-
Viruses, notably airborne viruses, are difficult to collect and detect because of the low concentrations of environmental microorganisms. Bacteriophages are frequently used in air experiments as suitable surrogates for human and animal viruses[1]. Bacteriophages are non-pathogenic, so they are safe for laboratory workers and do not require specialized biological protection measures. Bacteriophages can be prepared at high titers using simple and low-cost methods. The Phi-X174 and MS2 bacteriophages have been explored as viral aerosol models[2]. Staphylococcus albus (8032), which serves as the Chinese Standard indicator microorganism for air disinfection effect evaluation, is unsuitable in representing the characteristics of airborne viruses. However, few studies have addressed the use of indicator microorganisms for viruses in air disinfection effect evaluation. In this study, we have analyzed the distribution and deposition characteristics of bacteriophage Phi-X174 and MS2 and Staphylococcus albus aerosol in a series of experiments. The objective is to develop suitable methods for bacteriophage generation and aerosol collection, and to determine the best model virus for air disinfection effect evaluation.
The bacteriophage aerosol was studied in a 20 m3 chamber [4 m (L) × 2 m (W) × 2.5 m (H)] placed in a Class II biological safety cabinet. The particle counter was placed in the chamber to measure the particle size distribution and concentration. The bacteriophage or Staphylococcus albus suspension was aerosolized using a TK-3 nebulizer at a flow rate of 8 L/min for 1 and 10 min. The bacteriophage and Staphylococcus albus concentration in the nebulizer was 108–109 PFU/mL and 103–104 CFU/mL, respectively. The bacteriophage aerosol samples were collected using an AGI-30 impinger and SKC biosampler operated at a flow rate of 10 L/min for 3 min. The impinger contained 20 mL collection medium. Three collection media were considered: phosphate-buffered saline (PBS), nutrient broth, and SM buffer. The bacteriophage was enumerated using the double-agar-layer method. The Staphylococcus albus aerosol samples were collected using a six-stage viable particulate cascade impactor at a flow rate of 28.3 L/min. The nutrient agar plate was used as the growth medium. After sampling, the plate was incubated at 36 ± 1 °C for 48 h. Each of the experiments was repeated at least three times to confirm the validity of the results. All the data were subjected to Chi-square test using the SPSS16.0 for Windows statistical package.
The liquid impinger sampler has previously been shown to efficiently sample bio-aerosol[3]. Nutrient broth, PBS, and SM buffer were used as the collection medium for the bacteriophage aerosol. The bacteriophage concentrations using different impingers and collection media are given in Supplementary Tables S1–S2. The concentration of Phi-X174 and MS2 aerosols was dependent on the collection medium at a nebulized suspension concentration of 108 PFU/mL with nebulization for 1 min. The collection efficiency of the SM buffer was greater than that of the nutrient broth and PBS. The SM buffer contains protective ingredients which may reduce bacteriophage infectivity loss during impaction or collection. The SKC biosampler was more efficient than the AGI-30 impinger at a nebulized suspension concentration of 108–109 PFU/mL and nebulization for 1 min. The SKC biosampler exhibited better efficiency than AGI-30 impinger at a low bacteriophage concentration. Taking an overview of the data, the SKC biosampler was more suitable for low concentration aerosol sampling. But the AGI sampler has the benefits of simple operation and low cost, which are important practical considerations in terms of application. So the AGI-30 impinger exhibited greater efficiency for a nebulized suspension concentration of 109 PFU/mL and a nebulization time of 10 min.
Table S1. The bacteriophage concentration in the AGI-30 impinger with different collection mediums
Nebulized suspension Nebulization time Collection medium Nutrient broth PBS SM buffer 5.7 × 108 10 4.6 × 103 4.0 × 103 5.4 × 103 1.9 × 109 1 7.0 × 102 1.8 × 103 2.0 × 103 4.7 × 109 10 2.7 × 104 8.2 × 104 1.2 × 105 Table S2. The bacteriophage concentration of collection medium (SM buffer) in different impinger
Nebulized suspension Nebulization time Collection medium AGI SKC 2.9 × 108 1 5.6 × 102 8.4 × 102 4.6 × 108 10 4.6 × 103 4.8 × 103 4.9 × 109 1 4.9 × 103 6.8 × 103 3.9 × 109 10 3.3 × 104 2.4 × 104 SM buffer was used as the collection medium and AGI-30 impinger was used as aerosol sampler for study on distribution and deposition characteristics of Phi-X174 and MS2 Bacteriophages. The bacteriophage aerosol concentration exceeded 104 PFU/mL at a nebulized suspension concentration of 109 PFU/mL with nebulization for 10 min, which is satisfactory for the evaluation of an air disinfection effect (Table 1). Nebulization time has an effect on the bacteriophage aerosol distribution. A higher initial concentration of nebulized suspension and longer nebulization time result in an increase in the bacteriophage aerosol sampled in the air chamber. There was no significant difference in the Phi-X174 and MS2 aerosol concentration at a nebulized suspension concentration of 108–109 PFU/mL with nebulization for 10 min. Minor differences were observed, caused by the sampling devices employed and experimental operation.
Table 1. The bacteriophage concentration of collection medium in the different nebulization time and nebulized suspension concentration
Nebulization time (min) Phi-X174 MS2 Nebulized suspension Collection medium Nebulized suspension Collection medium 1 2.9 × 108 5.6 × 102 2.2 × 108 3.9 × 101 1 4.9 × 109 4.9 × 103 1.9 × 109 2.0 × 103 10 5.7 × 108 5.4 × 103 4.6 × 108 4.6 × 103 10 2.4 × 109 3.3 × 104 4.1 × 109 8.5 × 104 The size distribution of the bacteriophage aerosol was monitored for a nebulized suspension concentration of 109 PFU/mL and nebulization time of 10 min. In repeated tests, there was no significant difference in the bacteriophage Phi-X174 and MS2 aerosol particle size distributions, as shown in Supplementary Table S3. With respect to size distribution, more than 97% of the bacteriophage aerosols exhibited particles smaller than 2.5 μm, and the particle size of more than 80% of the bacteriophage aerosols was less than 1.0 μm.
Table S3. The number percentage of bacteriophage aerosol in difference particle size
Particle size (μm) 0.3−0.5 0.5−1 1−2.5 2.5−5 5−10 > 10 Phi-X174 55.0% 28.0% 14.4% 2.1% 0.4% 0.2% MS2 55.5% 27.7% 14.0% 2.0% 0.5% 0.2% The concentration of bacteriophage aerosol was uniformly distributed in the four sample sites at 30 min and 60 min after aerosol generation (Figure 1). Large differences were observed in the initial bacteriophage (0 min) aerosol concentration at different sample sites. The distribution approached uniformity with extended aerosol generation time. The Phi-X174 aerosol distribution in different sample sites at 30 and 60 min after aerosol generation showed greater uniformity than MS2. The Phi-X174 and MS2 aerosol size distribution was essentially equivalent.
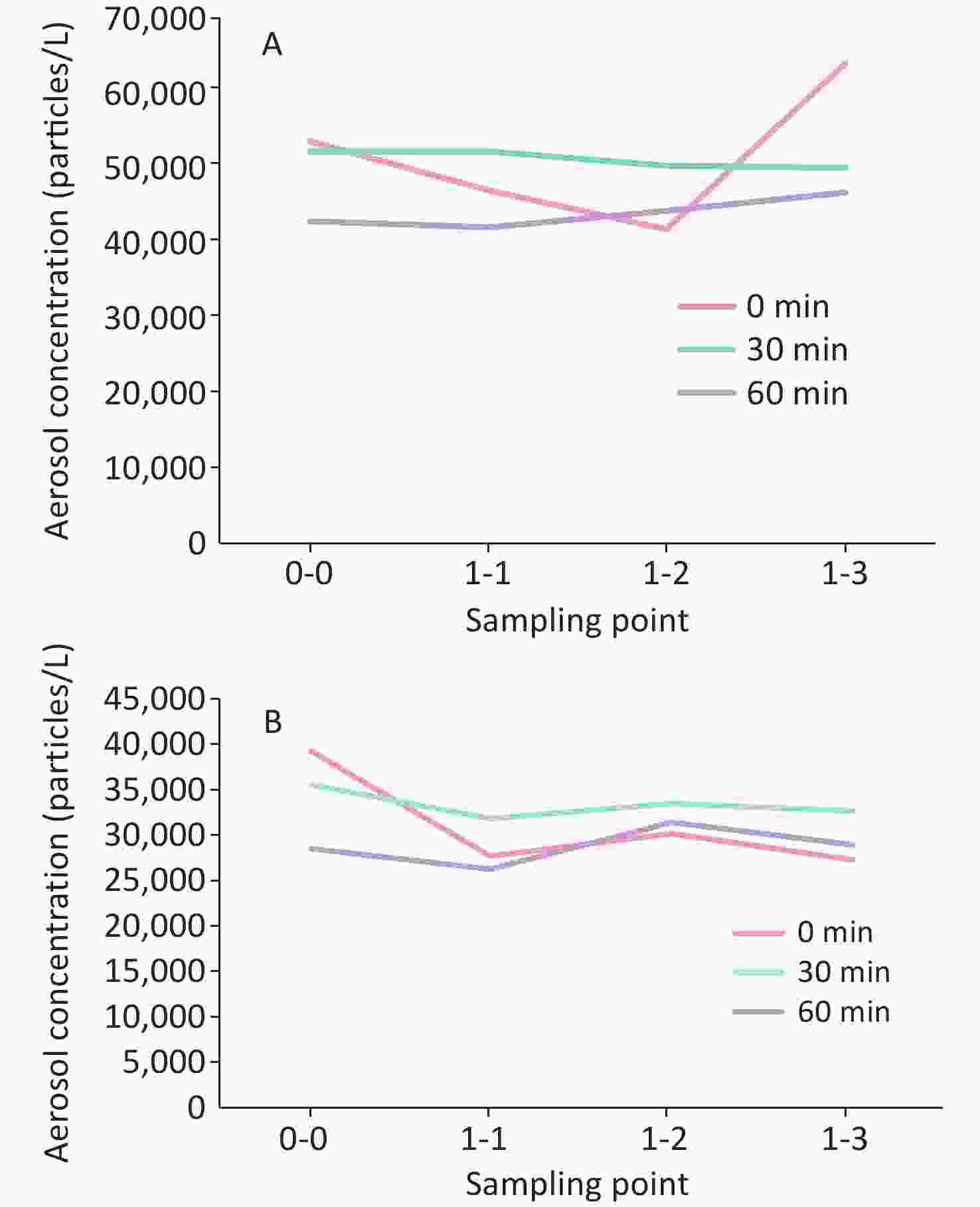
Figure 1. Bacteriophage Phi-X174(A) and MS2(B) aerosol disitribution in differenct sample sites of chamber at 0, 30 and 60 min after aerosol generation. Sampling point 0-0 was located in the center of the air chamber at floor level; sampling point 1-2 was 1 m vertically from 0-0; sampling points 1-1 and 1-3 were located 1 m horizontally from 1-2.
The deposition rate of the bacteriophage aerosol increased with an increase in the particle size (Supplementary Figure S1). The deposition rate of bacteriophage Phi-X174 aerosol was lower than that of MS2 at an initial aerosol concentration greater than 104 PFU/m3 (Figure 2). The deposition rate of the Staphylococcus albus aerosol at 60 min was more stable than observed at 30 min (Supplementary Figure S2). Some bacteriophages display structural characteristic similar to many human and animal viruses. The deposition rate is an important characteristic of a model virus[4]. The deposition rate of bacteriophage aerosol ranged from 32.1% to 98.7% when the initial concentration was lower than 104 PFU/m3. The deposition rate of the MS2 aerosol was higher than Phi-X174 when the initial concentration was lower than 104 PFU/m3 at 30 min after aerosol generation. The distribution and deposition of the bacteriophage aerosols exhibited some deviation and randomness at lower initial concentrations. The results suggest that both Phi-X174 and MS2 bacteriophages can serve as good surrogates for experimentally evaluating the disinfection of air viruses.
In China, Staphylococcus albus serves as indicator microorganism in air disinfection effect evaluation (WS/T 10009-2023). However, the current Chinese Standard is not applicable in representing the characteristics of airborne viruses. The Staphylococcus albus aerosols with a particle size smaller than 4.7 μm accounted for 80% of those analyzed where 29% of aerosols had a particle size smaller than 2.1 μm. The Staphylococcus albus aerosol particles were larger than the bacteriophage aerosols but the deposition rate of Staphylococcus albus was lower. This may be attributed to a bacteriophage resistance that was lower than bacteria[5]. The bacteriophage was inactivated during aerosolization and collection[6].
This study provides a comprehensive set of data that characterize the distribution and deposition characteristics of Phi-X174 and MS2 bacteriophages in an air chamber. The results have indicated that Phi-X174 is more suitable as a model virus for air virus disinfection effect evaluation.
doi: 10.3967/bes2025.032
Experimental Study on Distribution and Deposition Characteristics of Phi-X174 and MS2 Bacteriophages in an Air Chamber
-
None declared.
This study did not involve human or animal subjects, and thus, no ethical approval was required.
注释:1) Competing Interests: 2) Ethics: -
Figure 1. Bacteriophage Phi-X174(A) and MS2(B) aerosol disitribution in differenct sample sites of chamber at 0, 30 and 60 min after aerosol generation. Sampling point 0-0 was located in the center of the air chamber at floor level; sampling point 1-2 was 1 m vertically from 0-0; sampling points 1-1 and 1-3 were located 1 m horizontally from 1-2.
S1. The bacteriophage concentration in the AGI-30 impinger with different collection mediums
Nebulized suspension Nebulization time Collection medium Nutrient broth PBS SM buffer 5.7 × 108 10 4.6 × 103 4.0 × 103 5.4 × 103 1.9 × 109 1 7.0 × 102 1.8 × 103 2.0 × 103 4.7 × 109 10 2.7 × 104 8.2 × 104 1.2 × 105 S2. The bacteriophage concentration of collection medium (SM buffer) in different impinger
Nebulized suspension Nebulization time Collection medium AGI SKC 2.9 × 108 1 5.6 × 102 8.4 × 102 4.6 × 108 10 4.6 × 103 4.8 × 103 4.9 × 109 1 4.9 × 103 6.8 × 103 3.9 × 109 10 3.3 × 104 2.4 × 104 Table 1. The bacteriophage concentration of collection medium in the different nebulization time and nebulized suspension concentration
Nebulization time (min) Phi-X174 MS2 Nebulized suspension Collection medium Nebulized suspension Collection medium 1 2.9 × 108 5.6 × 102 2.2 × 108 3.9 × 101 1 4.9 × 109 4.9 × 103 1.9 × 109 2.0 × 103 10 5.7 × 108 5.4 × 103 4.6 × 108 4.6 × 103 10 2.4 × 109 3.3 × 104 4.1 × 109 8.5 × 104 S3. The number percentage of bacteriophage aerosol in difference particle size
Particle size (μm) 0.3−0.5 0.5−1 1−2.5 2.5−5 5−10 > 10 Phi-X174 55.0% 28.0% 14.4% 2.1% 0.4% 0.2% MS2 55.5% 27.7% 14.0% 2.0% 0.5% 0.2% -
[1] Lee JH, Wu CY, Lee CN, et al. Assessment of iodine‐treated filter media for removal and inactivation of MS2 bacteriophage aerosols. J Appl Microbiol, 2009; 107, 1912−23. [2] Turgeon N, Toulouse MJ, Martel B, et al. Comparison of five bacteriophages as models for viral aerosol studies. Appl Environ Microbiol, 2014; 80, 4242−50. [3] Appert J, Raynor PC, Abin M, et al. Influence of suspending liquid, impactor type, and substrate on size-selective sampling of MS2 and adenovirus aerosols. Aerosol Sci Technol, 2012; 46, 249−57. [4] Kindermann J, Karbiener M, Leydold SM, et al. Virus disinfection for biotechnology applications: different effectiveness on surface versus in suspension. Biologicals, 2020; 64, 1−9. [5] Suwandi T, Nursolihati V, Sundjojo M, et al. The efficacy of high-volume evacuators and extraoral vacuum aspirators in reducing aerosol and droplet in ultrasonic scaling procedures during the COVID-19 pandemic. Eur J Dent, 2022; 16, 803−8. [6] Walker CM, Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ Sci Technol, 2007; 41, 5460−5. -




 下载:
下载:



 Quick Links
Quick Links