10. doi: 10.1016/j.mayocp.2018.02.019 [6] Axelson M, Setchell KDR. The excretion of lignans in rats -- evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett, 1981; 123, 337-42. [7] Rachoń D, Vortherms T, Seidlová-Wuttke D, et al. Uterotropic effects of dietary equol administration in ovariectomized Sprague-Dawley rats. Climacteric, 2007; 10, 416−26. doi: 10.1080/13697130701624757 [8] Guizoni DM, Vettorazzi JF, Carneiro EM, et al. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide, 2020; 94, 48−53. doi: 10.1016/j.niox.2019.10.008 [9] Löffler M, Fairbanks LD, Zameitat E, et al. Pyrimidine pathways in health and disease. Trends Mol Med, 2005; 11, 430−7. doi: 10.1016/j.molmed.2005.07.003 [10] Siddiqui A, Ceppi P. A non-proliferative role of pyrimidine metabolism in cancer. Mol Metab, 2020; 35, 100962. doi: 10.1016/j.molmet.2020.02.005 -




 下载:
下载:



 Quick Links
Quick Links